Abstract
Alkaline phosphatase (ALP) is an enzyme critical for physiological and pathological biomineralization. Experiments were designed to determine if ALP participates in formation of calcifying nanometer-sized particles (NPs) in vitro. Filtered homogenates of human calcified carotid artery, aorta and kidney stones were inoculated into cell culture medium containing 10% fetal bovine serum in the absence or presence of inhibitors of ALP or pyrophosphate. Calcific NP biofilm developed within one week after inoculation and their development was reduced by pyrophosphate and inhibitors of ALP. ALP protein and enzymatic activity were detected in washed NPs whether calcified or decalcified. Therefore, ALP activity is required for formation of calcifying NPs in vitro, as has previously been implicated during pathological calcification in vivo.
Keywords: Biologic nanoparticles, calcification, heterotopic mineralization, hydroxyapatite, nanobacteria
1. INTRODUCTION
Spherical nano-sized structures comprised partly of calcium and phosphorous, historically called matrix vesicles, are present at sites of physiological and pathological mineral deposition such as normal bone and soft-tissues in mammals and birds [1–3]. Formation of these matrix vesicles was considered to be a passive process resulting from pathological increases in plasma calcium and phosphate levels. However, it is now accepted that their formation is a cell-mediated, regulated process which is similar to that of normal osteogenesis, and which is modulated by a group of proteins which either promote or inhibit calcification. Release of these membrane-vesicles by chondrocytes, osteoblasts, odontoblasts and activated or apoptotic cells provides an extracellular environment which promotes the initial steps for generation of hydroxyapatite (HA) crystals, leading to the deposition of extracellular mineral [4–7].
Structures similar to matrix vesicles have also been identified in histological sections of human calcified vascular tissue, renal papillae and kidney stones, whereas they are absent in comparable uncalcified tissues [8–12]. When filtered homogenates of calcified human tissue, blood or saliva are placed into cell culture, a calcific biofilm develops which is composed of nanosized complexes of HA and protein similar in anatomical appearance to matrix vesicles, but previously described as either nanobacteria [8, 13, 14] or calcifying nanoparticles [9, 11, 12, 15, 16]. Due to the failure to consistently sequence unique nucleic acid from these vesicles/particles, and because development of nano-sized structures is dependent upon the chemical composition of the in vitro milieu, the term nanoparticles (NPs) is now preferred to describe these structures [17]. Because nano-sized structures are associated with sites of ectopic mineralization, study of the capacity of NPs to propagate and induce HA mineral deposition in vitro may provide insight into the biochemical processes of pathological mineralization.
When incubated under tissue culture conditions, NPs develop both as an adherent calcified biofilm and exist as free-floating "planktonic” forms [15]. Both structures contain an outer calcium phosphate shell and inner core composed of both mammalian [18, 19] and prokaryotic proteins [12]. Some of the NP-associated proteins are structural or carrier molecules, for example fetuin-A, also known as α2-HS-Glycoprotein, a circulating hepatocyte-derived calcification inhibitor [20–23]
Alkaline phosphatase (ALP) belongs to a group of enzymes which individually promotes or inhibits mineralization in a highly-regulated process [24–26]. ALP is a cell membrane-associated enzyme that hydrolyzes inorganic pyrophosphate (PPi), a potent suppressor of HA crystal growth. Hydrolysis of PPi yields inorganic phosphate (Pi), a substrate for HA mineral [27–29], and thus, contributes to regulation of normal bone formation as well as in pathological extraosseous mineralization. The association of ALP with physiological and pathological mineralization is supported by observations that mice deficient in the gene for tissue non-specific ALP develop severe hypophosphatasia and exhibit abnormal bone development [30]. Furthermore, cultured aortic smooth muscle cells derived from spontaneously hypertensive rats (SHR) develop extracellular calcification, whereas similar cells derived from normotensive animals do not. Gene expression of ALP protein is higher in the cells from the SHR animals than the normotensive controls [31]. Serum levels of Pi and ALP also are correlated with vascular and renal calcification in humans [32, 33]. Given the participation of ALP in calcification processes, experiments were conducted to test the hypothesis that in vitro formation of calcifying NPs derived from human diseased tissues requires ALP.
2. MATERIALS AND METHODS
2.1. Reagents and drugs
Beryllium sulfate tetrahydrate, levamisole-hydrochloride, sodium pyrophosphate, phenylmethanesulfonyl fluoride and hydroxyapatite suspension (in 0.001 M phosphate buffer, pH 6.8; approx. 25% solid) were obtained from Sigma Chemical, St. Louis, MO. Beta-Glycerophosphate was from Calbiochem, Gibbstown, NJ. Dulbecco’s Modified Eagle Medium (DMEM) was purchased from Mediatech Inc., Manassas, VA. Gamma-irradiated fetal bovine serum was from Atlanta Biologicals, Lawrenceville, GA. All other chemicals were from ThermoFisher Scientific Inc., Waltham, MA. Beryllium sulfate and levamisole stocks were prepared in water and DMEM, respectively. Water used for these experiments was double-distilled; all water and phosphate-buffered saline (PBS, pH 7.4) were filtered (0.2 µm) prior to use.
2.2. Preparation of NP from tissue isolates
Calcified and uncalcified human segments from sub-adventitial endarterectomy or full thickness pieces of abdominal aorta and carotid arteries were collected under aseptic conditions as waste during surgical procedures for vascular repair in accordance with the guidelines of the Institutional Review Board governing use of human materials at Mayo Clinic, Rochester, MN [9]. Each segment was placed into sterile phosphate-buffered saline (PBS) on ice, and sent to the laboratory for immediate processing within an hour of explantation. Segments with high, low and no calcification were collected from separate individuals. A piece (200–300 mg) was cut from each vascular segment, rinsed, placed into 2 ml PBS and finely minced with scissors. These pieces were disrupted using several strokes in a Potter-Elvehjem homogenizer. The sample was then centrifuged for 15 minutes at 2,500Xg to pellet debris. The supernatant was filtered successively through 1.2, 0.45, and 0.2 µm cellulose acetate filters (Whatman Inc., Piscataway, NJ). This filtered isolate was used for the culture of NP (see below).
To characterize the material remaining after the 0.2 µm filtration, the filtrate from some preparations was divided into two equal portions; one portion was placed into culture for NP propagation as described below. The other was centrifuged at 125,000Xg, 4°C for 60 min. The supernatant was removed, diluted in medium and placed in culture. A sample of high-speed pellet was examined by transmission electron microscopy for structural elements (i.e. matrix vesicles); the remainder of the pellet was re-suspended in medium and cultured.
Kidney stones classified as calcium phosphate kidney stones (strain AP11) after compositional analysis in the Mayo Clinic Metals Laboratory, were washed with distilled water, dried, pulverized using a mortar and pestle, and stored at 4°C until use. Pulverized stones were demineralized in 1N HCl for 10 minutes with constant stirring, then neutralized with 1N NaOH. This suspension was centrifuged at 60,000Xg (Sorvall Evolution-RC centrifuge, ThermoFisher) for 1 hr at 4°C, followed by re-suspension of the resulting pellet in DMEM. After vortexing, the sample was filtered, first through a No. 42 filter (Whatman Inc., Piscataway, NJ), then through a 0.2 µm filter. An aliquot of filtrated isolate was used to culture NP.
