Abstract
Background & Aims
An important component of enteric inhibitory neurotransmission is mediated by a purine neurotransmitter, such as adenosine 5’-triphosphate (ATP), binding to P2Y1 receptors and activating small conductance K+ channels. In murine colon ß-nicotinamide adenine dinucleotide (ß-NAD) is released with ATP and mimics the pharmacology of inhibitory neurotransmission better than ATP. Here ß-NAD and ATP were compared as possible inhibitory neurotransmitters in human and monkey colons.
Methods
A small-volume superfusion assay and HPLC with fluorescence detection were used to evaluate spontaneous and nerve-evoked overflow of ß-NAD, ATP and metabolites. Postjunctional responses to nerve stimulation, ß-NAD and ATP were compared using intracellular membrane potential and force measurements. Effects of ß-NAD on smooth muscle cells (SMCs) were recorded by patch clamp. P2Y receptor transcripts and proteins were assayed by RT-PCR.
Results
In contrast to ATP, overflow of ß-NAD evoked by electrical field stimulation correlated with stimulation frequency and was diminished by neurotoxins, tetrodotoxin and ω-conotoxin GVIA. Inhibitory junction potentials and responses to exogenous ß-NAD, but not ATP, were blocked by P2Y receptor antagonists suramin, PPADS, MRS2179 and MRS2500. ß-NAD activated non-selective cation currents in SMCs, but failed to activate outward currents.
Conclusions
ß-NAD meets the criteria for a neurotransmitter better than ATP in human and monkey colons and therefore may contribute to neural regulation of colonic motility. SMCs are unlikely targets for inhibitory purine neurotransmitters because dominant responses of SMCs were activation of net inward, rather than outward, current.
Keywords: enteric nerves, P2Y receptors, gastrointestinal motility
Introduction
Enteric neural control is essential for mixing and propulsion of luminal contents in the gastrointestinal (GI) tract.1 Motility disorders, including diabetic enteropathy, slow-transit constipation, inflammatory neuropathy, and ageing-associated motility disorders are linked, directly or indirectly, to defects in the neural control of the GI tract.2,3,4 However, underlying mechanisms for neurotransmitter actions and post-junctional targets for neurotransmitters are not entirely understood. Thus, accurate design and targeting of treatments for motility disorders has proven difficult.
An important component of enteric inhibitory neurotransmission is mediated by a purine neurotransmitter binding to P2Y1 purinoreceptors.5,6,7 Functional studies have typically suggested that adenosine 5’-triphosphate (ATP) is the inhibitory purinergic neurotransmitter in the gut.8 However, previous studies have largely assayed integrated responses in complex muscular tissues that are possibly mediated by mixed purine receptors. Few studies have measured release of neurotransmitters directly. ATP does not mimic the effects of the endogenous neurotransmitter9 in some cases, and many investigators refer to the neurotransmitter as a purine or “purine-like” substance.10,11,12,13,14
ß-nicotinamide adenine dinucleotide (ß-NAD) is another purine substance released by nerve stimulation in vascular and visceral smooth muscles and neuro-secretory cells.9,15,16,17,18 We found that nerve stimulation of murine colon evoked release of ß-NAD and ATP, and the pharmacological profile of ß-NAD mimicked the endogenous neurotransmitter better than ATP.9 Here we investigated whether ß-NAD serves as an inhibitory neurotransmitter in larger mammals, such as humans and non-human primates.
Materials and Methods
Tissue Preparation
Proximal colons of 94 Cynomolgus monkeys (Macaca fascicularis) of both sexes were obtained from Charles River Laboratories Preclinical Services (Reno, NV). Monkeys sedated with Ketamine (10 mg kg−1) and 0.7 ml Beuthanasia-D (Schering-Plough AH, Kenilworth, NJ, USA) were exsanguinated (Charles River Laboratories Institutional Animal Care and Use Committee).
Human colon samples were obtained as surgical waste from 48 male and female patients (ages 32-77) undergoing colon resections for neoplasm or diverticulitis at Renown Medical Center and St. Mary’s Hospital (Reno, NV), or Samsung Hospital (Seoul, South Korea). Tissues from a site distant to the disease-affected area were placed in oxygenated Krebs solution (10°C) containing (mmol/L): 118.5 NaCl; 4.2 KCl; 1.2 MgCl2; 23.8 NaHCO3; 1.2 KH2PO4; 11.0 dextrose; 1.8 CaCl2 (pH 7.4). Use of human colon was approved by Human Subjects Research Committees at Renown and St. Mary’s, and Samsung hospitals, and the Biomedical Institutional Review Board at University of Nevada, Reno. Tunica muscularis was processed as below after removal of mucosa and submucosa.
