Abstract
Periodontitis (progressive inflammatory disease characterized by alveolar bone loss, a major cause of tooth loss worldwide) is associated with both systemic osteoporosis and its milder form, osteopenia. Tetracyclines, by virtue of their non-antimicrobial proanabolic and anti-catabolic properties, are excellent candidate pharmaceuticals to simultaneously treat these local and systemic disorders. This paper reviews the foundational basic science and translational research which lead to a pivotal multicenter randomized clinical trial in postmenopausal women with both periodontitis and systemic (skeletal) osteopenia. This trial was designed primarily to examine whether subantimicrobial dose doxycycline (SDD) could reduce progressive alveolar (oral) bone loss associated with periodontitis and, secondarily, whether SDD could reduce systemic bone loss in the same subjects. This paper describes the efficacy and safety findings from this clinical trial and also outlines future directions using this promising and novel approach to manage both oral and systemic bone loss.
Keywords: subantimicrobial dose doxycycline, osteoporosis, osteopenia, periodontitis, postmenopausal women, collagenases
1. Introduction
Almost three decades ago, tetracyclines were unexpectedly found to inhibit collagenases and other host-derived matrix metalloproteinases (MMPs) and by a mechanism independent of their antimicrobial activity [1]. As a result of this landmark discovery, it became apparent that tetracyclines could be useful therapeutically to treat a wide array of diseases in which pathologically-elevated collagenase activity and concomitant collagen degradation are the hallmark features of disease pathogenesis. A number of disease conditions modulated by tetracyclines include, but are not limited to, periodontitis [2], osteoporosis [3], rheumatoid arthritis [4], cancer invasion and metastasis [5], sterile corneal ulcers [6], abdominal aortic aneurysms [7], the inflammatory skin disease, rosacea [8], and systemic inflammation associated with cardiovascular diseases [9].
The purpose of this review is to discuss using tetracyclines in a common clinical situation: the treatment of reduced bone mass both locally (e.g., alveolar [periodontal] bone during periodontitis) and systemically (e.g., hip and spine) in postmenopausal women with osteoporosis or its milder form, osteopenia. The application of tetracyclines to simultaneously treat both osteoporosis/osteopenia and periodontitis in humans is based on a strong foundation of basic science and translational studies. This paper will review these studies and describe the effects of subantimicrobial dose doxycycline (SDD) in postmenopausal osteopenic women with periodontitis (progressive inflammatory disease characterized by alveolar bone loss, which can lead to tooth loss if untreated) in a recent multicenter, randomized National Institutes of Health-supported human clinical trial. Finally, future directions will be considered.
2. Basic science foundation
After observing that tetracyclines inhibited mammalian collagenase activity by a non-antimicrobial mechanism, Golub and his collaborators then began to study the effect of tetracyclines on bone resorption in organ culture [10], osteoclast activity in cell culture [11], and alveolar bone loss in experimental periodontitis in rats [12,13]. The rationale was that collagenase and other MMPs participate in the degradation of type I collagen, the major constituent of the organic matrix of bone, as well as the destruction of other connective tissue constituents. Our group and others found that tetracyclines not only inhibited bone loss through inhibition of osteoclast-mediated bone resorption, but also by enhancing osteoblast activity, upregulation of type I collagen expression and increased bone formation [14-16]. This increase in bone formation induced by tetracyclines was observed in animal models of low-turnover bone loss (the osteopenic diabetic rat) [14-16] and high-turnover bone loss (ovariectomized rat model of osteoporosis; see below [17]). Tetracyclines can increase bone formation via the following mechanisms: 1) increasing both steady-state levels of type I procollagen mRNA and collagen synthesis [15]; 2) partially restoring osteoblast activity and bone matrix formation and mineralization depressed during disease (diabetes) [14]; and 3) increasing the number of active osteoblasts relative to inactive osteoblasts [16].
Our group also identified a number of mechanisms by which tetracyclines can inhibit MMPs and connective tissue breakdown. As described in two reviews [12,18], tetracyclines have the ability to: 1) inhibit already-active collagenase and MMPs in the extracellular matrix, a mechanism dependent on their Ca++ and Zn++ binding properties; 2) prevent the conversion of latent pro-collagenase/pro-MMPs into active forms, an effect independent of tetracyclines’ metal-binding properties; and 3) down-regulate the expression of procollagenase/pro MMPs, which appears to be related to tetracycline inhibition of pro-inflammatory mediators such as the cytokines interleukin (IL)-1β, tumor necrosis factor (TNF)-α and IL-6, as well as phospholipase A2/prostaglandin E2 and inducible nitric oxide synthase.
One tetracycline, chemically-modified tetracycline (CMT; chemically-modified tetracyclines are tetracyclines with different side chains removed and/or added to yield non-antimicrobial MMP inhibitors )-3, but not others, can directly and indirectly inhibit another category of tissue-destructive proteinases, the serine proteinases, particularly neutrophil elastase (other tetracyclines can only indirectly inhibit serine proteinases) [12,18,19]. These additional properties of tetracyclines confer further connective tissue and bone-sparing effects. Regarding the indirect effect on elastase, MMPs can partially degrade and inactivate the endogenous serine proteinase-inhibitor, α1-antitrypsin (also known as α1-proteinase inhibitor), in serum and other biologic fluids [18,20,21]. When tetracyclines block MMPs, α1-antitrypsin is spared and is then available to inhibit pathologically-elevated elastase activity.
Thus, multiple studies demonstrated that tetracyclines, by non-antimicrobial, anti-collagenolytic mechanisms, have the therapeutic potential to improve bone mass by inhibiting bone resorption and enhancing bone formation.
3. Translational studies
Because of the enormous clinical potential of these basic and mechanistic studies on the non-antimicrobial properties of tetracyclines, a pivotal translational study in ovariectomized, osteopenic aged rats was carried out by Williams et al. [22] at the National Institute on Aging. They demonstrated both proanabolic and anti-catabolic effects of minocycline in a model of high-turnover bone loss reflective of the changes that occur soon after the menopause. In this study, minocycline treatment, starting one day post-ovariectomy and continuing throughout the eight-week experiment, mitigated the effect of ovariectomy on trabecular bone. Based on dynamic histomorphometric analytical techniques, minocycline also was found to increase osteoid surface, mineralizing surface, mineral apposition rate and bone formation rate, and reduced eroded surface measurements. The authors concluded that the increase in systemic (femoral) bone mineral density (BMD) and positive effects on trabecular bone observed in these aged rats was likely due to both an increase in bone formation and a decrease in bone resorption. In contrast, aged rats treated with 17 Beta-estradiol demonstrated a decrease in bone resorption and suprisingly, unlike minocycline treatment, a decrease in bone formation, although the effect of the female sex hormone on bone resorption exceeded the effect on bone formation, resulting in a decrease in bone loss.
