Abstract
Mechanical and electrical properties of polycaprolactone fumarate-polypyrrole (PCLF-PPy) scaffolds were studied under physiological conditions to evaluate their ability to maintain material properties necessary for application as conductive nerve conduits. PC12 cells cultured on PCLF-PPy scaffolds were stimulated with regimens of 10 μA of constant or 20 Hz frequency current passed through the scaffolds for 1 h/day. PC12 cellular morphologies were analyzed by fluorescence microscopy after 48 h. PCLF-PPy scaffolds exhibited excellent mechanical properties at 37°C which would allow suturing and flexibility. The surface resistivity of the scaffolds was 2kΩ and the scaffolds were electrically stable during application of electrical stimulation (ES). In vitro studies showed significant increases in percentage of neurite bearing cells, number of neurites per cell and neurite length in the presence of ES compared to no ES. Additionally, extending neurites were observed to align in the direction of the applied current. This study shows that electrically conductive PCLF-PPy scaffolds possess material properties necessary for application as nerve conduits. Additionally, the capability to significantly enhance and direct neurite extension by passing electrical current through PCLF-PPy scaffolds renders them even more promising as future therapeutic treatments for severe nerve injuries.
Keywords: Electrical Stimulation, Polypyrrole, Nerve, PCLF, PC12 cells
1. Introduction
Nerve injuries caused by sharp objects such as broken glass or knives typically result in clean transections[1] that can be repaired by suturing the severed ends when tensionless approximation is possible. In order to attain functional recovery it is critical that the proximal nerve extends axons that cross into the distal endoneurial tubes, which occurs at a rate of 1–3 mm per day[2]. However, full functional recovery from primary neuropathy can not always be achieved[3]. Insurmountable gaps created by nerve retraction or extensive injuries resulting in segmental nerve loss require the use of autologous nerve grafts, typically from the sural nerve, to bridge the gap. However these grafts have several disadvantages, including donor site morbidity, insufficient donor nerve length, mismatch in diameter, limited availability, and the risk of a second surgical intervention. In order to find alternative treatment methods, nerve conduits have been fabricated from many types of synthetic and natural materials including FDA approved poly(lactic-co-glycolic acid) (PLGA)[4], polycaprolactone (PCL)[5] and collagen[6]. In addition to modifying the geometry, mechanical properties, degradation rates, and chemical compositions of the nerve conduits[7–14], the inclusion of biomolecules such as glial cell line-derived neurotrophic factor (GDNF) and nerve growth factor (NGF) in the conduits for controlled release to promote nerve regeneration have been investigated and have shown significant increases in axonal regrowth[15–17].
An alternative method of stimulating neuronal tissue regeneration includes the application of electrical stimulation (ES) treatments directly on severed nerves, which has rendered promising findings[2, 18–20]. This idea has been extended to applying ES treatments through nerve conduits made from electrically conductive materials to stimulate axonal regeneration. This has led to the investigation of polypyrrole (PPy), polythiophene, polyaniline[21–34], carbon nanotubes[35, 36], and conductive nanoparticle coatings[37] with regard to nerve conduit fabrication. Of the above mentioned, PPy is one of the most extensively investigated conductive materials for tissue engineering applications because it possesses good electrical conductivity, biocompatibility, high electrical stability, and ease of synthesis[38]. However its poor mechanical properties, lack of biodegradability, and difficulty associated with processing it into complex three dimensional structures limits its applicability as a biomaterial[39]. Therefore attempts have been made to combine PPy with materials that possess the desired material properties to obtain hybrid composites with excellent material properties and electrical conductivity. A recent study by Durgam et al. reported the synthesis of a block co-polymer composed of PPy and PCL[40]. This study demonstrated the composite material had good conductivity, biodegradability, and the ability to support PC12 cell proliferation. Electrospun PLGA nanofibers featuring good cytocompatibility have been coated with PPy, enabling the application of ES treatments on PC12 cells, which led to an increase in number and length of neurite extensions[33]. Huang et al. used the biodegradable chitosan-PPy composite to subject Schwann cells to ES treatment regimens enhancing cell proliferation but more importantly increasing neurotrophin secretion[41] In our previous work we reported the synthesis, characterization and biocompatibility of an electrically conductive composite material composed of PPy and polycaprolactone fumarate (PCLF), with the latter having been shown to possess excellent material properties that closely resemble the desired properties for nerve conduits. We demonstrated that PCLF-PPy hybrid materials promoted cell attachment and differentiation of PC12 cells and dorsal root ganglia explants over PCLF and used the study to determine the optimal PCLF-PPy composition for studies involving ES that are reported herein[39].
In this study the PCLF-PPy scaffolds were investigated further for their mechanical properties, stability of electrical conductive properties, degradation, swelling, and protein adsorption. In vitro experiments (Figure 1) were accomplished to determine the effects of different ES treatment regimens applied through the scaffolds on the percent of neurite bearing cells, number of neurites per cell and length of neurite extensions. Since a major concern of regenerating nerves is the crossing of proximal outgrowing axons with the desired trajectory towards and into the distal endoneurial tubes[42] the effect of ES treatments on the direction of neurite extensions in vitro was analyzed.
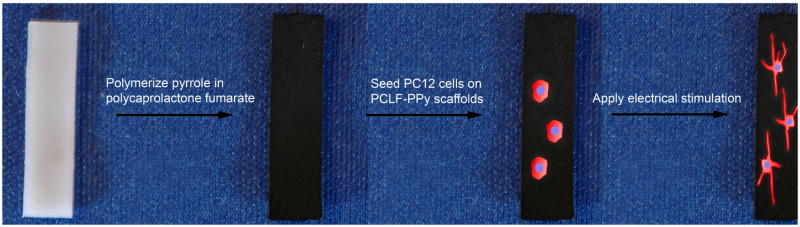
Figure 1.
Schematic overview of the project’s main steps: fabrication of PCLF-PPy composite scaffolds, cell seeding, and application of ES treatment regimens.
2. Materials and Methods
2.1 Synthesis of polycaprolactone fumarate-polypyrrole (PCLF-PPy)
2.1.1 Synthesis of polycaprolactone-fumarate (PCLF)
All chemicals were purchased from Fisher or Aldrich and used as is unless noted otherwise. PCLF with a number average molecular weight (Mn) of 17190 g/mol and a polydispersity index (PDI) of 1.43 was synthesized using previously published procedures[12, 39]. Molecular weights were characterized by gel permeation chromatography (GPC) using polystyrene standards of Mn 474, 1060, 2790, 6340, 10800, 18600, 39200 g/mol. The GPC system consisted of a Waters 2410 refractive index detector, 717 Plus autosampler, Styragel HR4E column, and a 515 HPLC pump. tetrahydrofuran (THF) was used as an eluent at a flow rate of 1 mL min−1.
2.1.2 PCLF scaffold fabrication
PCLF (3.0 g) was dissolved in methylene chloride (2 mL). 300 μL of photo-initiator phenyl bis(2,4,6-trimethylbenzoyl) phosphine oxide (BAPO or Irgacure 819) dissolved in methylene chloride (100 μg mL−1) was added. After gentle heating and vortexing, a viscous and homogenous fluid is obtained. Nerve conduits were fabricated similar to previous reports[11] by loading a 6 mL syringe with the PCLF resin and injecting it into a glass tube with an inner diameter of 3.1 mm and a length of 8.0 cm. A metal rod with a diameter of 1.9 mm was inserted into the glass tubes and held in place by end caps which also sealed the glass tube. PCLF thin films were fabricated by pouring the PCLF resin into molds consisting of two glass slides separated by a Teflon spacer measuring 13.5 × 15.5 cm2 with a thickness of 0.5 mm. Both molds containing the PCLF mixture were placed in a UV chamber for 1 h at λ = 315–380 nm to initiate cross-linking. The molds containing scaffolds were then cooled to 0°C to facilitate scaffold removal. Scaffolds were purified of un-crosslinked oligomers by extensive swelling in acetone and methylene chloride and dried under vacuum. The resulting tubes had 3.0 mm outer and 1.7 mm inner diameter. The films were 0.5 mm thick and were cut to dimensions of 25.0 × 6.0 mm.
