Abstract
Background
Fenofibrate significantly reduces circulating triglyceride (TG) concentrations, particularly in individuals with elevated levels. The purpose of the current study was to determine whether fenofibrate treatment reduces markers of oxidative stress, oxidized low density lipoprotein (ox-LDL) and 8-isoprostane (8-isoP), in a manner similar to TG where those with the highest levels show the greatest reductions.
Materials/Methods
The concentrations of TG, 8-isoP, and ox-LDL were measured in plasma before and after 3 weeks of fenofibrate treatment (160 mg/d) in a sub-cohort (n=187) of the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) study.
Results
Data were divided into tertiles as determined by pre-treatment values of the respective target. Fenofibrate treatment resulted in significant reductions in TG concentrations by 24.2% (p<0.0001), 41.9% (p<0.0001), and 46.6% (p<0.0001) in tertiles 1, 2, and 3, respectively. Significant reductions were also observed in ox-LDL of 7.2% (p=0.0096), 8.5% (p=0.0019) and 12.1% (p<0.0001) in tertiles 1, 2, and 3, respectively. Finally, fenofibrate treatment resulted in a 32.7% increase (p=0.0201) in 8-isoP levels in tertile 1, but a significant decrease of 34.4% (p<0.0001) in tertile 3.
Conclusions
This study is the largest to date to demonstrate that fenofibrate reduces oxidative stress and the first to show a suppressive effect on 8-isoP levels in individuals with a high oxidative burden following short term (3 wk) drug therapy. Those with the highest baseline levels of ox-LDL and 8-isoP showed the greatest reductions following fenofibrate treatment. Given the role of oxidative stress in atherosclerosis and coronary heart disease, our observations may partially explain the efficacy of fenofibrate in reducing cardiovascular events in select patients.
Keywords: Fenofibrate, atherosclerosis, isoprostane, ox-LDL, triglyceride, GOLDN
Introduction
The fibrate class of drugs is intended as a treatment for preventing atherosclerosis and reducing coronary heart disease (CHD) (1). As with other fibrates, fenofibrate has been clinically proven to be an effective agent to both lower serum triglycerides (TG) and raise serum high density lipoprotein (HDL) (2). However, clinical trials have produced mixed results in determining whether various fibrates reduce CHD. For example, Robins et al. (3) demonstrated gemfibrozil significantly reduces cardiovascular (CV) events in the VAHIT trial which targeted patients with a relatively low HDL and elevated TG while having only modest elevations in LDL. In contrast, primary analysis of the impact of fenofibrate either alone or in the presence of statins within the FIELD and ACCORD trials failed to demonstrate a statistically significantly reduction in primary CV related endpoints. In spite of these results on primary outcomes, additional analyses of both select secondary endpoints and specific patient sub-populations identify positive effects of fibrate use (4, 5). Based on these mixed outcomes, it is probable that fibrates affect risk factors for atherosclerosis and CHD beyond TG or HDL subgroups. Of growing interest among non-traditional markers of CV risk are those related to measures of oxidative stress, specifically, oxidized low density lipoprotein (ox-LDL) and 8-isoprostane (8-isoP).
An increase in oxidative stress, indicated by elevated levels of ox-LDL and 8-isoprostane (also known as 8-epi-PGF2α, 8-iso-PGF2α or 15-isoprostane F2t), has been widely reported in both atherosclerosis and CHD (6-10). Ox-LDL is the product of the oxidative conversion of LDL and has long been considered a biomarker of oxidative stress as well as a key component in the development of atherosclerosis. In addition, ox-LDL has been shown to mediate inflammation in the sub-endothelial space (11-13), potentially contributing to inflammation observed in the progression of atherosclerosis (14). Given the evidence that ox-LDL is a marker of oxidative stress, plays a role in inflammation, and co-localizes to atherosclerotic plaques, further study is required to assess whether ox-LDL is pharmacologically targeted by existing medications intended to reduce atherosclerosis such as fenofibrate.