Another important question to answer in animal studies prior to proceeding to human clinical trials was whether treatment with tetracyclines could ameliorate the effect of ovariectomy on both local (alveolar bone) and systemic bone loss. A study in rats by Golub et al. [17] addressed this issue, using an animal model of ovariectomy-induced, estrogen-deficiency osteoporosis. Two days after ovariectomy, both “mature” and “aged” ovariectomized rats were orally administered CMT-8 (the chemically-modified, nonantimicrobial analog of doxycycline) or vehicle alone on a daily basis. Pathologically-elevated gingival collagenase activity was seen in the ovariectomized rats and CMT-8 abrogated this effect. Ovariectomy resulted in both proximal tibial BMD loss and alveolar bone height loss compared to non-ovariectomized controls. Treatment with 2 mg/day CMT-8 by oral intubation for 90 days normalized both the alveolar bone height loss and the reduction of trabecular bone density in the tibia. This study thus demonstrated, in a classical animal model of postmenopausal osteoporosis, that a tetracycline can inhibit both oral and systemic bone loss.
4. Human clinical trials
The translational studies described above [17,22] demonstrated the following proanabolic and anticatabolic properties of tetracyclines: 1) stimulation of osteoblast activity and bone formation; 2) MMP inhibition; and 3) inhibition of bone resorption. Meanwhile, around this time, SDD was shown to be non-antimicrobial, effective and safe in a Phase III United States Food and Drug Administration (FDA) human multi-institutional, double-blind, placebo-controlled clinical trial. SDD reduced progressive periodontitis in this Phase III FDA clinical trial in a general population of chronic periodontitis subjects. As a result, SDD was approved by the FDA in 1998 to treat chronic periodontitis as an adjunct to mechanical debridement procedures (see Caton et al. in this issue) and SDD, including a recently FDA-approved, sustained-release SDD to treat the chronic inflammatory skin condition, rosacea, is the only MMP inhibitor drug approved for systemic use by the FDA to treat human diseases. Both osteoporosis and osteopenia have been associated with periodontitis [23-26], so therapeutic strategies that address both oral and systemic bone loss observed in periodontitis and osteoporosis, respectively, are desirable. These aforementioned properties made a compelling case for SDD as a novel pharmaceutical in the treatment of postmenopausal women who exhibit local, periodontal bone loss as well as systemic osteoporosis or osteopenia.
4.1 Pilot human clinical trial
As a preliminary study, we enrolled six postmenopausal osteoporotic/osteopenic women with a history of periodontitis. Three women received a cyclical SDD regimen for one year (four months “on,” four months “off,” and four months “on” drug) and three women received placebo in the same cyclical fashion adjunctive to periodontal maintenance therapy (debridement procedures to reduce the oral bacterial burden) [27]. The rationale for the cyclical regimen was based on a previous early study by Crout et al. [20] which demonstrated safety, MMP inhibition and clinical efficacy in periodontitis patients using a similar cyclical regimen. Computer-assisted densitometric image analysis (CADIA) was used to determine changes in alveolar bone density and alveolar bone height at posterior interproximal sites (Figure 1 and Figure 2). Relative clinical attachment levels (RCALs) were determined by Florida Disk Probe (Florida Probe Corporation, Gainesville, Florida) (Figure 3). RCAL is a clinical measurement of soft tissue changes and attachment levels and represents the “gold” standard for measuring periodontitis disease activity (periodontitis progression with time).
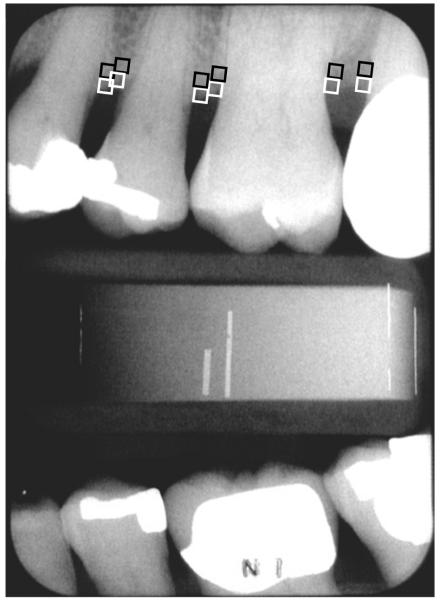
Figure 1. Representative dental radiograph showing locations for alveolar bone density measurements.
1 mm2 regions were measured at the alveolar crest (upper boxes; crestal area of interest) and immediately below the crestal area of interest (lower boxes; subcrestal area of interest) [30].
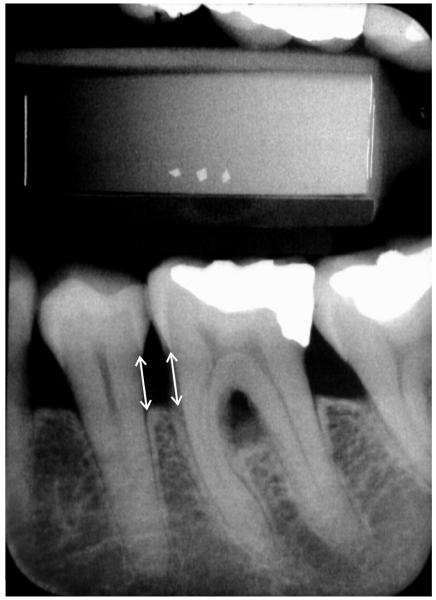
Figure 2. Representative dental radiograph showing alveolar bone height measurements.
Alveolar bone height was measured from the cemento-enamel junction to the alveolar crest at posterior interproximal sites [30]. This figure shows the alveolar bone height measurement on the mesial surface of the mandibular left first molar and the distal surface of the mandibular left second premolar.
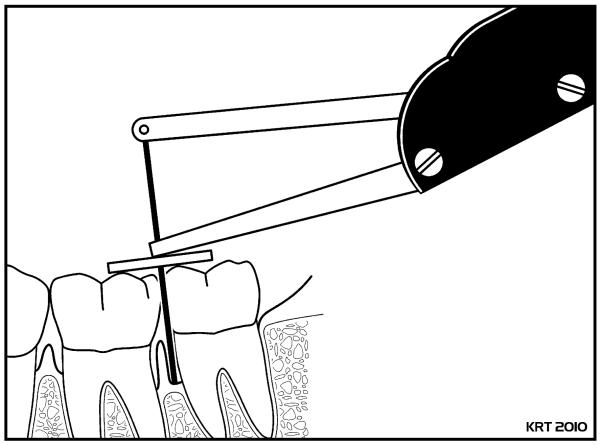
Figure 3. Diagrammatic representation of a relative clinical attachment level (RCAL) measurement on the mesial surface of a mandibular left second molar.
RCAL represents a measurement of the distance from the occlusal surface of a tooth to the base of the periodontal pocket and is recorded to the nearest 0.2 mm [29]. In the multicenter randomized SDD clinical trial in postmenopausal women with periodontitis and systemic osteopenia, RCAL measurements were made on posterior teeth (premolars and molars).
It should be recognized that periodontal disease (i.e., periodontitis) is episodic in nature [28]. Indeed, only a few percent of all clinically-detected periodontal lesions (i.e., periodontal pockets) are progressive or active at any given time; most are inactive or quiescent [29]. Based on a threshold of 0.4 mm (two times the standard deviation of replicate measurements), 6.0% of posterior interproximal sites in the placebo group lost alveolar bone height, while 1.7% of posterior interproximal sites in the SDD group lost alveolar bone height in our pilot human clinical trial. SDD also reduced alveolar bone density loss during the one-year protocol. Regarding RCAL, the placebo subjects demonstrated a greater frequency of 2.0 mm progressive RCAL loss than SDD subjects (2.8% vs. 0%, respectively) over the one-year study and a lower frequency of RCAL gain (0% vs. 2.4%, respectively). Taken together, these pilot data suggested that SDD reduced alveolar bone height loss, alveolar bone density loss, and RCAL loss and enhanced RCAL gain relative to placebo in postmenopausal women with systemic osteoporosis or osteopenia and periodontitis.