2.1.3 Synthesis of PCLF-PPy Composite Materials
PCLF-PPy materials were synthesized using previously published procedures[12, 39]. Briefly, PCLF scaffolds were placed in 200 mL of methylene chloride containing 20.0 g of benzoyl peroxide for 20 min and then air dried. A solution composed of 4.0 g (17 mmol) of naphthalene sulfonic acid (NSA), 5.6 g of distilled pyrrole and 200 mL of double distilled water (DDW) and one consisting of 6.0 g (17 mmol) of dodecylbenzenesulfonic acid (DBSA), 5.6 g distilled pyrrole and 200 mL of DDW were prepared. After adding the scaffolds, both solutions were cooled with ice and stirred for 24 h. The samples were then purified extensively by repeated swelling steps in acetone and methylene chloride and then dried under vacuum.
2.2 Material properties
2.2.1. Differential scanning calorimetry
Using a TA instruments Differential Scanning Calorimeter (DSC), which measures the temperature and heat flow associated with thermal transitions in a material, the melting temperature of the scaffolds was determined. After heating to 100 °C (to equalize the thermal-history), cooling to −80°C and re-heating to 150°C at a rate of 5°C min−1, the heat flow during the heating cycle was plotted over the temperature in order to display the melting temperature of the samples.
2.2.2. Tensile modulus
Using a razorblade equipped stencil, the PCLF-PPyNSA, PCLF-PPyDBSA and PCLF films were cut into a dog bone shape with a diameter of 2.1 mm. Half of the samples were kept in water at room temperature and half at 37°C in a water bath after previously had been heated to 50°C. Each scaffold was mounted on a TA instruments Dynamic Mechanical Analyzer (DMA) 2980 tension clamp. The force applied on the sample started at 0.02 Newton (N) and increased at a rate of 3.0 N/min until reaching either 18.0 N or the material’s failure point. Subsequently the tensile modulus of the scaffolds was determined by measuring the slope of the linear part of the stress/strain curve.
2.2.3. Flexural modulus
To analyze the three-point bending properties of the materials, the tube shaped PCLF-PPyNSA, PCLF-PPyDBSA and PCLF scaffolds were mounted on a TA instruments DMA 2980 three-point bending clamp and a vertical force starting at 0.02 N and increasing at a rate of 3.0 N/min until reaching 18 N was applied through a thin metal plate oriented in a vertical and perpendicular fashion to the samples. Half of the samples were kept at room temperature and half at 37°C after having been heated to 50°C. The TA instruments’ universal analysis software was used to identify the materials’ flexural modulus.
2.2.4. Suture pullout test
The pullout strength of the composite PCLF-PPy conduits were compared to PCLF conduits using previously reported procedures[11, 43]. All polymeric conduits were sutured using a 9-0 monofilament nylon suture. The scaffolds were submerged in a water bath with half of them being held at 37°C after having been heated to 50°C. They were then mounted on a TA instruments DMA 2980 tension clamp and a pulling force starting at 0.02 N and increasing at a rate of 0.5 N/min was applied on the sutures until the failure point of either the suture or the material was reached. Since in all attempts it was the suture to break first, the samples were additionally sutured with a 4.0 Vicryl (Ethicon) and the suture pullout tests were repeated using the above mentioned procedure. The pullout strength of the scaffolds was determined by plotting the force (N) applied on the suture over the displacement of the suture within the sample as previously reported[12] using the TA instruments’ Universal Analysis software.
2.2.5. Effect of ES on scaffold electrical stability and media temperature
In all experiments Dulbecco’s Modified Eagle’s Medium (DMEM) (Mediatech) supplemented with 10% heat inactivated horse serum, 5% heat inactivated fetal bovine serum, and 0.5% penicillin/streptomycin was used as media.
The PCLF-PPyNSA and PCLF-PPyDBSA scaffolds were cut to fit in 24-well tissue culture plates. Autoclaved medical grade silicon tubing with inner diameter of .95 cm and outer diameter of .1.6 cm fit tightly in the wells of the tissue culture plate. The silicon tubing walls were pierced by two platinum wires that exited the bottom of the silicon tube wall. When the tube with platinum wires was tightly pressed into the well on top of the scaffold an excellent contact between the scaffold and platinum wires was created, leaving no space for the media to be in contact with the wires. A small amount of Dow Corning® high vacuum grease was applied to the bottom of the silicone tubing before the tube was inserted into the well to ensure prevention of the media from coming into contact with the platinum wires. Then 1mL of media was added to each well. The tissue culture plates were placed in a 5% CO2 incubator at 37°C and the platinum wires hooked up to an A.M.P.I. Master 8 programmable electrical stimulator using alligator clamps attached to wires running out of the incubator. Direct current of 10 μA was applied on the scaffolds and the increase in resistance over the course of 100 h was measured using a Fluke 73 multimeter. The surface resistivity (Rs) of the samples was calculated using the following formula, where Rs = R × W/D. R is the measured resistance, W is the sample width and D is the distance between the two platinum wires. The increase in resistance of the samples was compensated for daily by adjusting the amount of voltage applied. Stimulation regimens investigated were 1 h of direct current per day, 1 min/hour, and 1 sec/min. To determine if the electrical current running through the samples increased the temperature of their surrounding, the temperature of the media was measured during an ES regimen of 8 h of constant direct current of 1 mA using a Traceable® thermometer.
2.2.6. Scaffold degradation and swelling
After weighing, PCLF-PPyNSA, PCLF-PPyDBSA and PCLF samples were placed in separate vials filled with phosphate buffered saline (PBS) at 37°C. Every week the PBS was aspirated, the samples were dried in a vacuum oven for 24 h and then weighed on a precision scale. This procedure was performed for 34 weeks. For the swelling test PCLF-PPyNSA, PCLF-PPyDBSA and PCLF samples were weighed (Wd, dry weight) and subsequently placed in separate vials containing PBS at 37°C. After 24 hours the weight (Ws, weight after swelling) was determined to calculate the swelling ratio (Ws−Wd)/Wd.
2.2.7. Protein adsorption
PCLF-PPyNSA, PCLF-PPyDBSA and PCLF films were incubated for 24 h at 37°C in media containing 50 ng/mL of nerve growth factor (NGF) (Harlan) or media without NGF. Samples with no media were used as control. Subsequently the solutions were aspirated and after washing them three times with PBS, each sample was submerged in 240 μL of sodium dodecyl sulfate (SDS) for 1 h. Using the Thermo Scientific Micro BCA Protein Assay kit, a working solution consisting of 5.0 mL of Micro BCA Reagent MA, 4.8 mL of Micro BCA Reagent MB and 0.2 mL of Micro BCA Reagent MC was prepared. Aliquots of 150 μL of SDS extract from each sample and standards of 200, 40, 20, 10, 5, 2.5, 1, 0.5, and 0 μg/mL of bovine serum albumin (BSA) were pipetted on a micro assay plate, mixed with 150 μL of the working solution mentioned above, covered with tin foil, and incubated at 37°C for 2 h. The micro array plate was placed in a Molecular Devices Spectra Max Plus spectrophotometer and the absorbance of the solutions was measured at 562 nm. Using the absorbance values of the BSA standards, a calibration curve was created and the protein absorption of the samples was calculated.
2.3 Electrical Stimulation of PC12 cells on PCLF-PPyNSA Scaffolds
To study the effect of ES on neurite extension, PC12 cells (ATCC) were cultured on PCLF-PPyNSA films while passing electrical stimuli through the scaffolds. The same set up as mentioned above for the conductivity experiments was used. Scaffolds, wires, and tubing were sterilized with 70% aqueous ethanol for 10 min, washed three times with sterile PBS and incubated for 24 h at 37 °C with each well filled with 1 mL of media containing 50 ng of NGF. The media was then removed and PC12 cells were seeded at a density of 50,000 cells/cm2 with each well being filled with 1 mL of fresh media and 50 ng of NGF.