The prostaglandin-like 8-isoP is the stable end product of the free-radical mediated non-enzymatic conversion of arachidonic acid; as with ox-LDL, it is considered a marker of lipid peroxidation and oxidative stress. Conversely, it is not known whether 8-isoP directly contributes to oxidative stress induced cell damage or inflammation, but it has been found to localize to foam cells as well as atherosclerotic plaques (7). Though isoprostanes have been shown to decrease with statin treatment (15, 16), it is currently unknown whether fenofibrate lowers elevated 8-isoP levels. The current investigation evaluates the effects of short-term fenofibrate treatment on ox-LDL and 8-isoP levels in a sub-cohort of the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) study.
Materials and methods
2.1 Study design
This study is a sub-population of the GOLDN study. GOLDN is a multi-center study in families with 3-generation pedigree. The study is funded by NIH and has two centers, one in Minneapolis, MN and the other in Salt Lake City, UT with the majority of the population as European Caucasians. The primary purpose is to investigate the interaction of genetic and environmental factors on response to fenofibrate treatment. The detailed design of the study is described elsewhere (17).
The study participants went through an initial screening and were asked to suspend lipid lowering drugs for four weeks. Fasting baseline blood before the fenofibrate therapy was collected on two consecutive days. The treatment was given as a 28-day supply of open label 160 mg fenofibrate tablets (TriCor®, Abbott Laboratories, Chicago, IL). The tablets were taken with a breakfast meal once daily for a minimum of 21 days. After the therapy, blood samples were again collected on two consecutive days. Plasma was immediately frozen at -80° C for all samples before and after fenofibrate treatment. Fasting lipid concentrations reported are the average of the two measurements on consecutive days, either before or after fenofibrate treatment.
2.2 Study population
Two sub-populations were selected from the GOLDN cohort. Ninety-six hypertriglyceridemic individuals (>150 mg/dL) were originally selected, as fenofibrate is prescribed for its triglyceride lowering effects. Oxidized LDL was measured in this sub-cohort; 8-isoP was not measured in these individuals due to an inadequate amount of plasma. To broaden the study population in terms of baseline characteristics, an additional ninety-two participants were randomly selected and both ox-LDL and 8-isoP were measured in these participants. Written informed consent was obtained from each participant at the screening visit. The protocol was approved by the Institutional Review Boards at the University of Minnesota, the University of Utah, and Tufts University.
2.3 Biochemical assays
Cholesterol was measured using a cholesterol esterase, cholesterol oxidase reaction (Chol R1, Roche Diagnostics Corporation, Indianapolis, IN). This same reaction was used to measure HDL-C after precipitation of the non-HDL-C fractions with magnetic 50,000 MW dextran sulfate and magnesium chloride. LDL-C was measured in plasma using a homogeneous direct assay manufactured by Genzyme Diagnostics (LDL Direct Liquid Select Reagent Kit, Equal Diagnostics, Exton, PA). Triglycerides were measured using a glycerol blanked enzymatic method (Trig/GB, Roche Diagnostics Corporation, Indianapolis, IN). All were determined on the Roche/Hitachi 911 Automatic Analyzer (Roche Diagnostics Corporation).
2.4 Oxidative markers
Ox-LDL was measured in plasma using the ox-LDL ELISA enzyme immunoassay kit from Mercodia AB (Uppsala, Sweden). The ELISA is a two-site enzyme immunoassay using the direct sandwich technique where two antibodies recognize different antigenic determinants on the oxidized apolipoprotein B molecule.
Plasma 8-isoprostane was separated from other eicosanoids and interfering substances using the 8-Isoprostane Affinity Purification Kit from Cayman Chemical (Ann Arbor, MI), and measurement of 8-isoprostane was completed with the 8-Isoprostane EIA kit, also from Cayman Chemical.
2.5 Statistical analyses
The population was divided into tertiles based on baseline values of the analyzed target: TG and ox-LDL (n=187); 8-isoP (n=92). Student t test was used to determine differences between baseline and post-treatment groups, with statistical significance considered at the P<0.05 level.
