Abstract
Previous investigations of the influence of paranoia on facial affect recognition in schizophrenia have been inconclusive as some studies demonstrate better performance for paranoid relative to non-paranoid patients and others show that paranoid patients display greater impairments. These studies have been limited by small sample sizes and inconsistencies in the criteria used to define groups. Here, we utilized an established emotion recognition task and a large sample to examine differential performance in emotion recognition ability between patients who were actively paranoid (AP) and those who were not actively paranoid (NAP). Accuracy and error patterns on the Penn Emotion Recognition test (ER40) were examined in 132 patients (64 NAP and 68 AP). Groups were defined based on the presence of paranoid ideation at the time of testing rather than diagnostic subtype. AP and NAP patients did not differ in overall task accuracy; however, an emotion by group interaction indicated that AP patients were significantly worse than NAP patients at correctly labeling neutral faces. A comparison of error patterns on neutral stimuli revealed that the groups differed only in misattributions of anger expressions, with AP patients being significantly more likely to misidentify a neutral expression as angry. The present findings suggest that paranoia is associated with a tendency to over attribute threat to ambiguous stimuli and also lend support to emerging hypotheses of amygdala hyperactivation as a potential neural mechanism for paranoid ideation.
Keywords: Paranoia, facial emotion recognition, amygdala, social cognition
1. Introduction
Emotion recognition impairments and their relationship to social and functional outcome are well established in schizophrenia (Couture et al. 2006; Kohler et al. 2009; Pinkham et al. 2007). It remains unclear however whether the degree of these impairments may differ between symptom based subgroups and specifically between patients who experience prominent paranoid symptoms and those who do not. Previous investigations of the influence of paranoia on facial affect recognition in schizophrenia have provided conflicting results. Some studies support an advantage for paranoid over non-paranoid patients (Chan et al. 2008; Davis and Gibson 2000; Lewis and Garver 1995; Phillips et al. 1999; Van't Wout et al. 2007), while others show the opposite pattern (An et al. 2006; Russell et al. 2007; Williams et al. 2007). Kline and colleagues (1992) note that this pattern may differ based on emotion, as their work showed that paranoid patients were more accurate for negative emotions but that groups did not differ in correctly labeling positive emotions.
These discrepancies may be partially explained by the fact that all of the above studies utilized relatively small samples (generally fewer than 20 individuals per group); however, methodological differences between studies also deserve consideration. Perhaps most importantly, many previous studies have defined the paranoid and non-paranoid subgroups based on diagnostic subtype rather than symptom ratings at the time of testing (An et al. 2006; Chan et al. 2008; Davis and Gibson 2000; Lewis and Garver 1995; Van't Wout et al. 2007). It is important to note that under DSM-IV-TR, patients do not require persecutory or paranoid delusions to be diagnosed with this subtype. Therefore, much of the previous work may not have examined paranoia per se. Additionally, the range of emotions studied and the tasks used to measure emotion recognition ability have varied widely between studies.
The potential role of paranoia in emotion recognition is also raised by several neuroimaging studies showing differences in amygdala functioning between individuals with prominent paranoid symptoms at the time of scanning and those without. The amygdala is thought to play a key role in emotion perception (Adolphs 2002; Gur et al. 2002; Loughead et al. 2008; Vuilleumier and Pourtois 2007) and has also been linked to the processing of threat (Ohman 2005) and complex social judgments (Adolphs et al. 1998; Winston et al. 2002). fMRI studies indicate that paranoid patients generally show reduced amygdala activation as compared to non-paranoid patients (Phillips et al. 1999; Pinkham, Hopfinger, Pelphrey et al. 2008; Pinkham, Hopfinger, Ruparel et al. 2008; Russell et al. 2007; Williams et al. 2004; Williams et al. 2007), a finding that would seem to contradict behavioral reports of increased recognition accuracy for paranoid patients. Of note, however, each of these imaging studies has assigned individuals to groups based on clinical ratings of paranoia as opposed to diagnostic subtype, and these studies have largely used tasks of implicit emotion recognition (e.g. asking participants to identify the gender of an emotional face) rather than the explicit identification of emotion utilized in behavioral tasks. While differences in task demands may partially explain the unexpected result of reduced amygdala activation in paranoid patients, tasks of explicit emotion recognition administered after scanning do show greater impairments in paranoid patients (Russell et al. 2007; Williams et al. 2004; Williams et al. 2007), suggesting that reduced amygdala activation may indeed be related to poorer performance.