2.3. Propagation and collection of NPs
The filtered isolate from either the vascular or kidney stone homogenates was diluted 1:20 in cell culture medium (DMEM) containing 10% γ–irradiated fetal bovine serum (FBS). Concentrations of Ca2+ and Pi were 1.8 mM and 0.9 mM, respectively, being derived from both the medium and FBS.
Filtered tissue homogenate (0.2 mL) was injected into culture medium (5 ml) and placed into 25 cm2 vented culture flasks and maintained in a humid 13% CO2 incubator at 37°C. After 2–3 week, the adherent calcific biofilm containing NPs was scraped from the bottom of each flask into the medium. The turbidity of the medium was then measured in Nephelometric Turbidity Units (NTU) using a Hach Model 2100N turbidimeter (Hach Co., Loveland, CO) which was used as an index of NP propagation. This methods measures total NPs, those from the biofilm and those which were free floating (planktonic) in the media. Random flasks were screened for bacterial contamination, including Mycoplasma, using a sensitive rapid PCR test, by the Mayo Clinic Microbiology Laboratory; all results were negative.
In some experiments, commercial synthetic HA crystals were added to the culture media (instead of tissue filtrate) and processed in parallel with flasks containing the tissue filtrate to serve as a non-biological control.
To determine whether ALP was required for NP propagation, the culture medium was modified by addition of either an ALP inhibitor (beryllium, 30 µM or levamisole 1mM), an ALP substrate (β-glycerophosphate, 5 mM) [34] or an inhibitor of apatite mineral deposition (PPi, 10 µM).
2.4. Transmission electron microscopy (TEM)
To examine structural elements, the 125,000Xg fraction of a freshly isolated artery was fixed in 3% glutaraldehyde overnight at 4°C and then embedded in Epon/Araldite resin, thin sectioned and transferred to a carbon-coated grid. The grid was then negatively stained with 2% uranyl acetate, dried, and examined using TEM (Tecnai T12, FEI Co., Hillsboro, OR). In other experiments, NPs propagated for 14 days were examined using whole mounts. The NPs were harvested then fixed as described above. A 25 µl drop of suspended NPs was transferred to a grid; each grid was then stained, dried and examined using TEM.
2.5. Enzyme activity, Protein isolation and Western blotting
ALP activity in medium and in harvested NPs before and after decalcification was measured colorimetrically as the hydrolysis of p-nitrophenyl phosphate at pH 9.8 according to the manufacturer’s instructions (Sigma, Inc.). To decalcify NPs, the harvested NPs were suspended in 0.5M EDTA with continuous shaking overnight at 4°C. Decalcified NPs (dNPs) were then pelleted by centrifugation at 60,000Xg and washed twice in PBS.
To measure protein, pellet of decalcified NPs was suspended in PBS containing 1mM phenylmethanesulfonyl fluoride and the resulting suspension was sonicated for 20 minutes on ice (10% wave intensity), followed by centrifugation at 8,000Xg for 5 minutes at 4°C. Total protein in the supernatant was determined colorimetrically using a kit based on bicinchoninic acid (BCA; Pierce Biotechnology, Rockford, IL); individual proteins were resolved via SDS-PAGE under reducing conditions and these resulting bands were identified via Coomassie brilliant blue staining. For Western blotting, gels were destained, electro-transferred to a PVDF membrane, and probed using a polyclonal primary antibody raised in goats which maps an epitope within an internal region of ALP of human origin (ALP I-12; Santa Cruz Biotechnology, Santa Cruz, CA), followed by donkey anti-goat IgG-HRP (Santa Cruz).
2.6. Statistics
Statistical data are presented as mean ± SEM; n = the number of times the experiment was repeated using filtered tissue homogenates from different patients. The Student’s t-test was used to determine differences from control conditions; statistical significance was accepted at P< 0.05. One exception was the initial survey of various tissues to identify possible sources of NPs (n=1 each).
3. RESULTS
3.1. Absence of matrix vesicles in tissue homogenates which proprogate NPs
The filtered homogenate prepared from calcified vascular tissue contained structures similar in size and appearance to matrix vesicles previously isolated from human atherosclerotic aorta (Fig. 1) [35]. Supernatant resulting from the125,000Xg centrifugation of the homogenate retained the capacity to propagate NPs in culture, whereas the pelleted fraction that contained matrix vesicle-like structures from the homogenate did not (Fig. 1).
Figure 1.
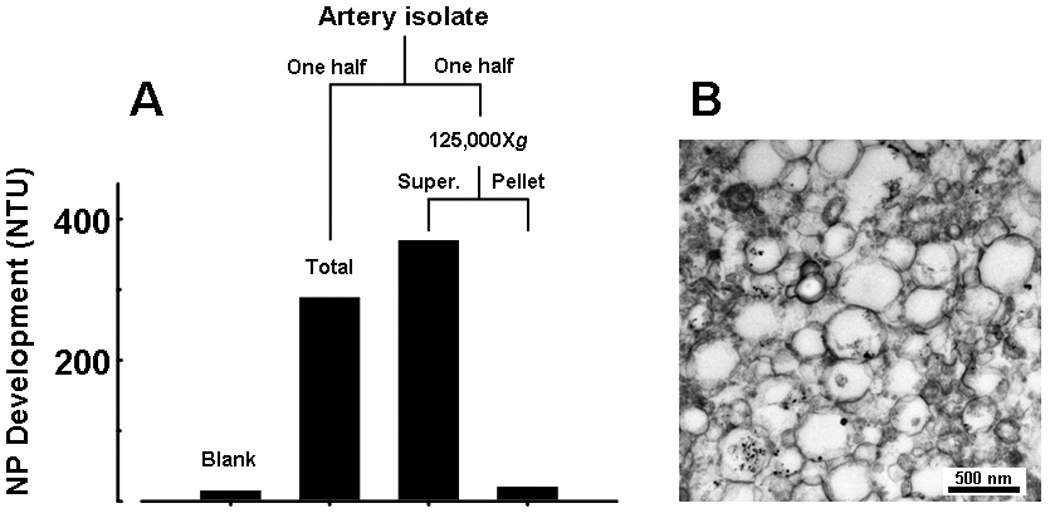
NPs propagate in vitro from explanted calcific carotid artery isolates after removal of nano-sized components. Fresh artery tissue was prepared as described in Methods, 0.2 µm-filtered and divided in half. One half was added to 5 ml medium and cultured for 3 weeks (Total); the other half was centrifuged at 125,000Xg and the resulting supernate and pellet were cultured separately. A: NP development after 3 weeks in culture: NPs readily propagated from complete sample (Total homog); propagation was similar in replicate sample devoid of structural components (Supernate), but was absent in fraction containing these components (Pellet). B: TEM micrograph showing pelleted structures in size range of matrix vesicles and apoptotic bodies.
3.2. Evidence for ALP activity in the culture medium
ALP activity in freshly-prepared DMEM containing 10% FBS, measured colorimetrically with p-nitrophenol phosphate was 0.02 U/ml; this activity decreased by 91% with addition of 1 mM levamisole (not shown). Increases in turbidity of the media were used as an indirect measure of NP formation. The turbidity of DMEM plus 10% γ– irradiated FBS incubated under culture conditions for 14 days increased significantly with the addition of 5 mM β-glycerophosphate, a non-biological substrate for ALP. (Fig. 2). Concurrent addition of levamisole to β-glycerophosphate-supplemented medium reduced the turbidity to control levels (Fig. 2).
Figure 2.