Results
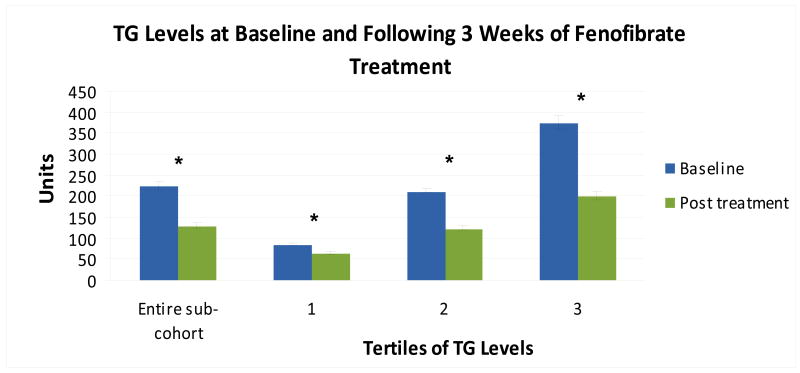
TG levels at baseline and following fenofibrate treatment are shown in Figure 1. Data from the entire sub-cohort are analyzed and also stratified by tertiles according to baseline values of each target. Fenofibrate treatment resulted in a significant 42.3% mean decrease in TG levels (p<0.0001) for all participants in this sub-cohort of GOLDN, but the size of the decreases were found to be highly correlated with baseline triglyceride levels, ranging from 24.6% in tertile 1 to 46.6% in tertile 3.
Figure 1.
TG levels at baseline and following 3 weeks of fenofibrate treatment (160 mg/d) in a subcohort of the GOLDN study (n=187). Tertiles were determined by baseline TG values. Values are expressed as mean ±SEM * P value < 0.05 for t- test comparing baseline and post-treatment values.
The effects of fenofibrate treatment on markers of oxidative stress ox-LDL and 8-isoP are shown in Figure 2. Following fenofibrate treatment, ox-LDL decreased 9.8% (p<0.0001) on average for the entire sub-cohort and decreases were observed for all tertiles of ox-LDL (Fig. 2a). Similar to triglycerides, the size of the decreases were dependent on baseline levels: a significant 7.2% mean decrease in tertile 1 (p=0.0096); a significant 8.5% mean decrease in tertile 2 (p=0.0019); and finally, a significant 12.1% mean decrease in tertile 3 (p<0.0001). For 8-isoP (Fig. 2b), all participants showed a significant 12.6% mean decrease following fenofibrate treatment. After stratifying into tertiles, individuals with the lowest baseline levels (tertile 1), showed a significant 32.7% mean increase (equal to 6.51 pg/mL) following fenofibrate treatment (p=0.0201). In contrast, a significant 34.4% mean decrease was observed in tertile 3 (p<0.0001). No significant difference was observed in tertile 2. Overall, TG levels and oxidative markers showed the greatest post-treatment reductions in individuals with the highest baseline levels.
Figure 2.
Levels of oxidative markers (a) 8-isoP (n=92) and (b) ox-LDL (n=187) at baseline and following 3 weeks of fenofibrate treatment (160 mg/d) in a subcohort of the GOLDN study. Tertiles were determined by baseline values of the respective target. Values are expressed as mean ±SEM * P value < 0.05 for t- test comparing baseline and post-treatment values.
A positive correlation was found between pre-treatment levels of ox-LDL and pre-treatment levels of LDL with a Pearson correlation coefficient of 0.653 (Fig. 3a). However, post-treatment levels of ox-LDL and LDL did not show any correlation (Fig. 3b). Similar analysis conducted on 8-isoP demonstrated no correlations between levels of 8-isoP and levels of ox-LDL (data not shown).
Figure 3.
Correlations between: a) baseline levels of ox-LDL vs. LDL; b) change in ox-LDL vs. change in LDL after treatment.