In an effort to complement the neuroimaging findings, and to address the discrepancies in previous behavioral studies, we utilized an established emotion recognition task and a large sample to assess facial affect recognition abilities in patients with schizophrenia who were actively paranoid and those who were not actively paranoid. Groups were defined based on the presence of paranoid ideation at the time of testing rather than diagnostic subtype. Based on the above-mentioned findings of reduced amygdala activation in paranoid patients, we hypothesized that actively paranoid patients would be impaired relative to patients who were not actively paranoid in emotion identification accuracy.
2. Method
2.1 Participants
Archival data from 132 individuals with schizophrenia were utilized for the present investigation. All individuals were volunteers at the Schizophrenia Research Center of the University of Pennsylvania Medical Center who had provided written informed consent to participate in studies that were approved by the University of Pennsylvania ethics review board. Diagnoses were confirmed with the Diagnostic Interview for Genetics Studies (DIGS; (Nurnberger et al., 1994) and medical history information. Only patients receiving a diagnosis of schizophrenia were included in the current sample.
Symptom severity at the time of testing was assessed with the Scale for Assessment of Negative Symptoms (SANS, 25 items; Andreasen 1984a) and the Scale for Assessment of Positive Symptoms (SAPS, 34 items; Andreasen 1984b). For both scales, interviewers specify the severity of symptoms by assigning a rating between 0 and 5, with higher ratings indicating greater severity. Ratings on the persecutory delusions item of the SAPS were used to divide patients into two groups: actively paranoid (AP) and not actively paranoid (NAP). Individuals scoring 2 or greater, indicating the presence of paranoid ideation, were included in the AP group (N=68), and individuals scoring 0, indicating absence of paranoid ideation, constituted the NAP group (N=64). No individuals received a score of 1, which would indicate questionable levels of paranoia. Groups did not significantly differ in gender (χ2=.004, p=.95), ethnicity (χ2=1.64, p=.44), age (t(130)=.64, p=.53), education (t(130)=1.44, p=.15) or parental education (maternal education: t(121)=1.16, p=.25 and paternal education: t(115)=1.51, p=.14). AP patients did show greater severity of negative (t(130)=2.22, p=.028) and positive symptoms (t(130)=7.45, p<.001) as indexed by the sum of SANS and SAPS global ratings. The difference in positive symptoms remained even when omitting global ratings on the delusion subscale (t(130)=4.67, p<.001). Finally, mean Chlorpromazine equivalent dose (Woods 2003) did not significantly differ between groups (t(130)=.54, p=.59). Participant demographic information is provided in Table 1.
Table 1.
Participant Demographic Information
| AP (n=68) | NAP (n=64) | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Gender | ||
| Male | 45 | 42 |
| Female | 23 | 22 |
| Ethnicity | ||
| Caucasian | 32 | 31 |
| African American | 33 | 28 |
| Asian | 2 | 5 |
| Age | 35.24 (10.49) | 33.98 (12.06) |
| Education | 12.69 (2.21) | 13.28 (2.49) |
| Maternal Education | 13.00 (3.11) | 13.61 (2.69) |
| Paternal Education | 12.95 (3.94) | 14.02 (3.74) |
| SANS | 7.62 (4.18) | 5.94 (4.52) |
| SAPS | 6.94 (3.35) | 2.64 (3.28) |
| SAPS (omitting delusion subscale) | 4.03 (2.72) | 1.92 (2.46) |
| Chlorpromazine Equivalent | 454.10 (418.68) | 492.42 (404.02) |
Abbreviations: AP=actively paranoid, NAP=not actively paranoid
Note: Ethnicity information was missing for one patient in the AP group. Maternal education was missing for 6 individuals in the AP group and 3 in the NAP group. Paternal education was missing for 9 and 6 individuals in the AP and NAP groups, respectively. SANS and SAPS scores are presented as the sum of the global scores with 0–20 as the range of possible scores on both scales.