ES treatments for 1 h/day of constant direct current and 20 Hz direct current with the pulse width of the square wave resembling half the length of the interval were applied over two days. For all experiments a current of 10 μA was applied, corresponding to a surface current density of 7.2 μA/cm2. PC12 cellular morphologies were imaged using fluorescence microscopy. The cells were stained according to previously described methods[39] using 1% rhodium phalloidin (Invitrogen, CA) and DAPI (4′,6-diamidino-2-phenylindole) - stain (Vector). Samples were imaged on an LSM 510 inverted confocal microscope at wavelengths of 368 nm and 488 nm and 300 cells were analyzed for each ES treatment regimen using NIH Image J software. The percentage of neurite bearing cells and the number of neurites per cell (taking into consideration only those cells with at least one neurite) were calculated. In order to be considered a neurite, a cell extension had to be longer than 5 μm measuring from the tip to the middle of its base[44]. For branched neurites the junction with the longest neurite was considered to be the base of the neurite branch[40]. Additionally the neurite length and neurite directionality were examined.
2.4. Statistics
All the material property characterization and cell culture experiments were run in triplicates unless otherwise noted. Using StatView (version 5.0.1.0, SAS Institute, Inc, Cary, NC) a single factor analysis of variance (ANOVA) was performed to determine the significance of the results. Bonferroni’s method was used for multiple comparison tests when the global F-test was positive at an alpha level of 0.05 to determine differences among the experimental groups. The neurite length was not normally distributed, therefore the median value for each regimen was calculated and the Mann-Whitney U test was employed for statistical analysis.
3. Results
3.1. Mechanical properties
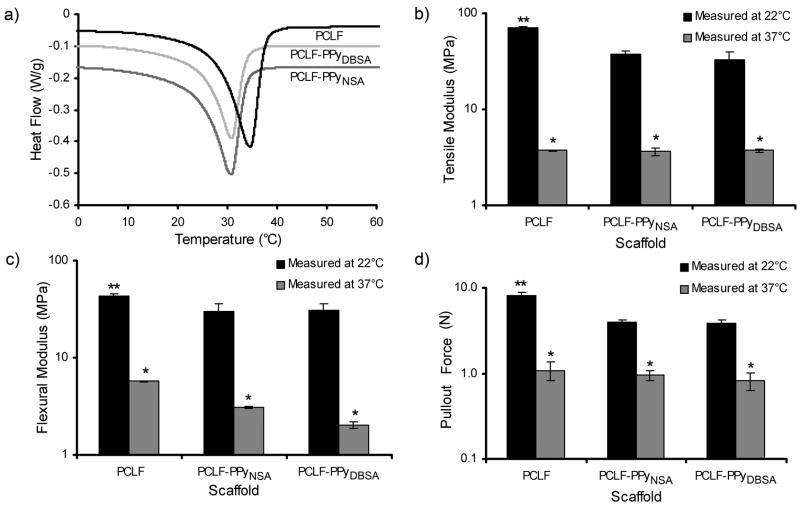
PCLF based materials possess melting transitions (Tm) between room temperature and physiological temperatures as shown in Figure 2a. This transition signifies the transition from crystalline to the amorphous state and can have profound impacts on material properties. Additionally, Figure 2a shows the incorporation of PPy into PCLF lowers this transition from 34.6°C for PCLF to 30.8°C for PCLF-PPy.
Figure 2.
Effect of thermal transition of the materials on mechanical properties of PCLF, PCLF-PPyNSA and PCLF-PPyDBSA scaffolds at room temperature and 37°C, measured by a) differential scanning calorimetry, (b) tensile test, (c) 3-point bending, and (d) suture pullout test. *denotes significant differences (p <0.01) in material properties between room temperature and 37°C. **denotes significant differences (p <0.01) between PCLF-PPy and PCLF at room temperature.
Properties critical for application as nerve conduits including tensile modulus, flexural modulus, and suture pullout strength were measured for PCLF and PCLF-PPy materials at 37° and room temperature (Figure 2b–d). The tensile modulus (Figure 2b) of PCLF decreased from 71.6 MPa at room temperature to 3.7 MPa at 37°C. PCLF-PPyNSA and PCLF-PPyDBSA decreased from 37.5 and 33.3 MPa to 3.6 and 3.7 MPa, respectively. All decreases in tensile modulus were found to be significant (p <0.01). The tensile modulus was found to be significantly higher (p <0.01) for PCLF than either PCLF-PPyNSA or PCLF-PPyDBSA at room temperature, but no significant difference was found for the tensile modulus between samples when measured at 37°C. The flexural modulus at room temperature for PCLF, PCLF-PPyNSA and PCLF-PPyDBSA was 42.7, 30.3, and 31.1 MPa, respectively, and decreased significantly (p <0.01) to 5.7, 3.1, and 1.9 MPa upon warming to 37°C. Significant differences (p<0.01) in the flexural modulus were observed between composite materials and PCLF at room temperature, but not at 37°C.
Suture pullout tests were performed to determine if PCLF and PCLF-PPy materials could fail as a result of having sutures tear out of the material at room temperature or more importantly at 37°C. When initially performing the suture pullout tests using a 9.0 nylon suture, the largest and strongest suture typical of what would be used in a clinical setting for repairing nerve with a size equivalent to the scaffold tube, the conduits in both the crystallized and amorphous state could bear more force than the sutures themselves, resulting in the suture failing and not the material in all cases. When tested with a stronger 4-0 suture, PCLF-PPyNSA and PCLF-PPyDBSA scaffolds exhibited pullout strengths of 4.0 and 3.9 N which was lower compared to the 8.2 N for the PCLF samples (p <0.01 for both cases). However at 37°C all sample types showed no significant difference in pullout strengths: PCLF-PPyNSA 1.0 N, PCLF-PPyDBSA 0.8 N and PCLF 1.1 N (Figure 2d).
3.2. Effects of ES on the scaffold’s conductive stability and media temperature
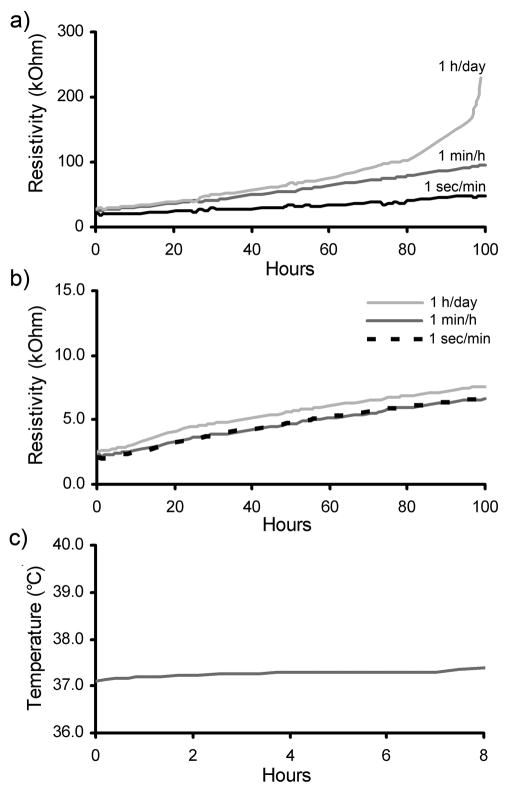
The effect of passing of electrical current through PCLF-PPy scaffolds in the presence of media was investigated to determine the conductive stability and temperature increase of the local environment. Figure 3a,b show the increase in surface resistivity over the course of 100 h when different electrical stimulation regimens of 10 μA were applied through PCLF-PPyDBSA and PCLF-PPyNSA scaffolds. The initial Rs of the different PCLF-PPyNSA samples was 2 kΩ which was much lower than the 25 kΩ of the PCLF-PPyDBSA scaffolds. When current was applied through PCLF-PPyNSA scaffolds, there was almost no difference between the linear increases of surface resistivity over time for the different stimulation regimens. PCLF-PPyDBSA showed a steeper increase in resistivity for 1 min/h stimulation regimen when compared to 1 sec/min stimulation regimen. A daily application of 10 μA of current for 1 h on PCLF-PPyDBSA samples led to a nearly exponential increase of surface resistivity after 80 h. Overall the PCLF-PPyNSA scaffolds exhibited a better electrical stability and conductivity than PCLF-PPyDBSA. Because excessive increases in temperature can be detrimental to the local cellular environment, we measured the increase in temperature of the media over the course of 8 h with constant electrical stimulation of 1mA. Figure 3c shows that the temperature of the surrounding media remained stable over the entire course of the experiment and did not rise for more than 0.3°C.