4.2 Multicenter clinical trial design: hypothesis
The basic science and translational foundation coupled with this pilot study lead us to establish a large-scale, multi-center clinical trial [30]) which was funded by the dental research division of the National Institutes of Health (National Institute of Dental and Craniofacial Research). The following hypothesis was tested: tetracyclines (namely SDD), by non-antimicrobial properties, can reduce bone loss in the periodontium (and skeletal tissues as well), improve clinical parameters of periodontitis, and reduce levels of biochemical mediators of collagen and bone breakdown in the gingival crevicular fluid (GCF; serum inflammatory exudate found in the sulcus or periodontal pocket) and serum in estrogen-deficient postmenopausal women with periodontitis and osteopenia of either the lumbar spine or femoral neck. For ethical reasons, osteoporotic subjects had to be excluded from enrollment in this trial, as they needed to be treated with conventional therapies such as bisphosphonates, selective estrogen receptor modulators or parathyroid hormone.
4.2.1. Inclusion and exclusion criteria
This hypothesis was tested in a two-center (University of Nebraska Medical Center College of Dentistry and Stony Brook University School of Dental Medicine), placebo-controlled, double-blind randomized clinical trial (RCT) of SDD (20 mg twice daily) in postmenopausal women. The following list comprises the inclusion criteria: 45-70 years of age at telephone screening; postmenopausal for at least 6 months and not receiving hormone replacement therapy; osteopenic (i.e., reduced bone mass) at the lumbar spine or femoral neck (BMD T-score between −1.0 and −2.5 inclusive, based on dual-energy x-ray absorptiometry scores); undergoing periodontal maintenance treatment for generalized moderate to advanced chronic periodontitis; willingness to provide consent; and having at least 9 posterior teeth and demonstrating evidence of periodontitis (i.e., at least two sites with probing depths 5 mm or greater, 5 mm of clinical attachment loss or greater, and bleeding on probing). Subjects were excluded if they had: an allergy or hypersensitivity to tetracyclines; diseases or received regular drug therapy that would affect the inflammatory or immune response (e.g., chronic use of non-steroidal anti-inflammatory drugs) or bone remodeling (e.g., prescription estrogens, bisphosphonates, calcitonin, and steroids); a requirement for antibiotic premedication; diabetes; active periodontal therapy within the past year; normal BMD at the lumbar spine and femoral neck (T-score above −1.0), or osteoporosis of the lumbar spine or femoral neck (T-score less than −2.5).
4.2.2. Recruitment
Subjects were recruited from Nebraska and Long Island, New York from private periodontal and general dentistry practices, from the Stony Brook University and University of Nebraska Medical Center dental school patient pools, and advertisements. The study protocol was reviewed and approved by the Institutional Review Boards from both institutions. One hundred twenty-eight eligible subjects were randomized, 75 at the University of Nebraska Medical Center College of Dentistry and 53 at the Stony Brook University School of Dental Medicine. Sixty-four subjects were randomized to each of the two treatment arms (SDD and placebo). All subjects received calcium and vitamin D supplements (1200 mg of calcium and 400 I.U. of vitamin D daily) and all subjects received periodontal maintenance (mechanical debridement) every three to four months throughout the clinical trial. Periodontal maintenance was provided by the subjects’ own dental care providers and at no cost to the subjects.
4.3. Multicenter clinical trial: oral radiographic, oral clinical and systemic bone mineral density efficacy findings
Based on overall, intent-to-treat statistical analyses, SDD did not significantly reduce alveolar bone loss (Table 1) [30]. Intent-to-treat analyses compare subject groups based on the treatment allocation, regardless of protocol deviations, subject compliance or subject withdrawal. In addition, all sites were included in the intent-to-treat statistical analyses, including both healthy sites (which would not be expected to improve with treatment) and pathologically-elevated periodontal pocket sites. Most importantly, among sites with baseline probing depths 5 mm or greater (i.e., pathologically-elevated periodontal pocket sites exhibiting moderate to advanced periodontitis), alveolar bone density loss was significantly reduced in the SDD group relative to placebo. This finding is noteworthy because this statistically-significant SDD treatment effect was observed in periodontally-diseased sites. Furthermore, among non-smokers (who comprised 80% of the 128 randomized subjects), SDD subjects experienced significantly less alveolar bone density loss than placebo subjects. Finally, reduced alveolar bone height loss was observed in SDD subjects relative to placebo among women more than 5 years postmenopausal and among subjects who adhered to the protocol.
Table 1.
Oral radiographic findings in SDD clinical trial over two years [30]
| In the overall intent-to-treat analysis, SDD did not significantly reduce alveolar bone density loss (OR = 0.84 [SDD relative to placebo], 95% CI: 0.65 to 1.08, p=0.2) or alveolar bone height loss (OR = 0.82 [SDD relative to placebo], 95% CI: 0.62 to 1.08, p=0.2) |
| In subgroup analyses: |
| SDD reduced alveolar bone density loss among sites with baseline probing depths ≥ 5 mm (difference in mean CADIA change [SDD-placebo] = 2.45 CADIA Units, 95% CI: 0.85 to 4.04, p=0.003) |
| SDD reduced alveolar bone density loss in non-smokers (difference in mean CADIA change [SDD-placebo] = 1.13 CADIA Units, 95% CI: 0.003 to 2.25, p=0.05) |
| SDD reduced the odds of more progressive alveolar bone height loss by 29% in women more than 5 years postmenopausal (OR =0.71 [SDD relative to placebo], 95% CI: 0.50 to 0.99, p=0.05) |
| SDD reduced the odds of more progressive alveolar bone height loss by 36% in per-protocol analyses (OR =0.64 [SDD relative to placebo] 95% CI: 0.43 to 0.95, p=0.03) |
Based on overall, intent-to-treat analyses, SDD reduced progressive clinical attachment loss (a soft tissue measure of periodontitis activity or progression with time, as opposed to non-active or non-progressive periodontal pockets) in this study population (Table 2) [29]. In addition, among non-smokers and protocol-adherent subjects, SDD reduced bleeding on probing. This reduction in soft tissue inflammation is consistent with the anti-inflammatory properties of tetracyclines, which have been shown to reduce IL-1 [31], IL-6 [32], TNF-α [33], and MMP activity [18]. Finally, among subjects not taking significant concomitant medications, SDD reduced the odds of more progressive periodontal disease based on probing depth, another soft tissue measure of periodontitis.
Table 2.