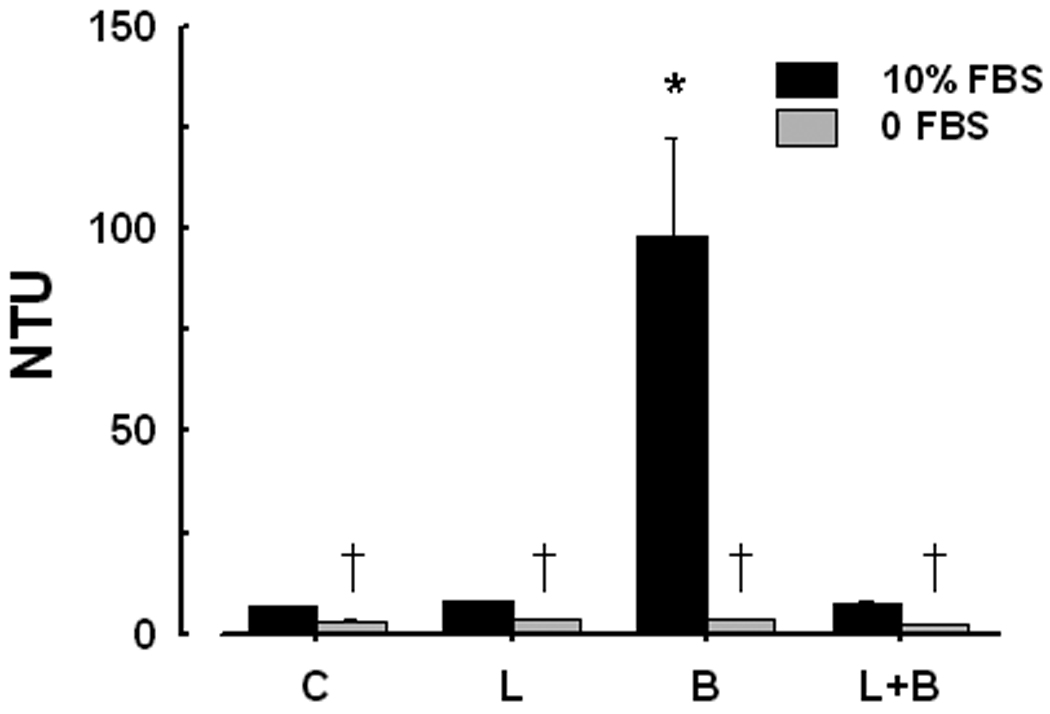
Changes in turbidity of the media (DMEM plus 10% FBS; dark bars) expressed as Nephelometric Turbidity Units (NTU) in the absence (control, C) and presence of ALP substrate β-glycerophosphate (5 mM; B) and ALP inhibitor levamisole (1 mM; L) after 14 days of incubation. Turbidity of paired samples of DMEM without FBS (light bars) was near background. Statistically significant difference. (P < 0.05) from: *, 10% FBS control; †, corresponding 10% FBS sample.
3.3. NPs propagated in vitro from isolates of calcified tissue require ALP activity
Turbidity of media (NP formation) increased when seeded with filtered supernatant derived from a 2,500Xg centrifugation of calcified tissues homogenates (calcified aortic aneurysm, carotid artery and kidney stone), but not from a non-calcified aneurysm (Fig. 3). The competitive ALP inhibitor, beryllium (30 µM), or an inhibitor of apatite mineral deposition, PPi (10 µM), reduced turbidity (NP formation) to 6% – 28% of levels observed in flasks without inhibitors (controls, Fig. 3).
Figure 3.
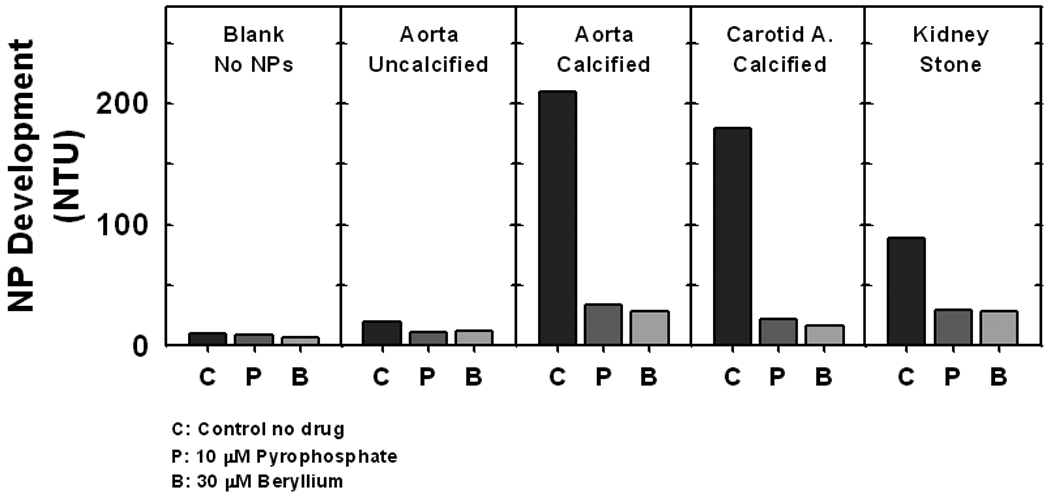
Propagation of NPs in vitro from isolates of explanted vascular tissue and calcium phosphate kidney stones. Filtered supernatant from the 2,500g spin of tissue homogenates were seeded into flasks. After 14 days in culture, the turbidity of the medium containing total NPs (in Nephelometric Turbidity Units; NTU) was measured and used as an index of NP propagation. NPs propagated from all calcified tissues but not from an uncalcified aorta. Propagation was attenuated by PPi (10 µM), an inhibitor of hydroxyapatite mineral deposition, and by the ALP inhibitor beryllium (30 µM). n=1 each.
To confirm these observations using other calcified tissue isolates and other ALP inhibitors, kidney stone-derived homogenates were seeded into flask containing DMEM and FBS. Turbidity of media without tissue homogenates (NPs;controls) and of media seeded with kidney stone homogenates was low at baseline (day 1; 2.7 ± 0.1 and 4.7 ± 0.3 NTU, respectively; n = 5 each; Fig. 4). After 21 days, turbidity of media seeded with kidney stone homogenates significantly increased (296.2 ± 33.4 NTU, n = 5) compared to the turbidity of control flasks (6.1 ± 0.2 NTU, n = 5). This increase was significantly attenuated in the presence of levamisole (1 mM; 23.1 ± 0.4 NTU, n = 5). NPs that formed under control conditions ranged in size from 150–300 nm, with crystals on the exterior structure (Fig. 4b). However, NPs cultured in the presence of levamisole appeared smaller with less associated crystal (Fig. 4b). Previous studies demonstrated that these crystals are composed of calcium phosphate hydroxyapatite [9].
Figure 4.