Figure 3.
Surface resistivity (Rs) over the course of 100 h when different stimulation regimens at an intensity of 10 μA are applied on PCLF-PPyDBSA samples (a) and PCLF-PPyNSA samples (b) incubated in media. Figure 3c shows the temperature increase of the surrounding media when 1 mA of direct current is constantly applied for 8 h.
3.3. Degradation and swelling
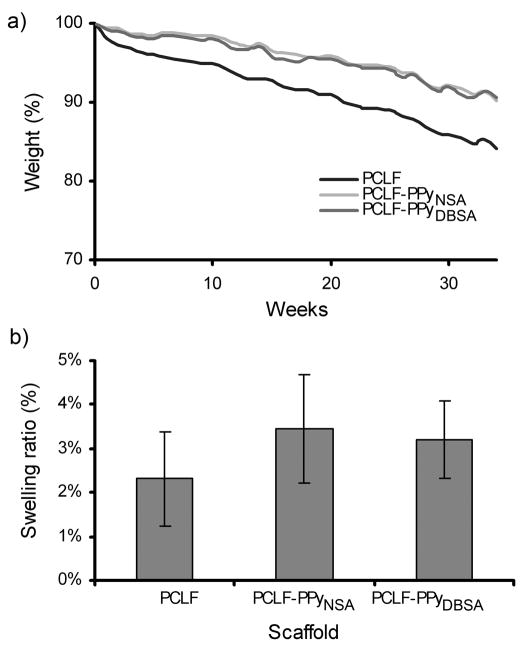
To determine the effect of PPy incorporation on the degradation of PCLF, the scaffolds were submerged in PBS and the percent weight loss was measured over 34 weeks. PCLF scaffolds showed a constant degradation rate resulting in 84% of the material remaining after 34 weeks. PCLF-PPyNSA and PCLF-PPyDBSA samples degraded slightly slower than PCLF with 90% of weight remaining, thus exhibiting a lower degradation rate than PCLF (Figure 4a). The 2.3%, 3.5%, and 3.2% relative weight increase for PCLF, PCLF-PPyNSA, and PCLF-PPyDBSA scaffolds, respectively, in the swelling experiment yielded no statistically significant difference between the groups and indicated that all tested scaffolds do not swell considerably in an aqueous solution (Figure 4b).
Figure 4.
a) Percent weight loss of PCLF, PCLF-PPyNSA and PCLF-PPyDBSA samples incubated at 37°C in PBS for 34 weeks. b) Percent weight gain of scaffolds after 24h swelling in PBS at 37°C. No statistically significant difference was found between different scaffolds.
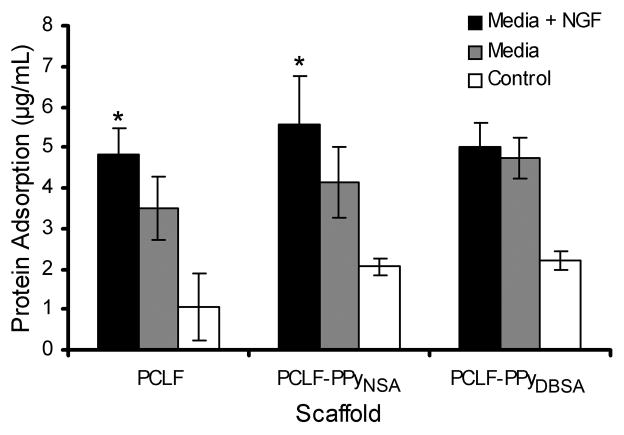
3.4. Protein adsorption
Protein adsorption to scaffold surfaces is a critical step before cells attach to the scaffold surface. The results of protein adsorption on the scaffold surfaces from media and from media containing nerve growth factor are presented in Figure 5. PCLF-PPyNSA and PCLF-PPyDBSA samples submerged in media with or without NGF showed similar protein adsorption amounts and patterns as the PCLF samples. Submersion in media resulted in 4.2 μg/mL of adsorbed protein for the PCLF-PPyNSA samples, 4.7 μg/mL for PCLF-PPyDBSA and 3.5 μg/mL for PCLF. When incubated in media containing 50 ng/ml of NGF, the protein adsorption was 5.6 μg/mL for the PCLF-PPyNSA samples, 5.0 μg/mL for PCLF-PPyDBSA and 4.8 μg/mL for PCLF, thus showing a significant increase in protein adsorption for the PCLF-PPyNSA (p =0.026) and PCLF samples (p =0.039) when NGF is added to the media. No differences were observed between scaffold types.
Figure 5.
Protein adsorption on PCLF, PCLF-PPyNSA and PCLF-PPyDBSA scaffolds after incubation for 24 h in media with or without NGF. Samples with no media were used as control. *denotes significant differences (p <0.05) in the amount of protein adsorbed on scaffolds in media with NGF compared to scaffolds incubated in media without NGF.
3.5. Electrical stimulation of PC12 cells on PCLF-PPyNSA scaffolds
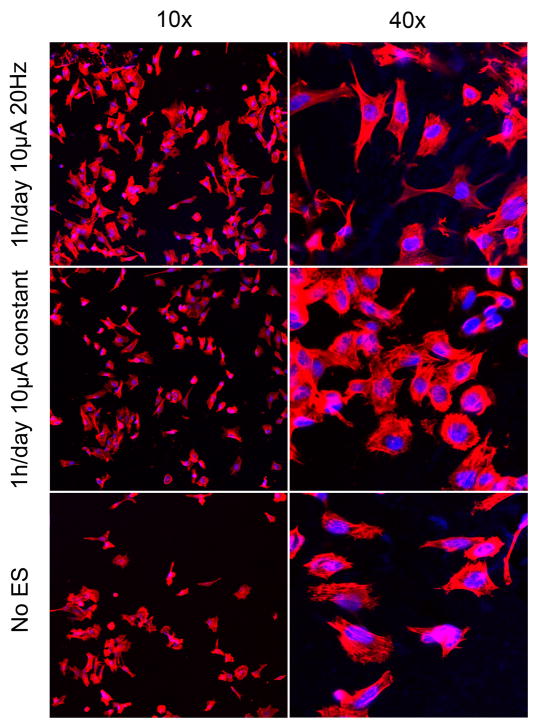
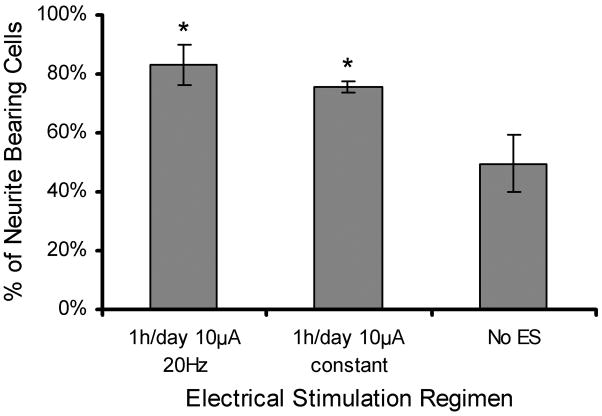
PC12 cells were chosen as the model cell line for the initial studies involving electrical stimulation because they are a commonly used cell for nerve regeneration studies, and there is ample literature precedence to compare the results of electrical stimulation treatments. Figure 6 shows fluorescence microscopy images of PC12 cells at 10× and 40×. The 10× images show typical images used to analyze neurite extensions through NIH image J software. Distinctly more and longer neurites can be seen when ES was applied than without electrical stimulation. The percentage of PC12 cells bearing neurites was 75.7% for 1 h/day of 10 μA of constant current and 83.0% for 1 h/day of 10 μA of 20 Hz frequency. These results were significantly higher (p <0.01) than the 49.7% observed with no ES. No statistically significant difference was observed between ES treatment regimens (Figure 7). For neurite bearing cells, the average number of neurites per cell when applying different stimulation regimens was measured (Figure 8a). Compared to cells not exposed to ES, PC12 cells that received ES treatment demonstrated an increase in amount of neurites per cell from 1.8 to 2.7 for stimulation of 20Hz and 2.2 for constant stimulation (p <0.01). Figure 8b shows the distribution of the number of neurites per cell. Of the neurite bearing PC12 cells cultured in the absence of ES 46% had 1 neurite, 82% had two or less and 96% had 3 or less neurites. ES stimulation shifted the distribution to higher numbers of cells bearing 2 or more neurites. 32% had one neurite, 31% had two neurites, 20% had three neurites, and 16% of the PC12 cells exposed to constant stimulation had 4 or more neurites, up from the 5% observed with no ES. PC12 cells cultured in the presence of 20 Hz ES exhibited the most substantial shift in number of neurites per cell. Only 17% of these PC12 cells had one neurite, while 30% had 4 or more neurites. Figure 8c shows a 40× image of one PC12 cell having been subject to 1 h/day of 10 μA 20Hz ES and bearing multiple neurite extensions.