Oral clinical findings in SDD clinical trial [29]
| In the overall intent-to-treat analysis, SDD reduced the odds of more progressive periodontitis over two years by 19% based on attachment level (gold standard clinical measurement for periodontitis) (OR=0.81 [SDD relative to placebo], 95% CI: 0.67 to 0.97, p=0.03) |
| In subgroup analyses: |
| SDD reduced the odds of bleeding on probing over two years by 30% in non- smokers (OR = 0.70 [SDD relative to placebo], 95% CI: 0.50 to 1.00, p = 0.05) |
| SDD reduced the odds of bleeding on probing over two years by 34% in protocol- adherent subjects (OR = 0.66 [SDD relative to placebo], 95% CI: 0.44 to 1.00, p = 0.05) |
| Among subjects not taking concomitant medications, SDD reduced the odds of more progressive periodontal disease based on probing depth at two years by 43% (OR = 0.57 [SDD relative to placebo], 95% CI: 0.34 to 0.97, p = 0.04) |
There was no difference between placebo and SDD groups with respect to systemic BMD loss over the two-year clinical trial [30]. However, the BMD loss in both SDD-treated and placebo-treated groups was on the order of tenths of a percent per year, lower than expected. This minimal bone loss in each group is likely related to two phenomena: 1) subjects had systemic osteopenia, which constitutes reduced bone mass but not pathologically-reduced bone mass associated with osteoporosis; and 2) each group was treated with an optimum dose of calcium and vitamin D. Adequate calcium intake reduces bone loss in postmenopausal women [34]. It is likely that osteoporotic subjects would have experienced increased local (alveolar) and systemic (lumbar spine and femur) bone loss relative to osteopenic subjects over the two-year period; thus, there likely would have been greater disease activity to treat pharmaceutically.
4.4. Multicenter clinical trial: efficacy findings in GCF (local biological fluid)
Regarding biochemical findings in the GCF, SDD significantly reduced GCF collagenase activity and MMP-8 (collagenase-2) over the two-year protocol based on intent-to-treat analyses (Table 3) [35]. Previous studies had demonstrated short-term reductions (i.e., several weeks to several months) in GCF collagenase activity by SDD. However, our clinical trial showed the most prolonged (i.e., two-year) reduction in GCF collagenase activity by SDD ever reported in the scientific literature. Second, SDD effects on GCF collagenase activity were positively and statistically significantly associated (p<0.001), at all time points (see Table 3), with SDD effects on C-telopeptide to helix (ICTP), a pyridinoline-crosslink-containing degradation fragment of the C-terminal telopeptide region of type I collagen and a biomarker of bone resorption. Third, these effects were observed in subjects manifesting both local bone loss (periodontitis) and systemic bone loss (osteopenia); previous studies included general periodontitis populations and did not examine specific vulnerable subjects like postmenopausal, estrogen-deficient women. We also found that SDD decreased IL-1β, a pro-inflammatory cytokine and a biomarker associated with bone resorption, in women more than 5 years postmenopausal; in this same subgroup, SDD also reduced alveolar bone height loss. The biochemical effect of SDD thus corresponded well to the clinical effect.
Table 3.
GCF findings in SDD clinical trial over two years [35]
| In the overall intent-to-treat analysis, SDD reduced median GCF collagenase activity by 22% compared to placebo (95% CI: 37% lower to 5% lower, p=0.01) |
| In the overall intent-to-treat sample, SDD reduced median GCF ICTP levels by 16% compared to placebo (p=0.08). However, when 3 extreme baseline values were deleted (2 values in the placebo group and 1 in the SDD group), SDD significantly reduced GCF ICTP by 19% compared to placebo (95% CI: 33% lower to 2% lower, p=0.03). |
| In the overall intent-to-treat sample, SDD significantly reduced the odds of elevated MMP-8 values (relative to placebo subjects) by 60% (OR=0.40, 95% CI: 0.21 to 0.77, p=0.006). |
| Collagenase activity and ICTP in the GCF were significantly and linearly related with positive correlation coefficients (r = 0.62, 0.52 and 0.50 for baseline, 1-year and 2-year time periods, respectively and all three r values were highly statistically significant [p < 0.001]). In general, the higher the values for GCF collagenase activity, the greater the level of bone collagen breakdown products (ICTP). |
| In subgroup analyses: |
| SDD reduced median IL-1β levels by 51% among subjects more than 5 years postmenopausal (OR=0.49; 95% CI: 76% lower to 1% lower; p=0.05). |
Indeed, the oral radiographic findings observed in the alveolar bone and the soft tissue findings in the periodontal pockets corresponded extremely well to the biochemical findings observed in the GCF of these postmenopausal women. Regarding alveolar bone, since type I collagen constitutes 90% of the organic bone matrix, reduction in total collagenase activity and, specifically, a reduction in MMP-8, the predominant collagenase found in GCF which accounts for approximately 80% of total GCF collagenase activity, offers a compelling explanation for the diminished alveolar bone loss observed in subgroups of postmenopausal women. Furthermore, SDD’s reduction of GCF ICTP, which is believed to reflect, at least in part, degradation of the triple helical collagen molecule in bone matrix, represents another potential mechanism of alveolar bone preservation. The decrease in periodontitis progression based on soft tissue measurements (attachment levels) can be explained on the basis of collagen stabilization in the periodontal pocket, thereby resulting in reduced periodontal probe penetration into the gingival connective tissue and a gain in attachment level. Likewise, the reduction in bleeding on probing observed in subgroups can be explained by the anti-inflammatory properties of SDD as well as the reduction of collagenase activity, which results in less collagen degradation, more intact collagen, and increased resistance to periodontal probe penetration into the gingival connective tissue. The less than-complete reduction in GCF collagenase activity and MMP-8 (produced mainly by neutrophils) is desirable [36], as MMPs play important physiological roles in connective tissue turnover and in the host response to periodontal infection by processing growth factors and anti-inflammatory cytokines. The reduction in collagenase activity observed likely represents a diminution in pathologically-elevated levels of this family of enzymes, with preservation of collagenase activity for physiological functions.
4.5. Multicenter clinical trial: efficacy findings in serum (systemic biological fluid)
Consistent with the finding observed in GCF, SDD also significantly reduced the serum bone resorption biomarker ICTP (in subgroups of patients; Table 4) [37]. In postmenopausal women within 5 years of menopause and in women not taking significant concomitant medications (e.g., non-steroidal anti-inflammatory drugs or medications which affect bone metabolism), SDD significantly reduced ICTP and marginally reduced C-telopeptide cross-link of type I collagen (CTX), a deoxypyridinoline-containing degradation fragment of the C-terminal telopeptide region of type I collagen. To our knowledge, ours is the first clinical trial to examine the effects of SDD on biomarkers of systemic (as opposed to only local) bone turnover. Although SDD significantly reduced a serum biomarker of bone resorption in subgroups, SDD did not have a significant effect in this clinical trial on serum levels of bone-specific alkaline phosphatase (BSAP; biomarker of bone formation) and osteocalcin (biomarker of bone turnover). Had the subjects been osteoporotic or had a more potent tetracycline been utilized (see future directions below), it is possible that SDD would have had a proanabolic effect in serum (i.e., increased serum BSAP). SDD’s potential to increase the rate of bone formation, as measured in GCF in humans, has been suggested by Golub et al. [35,38].
Table 4.