LDL particle size showed a non-significant increase of 0.452 nm following fenofibrate treatment (p=0.056, data not shown). Though significant inverse associations were observed between LDL particle size and ox-LDL levels at baseline (-0.464, p<0.0001) (Figure 3c) and following fenofibrate treatment (-0.327, p<0.0001) (Figure 3d), no significant correlation was observed between Δ LDL particle size and Δ ox-LDL levels (data not shown).
Discussion
The current investigation explores the benefits of short-term fenofibrate treatment, specifically its effects on two markers of oxidative stress, ox-LDL and 8-isoP. As expected, fenofibrate treatment significantly lowered TG and ox-LDL with the greatest reductions observed in individuals with the highest baseline values. We show for the first time that fenofibrate treatment significantly lowers 8-isoP levels, but only in individuals with the highest baseline values.
From a clinical standpoint, fenofibrate is used primarily to treat hypertriglyceridemia; ATPIII guidelines define abnormal TG as ≥ 150 mg/dL, reduced from the previous guideline of 200 mg/dL. Here we show that the magnitude of the reduction in TG is directly related to baseline TG level. In addition, we observed that fenofibrate reduces TG, 8-isoP, and ox-LDL in a comparable manner. Importantly, previous studies may have been too small to test for this effect of fenofibrate on ox-LDL or 8-isoP.
Though we hypothesized that the reduction in ox-LDL may be a result of reduced availability of LDL, we found no such relationship (Figure 3b). However, a number of alternative mechanisms by which fenofibrate may reduce ox-LDL have been proposed. Both glutathione peroxidase-1 (GPx1) and paraoxonase 1 (PON1) have been hypothesized to be the vehicles for the anti-oxidant effects of fenofibrate (18). Tkac et al. (19) showed that 12 weeks of fenofibrate treatment resulted in an 80% increase in activity of GPx1 and a corresponding decrease in ox-LDL. In addition, Phuntuwate et al. (20) showed that increases in PON1 concentration and activity were negatively correlated to the reduction in ox-LDL levels following 12 weeks of fenofibrate treatment (160 mg/d). Apart from its effects on these two enzymes, fenofibrate is known to cause an increase in LDL particle size, and larger LDL particle size may be less susceptible to oxidation. In the current study, we found an inverse association of LDL particle size with ox-LDL levels at baseline and following treatment, yet we observed no association between Δ LDL particle size and Δ ox-LDL levels. Further studies are needed to determine whether the fenofibrate-mediated reduction in ox-LDL is due in part to increased LDL particle size.
Apart from ox-LDL, we found paradoxical effects of fenofibrate on 8-isoP, depending on baseline levels. We observed that fenofibrate treatment resulted in a modest but significant increase in 8-isoP in individuals with the lowest levels (tertile 1) and a significant 34% decrease in those with the highest baseline levels (tertile 3). Previous studies found no effect of fenofibrate on 8-isoP (21, 22), but the study populations were most likely too small (n=18 and 19, respectively) to divide individuals into tertiles. Consequently, the effect of fenofibrate on 8-isoP in individuals with high baseline concentrations may have been masked in these studies. By stratifying our population into tertiles, the current study demonstrates the efficacy of fenofibrate in significantly lowering 8-isoP in those with relatively elevated baseline levels. The mechanism behind the modest increase in 8-isoP of 6.51 pg/mL for individuals with the lowest levels (tertile 1) is currently unknown. Further studies are required to understand this phenomenon and its clinical significance.
The current investigation does have a number of limitations. Though the present study shows that fenofibrate is effective in reducing oxidative stress markers in the short term, longitudinal clinical studies would better elucidate whether these changes are sustained and/or correlated with clinical outcomes. In addition, the experimental design of the GOLDN study did not include a placebo control, thus we cannot definitively conclude that all changes in ox-LDL and 8-isoP are due to fenofibrate treatment in this sub-cohort. Finally, GOLDN participant samples were not immediately assayed for 8-isoP, thus spontaneous generation or oxidation of the marker is a concern though results were within the confidence range of the assay. Nonetheless, validation of the current results may be warranted.