Figure 6.
Fluorescence microscopy of PC12 cells imaged at 10× and 40× magnification after undergoing different ES treatment regimens for 48 h.
Figure 7.
Percentage of neurite bearing cells seen in fluorescent microscopy when ES treatment regimens of 20Hz and constant current are applied on the PCLF-PPyNSA scaffolds. *denotes significant difference (p <0.01) from no ES.
Figure 8.
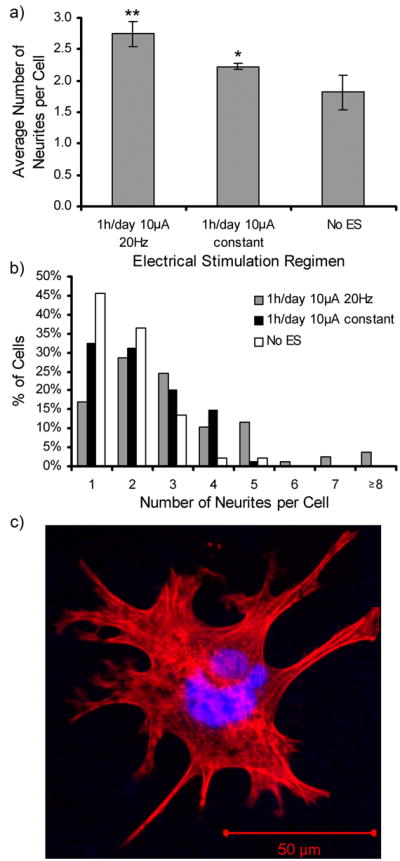
Average number of neurites per cell (a) and distribution of neurites (b) of PC12 cells cultured on PCLF-PPyNSA scaffolds under different ES regimens. Fluorescence microscopy of an exemplary image of a cell stimulated for 1 h/day with 10 μA 20 Hz ES bearing multiple neurites (c). *denotes significantly higher number of neurites per cell compared to no ES (p <0.01). **denotes significant differences between 20Hz frequency and constant ES (p <0.01).
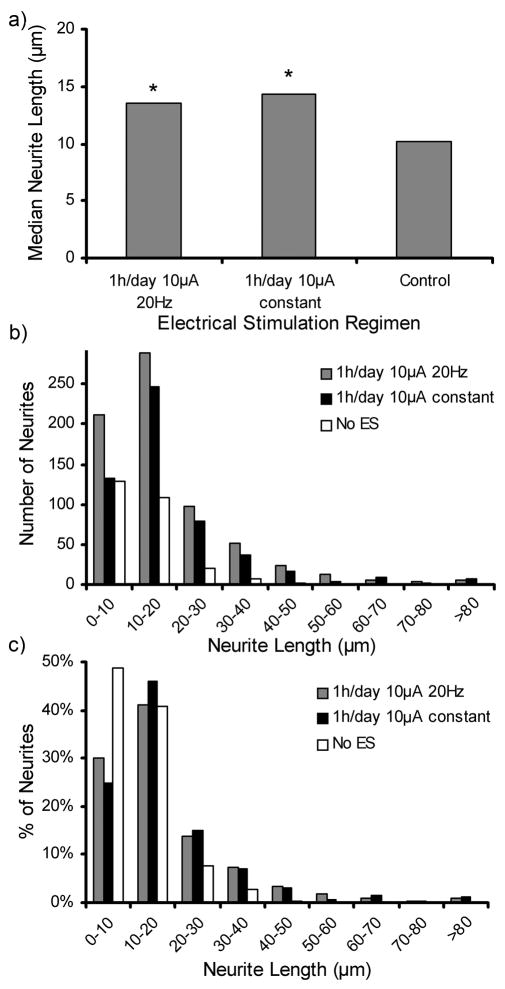
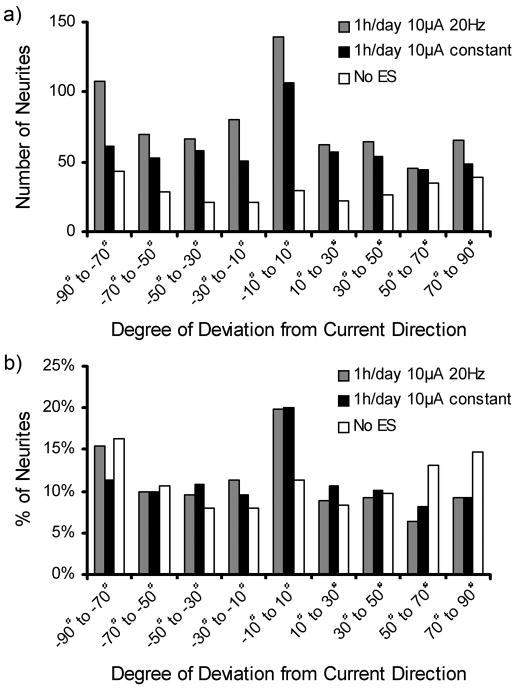
The median neurite length and the distribution of neurite lengths are shown in Figure 9. Cells stimulated with ES showed significant increases (p <0.01) in median neurite length from 10.2 μm with no ES to 14.4 μm for constant ES and 13.6 μm for 20 Hz ES (Figure 9a). No significant difference was observed between the two ES regimens. Figure 9b,c show the distribution of neurites measured for lengths of 10 μm ranges. Figure 9b shows that the 20 Hz ES treatments had the highest counts in most length categories of the neurite length distribution, consistent with the results from Figure 8b. Figure 9c shows the relative distribution where 90% of the neurites of PC12 cells cultured in the absence of ES had lengths of 0–20 μm. PC12 cells with either ES treatment exhibited 71% of cells having neurites of 0–20 μm, but they were distributed differently with constant and 20Hz ES having 46% and 41% of neurites ranging from 10–20 μm. The effect of ES on the direction at which neurites extend was investigated by measuring the angle of the neurites in relation to the direction of the applied current. Figure 10 displays the distribution of neurite alignment showing a doubling in the number of neurites within a range of ± 10° parallel to the current direction for both ES treatment regimens. When no ES was applied this peak was absent, and the angles at which the neurites were extending were evenly distributed. This indicates a preferential alignment of neurites with the direction of the stimulating current if ES is applied on cells.