Serum findings in SDD clinical trial [37]
| In the overall, intent-to-treat analysis, changes in serum biomarkers of bone resorption (ICTP and CTX), bone formation and turnover (bone-specific alkaline phosphatase and osteocalcin, respectively) were not significantly different between SDD and placebo. |
| In subgroup analyses: |
| In subjects who were postmenopausal ≤ 5 years at the baseline visit, SDD treatment significantly reduced serum ICTP (difference in mean values [SDD minus placebo] = −0.46 ng/ml, 95% CI: −0.71 to −0.21 ng/ml, p=0.0003) and marginally reduced serum CTX (ratio of median values [SDD:placebo], 0.71, 95% CI: 0.50 to 1.02, p=0.06) over two years. |
| In postmenopausal subjects not on significant concomitant medications, SDD treatment significantly reduced serum ICTP (difference in mean values [SDD minus placebo] = −0.30 ng/ml, 95% CI: −0.60 to 0.0045 ng/ml, p=0.05) and marginally reduced serum CTX (ratio of median values [SDD:placebo], 0.75, 95% CI: 0.56 to 1.01, p=0.06) at the two-year time point. |
Regarding mechanisms for inhibiting bone resorption, the first-reported mechanism for non-antimicrobial tetracycline effects was the ability of tetracyclines to block collagenase and other MMPs by osteoblasts, which are involved in degrading the osteoid required for osteoclasts to be attracted to the underlying calcified bone. SDD also may reduce bone resorption by inhibiting the expression by osteoblasts of pro-inflammatory cytokines, IL-1βand IL-6 [39], thereby decreasing the recruitment of osteoclasts to resorb bone. Finally, SDD may inhibit the expression of proinflammatory cytokine-induced Receptor Activator for Nuclear Factor κB Ligand (RANKL) by T-lymphocytes and B-lymphocytes [40], reducing the availability of RANKL to bind RANK on osteoclast precursors, thereby decreasing osteoclast differentiation and bone resorption [41].
Although seemingly unrelated, C-reactive protein (CRP), a biomarker of systemic inflammation and risk factor for cardiovascular disease [42], has been found recently [43] to reflect susceptibility to skeletal bone-deficiency disease (i.e., postmenopausal osteoporosis). Kim et al. [43] reported that urinary N-telopeptide cross-link of type I collagen, NTX, was positively correlated with serum high-sensitivity-CRP levels and also reported that women with higher serum concentrations of high-sensitivity-CRP had lower BMD. In our clinical trial, SDD significantly reduced median high-sensitivity-CRP (p=0.02) over two years compared to placebo [44] in this group of postmenopausal osteopenic women; this beneficial reduction of CRP is potentially important in this cohort, as postmenopausal women have been reported to be at increased risk for cardiovascular disease [45]. Based on the relationship between CRP and susceptibility to skeletal bone-deficiency disease, SDD’s reduction of CRP, a systemic biomarker of inflammation and an acute-phase reactant, may also, in the long-term, reduce bone turnover and prevent BMD loss.
4.6 Multicenter clinical trial: safety
Regarding safety, adverse event experiences did not differ significantly between SDD and placebo groups with the exception that significantly fewer SDD subjects experienced dermatological adverse events (e.g., rosacea, acne, rash) (Table 5) [30] during the course of the two-year clinical trial, which is consistent with clinical trials showing efficacy of SDD in patients with rosacea [8,46] and acne [47]. In addition, based on culture and sensitivity oral microbiological analyses [48], SDD did not exert any detectible effect on total anaerobic counts and total counts for actinomyces and streptococci (considered to be part of the normal subgingival flora). SDD did not result in the colonization or overgrowth by periodontal organisms or opportunistic organisms. Finally, there was no evidence of emergence of resistance in bacterial species originally found to be susceptible (in either the SDD or placebo groups), and there was no evidence of development of cross-antibiotic or multi-antibiotic resistance (other antibiotics tested included amoxicillin, erythromycin, clindamycin, minocycline and tetracycline).
Table 5.
| Overall, adverse event experiences were similar between SDD and placebo subjects. However, significantly fewer SDD subjects experienced a dermatologic adverse event (e.g., acne, itchy skin, rash and rosacea) at some time during the clinical trial compared to placebo subjects (2% versus 17%, p = 0.002). |
| No significant antimicrobial effect on the subgingival oral flora was detected following two-year SDD treatment relative to baseline or compared to placebo. |
5. Future directions and conclusions
The two-year clinical trial described above demonstrated beneficial clinical therapeutic effects of SDD in postmenopausal women exhibiting both periodontitis (local bone loss) and osteopenia (systemic bone loss) that are consistent with the biochemical findings in their biologic fluids, GCF and serum. In this long-term clinical trial, SDD was clearly anti-resorptive, as SDD reduced alveolar bone height loss and alveolar bone density loss (in subgroups) [30] and reduced serum ICTP and CTX, biomarkers of bone resorption (also in subgroups) [37]. Furthermore, SDD reduced periodontitis progression (in the entire intent-to-treat population), as measured by clinical attachment levels over the 2-year time period, and decreased gingival inflammation, as measured by bleeding on probing, in subgroups [29]. Finally, SDD reduced GCF collagenase activity and, specifically, MMP-8 (collagenase-2) in the entire study population over two years [35]. This collagen stabilization and reduction in pathologically-excessive collagenase activity likely contributed to diminished periodontitis progression by reducing periodontal probe penetration (a diagnostic measure of disease severity) into the gingival connective tissue. This effect is important as continued progression of the periodontal lesion can ultimately lead to tooth loss; furthermore, periodontitis has been associated with increased risk for medical diseases such as coronary artery disease [49].
One disadvantage of the SDD formulation is the required twice-daily dosing regimen. A once daily formulation would result in better patient compliance. In this regard, doxycycline is now available (approved by the United States FDA in 2006) as a 40 mg sustained-release, once-daily dose to treat the chronic inflammatory skin disease, rosacea [46]; this formulation has similar pharmacokinetics to SDD (e.g., similar peak blood levels) with the added advantage of once-daily dosing. As a third formulation of a non-antimicrobial tetracycline, although not yet approved as a prescription drug, once-daily administration of a chemically-modified tetracycline, CMT-3, also has significant therapeutic potential to treat osteoporotic bone loss. CMT-3 has a long half-life and is highly lipophilic.
CMT-3 (6-demethyl-6-deoxy-4-dedimethylaminotetracycline) is the only chemically-modified, non-antimicrobial analog of this antibiotic ever administered to humans. Studies involving in vitro, cell culture, and in vivo animal models of connective tissue-destructive, including bone-deficiency diseases, have generally indicated that CMT-3 is more potent than most other CMTs or doxycycline (an exception is the non-antimicrobial analog of doxycycline, CMT-8, which is discussed below). As examples, CMT-3 has been found to be a more potent inhibitor of: (i) MMP activity with lower IC50 (the concentration of the compound required to inhibit 50% of the enzyme/proteinase activity) than doxycycline and many CMTs; (ii) proinflammatory cytokine production in cell culture; and (iii) alveolar bone loss and gingival tissue MMPs in rat models of type I diabetes and P. gingivalis-and endotoxin-induced periodontal breakdown [18,19,50]. In part, at least, this relatively strong efficacy in vivo reflects superior pharmacokinetics of CMT-3 in rats and humans including a long serum half-life after oral administration [51-52]. Based on these characteristics, and other characteristics of efficacy, CMT-3 has been tested in phase I and phase II clinical trials in cancer patients [52-53]. In a study on patients with Kaposi’s sarcoma, Dezube et al. [53] reported that 50 mg of CMT-3 administered orally once daily resulted in a 41% therapeutic response rate based on serial tumor assessments; these patients showed partial and, in some cases, a complete resolution of these lesions assessed by skin biopsy. Also, patients receiving CMT-3 showed a statistically significant reduction in the plasma biomarkers MMP-2 and MMP-9.