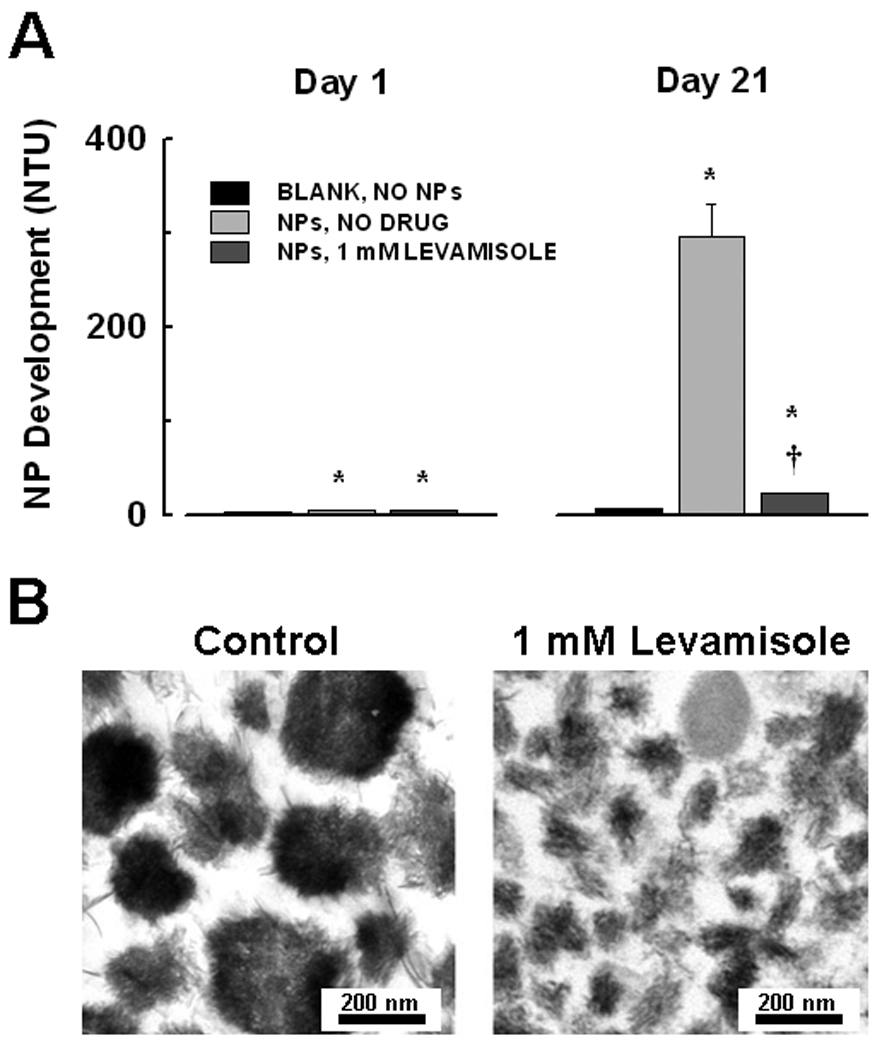
Inhibition of NP propagation in vitro by levamisole. A: Kidney stone derived-NPs were cultured in flasks in DMEM/10% FBS for 21 days. Baseline (day 1) turbidity (NTU) of NP-seeded medium was slightly above that of unseeded blanks. After 21 days, turbidity increased significantly in seeded flasks. Turbidity was significantly reduced in the presence of 1 mM levamisole, an ALP inhibitor. Mean ± SEM, n = 5. Significant difference (P < 0.05) from corresponding: *, blank; †, untreated seeded sample. B: Inhibition of ALP reduces NP mineralization in vitro. Transmission electron micrographs of NPs derived from a calcified human aortic aneurysm taken after propagation in DMEM/10% FBS for 3 weeks in the absence (control, left) or presence (right) of 1 mM levamisole. The deposition of crystals seen in control NPs, shown by previous work to be hydroxyapatite [9], was reduced by levamisole, thus resulting in smaller sized NPs.
3.4. ALP activity in NPs
ALP activity was detected in NPs harvested after 14 days in culture and washed three times with physiological saline to remove adherent proteins (Fig. 5). ALP activity also was present, although reduced, in NPs after decalcification with 0.5M EDTA. HA crystals incubated in DMEM containing 10% FBS under similar conditions and processed in the same way also contained measurable but low ALP activity. However, ALP activity was near background levels in HA crystals incubated in DMEM without FBS (Fig. 5).
Figure 5.
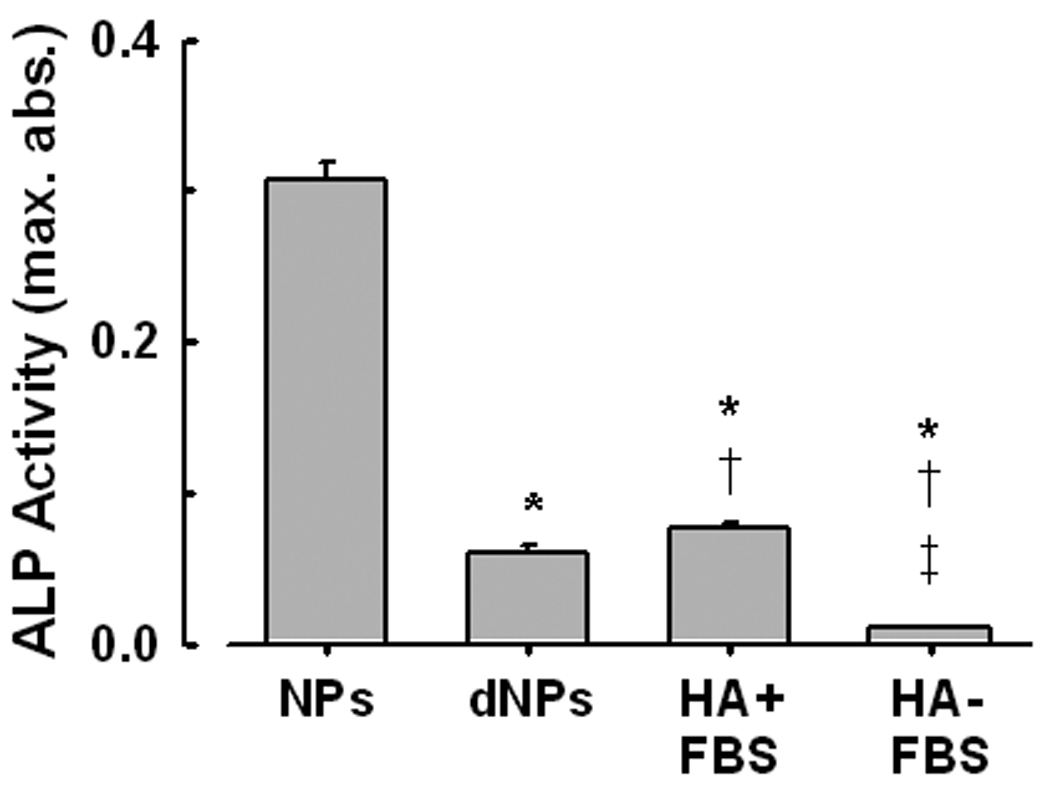
ALP activity measured in kidney stone-derived NPs propagated for 14 days in DMEM/10% FBS. NPs were isolated and washed in cold PBS before assay. ALP activity was measured in the isolated NPs before and after demineralization (using 0.5M EDTA; dNPs), and in commercial hydroxyapatite crystals incubated under similar conditions (HA+FBS). ALP activity was at background levels when FBS was omitted (HA-FBS) from the culture. Mean ± SEM, n = 5 each. Sig. Dif. (P < 0.05) from: *, NPs (calcified); †, dNPs; ‡, HA+FBS.
3.5. Immunological detection of ALP antigen
In a typical experiment approximately 250 µg of protein was recovered after decalcification of one flask of NPs. Numerous protein bands that stained with Comassie-blue were detected by SDS-PAGE of decalcified NP pellets (Fig. 6A).