Figure 9.
a) Median neurite length of PC12 cells cultured on PCLF-PPyNSA scaffolds under ES treatment regimens of 20Hz and constant current. Figure 9b,c show the neurite length distribution in terms of total neurite counts (b) and in relative values (c) for different ES treatments applied. *denotes significant difference compared to cells cultured in the absence of ES (p <0.01).
Figure 10.
With the direction of the current defined as 0° the distribution of the neurite alignment is displayed for ES treatment regimens of 20Hz and constant current in terms of absolute neurite counts (a) and relative values (b).
4. Discussion
Materials used to fabricate nerve conduits require specific material properties that make them suitable for application in the animal model and eventual clinical trials. A critical feature for nerve conduits is their mechanical properties. The implanted nerve tubes have to be flexible and comply with tensile and bending stress especially when situated in the proximity of joints while at the same time they have to withstand the pulling forces typically exerted by a 9-0 nerve suture. PCLF-PPy scaffolds exhibit excellent suture pullout strengths, and unique thermal transitions that occur near the physiological temperature of 37 °C. By controlling the molecular weight of PCLF the thermal transitions, crystallinity and resulting mechanical properties of cross-linked PCLF can be manipulated as previously reported[45]. Therefore warming PCLF and the composite PCLF-PPy scaffolds to 37 °C turned them from semi-crystalline to an amorphous state, which significantly increased their flexibility while still providing enough strength to hold a nerve suture.
The biodegradability of the nerve conduit is very important and a balance between providing support, guidance, and degradation is critical. The degradation of the conduit can prevent the regenerating nerve from being compressed and limits chronic foreign body reactions[46]. Also the conduit should not degrade too quickly, because it has to maintain its mechanical integrity to provide nerve guidance. Additionally, large amounts of small degradation products might increase the osmotic pressure of the conduit causing increased material swelling[7, 47] which in general can lead to deleterious compression of the nerve[46]. According to our results, PCLF-PPy scaffolds meet these requirements featuring a lack of swelling when submerged in aqueous solvent and slow but consistent degradation rates, thus being able to provide a strong guidance to outgrowing axons, without leading to compression over time.
The main interest in PCLF-PPy scaffolds is their electrical conductivity and being able to pass electrical current through them to stimulate growing nerve cells. Based on previous results PCLF-PPy networks doped with NSA and DBSA anions[39] were selected because they demonstrated best material properties and most extensive cell attachment. The Rs for PCLF-PPyNSA was shown to be as low as 2 kΩ thus exhibiting a similar conductivity as recently reported for other PPy composite materials[33, 41]. Additionally, the scaffolds proved to be electrically stable when different ES treatment regimens were applied, especially if NSA was the utilized dopant. This combined with the fact that compared to PCLF-PPyDBSA PCLF-PPyNSA samples were able to adsorb a higher amount of NGF to their surface led to the conclusion to use this type of scaffold for the cell culture experiments.
PC12 cells were electrically stimulated with 10 μA of current for 1 h per day. The current was either constant direct current or direct current with a frequency of 20 Hz. The amount of current, 10 μA, was chosen because it renders a surface current density of 7.2 μA/cm2 which induced the most favorable cell response from a previous study by Zhang et al.[48]. The frequency of 20 Hz was chosen because 20 Hz is considered an effective frequency for stimulating nerve regeneration in the rat model[2, 49]and in human[49, 50]. Using ES with a frequency of 20 Hz instead of constant current may be advantageous because it resembles average firing frequencies of motor neurons[50]. Even though the electrical stability of the samples was tested with three different time regimens, the cells were only subject to the 1 h/day ES treatment because it is a commonly used regimen when trying to stimulate neuronal regeneration in vivo[19, 49, 51].
Image analysis of PC12 stimulated with a frequency of 20 Hz revealed an impressive 67% increase in the percentage of neurite bearing cells, dramatically higher than the 5% recently achieved in another study with similar PPy-PCL scaffolds[40]. Using PPy coated PLGA nanofibers to apply ES treatments on PC12 cells, an even higher relative increase of 92% was reported, however, in that case no increase in numbers of neurites per cell was found[33]. In comparison, when treatments of 1 h/day 10 μA 20 Hz ES were applied on cells seeded on PCLF-PPyNSA samples the average number of neurites per cell increased by 52%. Considering the length of the extending neurites when electrical stimulation regimens are applied relative increases of 30%[40], 48.8%[33], 50%[24], and 90.5%[52] are reported in the literature. The 33.0% increase achieved when 10 μA 20 Hz ES were applied on PCLF-PPyNSA scaffolds might therefore seem low at first sight, however it has to be taken into consideration that the increase in cells bearing multiple neurites leads to a shift of the median neurite length to lower values, because the neurites grow longer, but at the same time many new short ones appear. This explanation is corroborated by the observation that constant ES, which had a lower relative increase of the average number of neurites per cell showed a higher relative increase of 41.0% in the median neurite length. As mentioned by Lee et al. the exact mechanism of action of ES on neurons is not fully understood, but might involve increased adsorption of the extracellular matrix protein fibronectin to the scaffold surface[53], changes in membrane potential[54], and the vectored accumulation of surface glycoproteins[55]. According to our results, pulsed ES treatments of 20 Hz led to a significant increase of the number of neurites per cell when compared to constant stimulation. Considering the widely accepted use of pulsed ES treatments of 20 Hz to aid nerve regeneration in vivo[2, 49, 50] and the finding that PC12 cells show increased viability when stimulated with pulsed rather than constant treatments[37], the use of pulsed ES in future studies for enhancing neurite extension on electrically conductive scaffolds might lead to improved results.
Since outgrowing axons need to find the desired trajectory towards and into the distal endoneurial tubes[42], micropatterned surfaces[42, 56], microchannels[57] or aligned nanofibers[58, 59] have been investigated for their potential to guide outgrowing nerve axons, rendering promising results. When analyzing the orientation of neurite extension in this study, alignment with the current direction was found. Combined with the fact that electrical fields are able to influence the direction of neurite extension in general[55, 60–62], this finding indicates an additional method to guide axon outgrowth within nerve conduits by applying electrical current.
5. Conclusion
Electrically conductive scaffolds composed of PCLF-PPy were investigated to determine whether their material properties are suitable for use as nerve conduits. PCLF-PPy materials were shown to maintain mechanical properties necessary for nerve conduit applications. PCLF-PPy materials in the amorphous state had increased flexibility while still maintaining the strength required to hold a suture. PCLF-PPyNSA had excellent electrical stability required for application of extended electrical stimulation regimens. PC12 cells were stimulated for 1 h/day with 10 μA of direct current for 2 days and the effect of constant versus 20 Hz frequency was compared. It was determined that 20 Hz stimulation increased the number of neurites per cell compared to constant stimulation, but the two ES treatments also resulted in similar percents of neurite bearing cells and neurite lengths. Also the alignment of neurites parallel to the direction of applied current shows that ES can be used a guidance cue to direct neurite extension. Both ES treatments were shown to be significantly better than no ES in all categories for increasing neurite extension.