2.2 Measure
The Penn Emotion Recognition Task (ER40; Kohler et al. 2005) is comprised of 40 photographs of actors expressing one of four basic emotions (happiness, sadness, anger, fear) or a neutral expression. Photos are presented in a random order, and participants are asked to identify the emotion expressed by each face. Stimuli are displayed until the participant makes a response, and both the response and response time are recorded. The ER-40 demonstrates sound convergent and discriminant validity and adequate test-retest reliability (r=.80)(Carter et al. 2009). Actor identity does not repeat, and the task is counterbalanced for actor sex.
2.3 Statistical Analyses
Group differences in emotion recognition accuracy and response time were assessed using two repeated measures Analyses of Variance (ANOVA) with emotion (happiness, sadness, anger, fear and neutral) as the within-subjects factor and group (AP vs. NAP) as the between-subjects factor. Significant main effects and interactions were probed with follow-up univariate tests, and where Mauchly’s test indicated that the assumption of sphericity had been violated, Greenhouse-Geisser corrections were utilized. A logarithmic transformation was applied to response time to normalize the distribution of these data. Finally, given that groups differed in severity of negative and positive symptoms, the sum of global SANS and SAPS ratings (excluding the delusion subscale) were used as covariates in all analyses.
3. Results
3.1 Primary Analyses
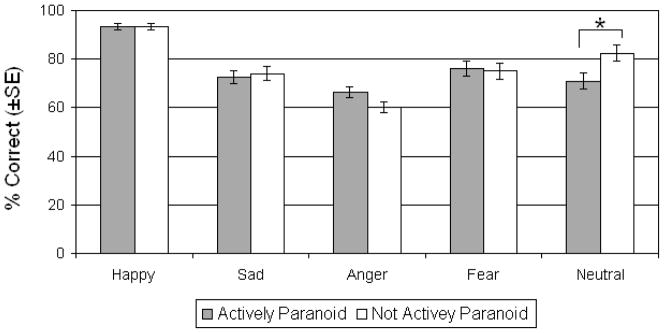
Contrary to our prediction that AP patients would be impaired relative to NAP patients on emotion recognition accuracy, the main effect of group was not significant (F(1,128)=.26, p=.61, ηp2=.002) indicating that the two patient groups did not differ overall. There was however a main effect of emotion (F(3.16,404.43)=9.12, p<.001, ηp2=.066) such that accuracy for happy was better than all other emotions (p<.001 for all comparisons) and accuracy for anger was worse than all other emotions (p<.001 for all comparisons). More importantly, the group by emotion interaction was also significant (F(3.16,404.43)=3.27, p=.019, ηp2=.025). Follow-up univariate tests demonstrated that this interaction was driven by a significant difference between groups only for the accurate identification of neutral expressions (F(1,128)=5.80, p=.017, ηp2=.043) in which NAP patients out performed AP patients (Figure 1).
Figure 1.
Emotion Recognition Accuracy. Mean (±SE) percent correct for actively paranoid and non-paranoid patients with schizophrenia. Asterisks denote significant group differences at p<.05.
The analysis of response time revealed a main effect of emotion (F(4,125)=11.75, p<.001, ηp2=.27) indicating that participants were slowest in responding to fear (p<.001 for all comparisons) and fastest in responding to happy expressions (p<.001 for all comparisons). The main effect of group was not significant (F(1,128)=.34, p=.56, ηp2=.003), nor was the group by emotion interaction (F(4,125)=.66, p=.62, ηp2=.021). Response time data are provided in Table 2.
Table 2.