To our knowledge, this study is the largest to demonstrate that fenofibrate reduces oxidative stress and the first to show that the drug lowers 8-isoP levels following short term (3 week) drug therapy. Moreover, we demonstrate that fenofibrate lowers 8-isoP and ox-LDL in a manner similar to TG, where the greatest reductions are seen in those with the highest baseline values. Further studies are required to determine the mechanisms by which fenofibrate reduces 8-isoP and ox-LDL in individuals with elevated levels and whether this reduction improves outcomes in a clinical setting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keating GM, Croom KF. Fenofibrate: a review of its use in primary dyslipidaemia, the metabolic syndrome and type 2 diabetes mellitus. Drugs. 2007;67:121–153. doi: 10.2165/00003495-200767010-00013. [DOI] [PubMed] [Google Scholar]
- 2.Birjmohun RS, Hutten BA, Kastelein JJ, Stroes ES. Efficacy and safety of high-density lipoprotein cholesterol-increasing compounds: a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2005;45:185–197. doi: 10.1016/j.jacc.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Robins SJ, Collins D, Wittes JT, Papademetriou V, Deedwania PC, Schaefer EJ, McNamara JR, Kashyap ML, Hershman JM, Wexler LF, Rubins HB. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA. 2001;285:1585–1591. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 4.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesaniemi YA, Sullivan D, Hunt D, Colman P, d'Emden M, Whiting M, Ehnholm C, Laakso M. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 5.Fruchart JC, Sacks FM, Hermans MP. Implications of the ACCORD Lipid study: perspective from the Residual Risk Reduction Initiative (R(3)i) Curr Med Res Opin. 2010 doi: 10.1185/03007995.2010.489341. [DOI] [PubMed] [Google Scholar]
- 6.Nishi K, Itabe H, Uno M, Kitazato KT, Horiguchi H, Shinno K, Nagahiro S. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler Thromb Vasc Biol. 2002;22:1649–1654. doi: 10.1161/01.atv.0000033829.14012.18. [DOI] [PubMed] [Google Scholar]
- 7.Pratico D, Iuliano L, Mauriello A, Spagnoli L, Lawson JA, Rokach J, Maclouf J, Violi F, FitzGerald GA. Localization of distinct F2-isoprostanes in human atherosclerotic lesions. J Clin Invest. 1997;100:2028–2034. doi: 10.1172/JCI119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassalle C, Botto N, Andreassi MG, Berti S, Biagini A. Evidence for enhanced 8-isoprostane plasma levels, as index of oxidative stress in vivo, in patients with coronary artery disease. Coron Artery Dis. 2003;14:213–218. doi: 10.1097/01.mca.0000063504.13456.c3. [DOI] [PubMed] [Google Scholar]
- 9.Verhoye E, Langlois MR. Circulating oxidized low-density lipoprotein: a biomarker of atherosclerosis and cardiovascular risk? Clin Chem Lab Med. 2009;47:128–137. doi: 10.1515/CCLM.2009.037. [DOI] [PubMed] [Google Scholar]
- 10.Wang B, Pan J, Wang L, Zhu H, Yu R, Zou Y. Associations of plasma 8-isoprostane levels with the presence and extent of coronary stenosis in patients with coronary artery disease. Atherosclerosis. 2006;184:425–430. doi: 10.1016/j.atherosclerosis.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Daub K, Seizer P, Stellos K, Kramer BF, Bigalke B, Schaller M, Fateh-Moghadam S, Gawaz M, Lindemann S. Oxidized LDL-activated platelets induce vascular inflammation. Semin Thromb Hemost. 2010;36:146–156. doi: 10.1055/s-0030-1251498. [DOI] [PubMed] [Google Scholar]