Acknowledgments
This work was funded by NIH training grant 1T32AR056950-01, the NIH Loan Repayment Program, NIBIB PAR56212, and the Armed Forces Institute of Regenerative Medicine by DOD activity contract # W81XWH-08-2-0034.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Isaacs J. Treatment of acute peripheral nerve injuries: current concepts. J Hand Surg Am. 2010;35:491–7. doi: 10.1016/j.jhsa.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20:2602–8. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans GR. Challenges to nerve regeneration. Semin Surg Oncol. 2000;19:312–8. doi: 10.1002/1098-2388(200010/11)19:3<312::aid-ssu13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Yucel D, Kose GT, Hasirci V. Polyester based nerve guidance conduit design. Biomaterials. 31:1596–603. doi: 10.1016/j.biomaterials.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Chiono V, Vozzi G, Vozzi F, Salvadori C, Dini F, Carlucci F, et al. Melt-extruded guides for peripheral nerve regeneration. Part I: Poly(epsilon-caprolactone) Biomed Microdevices. 2009 May; doi: 10.1007/s10544-009-9321-9. Epub. [DOI] [PubMed] [Google Scholar]
- 6.Kemp SW, Syed S, Walsh W, Zochodne DW, Midha R. Collagen nerve conduits promote enhanced axonal regeneration, schwann cell association, and neovascularization compared to silicone conduits. Tissue Eng Part A. 2009;15:1975–88. doi: 10.1089/ten.tea.2008.0338. [DOI] [PubMed] [Google Scholar]
- 7.de Ruiter GC, Onyeneho IA, Liang ET, Moore MJ, Knight AM, Malessy MJA, et al. Methods for in vitro characterization of multichannel nerve tubes. J Biomed Mater Res A. 2007;84:643–51. doi: 10.1002/jbm.a.31298. [DOI] [PubMed] [Google Scholar]
- 8.de Ruiter GC, Spinner RJ, Malessy MJA, Moore MJ, Sorenson EJ, Currier BL, et al. Accuracy of motor axon regeneration across autograft, single-lumen, and multichannel poly(lactic-co-glycolic acid) nerve tubes. Neurosurgery. 2008;63:144–55. doi: 10.1227/01.NEU.0000335081.47352.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Ruiter GCW, Malessy MJA, Alaid AO, Spinner RJ, Engelstad JK, Sorenson EJ, et al. Misdirection of regenerating motor axons after nerve injury and repair in the rat sciatic nerve model. Exp Neurol. 2008;211:339–50. doi: 10.1016/j.expneurol.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore MJ, Friedman JA, Lewellyn EB, Mantilla SM, Krych AJ, Ameenuddin S, et al. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials. 2006;27:419–29. doi: 10.1016/j.biomaterials.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Yaszemski MJ, Knight AM, Gruetzmacher JA, Windebank AJ, Lu L. Photo-crosslinked poly(Îμ-caprolactone fumarate) networks for guided peripheral nerve regeneration: Material properties and preliminary biological evaluations. Acta Biomater. 2009;5:1531–42. doi: 10.1016/j.actbio.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Lu L, Gruetzmacher JA, currier BL, Yaszemski MJ. Synthesis and characterizations of biodegradable and crosslinkable poly(e-caprolactone fumarate), poly(ethylene glycol fumarate), and their amphiphilic copolymer. Biomaterials. 2006;27:832–41. doi: 10.1016/j.biomaterials.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Li XK, Cai SX, Liu B, Xu ZL, Dai XZ, Ma KW, et al. Characteristics of PLGA-gelatin complex as potential artificial nerve scaffold. Colloids Surf B Biointerfaces. 2007;57:198–203. doi: 10.1016/j.colsurfb.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Sun M, Kingham PJ, Reid AJ, Armstrong SJ, Terenghi G, Downes S. In vitro and in vivo testing of novel ultrathin PCL and PCL/PLA blend films as peripheral nerve conduit. J Biomed Mater Res A. 93:1470–81. doi: 10.1002/jbm.a.32681. [DOI] [PubMed] [Google Scholar]
- 15.Madduri S, Feldman K, Tervoort T, Papaloizos M, Gander B. Collagen nerve conduits releasing the neurotrophic factors GDNF and NGF. J Control Release. 143:168–74. doi: 10.1016/j.jconrel.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Madduri S, Papaloizos M, Gander B. Trophically and topographically functionalized silk fibroin nerve conduits for guided peripheral nerve regeneration. Biomaterials. 31:2323–34. doi: 10.1016/j.biomaterials.2009.11.073. [DOI] [PubMed] [Google Scholar]
- 17.Piquilloud G, Christen T, Pfister LA, Gander B, Papaloizos MY. Variations in glial cell line-derived neurotrophic factor release from biodegradable nerve conduits modify the rate of functional motor recovery after rat primary nerve repairs. Eur J Neurosci. 2007;26:1109–17. doi: 10.1111/j.1460-9568.2007.05748.x. [DOI] [PubMed] [Google Scholar]
- 18.Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002;22:6631–8. doi: 10.1523/JNEUROSCI.22-15-06631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon T, Udina E, Verge VM, de Chaves EI. Brief electrical stimulation accelerates axon regeneration in the peripheral nervous system and promotes sensory axon regeneration in the central nervous system. Motor Control. 2009;13:412–41. doi: 10.1123/mcj.13.4.412. [DOI] [PubMed] [Google Scholar]
- 20.Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VMK. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol. 2007;205:347–59. doi: 10.1016/j.expneurol.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 21.Ateh DD, Navsaria HA, Vadgama P. Polypyrrole-based conducting polymers and interactions with biological tissues. J R Soc Interface. 2006;3:741–52. doi: 10.1098/rsif.2006.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen SJ, Wand DY, Yuan CW, Wany XD, Zhang PY, Gu XS. Template synthesis of the polypyrrole tube and its bridging in vivo sciatic nerve regeneration. J Mater Sci Lett. 2000;19:2157–9. [Google Scholar]
- 23.Gomez N, Lee JY, Nickels JD, Schmidt CE. Micropatterned polypyrrole: a combination of electrical and topographical characteristics for the stimulation of cells. Adv Funct Mater. 2007;17:1645–53. doi: 10.1002/adfm.200600669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez N, Schmidt CE. Nerve growth factor-immobilized polypyrrole: bioactive electrically conducting polymer for enhanced neurite extension. J Biomed Mater Res A. 2007;81:135–49. doi: 10.1002/jbm.a.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J-W, Serna F, Nickels J, Schmidt CE. Carboxylic acid-functionalized conductive polypyrrole as a bioactive platform for cell adhesion. Biomacromolecules. 2006;7:1692–5. doi: 10.1021/bm060220q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J-W, Serna F, Schmidt CE. Carboxy-endcapped conductive polypyrrole:biomimetic conducting polymer for cell scaffolds and electrodes. Langmuir. 2006;22:9816–9. doi: 10.1021/la062129d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao C, Zhu A, Wu Q, Chen X, Kim J, Shen J. New biocompatible polypyrrole-based films with good blood compatibility and high electrical conductivity. Colloids Surf B Biointerfaces. 2008;67:41–5. doi: 10.1016/j.colsurfb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Neoh KG, Lau KKS, Wang VVT, Kang ET, Tan KL. Structure and degradation behavior of polypyrrole doped with sulfonate anions and different sizes subject to undoping-redoping cycles. Chem Mater. 1996;8:167–72. [Google Scholar]
- 29.Olayo R, Rios C, Salgado-Ceballos H, Cruz GJ, Morales J, Olayo MG, et al. Tissue spinal cord response in rats after implants of polypyrrole and polyethylene glycol obtained by plasma. J Mater Sci Mater Med. 2008;19:817–26. doi: 10.1007/s10856-007-3080-z. [DOI] [PubMed] [Google Scholar]
- 30.Shi G, Zhang Z, Rouabhia M. The regulation of cell functions electrically using biodegradable polypyrrole-polylactide conductors. Biomaterials. 2008;29:3792–8. doi: 10.1016/j.biomaterials.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Shustak G, Gadzinowski M, Slomkowski S, Domb AJ, Mandler D. A novel electrochemically synthesized biodegradable thin film of polypyrrole-polyethyleneglycol-polylactic acid nanoparticles. New J Chem. 2007;31:163–8. [Google Scholar]