Emotion Recognition Response Time (seconds)
| AP (n=68) | NAP (n=64) | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Happy | 3.32 (2.06) | 2.73 (1.29) |
| Sad | 3.92 (2.18) | 3.76 (1.89) |
| Anger | 4.55 (2.77) | 4.08 (1.89) |
| Fear | 5.04 (4.12) | 3.99 (2.20) |
| Neutral | 4.15 (2.39) | 3.74 (3.01) |
Abbreviations: AP=actively paranoid, NAP=not actively paranoid
3.2 Post-hoc Analyses
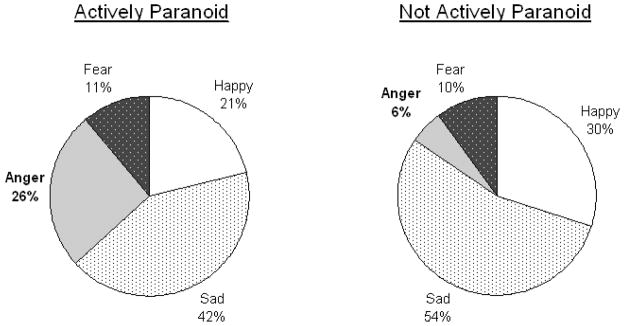
To explore the group difference in correctly labeling neutral expressions, we conducted a post-hoc analysis of error patterns on neutral stimuli. A 2 (group: AP vs. NAP) x 4 (error type: angry vs. fear vs. happy vs. sad) repeated measures ANOVA conducted on incorrect responses to neutral faces revealed only a significant group by error type interaction (F(2.38,202.2)=2.881, p=.049, ηp2=.033). Follow-up univariate tests indicated that AP patients were significantly more likely than NAP patients to identify a neutral face as angry (F(1,85)=12.05, p=.001, ηp2=.124). Groups did not differ on any other type of error (Figure 2).
Figure 2.
Error Patterns for Neutral Expressions. Mean percentage of incorrect responses to neutral expressions that were wrongly labeled happy, sad, anger, or fear. Bold labels indicate significant differences between groups at p<.01.
4. Discussion
The current study assessed potential differences in emotion recognition ability between actively paranoid and non-paranoid patients with schizophrenia. Patients who were paranoid at the time of testing, relative to those who were not, showed a specific impairment in the ability to accurately identify neutral facial expressions. An analysis of error patterns revealed that this impairment was due to a greater tendency for AP patients to incorrectly attribute anger to neutral faces. These findings are consistent with previous behavioral studies showing an emotion recognition advantage for non-paranoid patients (An et al. 2006; Russell et al. 2007; Williams et al. 2007) and clarify this work by demonstrating that impairments in AP patients are limited to neutral expressions. The over attribution of anger to neutral expressions is also consistent with work suggesting that delusional patients misattribute salience to neutral stimuli (Holt et al. 2006) and hypotheses that paranoid ideation is related to heightened perception of threat, particularly in ambiguous situations (Green and Phillips 2004; Phillips et al. 2000). This latter finding may have important implications for the day-to-day social functioning of individuals with paranoia. Viewed within the framework of Couture and colleagues (2006), early misperceptions of facial emotion can lead to a cascade of negative attributions and resulting negative social interactions. Thus, it is possible that the tendency for AP patients to over attribute anger could play an important role in social impairment.
The present findings may also be informative for understanding findings of differential amygdala abnormalities in paranoid and non-paranoid patients. As noted above, several studies have demonstrated that paranoid patients show hypoactivation of the amygdala as compared to non-paranoid patients. Given that the amygdala is implicated in threat perception and that paranoia can be conceptualized as continually perceiving environmental threat, this finding seems somewhat counterintuitive. It is important to note, however, that our interpretations of these neuroimaging findings are limited by the fact that the vast majority of analyses of blood oxygenation level dependent (BOLD) imaging rely on a contrast between activation occurring during a specific event and activation that occurs during non-events, or a baseline. This renders it difficult to determine if reduced activations in paranoid patients reflect true reductions in neural responses to stimuli or differences in baseline activity that influence the BOLD estimate. It is therefore possible that paranoid individuals may actually show hyperactivation of the amygdala that results in a high baseline and subsequent ceiling effect that limit the signal change detected by BOLD techniques.