Figure 6.
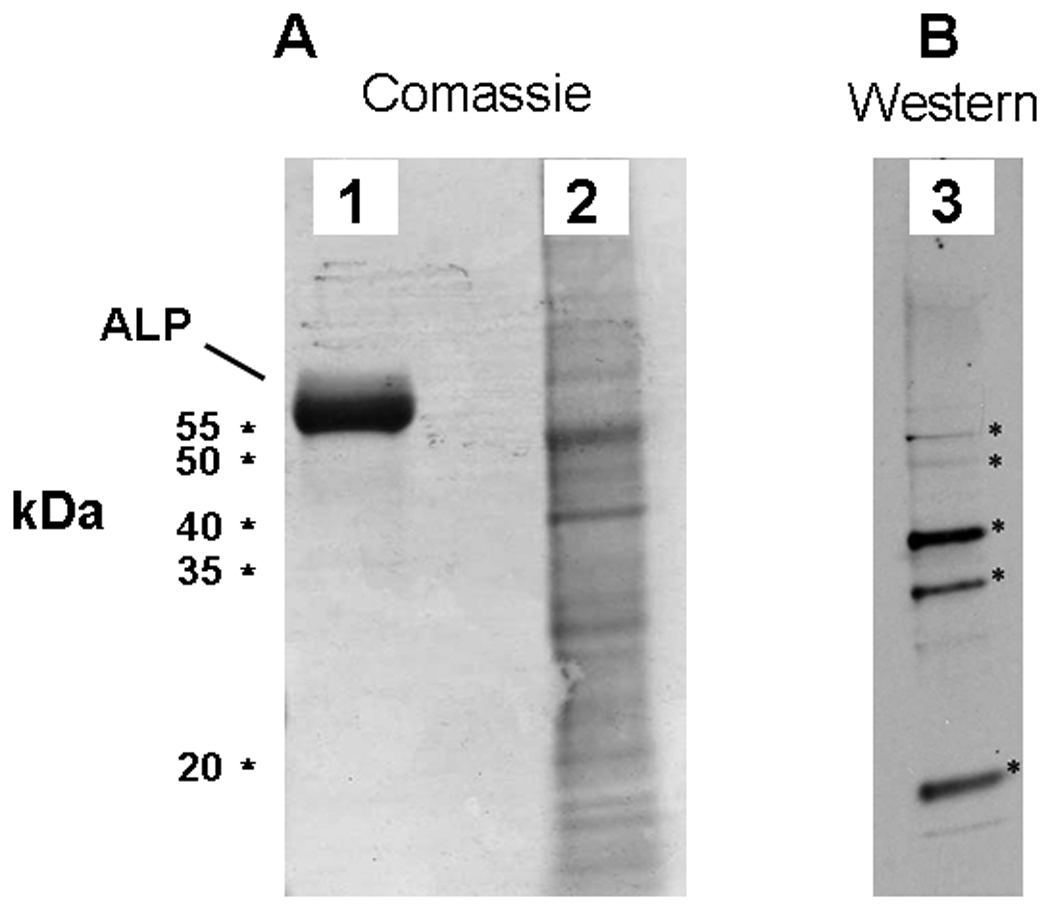
ALP associates with NPs in vitro. A: Coomassie brilliant blue stained SDS-PAGE gel comparing protein profiles of a decalcified NP isolate (2) and an ALP standard (1). B: Western blot of comparable NP isolate probed with a polyclonal antibody against an internal region of human ALP. Five of the most prominent bands recognized by the antibody (indicated by stars) were cut from a companion gel and subjected to MS/MS analysis for determination of NP protein.
A commercial antibody raised against human ALP recognized at least 5 bands in decalcified NP lysate by Western blotting, 2 of these bands migrated to the approximate molecular weight of human or bovine ALP, and 3 others of smaller molecular weight (Fig. 6B). The bands recognized by the anti-ALP antibody were then cut from a duplicate gel for further analysis in order to identify the proteins by trypsin digestion and tandem mass spectrometry. Neither eukaryotic nor prokaryotic ALP was detected via this analysis, even though these bands were recognized by the ALP antibody.
4. DISCUSSION
This study confirms that calcifying biologic NPs form in vitro from filtered homogenates of calcified vascular tissue and kidney stones, but not from non-calcified vascular tissue [9, 12]. It is unlikely that NPs studied in the present experiments were matrix vesicles since NPs could not be propagated from pelleted fractions resulting from high speed centrifugation of tissue homogenates whereas NPs did propagate from the corresponding supernatant. Furthermore, although ALP is expressed in matrix vesicles derived from various cells [7, 38, 39], it is absent or present at low levels in matrix vesicles isolated from fresh atherosclerotic blood vessels. These vesicles are enriched in ATPase, AMPase and NTP pyrophosphohydrolase. These other hydrolases regulate calcification [35, 40].
In contrast, results of this study provide evidence that ALP is required for propagation of NP derived from homogenates of calcified tissue. Found in most species from bacteria to man, ALP is a ubiquitous dimeric enzyme which catalyzes hydrolysis of phosphomonoesters, with release of Pi [24] and is implicated in the formation of renal stones and in arterial calcification [27, 28]. Development and propagation of NPs from these tissues were inhibited by both beryllium and levamisole, inhibitors of ALP [26, 29, 41]. Beryllium, a competitive ALP inhibitor [42] reduces mineralization as well as corresponding ALP activity [41]. In contrast, levamisole, an uncompetitive ALP inhibitor [36, 37] binds to the ALP/substrate complex. In the present study, levamisole reduced NP formation and inhibited ALP activity by similar degrees (91%) in addition to reducing the amount of apatite mineral in association with NPs as determined by TEM. To our knowledge, neither beryllium nor levamisole has been shown to inhibit mineralization via a direct effect on crystal growth. ALP activity in culture medium containing 10% FBS, as used in this study, was measured at 0.02 U/ml, sufficient to hydrolyze 5 mM β-glycerophosphate and increase medium turbidity, most likely due to precipitation of calcium phosphate [34]. The lack of change in turbidity in β-glycerophosphate-treated medium in the presence of levamisole confirms the capacity of antagonists of ALP to modulate NP propagation.
Exogenous PPi was used in this study as a tool to inhibit apatite mineral deposition. The effect of endogenous PPi on mineralization appears to be concentration-dependent and bimodal [43]. Lower concentrations (< 0.1mM) favor mineralization because PPi is efficiently hydrolyzed by ALP liberating Pi, making it available for formation of apatite. However, at supraphysiological concentrations, as used in the present study, a large proportion of PPi remains unhydrolyzed and available to bind HA thereby inhibiting mineralization.
Electron microscopic examination of calcified vascular tissue sections by energy-dispersive x-ray analysis indicated that the mineral present in these segments was in the form of calcium phosphate HA [9]. NPs that develop in vitro also appear to be complexes of proteins together with calcium phosphate HA with no evidence of carbonated HA or of other minerals [8, 9, 44]. In the present study, the mineral content of NPs developed under various pH or calcium concentrations was not examined.
This study extends previous findings by demonstrating that addition of PPi at a concentration which suppresses calcification in vitro [27, 45] inhibits formation of this calcified shell, whereas ALP activity promotes it. Certain plasma proteins bind to HA [46], and several groups including ours have shown that two of these, fetuin-A and albumin, are localized within NPs [18, 19, 44]. The present results also suggest that ALP may bind to HA since ALP activity was reduced by demineralization of the NPs. Indeed, ALP activity was present with commercial apatite crystals that were incubated in DMEM containing 10% FBS.