- 32.Wang X, Gu X, Yuan C, Chen S, Zhang P, Zhang T, et al. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J Biomed Mater Res A. 2003;68:411–22. doi: 10.1002/jbm.a.20065. [DOI] [PubMed] [Google Scholar]
- 33.Lee JY, Bashur CA, Goldstein AS, Schmidt CE. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials. 2009;30:4325–35. doi: 10.1016/j.biomaterials.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi G, Rouabhia M, Wang Z, Dao LH, Zhang Z. A novel electrically conductive and biodegradable composite made of polypyrrole nanoparticles and polylactide. Biomaterial. 2004;25:2477–88. doi: 10.1016/j.biomaterials.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 35.Abarrategi A, Gutierrez MC, Moreno-Vicente C, Hortiguela MJ, Ramos V, Lopez-Lacomba JL, et al. Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials. 2008;29:94–102. doi: 10.1016/j.biomaterials.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 36.Edwards SL, Church JS, Werkmeister JA, Ramshaw JA. Tubular micro-scale multiwalled carbon nanotube-based scaffolds for tissue engineering. Biomaterials. 2009;30:1725–31. doi: 10.1016/j.biomaterials.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 37.Park JS, Park K, Moon HT, Woo DG, Yang HN, Park K-H. Electrical pulsed stimulation of surfaces homogeneously coated with gold nanoparticles to induce neurite outgrowth of PC12 cells. Langmuir. 2009;25:451–7. doi: 10.1021/la8025683. [DOI] [PubMed] [Google Scholar]
- 38.Guimard NK, Gomez N, Schmidt CE. Conducting polymers in biomedical engineering. Prog Polym Sci. 2007;32:876–921. [Google Scholar]
- 39.Runge MB, Dadsetan M, Baltrusaitis J, Knight AM, Ruesink T, Lazcano EA, et al. The development of electrically conductive polycaprolactone fumarate-polypyrrole composite materials for nerve regeneration. Biomaterials. 2010;31:5916–26. doi: 10.1016/j.biomaterials.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durgam H, Sapp S, Deister C, Khaing Z, Chang E, Luebben S, et al. Novel Degradable Co-polymers of Polypyrrole Support Cell Proliferation and Enhance Neurite Out-Growth with Electrical Stimulation. J Biomater Sci Polym Ed. 2010 June; doi: 10.1163/092050609X12481751806330. Epub. [DOI] [PubMed] [Google Scholar]
- 41.Huang J, Hu X, Lu L, Ye Z, Zhang Q, Luo Z. Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers. Journal of Biomedical Materials Research - Part A. 2010;93:164–74. doi: 10.1002/jbm.a.32511. [DOI] [PubMed] [Google Scholar]
- 42.Yao L, Wang S, Cui W, Sherlock R, O’Connell C, Damodaran G, et al. Effect of functionalized micropatterned PLGA on guided neurite growth. Acta Biomater. 2009;5:580–8. doi: 10.1016/j.actbio.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Wang S, Kempen DH, Simha NK, Lewis JL, Windebank AJ, Yaszemski MJ, et al. Photo-cross-linked hybrid polymer networks consisting of poly(propylene fumarate) and poly(caprolactone fumarate): controlled physical properties and regulated bone and nerve cell responses. Biomacromolecules. 2008;9:1229–41. doi: 10.1021/bm7012313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leach JB, Brown XQ, Jacot JG, Dimilla PA, Wong JY. Neurite outgrowth and branching of PC12 cells on very soft substrates sharply decreases below a threshold of substrate rigidity. J Neural Eng. 2007;4:26–34. doi: 10.1088/1741-2560/4/2/003. [DOI] [PubMed] [Google Scholar]
- 45.Wang S, Yaszemski MJ, Gruetzmacher JA, Lu L. Photo-crosslinked poly(Îμ-caprolactone fumarate) networks: Roles of crystallinity and crosslinking density in determining mechanical properties. Polymer. 2008;49:5692–9. doi: 10.1016/j.polymer.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Ruiter GC, Malessy MJ, Yaszemski MJ, Windebank AJ, Spinner RJ. Designing ideal conduits for peripheral nerve repair. Neurosurg Focus. 2009;26:E5. doi: 10.3171/FOC.2009.26.2.E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.den Dunnen WF, van der Lei B, Robinson PH, Holwerda A, Pennings AJ, Schakenraad JM. Biological performance of a degradable poly(lactic acid-epsilon-caprolactone) nerve guide: influence of tube dimensions. J Biomed Mater Res. 1995;29:757–66. doi: 10.1002/jbm.820290612. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Rouabhia M, Wang Z, Roberge C, Shi G, Roche P, et al. Electrically conductive biodegradable polymer composite for nerve regeneration: electricity-stimulated neurite outgrowth and axon regeneration. Artif Organs. 2007;31:13–22. doi: 10.1111/j.1525-1594.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 49.Gordon T, Brushart TM, Chan KM. Augmenting nerve regeneration with electrical stimulation. Neurol Res. 2008;30:1012–22. doi: 10.1179/174313208X362488. [DOI] [PubMed] [Google Scholar]
- 50.Gordon T, Chan KM, Sulaiman OA, Udina E, Amirjani N, Brushart TM. Accelerating axon growth to overcome limitations in functional recovery after peripheral nerve injury. Neurosurgery. 2009;65:A132–44. doi: 10.1227/01.NEU.0000335650.09473.D3. [DOI] [PubMed] [Google Scholar]
- 51.Ahlborn P, Schachner M, Irintchev A. One hour electrical stimulation accelerates functional recovery after femoral nerve repair. Exp Neurol. 2007;208:137–44. doi: 10.1016/j.expneurol.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt CE, Shastri VR, Vacanti JP, Langer R. Stimulation of neurite outgrowth using an electrically conductive polymer. Proc Natl Acad Sci USA. 1997;94:8948–53. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kotwal A, Schmidt CE. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials. 2001;22:1055–64. doi: 10.1016/s0142-9612(00)00344-6. [DOI] [PubMed] [Google Scholar]
- 54.Patel NB, Poo MM. Perturbation of the direction of neurite growth by pulsed and focal electric fields. J Neurosci. 1984;4:2939–47. doi: 10.1523/JNEUROSCI.04-12-02939.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel N, Poo MM. Orientation of neurite growth by extracellular electric fields. J Neurosci. 1982;2:483–96. doi: 10.1523/JNEUROSCI.02-04-00483.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J, McNally H, Shi R. Enhanced neurite alignment on micro-patterned poly-L-lactic acid films. J Biomed Mater Res A. 2008;87:392–404. doi: 10.1002/jbm.a.31814. [DOI] [PubMed] [Google Scholar]
- 57.Mahoney MJ, Chen RR, Tan J, Saltzman WM. The influence of microchannels on neurite growth and architecture. Biomaterials. 2005;26:771–8. doi: 10.1016/j.biomaterials.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 58.Corey JM, Lin DY, Mycek KB, Chen Q, Samuel S, Feldman EL, et al. Aligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growth. J Biomed Mater Res A. 2007;83:636–45. doi: 10.1002/jbm.a.31285. [DOI] [PubMed] [Google Scholar]
- 59.Schnell E, Klinkhammer K, Balzer S, Brook G, Klee D, Dalton P, et al. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials. 2007;28:3012–25. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 60.Erskine L, McCaig CD. Growth cone neurotransmitter receptor activation modulates electric field-guided nerve growth. Dev Biol. 1995;171:330–9. doi: 10.1006/dbio.1995.1285. [DOI] [PubMed] [Google Scholar]
- 61.Davenport RW, McCaig CD. Hippocampal growth cone responses to focally applied electric fields. J Neurobiol. 1993;24:89–100. doi: 10.1002/neu.480240108. [DOI] [PubMed] [Google Scholar]
- 62.Cork RJ, McGinnis ME, Tsai J, Robinson KR. The growth of PC12 neurites is biased towards the anode of an applied electrical field. J Neurobiol. 1994;25:1509–16. doi: 10.1002/neu.480251204. [DOI] [PubMed] [Google Scholar]