- 12.Khan BV, Parthasarathy SS, Alexander RW, Medford RM. Modified low density lipoprotein and its constituents augment cytokine-activated vascular cell adhesion molecule-1 gene expression in human vascular endothelial cells. J Clin Invest. 1995;95:1262–1270. doi: 10.1172/JCI117776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehr HA, Olofsson AM, Carew TE, Vajkoczy P, von Andrian UH, Hubner C, Berndt MC, Steinberg D, Messmer K, Arfors KE. P-selectin mediates the interaction of circulating leukocytes with platelets and microvascular endothelium in response to oxidized lipoprotein in vivo. Lab Invest. 1994;71:380–386. [PubMed] [Google Scholar]
- 14.Fan J, Watanabe T. Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb. 2003;10:63–71. doi: 10.5551/jat.10.63. [DOI] [PubMed] [Google Scholar]
- 15.De Caterina R, Cipollone F, Filardo FP, Zimarino M, Bernini W, Lazzerini G, Bucciarelli T, Falco A, Marchesani P, Muraro R, Mezzetti A, Ciabattoni G. Low-density lipoprotein level reduction by the 3-hydroxy-3-methylglutaryl coenzyme-A inhibitor simvastatin is accompanied by a related reduction of F2-isoprostane formation in hypercholesterolemic subjects: no further effect of vitamin E. Circulation. 2002;106:2543–2549. doi: 10.1161/01.cir.0000038500.43292.d7. [DOI] [PubMed] [Google Scholar]
- 16.Desideri G, Croce G, Tucci M, Passacquale G, Broccoletti S, Valeri L, Santucci A, Ferri C. Effects of bezafibrate and simvastatin on endothelial activation and lipid peroxidation in hypercholesterolemia: evidence of different vascular protection by different lipid-lowering treatments. J Clin Endocrinol Metab. 2003;88:5341–5347. doi: 10.1210/jc.2003-030724. [DOI] [PubMed] [Google Scholar]
- 17.Lai CQ, Arnett DK, Corella D, Straka RJ, Tsai MY, Peacock JM, Adiconis X, Parnell LD, Hixson JE, Province MA, Ordovas JM. Fenofibrate effect on triglyceride and postprandial response of apolipoprotein A5 variants: the GOLDN study. Arterioscler Thromb Vasc Biol. 2007;27:1417–1425. doi: 10.1161/ATVBAHA.107.140103. [DOI] [PubMed] [Google Scholar]
- 18.Holtzman JL. Atherosclerosis and Oxidant Stress. Springer; US: 2008. The Role of Glutathione Pathways in the Prevention of Atherosclerosis; pp. 211–226. [Google Scholar]
- 19.Tkac I, Molcanyiova A, Javorsky M, Kozarova M. Fenofibrate treatment reduces circulating conjugated diene level and increases glutathione peroxidase activity. Pharmacol Res. 2006;53:261–264. doi: 10.1016/j.phrs.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Phuntuwate W, Suthisisang C, Koanantakul B, Chaloeiphap P, Mackness B, Mackness M. Effect of fenofibrate therapy on paraoxonase1 status in patients with low HDL-C levels. Atherosclerosis. 2008;196:122–128. doi: 10.1016/j.atherosclerosis.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Hodgson JM, Watts GF, Playford DA, Burke V, Croft KD. Coenzyme Q10 improves blood pressure and glycaemic control: a controlled trial in subjects with type 2 diabetes. Eur J Clin Nutr. 2002;56:1137–1142. doi: 10.1038/sj.ejcn.1601464. [DOI] [PubMed] [Google Scholar]
- 22.Hogue JC, Lamarche B, Tremblay AJ, Bergeron J, Gagné C, Couture P. Differential effect of atorvastatin and fenofibrate on plasma oxidized low-density lipoprotein, inflammation markers, and cell adhesion molecules in patients with type 2 diabetes mellitus. Metabolism. 2008 Mar;57(3):380–6. doi: 10.1016/j.metabol.2007.10.014. [DOI] [PubMed] [Google Scholar]