Numerous proteins were identified in NPs by SDS-PAGE; five of these proteins were recognized by an antibody to human ALP in Western blots. However, attempts to identify the source of the ALP antigen i.e., mammalian vs prokaryotic, by mass spectroscopy, were unsuccessful. Due to the data-dependent nature of this type of analysis, it is apparent that ALP is not the major protein in the bands recognized by the antibody, and a more purified sample will need to be submitted for analysis.
Several bone-related proteins are potential candidates for involvement in NP mineralization and propagation including fetuin-A [47], matrix Gla protein [48], osteopontin [49], and osteoprotegerin [50], all of which have been shown to be active participants in pathological calcification, and importantly, are regulated at the gene level. Others have provided evidence that NPs contain serum proteins, including albumin and fetuin-A, which assemble through common bio and physical chemical interactions, but have dismissed their pathogenic potential [16, 18, 19, 51].
It is not yet known if ALP activity associated with calcifying NPs derived from human diseased tissue is derived entirely from eukaryotic sources. The recent finding of the prokaryotic elongation factor-Tu (EF-Tu) in calcifying NPs suggests that bacterial proteins may participate in these events as well, perhaps entering the system as fragments or remnants of viable bacteria from tissue homogenates [15, 44]. Interestingly, EF-Tu can be localized at the cell surface and has a capacity to induce a pro-inflammatory response [52]. If similar events occur in the blood, NPs could be pathogenic factors in humans as they were found to be when injected into rabbits with vascular injury [53, 54]. These results suggest that propagation of calcific NP-biofilm from human calcified disease tissue is not simply nonspecific protein precipitation since both ALP activity and ongoing calcification appear to be required. Therefore, development of calcifying NPs may involve a complex sequence of events in which ALP, one component protein, acts locally in a calcium-rich solution (blood, urine, DMEM) to create an environment conducive to mineralization, i.e., hydrolysis of PPi with concomitant increase in Pi levels (Fig. 7). Once formed, calcified NPs can circulate in the blood, bind to sites of injury and promote calcification. Formation and circulation of NPs associated with hyrdoxyapatite in vivo could represent a novel mechanism that promotes soft tissue calcification in humans [55].
Figure 7.
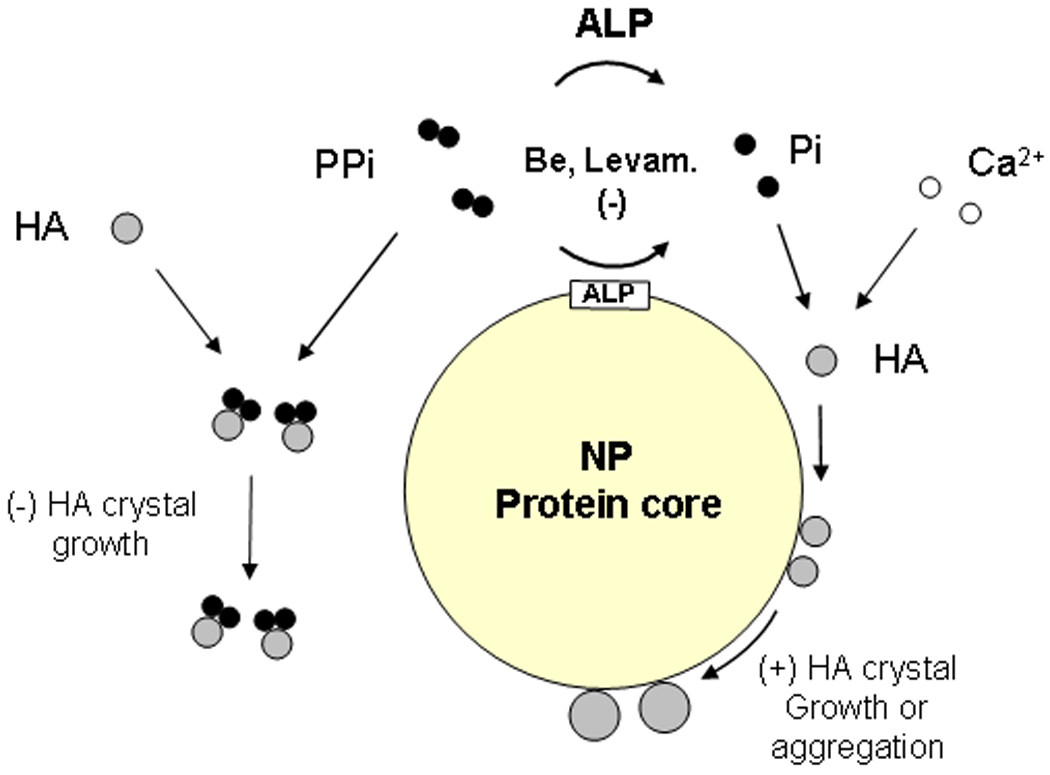
Possible relationships among proteins involved with formation and mineralization of NPs. Fetuin-A and albumin of likely bovine origin and other proteins with known affinity for calcium phosphate crystals have been identified in the protein core of NPs. Proteins of prokaryotic origin, considered to be important factors in metabolism, such as EF-Tu and EF-G, also appear to be present. ALP, which is associated with NPs and in plasma/extracellular space, hydrolyzes PPi and other substrates, including nucleotide triphosphates. The hydrolysis is inhibited by beryllium and levamisole. The liberated Pi, together with Ca2+ forms small HA crystals. PPi binds to some of the crystals, inhibiting their growth, whereas other crystals bind to the NP protein(s). Crystals bound to certain NP proteins may continue to grow by increasing mineral deposition. Alternatively, NPs may aggregate, forming larger protein/mineral complexes.
5. CONCLUSION
Self-propagating biologic NPs with the capacity to accumulate hydroxyapatite mineral developed in vitro from 0.2 µm-filtered isolates of calcified but not from non-calcified human vascular tissues and calcium phosphate kidney stones. NPs appear to be comprised of a mixture of proteins and hydroxyapatite. One such protein was ALP, an enzyme which is critical to NP development. NPs propagated in vitro utilized ALP derived partly from the serum present in the culture medium. However, NPs themselves possessed ALP activity, not all of which was complexed with mineral. Biologic NPs are localized to calcific regions of vascular tissue in situ, and ALP is up-regulated in diseases such as chronic kidney disease which is characterized by vascular calcification. Thus, propagation of NPs in vitro is a useful tool to examine the possible role of NPs in heterotopic mineralization, and the function of ALP as an active component of the mineralization process.