A similar non-antimicrobial chemically-modified compound, CMT-8, also showed superior in vitro evidence of MMP inhibition and potent efficacy in vivo. As described earlier in this paper, Golub et al [17] reported that oral administration of this compound in a standard animal model of post-menopausal osteoporosis, the ovariectomized aged rat, dramatically reduced the severity of skeletal (tibia) bone density loss as well as reduced alveolar (periodontal) bone loss; these bone changes paralleled the reduction in activity of gingival MMPs, collagenase and gelatinase, indicating decreased severity of periodontal disease. Although CMT-8, like CMT-3, has also demonstrated impressive pharmacokinetics in the rat when administered orally, this compound (unlike CMT-3) has not been tested in human subjects.
A concern about the CMTs as potential therapeutic agents, with greater efficacy than SDD, is the side-effect of photosensitivity, already seen in clinical trials testing CMT-3. A significant incidence of sunburn has been seen in humans administered relatively high oral doses, 50-150 mg/day [52-53]. In contrast, in a preliminary study on subjects administered a much lower oral dose of CMT-3, 10 mg/day, this phenomenon was not observed, yet the data indicated a reduction in biomarkers of collagen degradation and bone resorption (MMP-8 and IL-1β levels) in the GCF of lesions (pockets) in patients with periodontitis [54]. However, much more study is necessary to determine the therapeutic potential of this substantially lower dose of CMT-3 for both local (periodontal) and systemic bone-deficiency (and other) diseases.
With these concerns in mind, newer compounds which are NOT tetracycline molecules, but which are designed to incorporate a putative active site on the tetracycline and CMT compounds (i.e., the β-diketone, metal ion [calcium and zinc]-binding site at carbon-11 and carbon-12), are now being tested [55]. These new compounds, called the “PEZBINs” (poly-enolic/phenolic zinc-binding molecules), are showing evidence of efficacy: (i) in vitro, as inhibitors of the proteinase activity of MMPs including MMP-8 (collagenase-2), MMP-13 (collagenase-3), MMP-2 (gelatinase A), and MMP-9 (gelatinase B); (ii) in cell culture as inhibitors of the production of proinflammatory cytokines including IL-1β, TNF-α, IL-6, monocyte chemotactic protein-1, vascular endothelial growth factor; and (iii) in vivo in the diabetic rat model as inhibitors of MMPs, cytokines, and alveolar bone loss (and, perhaps, skeletal bone loss as well; currently under investigation).
In spite of these novel approaches, it should be remembered that the already FDA-approved SDD formulations for treatment of periodontitis and for the chronic inflammatory skin disease, rosacea, have shown a very high level of safety even when administered over a long period of time [30, 48]. Moreover, as recently described by Golub et al. [37], this 2-year regimen of SDD reduced the levels of bone resorption biomarkers, ICTP and CTX, in the serum of subgroups of post-menopausal women with osteopenia (lumbar spine and/or femoral neck) and periodontal bone loss with no detrimental effect on biomarkers of bone formation. These data suggest the potential of this safe medication to reduce the risk of conversion of bone loss associated with osteopenia into the more serious bone deficiency disease, osteoporosis. Furthermore, in this patient cohort from the two-year SDD clinical trial, we have found, for the first time, that increases in serum bone formation, resorption and turnover biomarkers, consistent with high-turnover bone loss in postmenopausal women, were associated with not only systemic bone loss, but alveolar bone loss as well, suggesting that oral (periodontal) and systemic bone loss are linked [56-57]. Thus, it is recommended that future clinical trials continue to target simultaneous treatment of oral (periodontal) and systemic bone loss with MMP-inhibitor compounds in periodontitis subjects with either osteopenia or osteoporosis.
Acknowledgements
The human multicenter clinical trial described in this review was supported by Grant Number R01DE012872 from the National Institute of Dental & Craniofacial Research (Dr. Jeffrey B. Payne, PI and Dr. Lorne M. Golub, Co-PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental & Craniofacial Research or the National Institutes of Health. SDD and placebo tablets were provided by CollaGenex Pharmaceuticals, Inc. (Newtown, PA).
Abbreviations
- BMD
bone mineral density
- BSAP
bone-specific alkaline phosphatase
- CMT
chemically-modified tetracycline
- CADIA
computer-assisted densitometric image analysis
- CRP
C-reactive protein
- CTX
C-telopeptide cross-link of type I collagen
- ICTP
C-telopeptide to helix cross-linked fragment of type I collagen
- FDA
Food and Drug Administration
- GCF
gingival crevicular fluid
- IL
interleukin
- MMP
matrix metalloproteinase
- NTX
N-telopeptide cross-link of type I collagen
- RCT
randomized clinical trial
- RANKL
Receptor Activator for Nuclear Factor κB Ligand
- RCAL
relative clinical attachment level
- SDD
subantimicrobial dose doxycycline
- TNF
tumor necrosis factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Golub LM, Lee HM, Lehrer G, Nemiroff A, McNamara TF, Kaplan R, et al. Minocycline reduces gingival collagenolytic activity during diabetes: Preliminary observations and a proposed new mechanism of action. J Periodontal Res. 1983;18:516–26. doi: 10.1111/j.1600-0765.1983.tb00388.x. [DOI] [PubMed] [Google Scholar]
- [2].Caton JG, Ciancio SG, Blieden TM, Bradshaw M, Crout RJ, Hefti AF, et al. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol. 2000;71:521–32. doi: 10.1902/jop.2000.71.4.521. [DOI] [PubMed] [Google Scholar]
- [3].Payne JB, Reinhardt RA. Potential application of low-dose doxycycline to treat periodontitis in postmenopausal women. Adv Dent Res. 1998;12:166–9. doi: 10.1177/08959374980120011401. [DOI] [PubMed] [Google Scholar]
- [4].O’Dell JR, Elliott JR, Mallek JA, Mikuls TR, Weaver CA, Glickstein S, et al. Treatment of early seropositive rheumatoid arthritis: doxycycline plus methotrexate versus methotrexate alone. Arthritis Rheum. 2006;54:621–7. doi: 10.1002/art.21620. [DOI] [PubMed] [Google Scholar]
- [5].Lokeshwar BL, Selzer MG, Zhu BQ, Block NL, Golub LM. Inhibition of cell proliferation, invasion, tumor growth and metastasis by an oral non-antimicrobial tetracycline analog (COL-3) in a metastatic prostate cancer model. Int J Cancer. 2002;98:297–309. doi: 10.1002/ijc.10168. [DOI] [PubMed] [Google Scholar]