Two recent studies, one using BOLD imaging (Hall et al. 2008) and the other using PET imaging (Fernandez-Egea et al. 2010), provide support for the hypothesis that individuals with schizophrenia show increased amygdala responses. While these studies did not specifically examine paranoid and non-paranoid subgroups, Hall and colleagues reported that patients did not differ from controls in amygdala responses to fearful faces but that they did show abnormally greater amygdala activation in response to neutral faces. Similarly, using PET imaging, which does not follow the same potential limitations as BOLD imaging, Fernandez-Egea et al. found that patients showed a non-specific hyperactivation of the amygdala as compared to control participants. Consistent with the proposal of Aleman and Kahn (2005) that high tonic levels of amygdala activation may result in assigning significance to neutral stimuli, over activation of the amygdala in paranoia would offer a potential causal mechanism for the results of the present study. Additionally, when considering the limitations of most current BOLD imaging analyses, generalized amygdala hyperactivation in paranoia would help explain previous imaging findings of reduced amygdala activation in this group. Further work is needed to substantiate these hypotheses; however, such work could be meaningful as several pharmacological agents show promise for attenuating amygdala responses during emotion processing (Pinkham et al., 2007).
While this study offers new information about emotion recognition impairments in actively paranoid and non-paranoid individuals with schizophrenia, some limitations should be considered. First, potential group differences in cognitive ability were not assessed. Given that the impairments seen in the AP group were specific to neutral faces and did not generalize to all emotions, it is unlikely that cognitive impairments account for the present results; however, this possibility cannot be fully ruled out. Second, the present investigation assessed recognition for only the basic emotional expressions of happiness, sadness, anger, fear and neutral. It is possible that additional group differences would have emerged if more complex emotional displays were included. Finally, the AP group was significantly more symptomatic than the NAP group suggesting a greater level of psychopathology. While we did statistically control for this difference in our analyses and groups did not differ in response time or overall task accuracy, it is possible that an additional third factor related to symptom severity may have contributed to the results. Notwithstanding these limitations, the present study highlights the utility of considering symptom-based subgroups in efforts to understand social cognitive impairments in schizophrenia and lends support to work showing behavioral and neural differences between paranoid and non-paranoid patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12(2):169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393(6684):470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Aleman A, Kahn RS. Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Prog Neurobiol. 2005;77(5):283–298. doi: 10.1016/j.pneurobio.2005.11.005. [DOI] [PubMed] [Google Scholar]
- An SK, Lee E, Kim JJ, Namkoong K, Kang JI, Jeon JH, et al. Greater impairment in negative emotion evaluation ability in patients with paranoid schizophrenia. Yonsei Med J. 2006;47(3):343–353. doi: 10.3349/ymj.2006.47.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1984a. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1984b. [Google Scholar]
- Carter CS, Barch DM, Gur R, Gur R, Pinkham A, Ochsner K. CNTRICS final task selection: social cognitive and affective neuroscience-based measures. Schizophr Bull. 2009;35(1):153–162. doi: 10.1093/schbul/sbn157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Wong R, Wang K, Lee TM. Emotion recognition in Chinese people with schizophrenia. Psychiatry Res. 2008;157(1–3):67–76. doi: 10.1016/j.psychres.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(Suppl 1):S44–63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PJ, Gibson MG. Recognition of posed and genuine facial expressions of emotion in paranoid and nonparanoid schizophrenia. J Abnorm Psychol. 2000;109(3):445–450. [PubMed] [Google Scholar]
- Fernandez-Egea E, Parellada E, Lomena F, Falcon C, Pavia J, Mane A, et al. 18FDG PET study of amygdalar activity during facial emotion recognition in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260(1):69–76. doi: 10.1007/s00406-009-0020-6. [DOI] [PubMed] [Google Scholar]