ACKNOWLEDGEMENTS
Grant support: National Institutes of Health HL 88988, the John E. Fetzer Memorial Trust, and the Ministry of Education and Science Technology (R13-2007-019-00000-0), Republic of South Korea.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenhill NS, Presland MR, Rogers KM, Stehbens WE. X-ray microanalysis of mineralized matrix vesicles of experimental saccular aneurysms. Exp Mol Pathol. 1985;43:220–232. doi: 10.1016/0014-4800(85)90042-5. [DOI] [PubMed] [Google Scholar]
- 2.Kim KM. Calcification of matrix vesicles in human aortic valve and aortic media. Federation Proc. 1976;35:156–162. [PubMed] [Google Scholar]
- 3.Anderson HC, Reynolds JJ. Pyrophosphate stimulation of calcium uptake into cultured embryonic bones. Fine structure of matrix vesicles and their role in calcification. Dev Biol. 1973;34:211–227. doi: 10.1016/0012-1606(73)90351-5. [DOI] [PubMed] [Google Scholar]
- 4.Wu LNY, Sauer GR, Genge BR, Valhmu WB, Wuthier RE. Effects of analogues of inorganic phosphate and sodium ion on mineralization of matrix vesicles isolated from growth plate cartilage of normal rapidly growing chickens. J Inorganic Biochem. 2003;94:221–235. doi: 10.1016/s0162-0134(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 5.Boyan BD, Schwartz ZVI, Carnes DL, Jr, Ramirez V. The effects of vitamin D metabolites on the plasma and matrix vesicle membranes of growth and resting cartilage cells in vitro. Endocrinology. 1988;122:2851–2860. doi: 10.1210/endo-122-6-2851. [DOI] [PubMed] [Google Scholar]
- 6.Kirsch T, Nah H-D, Shapiro IM, Pacifici M. Regulated production of mineralizationcompetent matrix vesicles in hypertrophic chondrocytes. J Cell Biol. 1997;137:1149–1160. doi: 10.1083/jcb.137.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson HC. Molecular biology of matrix vesicles. Clin Orthop Relat Res. 1995;314:266–280. [PubMed] [Google Scholar]
- 8.Kajander EO, Ciftcioglu N. Nanobacteria: An alternative mechanism for pathogenic intra- and extracellular calcification and stone formation. Proc Natl Acad Sci USA. 1998;95:8274–8279. doi: 10.1073/pnas.95.14.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller VM, Rodgers G, Charlesworth JA, Kirkland B, Severson SR, Rasmussen TE, Yagubyan M, et al. Evidence of nanobacterial-like structures in human calcified arteries and cardiac valves. Am J Physiol: Heart Circ Physiol. 2004;287:H1115–H1124. doi: 10.1152/ajpheart.00075.2004. [DOI] [PubMed] [Google Scholar]
- 10.Shiekh FA, Khullar M, Singh SK. Lithogenesis: Induction of renal calcifications by nanobacteria. Urol Res. 2006;34:53–57. doi: 10.1007/s00240-005-0034-0. [DOI] [PubMed] [Google Scholar]
- 11.Ciftcioglu N, Vejdani K, Lee O, Mathew G, Aho KM, Kajander EO, McKay DS, et al. Association between Randall's plaque and calcifyng nanoparticles. International Journal of Nanomedicine. 2008;3:105–115. doi: 10.2147/ijn.s2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar V, Farell G, Yu S, Harrington S, Fitzpatrick L, Rzewuska E, Miller VM, et al. Cell biology of pathologic renal calcification: contribution of crystal transcytosis, cellmediated calcification, and nanoparticles. J Investig Med. 2006;54:412–424. doi: 10.2310/6650.2006.06021. [DOI] [PubMed] [Google Scholar]
- 13.Folk RL. Nannobacteria in the natural environment and in medicine. Alpe Adria Microbiol J. 1998;7:87–95. [Google Scholar]
- 14.Ciftcioglu N, Pelttari A, Kajander EO. Extraordinary growth phases of Nanobacteria isolated from mammalian blood. SPIE. 1997;3111:429–435. [Google Scholar]
- 15.Schwartz MK, Hunter LW, Huebner M, Lieske JC, Miller VM. Characterization of biofilm formed by human-derived nanoparticles. Nanomedicine. 2009;4:931–941. doi: 10.2217/nnm.09.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cisar JO, Xu D-Q, Thompson J, Swaim W, Hu L, Kopecko DJ. An alternative interpretation of nanobacteria-induced biomineralization. Proc Natl Acad Sci USA. 2000;97:11511–11515. doi: 10.1073/pnas.97.21.11511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young JD, Martel J. The Rise and Fall of Nanobacteria. Sci Am. 2010;302:52–59. doi: 10.1038/scientificamerican0110-52. [DOI] [PubMed] [Google Scholar]
- 18.Young JD, Martel J, Young L, Wu CY, Young A, Young D. Putative nanobacteria represent physiological remnants and culture by-products of normal calcium homeostasis. PLoS ONE. 2009;4:e4417. doi: 10.1371/journal.pone.0004417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raoult D, Drancourt M, Azza S, Nappex C, Guieu R, Rolain J, Fourquet P, et al. Nanobacteria Are Mineralo Fetuin Complexes. PLoS Pathogens. 2008;4:e41. doi: 10.1371/journal.ppat.0040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schinke T, Amendt C, Trindl A, Poschke O, Muller-Esterl W, Jahnen-Dechent W. The serum protein alpha2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J Biol Chem. 1996;271:20789–20796. doi: 10.1074/jbc.271.34.20789. [DOI] [PubMed] [Google Scholar]
- 21.Price PA, Lim JE. The inhibition of calcium phosphate precipitation by fetuin is accompanied by the formation of a fetuin-mineral complex. J Biol Chem. 2003;278:22144–22152. doi: 10.1074/jbc.M300744200. [DOI] [PubMed] [Google Scholar]
- 22.Heiss A, DuChesne A, Denecke B, Grotzinger J, Yamamoto K, Renne T, Jahnen-Dechent W. Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J Biol Chem. 2003;278:13333–13341. doi: 10.1074/jbc.M210868200. [DOI] [PubMed] [Google Scholar]
- 23.Wu C-Y, Martel J, Young D, Young JD. Fetuin-A/Albumin-Mineral Complexes Resembling Serum Calcium Granules and Putative Nanobacteria: Demonstration of a Dual Inhibition-Seeding Concept. PLoS ONE. 2009;4:e8058. doi: 10.1371/journal.pone.0008058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman JE. Structure and mechanism of alkaline phosphatase. Annu Rev Biophys Biomol Struct. 1992;21:441–483. doi: 10.1146/annurev.bb.21.060192.002301. [DOI] [PubMed] [Google Scholar]
- 25.Schoppet M, Shanahan CM. Role for alkaline phosphatase as an inducer of vascular calcification in renal failure? Kidney Int. 2008;73:989–991. doi: 10.1038/ki.2008.104. [DOI] [PubMed] [Google Scholar]
- 26.Lomashvili KA, Garg P, Narisawa S, Millan JL, O'Neill WC. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int. 2008;73:1024–1030. doi: 10.1038/ki.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lomashvili K, Cobbs S, Hennigar R, Hardcastle K, O'Neill W. Phosphate-Induced Vascular Calcification: Role of Pyrophosphate and Osteopontin. J Am Soc Nephrol. 2004;15:1392–1401. doi: 10.1097/01.asn.0000128955.83129.9c. [DOI] [PubMed] [Google Scholar]
- 28.Moochhala SH, Sayer JA, Carr G, Simmons NL. Renal calcium stones: insights from the control of bone mineralization. Exp Physiol. 2008;93:43–49. doi: 10.1113/expphysiol.2007.040790. [DOI] [PubMed] [Google Scholar]