- [6].Golub LM, Suomalainen K, Sorsa T. Host modulation with tetracyclines and their chemically-modified analogues. Curr Opin Dent. 1992;2:80–90. [PubMed] [Google Scholar]
- [7].Xiong W, Knispel RA, Dietz HC, Ramirez F, Baxter BT. Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome. J Vasc Surg. 2008;47:166–72. doi: 10.1016/j.jvs.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sanchez J, Somolinos AL, Almodovar PI, Webster G, Bradshaw M, Powala C. A randomized, double-blind, placebo-controlled trial of the combined effect of doxycycline hyclate 20-mg tablets and metronidazole 0.75% topical lotion in the treatment of rosacea. J Am Acad Dermatol. 2005;53:791–7. doi: 10.1016/j.jaad.2005.04.069. [DOI] [PubMed] [Google Scholar]
- [9].Brown DL, Desai KK, Vakili BA, Nouneh C, Lee HM, Golub LM. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler Thromb Vasc Biol. 2004;24:733–8. doi: 10.1161/01.ATV.0000121571.78696.dc. [DOI] [PubMed] [Google Scholar]
- [10].Gomes BC, Golub LM, Ramamurthy NS. Tetracyclines inhibit parathyroid hormone-induced bone resorption in organ culture. Experientia. 1984;40:1273–5. doi: 10.1007/BF01946671. [DOI] [PubMed] [Google Scholar]
- [11].Sasaki T, Ohyori N, Debari K, Ramamurthy NS, Golub LM. Effects of chemically modified tetracycline, CMT-8, on bone loss and osteoclast structure and function in osteoporotic states. Ann NY Acad Sci. 1999;878:347–60. doi: 10.1111/j.1749-6632.1999.tb07694.x. [DOI] [PubMed] [Google Scholar]
- [12].Golub LM, Evans RT, McNamara TF, Lee HM, Ramamurthy NS. A non-antimicrobial tetracycline inhibits gingival matrix metalloproteinases and bone loss in Porphyromonas gingivalis–induced periodontitis in rats. Ann NY Acad Sci. 1994;732:96–111. doi: 10.1111/j.1749-6632.1994.tb24728.x. [DOI] [PubMed] [Google Scholar]
- [13].Ramamurthy NS, Rifkin BR, Greenwald RA, Xu JW, Liu Y, Turner G, et al. Inhibition of matrix metalloproteinase-mediated periodontal bone loss in rats: a comparison of 6 chemically modified tetracyclines. J Periodontol. 2002;73:726–34. doi: 10.1902/jop.2002.73.7.726. [DOI] [PubMed] [Google Scholar]
- [14].Bain S, Ramamurthy NS, Impeduglia T, Scolman S, Golub LM, Rubin C. Tetracycline prevents cancellous bone loss and maintains near-normal rates of bone formation in streptozotocin diabetic rats. Bone. 1997;21:147–53. doi: 10.1016/s8756-3282(97)00104-x. [DOI] [PubMed] [Google Scholar]
- [15].Craig RG, Yu Z, Xu L, Barr R, Ramamurthy N, Boland J, et al. A chemically-modified tetracycline inhibits streptozotocin-induced diabetic depression of skin collagen synthesis and steady-state type I procollagen mRNA. Biochim Biophys Acta. 1998;1402:250–60. doi: 10.1016/s0167-4889(98)00008-1. [DOI] [PubMed] [Google Scholar]
- [16].Sasaki T, Ramamurthy NS, Golub LM. Tetracycline administration increases collagen synthesis in osteoblasts of streptozotocin-induced diabetic rats: a quantitative autoradiographic study. Calcif Tissue Int. 1992;50:411–19. doi: 10.1007/BF00296771. [DOI] [PubMed] [Google Scholar]
- [17].Golub LM, Ramamurthy NS, Llavaneras A, Ryan ME, Lee HM, Liu Y, et al. A chemically modified nonantimicrobial tetracycline (CMT-8) inhibits gingival matrix metalloproteinases, periodontal breakdown, and extra-oral bone loss in ovariectomized rats. Ann NY Acad Sci. 1999;878:290–310. doi: 10.1111/j.1749-6632.1999.tb07691.x. [DOI] [PubMed] [Google Scholar]
- [18].Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- [19].Nieman GF, Zerler BR. A role for the anti-inflammatory properties of tetracyclines in the prevention of acute lung injury. Curr Med Chem. 2001;8:317–25. doi: 10.2174/0929867013373570. [DOI] [PubMed] [Google Scholar]
- [20].Crout RJ, Lee HM, Schroeder K, Crout H, Ramamurthy NS, Wiener M, et al. The “cyclic” regimen of low-dose doxycycline for adult periodontitis: a preliminary study. J Periodontol. 1996;67:506–14. doi: 10.1902/jop.1996.67.5.506. [DOI] [PubMed] [Google Scholar]
- [21].Lee HM, Golub LM, Chan D, Leung M, Schroeder K, Wolff M, et al. alpha 1-Proteinase inhibitor in gingival crevicular fluid of humans with adult periodontitis: serpinolytic inhibition by doxycycline. J Periodontal Res. 1997;32:9–19. doi: 10.1111/j.1600-0765.1997.tb01377.x. [DOI] [PubMed] [Google Scholar]
- [22].Williams S, Wakisaka A, Zeng QQ, Barnes J, Martin G, Wechter WJ, et al. Minocycline prevents the decrease in BMD and trabecular bone in ovariectomized aged rats. Bone. 1996;19:637–44. doi: 10.1016/s8756-3282(96)00302-x. [DOI] [PubMed] [Google Scholar]
- [23].Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int. 1999;10:34–40. doi: 10.1007/s001980050191. [DOI] [PubMed] [Google Scholar]
- [24].Reinhardt RA, Payne JB, Maze CA, Patil KD, Gallagher SJ, Mattson JS. Influence of estrogen and osteopenia/osteoporosis on clinical periodontitis in postmenopausal women. J Periodontol. 1999;70:823–8. doi: 10.1902/jop.1999.70.8.823. [DOI] [PubMed] [Google Scholar]
- [25].Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol. 2000;71:1492–8. doi: 10.1902/jop.2000.71.9.1492. [DOI] [PubMed] [Google Scholar]
- [26].Inagaki K, Kurosu Y, Yoshinari N, Noguchi T, Krall EA, Garcia RI. Efficacy of periodontal disease and tooth loss to screen for low bone mineral density in Japanese women. Calcif Tissue Int. 2005;77:9–14. doi: 10.1007/s00223-004-0275-x. [DOI] [PubMed] [Google Scholar]
- [27].Payne JB, Reinhardt RA, Nummikoski PV, Golub LM. Doxycycline effects on oral bone loss in postmenopausal women. J Dent Res. 2001;80 Spec Iss A. abstract 159. [Google Scholar]
- [28].Goodson JM, Tanner AC, Haffajee AD, Sornberger GC, Socransky SS. Patterns of progression and regression of advanced destructive periodontal disease. J Clin Periodontol. 1982;9:472–81. doi: 10.1111/j.1600-051x.1982.tb02108.x. [DOI] [PubMed] [Google Scholar]
- [29].Reinhardt RA, Stoner JA, Golub LM, Wolff MS, Lee HM, Meinberg TA, et al. Efficacy of sub-antimicrobial dose doxycycline in postmenopausal women: clinical outcomes. J Clin Periodontol. 2007;34:768–75. doi: 10.1111/j.1600-051X.2007.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Payne JB, Stoner JA, Nummikoski PV, Reinhardt RA, Goren AD, Wolff MS, et al. Subantimicrobial dose doxycycline effects on alveolar bone loss in post-menopausal women. J Clin Periodontol. 2007;34:776–87. doi: 10.1111/j.1600-051X.2007.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Solomon A, Rosenblatt M, Li DQ, Liu Z, Monroy D, Ji Z, et al. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Invest Ophthalmol Vis Sci. 2000;41:2544–57. [PubMed] [Google Scholar]