- Green MJ, Phillips ML. Social threat perception and the evolution of paranoia. Neurosci Biobehav Rev. 2004;28(3):333–342. doi: 10.1016/j.neubiorev.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, et al. Brain activation during facial emotion processing. Neuroimage. 2002;16(3 Pt 1):651–662. doi: 10.1006/nimg.2002.1097. [DOI] [PubMed] [Google Scholar]
- Hall J, Whalley HC, McKirdy JW, Romaniuk L, McGonigle D, McIntosh AM, et al. Overactivation of fear systems to neutral faces in schizophrenia. Biol Psychiatry. 2008;64(1):70–73. doi: 10.1016/j.biopsych.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Titone D, Long LS, Goff DC, Cather C, Rauch SL, et al. The misattribution of salience in delusional patients with schizophrenia. Schizophr Res. 2006;83(2–3):247–256. doi: 10.1016/j.schres.2005.12.858. [DOI] [PubMed] [Google Scholar]
- Kline JS, Smith JE, Ellis HC. Paranoid and nonparanoid schizophrenic processing of facially displayed affect. J Psychiatr Res. 1992;26(3):169–182. doi: 10.1016/0022-3956(92)90021-f. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Anselmo–Gallagher G, Bilker W, Karlawish J, Gur RE, Clark CM. Emotion–discrimination deficits in mild Alzheimer disease. Am J Geriatr Psychiatry. 2005;13(11):926–933. doi: 10.1176/appi.ajgp.13.11.926. [DOI] [PubMed] [Google Scholar]
- Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial Emotion Perception in Schizophrenia: A Meta-analytic Review. Schizophr Bull. 2009 doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SF, Garver DL. Treatment and diagnostic subtype in facial affect recognition in schizophrenia. J Psychiatr Res. 1995;29(1):5–11. doi: 10.1016/0022-3956(94)00033-n. [DOI] [PubMed] [Google Scholar]
- Loughead J, Gur RC, Elliott M, Gur RE. Neural circuitry for accurate identification of facial emotions. Brain Res. 2008;1194:37–44. doi: 10.1016/j.brainres.2007.10.105. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863–844. [DOI] [PubMed] [Google Scholar]
- Ohman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30(10):953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Senior C, David AS. Perception of threat in schizophrenics with persecutory delusions: an investigation using visual scan paths. Psychol Med. 2000;30(1):157–167. doi: 10.1017/s0033291799001397. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams L, Senior C, Bullmore ET, Brammer MJ, Andrew C, et al. A differential neural response to threatening and non-threatening negative facial expressions in paranoid and non-paranoid schizophrenics. Psychiatry Res. 1999;92(1):11–31. doi: 10.1016/s0925-4927(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Gur RE, Gur RC. Affect recognition deficits in schizophrenia: neural substrates and psychopharmacological implications. Expert Rev Neurother. 2007;7(7):807–816. doi: 10.1586/14737175.7.7.807. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008;99(1–3):164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Ruparel K, Penn DL. An investigation of the relationship between activation of a social cognitive neural network and social functioning. Schizophr Bull. 2008;34(4):688–697. doi: 10.1093/schbul/sbn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell TA, Reynaud E, Kucharska-Pietura K, Ecker C, Benson PJ, Zelaya F, et al. Neural responses to dynamic expressions of fear in schizophrenia. Neuropsychologia. 2007;45(1):107–123. doi: 10.1016/j.neuropsychologia.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Van't Wout M, van Dijke A, Aleman A, Kessels RP, Pijpers W, Kahn RS. Fearful faces in schizophrenia: the relationship between patient characteristics and facial affect recognition. J Nerv Ment Dis. 2007;195(9):758–764. doi: 10.1097/NMD.0b013e318142cc31. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45(1):174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Williams LM, Das P, Harris AW, Liddell BB, Brammer MJ, Olivieri G, et al. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am J Psychiatry. 2004;161(3):480–489. doi: 10.1176/appi.ajp.161.3.480. [DOI] [PubMed] [Google Scholar]
- Williams LM, Das P, Liddell BJ, Olivieri G, Peduto AS, David AS, et al. Fronto-limbic and autonomic disjunctions to negative emotion distinguish schizophrenia subtypes. Psychiatry Res. 2007;155(1):29–44. doi: 10.1016/j.pscychresns.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O'Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci. 2002;5(3):277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]