- 29.Huang MS, Sage AP, Lu J, Demer LL, Tintut Y. Phosphate and pyrophosphate mediate PKA-induced vascular cell calcification. Biochem Biophys Res Commun. 2008;374:553–558. doi: 10.1016/j.bbrc.2008.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narisawa S, Frohlander N, Millan JL. Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev Dyn. 1997;208:432–446. doi: 10.1002/(SICI)1097-0177(199703)208:3<432::AID-AJA13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Kanemaru K, Seya K, Miki I, Motomura S, Furukawa KI. Calcification of Aortic Smooth Muscle Cells Isolated From Spontaneously Hypertensive Rats. Journal of Pharmacological Sciences. 2008;106:280–286. doi: 10.1254/jphs.fp0072013. [DOI] [PubMed] [Google Scholar]
- 32.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of Serum Phosphorus and Calcium X Phosphate Product With Mortality Risk in Chronic Hemodialysis Patients: A National Study. Am J Kidney Dis. 1998;31:607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 33.Shantouf R, Kovesdy CP, Kim Y, Ahmadi N, Luna A, Luna C, Rambod M, et al. Association of Serum Alkaline Phosphatase with Coronary Artery Calcification in Maintenance Hemodialysis Patients. Clinical Journal of the American Society of Nephrology. 2009;4:1106–1114. doi: 10.2215/CJN.06091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khouja HI, Bevington A, Kemp GJ, Russell RG. Calcium and orthophosphate deposits in vitro do not imply osteoblast-mediated mineralization: mineralization by betaglycerophosphate in the absence of osteoblasts. Bone. 1990;11:385–391. doi: 10.1016/8756-3282(90)90131-h. [DOI] [PubMed] [Google Scholar]
- 35.Hsu HHT, Camacho NP. Isolation of calcifiable vesicles from human atherosclerotic aortas. Atherosclerosis. 1999;143:353–362. doi: 10.1016/s0021-9150(98)00322-0. [DOI] [PubMed] [Google Scholar]
- 36.Van Belle H, Alkaline phosphatase I. Kinetics and inhibition by levamisole of purified isoenzymes from humans. Clin Chem. 1976;22:972–976. [PubMed] [Google Scholar]
- 37.Kozlenkov A, Le Du MH, Cuniasse P, Ny T, Hoylaerts MF, Millan JL. Residues determining the binding specificity of uncompetitive inhibitors to tissue-nonspecific alkaline phosphatase. J Bone Miner Res. 2004;19:1862–1872. doi: 10.1359/JBMR.040608. [DOI] [PubMed] [Google Scholar]
- 38.Matsuzawa T, Anderson HC. Phosphatases of epiphyseal cartilage studied by electron microscopic cytochemical methods. J Histochem Cytochem. 1971;19:801–808. doi: 10.1177/19.12.801. [DOI] [PubMed] [Google Scholar]
- 39.Chen NX, O'Neill KD, Chen X, Moe SM. Annexin-Mediated Matrix Vesicle Calcification in Vascular Smooth Muscle Cells. J Bone Miner Res. 2008;23:1798–1805. doi: 10.1359/JBMR.080604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu HHT, Camacho NP, Sun F, Tawfik O, Aono H. Isolation of calcifiable vesicles from aortas of rabbits fed with high cholesterol diets. Atherosclerosis. 2000;153:337–348. doi: 10.1016/s0021-9150(00)00425-1. [DOI] [PubMed] [Google Scholar]
- 41.Price PA, Toroian D, Chan WS. Tissue-nonspecific alkaline phosphatase is required for the calcification of collagen in serum: a possible mechanism for biomineralization. J Biol Chem. 2009;284:4594–4604. doi: 10.1074/jbc.M803205200. [DOI] [PubMed] [Google Scholar]
- 42.Rej R, Bretaudiere J-P. Effects of Metal Ions on the Measurement of Alkaline Phosphatase Activity. Clin Chem. 1980;26:423–428. [PubMed] [Google Scholar]
- 43.Garimella R, Bi X, Anderson HC, Camacho NP. Nature of phosphate substrate as a major determinant of mineral type formed in matrix vesicle-mediated in vitro mineralization: An FTIR imaging study. Bone. 2005;38:811–817. doi: 10.1016/j.bone.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 44.Shiekh FA, Charlesworth JA, Sung-Hoon K, Hunter LW, Jayachandran M, Miller VM, Lieske JC. Proteomic evaluation of biological nanoparticles isolated from human kidney stones and calcified arteries. Acta Biomaterialia. 2010;6:4065–4072. doi: 10.1016/j.actbio.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer JL. Can Biological Calcification Occur in the Presence of Pyrophosphate? Arch Biochem Biophys. 1984;231:1–8. doi: 10.1016/0003-9861(84)90356-4. [DOI] [PubMed] [Google Scholar]
- 46.Terkeltaub RA, Santoro DA, Mandel G, Mandel N. Serum and plasma inhibit neutrophil stimulation by hydroxyapatite crystals. Evidence that serum alpha 2-HS glycoprotein is a potent and specific crystal-bound inhibitor. Arthritis Rheum. 1988;31:1081–1089. doi: 10.1002/art.1780310901. [DOI] [PubMed] [Google Scholar]
- 47.Mori K, Emoto M, Araki T, Yokoyama H, Teramura M, Lee E, Motoyama K, et al. Association of serum fetuin-A with carotid arterial stiffness. Clin Endocrinol (Oxf) 2007;66:246–250. doi: 10.1111/j.1365-2265.2006.02716.x. [DOI] [PubMed] [Google Scholar]
- 48.Shanahan CM, Cary NRB, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Monckeberg's sclerosis: Evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
- 49.Nitschke Y, Hartmann S, Torsello G, Horstmann R, Seifarth H, Weissen-Plenz G, Rutsch F. Expression of NPP1 is regulated during atheromatous plaque calcification. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jono S, Ikari Y, Shioi A, Mori K, Miki T, Hara K, Nishizawa Y. Serum Osteoprotegerin Levels Are Associated With the Presence and Severity of Coronary Artery Disease. Circulation. 2002;106:1192–1194. doi: 10.1161/01.cir.0000031524.49139.29. [DOI] [PubMed] [Google Scholar]
- 51.Martel J, Ding-E Young J. Purported nanobacteria in human blood as calcium carbonate nanoparticles. PNAS. 2008;105:5549–5554. doi: 10.1073/pnas.0711744105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Granato D, Bergonzelli GE, Pridmore RD, Marvin L, Rouvet M, Corthesy-Theulaz IE. Cell surface-associated elongation factor Tu mediates the attachment of Lactobacillus johnsonii NCC533 (La1) to human intestinal cells and mucins. Infect Immun. 2004;72:2160–2169. doi: 10.1128/IAI.72.4.2160-2169.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz MA-K, Lieske JC, Kumar V, Farell-Baril G, Miller VM. Human-derived nanoparticles and vascular responses to injury in rabbit carotid arteries: proof of principle. International Journal of Nanomedicine. 2008;3:243–248. doi: 10.2147/ijn.s2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartz MK, Lieske JC, Hunter LW, Miller VM. Systemic Injection of Planktonic Forms of Mammalian-derived Nanoparticles Alters Arterial Response to Injury in Rabbits. AJP Heart & Circ. 2009;296:1434–1441. doi: 10.1152/ajpheart.00993.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sommer AP. Cytotoxicity of Calcium Phosphate Crystals and Human-Derived Nanoparticles: An Overlooked Link. Circ Res. 2010;106:e10. doi: 10.1161/CIRCRESAHA.110.221374. [DOI] [PubMed] [Google Scholar]