- [32].Kirkwood K, Martin T, Andreadis ST, Kim YJ. Chemically modified tetracyclines selectively inhibit IL-6 expression in osteoblasts by decreasing mRNA stability. Biochem Pharmacol. 2003;66:1809–19. doi: 10.1016/s0006-2952(03)00450-7. [DOI] [PubMed] [Google Scholar]
- [33].Gu Y, Lee HM, Sorsa T, Simon SR, Golub LM. Doxycycline inhibits mononuclear cell-mediated connective tissue breakdown. FEMS Immunol Med Microbiol. 2010;58:218–25. doi: 10.1111/j.1574-695X.2009.00625.x. [DOI] [PubMed] [Google Scholar]
- [34].North American Menopause Society The role of calcium in peri- and postmenopausal women: 2006 position statement of the North American Menopause Society. Menopause. 2006;13:862–77. doi: 10.1097/01.gme.0000243566.25205.0b. [DOI] [PubMed] [Google Scholar]
- [35].Golub LM, Lee HM, Stoner JA, Sorsa T, Reinhardt RA, Wolff MS, et al. Subantimicrobial-dose doxycycline modulates gingival crevicular fluid biomarkers of periodontitis in postmenopausal osteopenic women. J Periodontol. 2008;79:1409–18. doi: 10.1902/jop.2008.070623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sorsa T, Golub LM. Is the excessive inhibition of matrix metalloproteinases (MMPs) by potent synthetic MMP inhibitors (MMPIs) desirable in periodontitis and other inflammatory diseases? That is: ‘Leaky’ MMPIs vs. excessively efficient drugs. Oral Dis. 2005;11:408–9. doi: 10.1111/j.1601-0825.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- [37].Golub LM, Lee HM, Stoner JA, Reinhardt RA, Sorsa T, Goren AD, et al. Doxycycline effects on serum bone biomarkers in postmenopausal women. J Dent Res. 2010;89:644–9. doi: 10.1177/0022034510363367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Golub LM, Lee HM, Greenwald RA, Ryan ME, Sorsa T, Salo T, et al. A matrix metalloproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflamm Res. 1997;46:310–9. doi: 10.1007/s000110050193. [DOI] [PubMed] [Google Scholar]
- [39].Kirkwood KL, Golub LM, Bradford PG. Non-antimicrobial and antimicrobial tetracyclines inhibit IL-6 expression in murine osteoblasts. Ann NY Acad Sci. 1999;878:667–70. doi: 10.1111/j.1749-6632.1999.tb07757.x. [DOI] [PubMed] [Google Scholar]
- [40].Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, et al. B and T lymphocytes are the primary source of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006;169:987–98. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- [42].Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- [43].Kim BJ, Yu YM, Kim EN, Chung YE, Koh JM, Kim GS. Relationship between serum hsCRP concentration and biochemical bone turnover markers in healthy pre- and postmenopausal women. Clin Endocrinol. 2007;67:152–8. doi: 10.1111/j.1365-2265.2007.02853.x. [DOI] [PubMed] [Google Scholar]
- [44].Payne JB, Stoner JA, Lee HM, Golub LM. Subantimicrobial-dose-doxycycline effects on serum inflammatory biomarkers in postmenopausal-osteopenic-women with periodontitis. J Dent Res. 2009;88 Spec Iss A. abstract number 2916. [Google Scholar]
- [45].Rees M, Stevenson J. Primary prevention of coronary heart disease in women. Menopause Int. 2008;14:40–5. doi: 10.1258/mi.2007.007037. [DOI] [PubMed] [Google Scholar]
- [46].McKeage K, Deeks ED. Doxycycline 40 mg capsules (30 mg immediate-release/10 mg delayed-release beads): anti-inflammatory dose in rosacea. Am J Clin Dermatol. 2010;11:217–22. doi: 10.2165/11204850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [47].Skidmore R, Kovach R, Walker C, Thomas J, Bradshaw M, Leyden J, et al. Effects of subantimicrobial-dose doxycycline in the treatment of moderate acne. Arch Dermatol. 2003;139:459–64. doi: 10.1001/archderm.139.4.459. [DOI] [PubMed] [Google Scholar]
- [48].Walker C, Puumala S, Golub LM, Stoner JA, Reinhardt RA, Lee HM, et al. Subantimicrobial dose doxycycline effects on osteopenic bone loss: microbiologic results. J Periodontol. 2007;78:1590–1601. doi: 10.1902/jop.2007.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Renvert S, Ohlsson O, Pettersson T, Persson GR. Periodontitis a future risk for acute coronary syndrome? A follow up study over three years. J Periodontol. 2010 doi: 10.1902/jop.2010.090105. E pub ahead of print. [DOI] [PubMed] [Google Scholar]
- [50].Golub LM, Ramamurthy NS, McNamara TF, Greenwald RA, Rifkin BR. Tetracyclines inhibit connective tissue breakdown: new therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med. 1991;2:297–321. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- [51].Liu Y, Ramamurthy N, Marecek J, Lee HM, Chen JL, Ryan ME, et al. The lipophilicity, pharmacokinetics, and cellular uptake of different chemically-modified tetracyclines (CMTs) Curr Med Chem. 2001;8:243–52. doi: 10.2174/0929867013373525. [DOI] [PubMed] [Google Scholar]
- [52].Rudek MA, Figg WD, Dyer V, Dahut W, Turner ML, Steinberg SM, et al. Phase I clinical trial of oral COL-3, a matrix-metalloproteinase inhibitor, in patients with refractory metastatic cancer. J Clin Oncol. 2001;19:584–92. doi: 10.1200/JCO.2001.19.2.584. [DOI] [PubMed] [Google Scholar]
- [53].Dezube BJ, Krown SE, Lee JY, Bauer KS, Aboulafia DM. Randomized phase II trial of matrix metalloproteinase inhibitor COL-3 in AIDS-related Kaposi’s sarcoma: an AIDS malignancy consortium study. J Clin Oncol. 2006;24:1389–94. doi: 10.1200/JCO.2005.04.2614. [DOI] [PubMed] [Google Scholar]
- [54].Ryan ME, Lee HM, Tenzler R, Carnu OI, Eftekhari S, Dhami A, et al. Effects of short-term COL-3 on local biomarkers of periodontitis. J Dent Res. 2008;87 Spec Iss A. abstract number 0040. [Google Scholar]
- [55].Golub LM, Lee HM, Zhang Y, London L, Grewal J, Grewal S, et al. New collagenase (MMP) inhibitors for periodontitis and other diseases. J Dent Res. 2010;89 Spec Iss B. abstract number 1182. [Google Scholar]
- [56].Payne JB, Stoner JA, Lee HM, Nummikoski PV, Reinhardt RA, Valente R, Golub LM. Serum bone-resorption biomarkers: correlation with alveolar and systemic bone changes. J Dent Res. 2008;87 Spec Iss B. abstract number 1558. [Google Scholar]
- [57].Payne JB, Stoner JA, Lee HM, Nummikoski PV, Reinhardt RA, Golub LM. Association between serum-bone-formation/bone-turnover biomarkers and alveolar and systemic bone loss. J Dent Res. 2010;89 Spec Iss A. abstract number 1267. [Google Scholar]