Purine Overflow
Muscle strips (2 × 6 mm) were prepared from monkey and human colonic tunica muscularis. Pure circular muscles (CM) (2 × 6 mm) of monkey colon were prepared by peeling off longitudinal muscle (LM) with attached myenteric ganglia (MG). NADPH-diaphorase histochemistry (below) verified absence of MG in CM. Due to adhesions between smooth muscle layers in human colon, CM preparations were not viable.
Muscles (50-70 mg) were placed in 200-μl superfusion chambers equipped with platinum electrodes.9, 19 Nerves were stimulated by electrical field stimulation (EFS; 480 pulses; 0.3 ms (whole muscle, WM) or 0.5 ms (CM); 4 Hz or 16 Hz; supramaximal voltage). Superfusates were collected before and during EFS and processed for derivatization to 1,N6-etheno-purines.20,21 ATP, ADP, AMP and adenosine (ADO) were converted to their 1,N6-etheno-derivatives eATP, eADP, eAMP, and eADO, respectively.21 ß-NAD, ADPR and cADPR all derivatized to eADPR.15
HPLC Assay of Etheno-purines
Reverse-phase gradient HP1100 liquid chromatography equipped with fluorescence detector (Agilent Technologies, Wilmington, DE) was used as previously described.21,15 Amounts of nucleotides/nucleosides were calculated against standards. Results, normalized for sample volume and tissue weight, were expressed as femtomoles per milligram of tissue wet weight (fmol/mg).
HPLC Fraction Analysis
The compounds forming eADPR were determined by HPLC fraction analysis.9,15 Superfusates from 28 chambers were combined, concentrated and analyzed by HPLC. Samples were collected according to retention time (cADPR and ATP 7.0–7.4 min, ADPR 8.3–8.7 min, and ß-NAD 10.3–10.7 min) and were etheno-derivatization and reanalyzed by HPLC for eADPR.
Electrophysiology and contractions
Muscles (10 × 10 mm) were perfused with oxygenated Krebs solution (mmol/L): NaCl 118.5; KCl 4.5; MgCl2 1.2; NaHCO3 23.8; KH2PO4 1.2; dextrose 11.0; CaCl2 2.4, pH 7.4, at 37±0.5°C, and CM cells were impaled with glass microelectrodes in the presence of nifedipine (1 μmol/L) to reduce movement.9,22 Parallel platinum electrodes delivered EFS (0.5ms) from a Grass S48 stimulator (Quincy, MA, USA).
Receptor antagonists were perfused into the recording chamber. ATP and ß-NAD, were applied (10 ms pulses at 10 psi) via picospritzer micropipettes (Picospritzer; General Valve, East Hanover, NJ) positioned close to electrical recording sites.
Single colonic smooth muscle cells (SMCs) were prepared from human and monkey colon as described23. Membrane currents were recorded by patch clamp (on-cell recording or permeabilized-patch conditions with amphotericin B) 23. Currents were amplified (Axopatch 200B, Axon Instruments) and digitized (12-bit A/D converter; Digidata 1322A, Axon Instruments) on-line via pCLAMP software (V9.2.0.09, Axon Instruments). Data were sampled at 2 kHz (whole cell) or 5 kHz (single channel recordings).
Bath solution (CaPSS) for whole-cell recording contained (mmol/L): 5 KCl, 135 NaCl, 2 CaCl2, 10 glucose, 1 MgCl2, and 10 HEPES, adjusted to pH 7.4 with Tris. Pipette solution contained (mmol/L): 110 Cs-aspartate, 30 TEACl, 0.1 EGTA, 10 HEPES, pH7.2. For single channel recordings, bath solution contained high K+ (HK, mmol/L): 150 KCl, EGTA 1, and 10 HEPES, pH 7.4. The pipette solution in single channel recordings was CaPSS.
Muscle strips (1 × 10 mm) were prepared for contractile studies and attached to Fort 10 force transducers (World Precision Instruments, Sarasota, Fl) for force measurments.9,22
Expression of P2Y receptors
P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11 receptor transcripts were measured in monkey and human colons. Detailed methods are given in Supporting Information (Supplementary Materials and Table 1S).
NADPH Diaphorase Histochemistry
CMs were checked for MG using NADPH diaphorase histochemistry as described.24, 25 After staining, muscles were mounted in Aquamount (Lerner Laboratories, Pittsburgh, PA) and examined with a Zeiss Axiovert 200M and a Hamamatsu C5810 camera.
Data Analysis
Data presented are means ± SEM. In neurotransmitter overflow experiments means are compared by two-tailed, unpaired t tests (GraphPadPrism, GraphPad Software, San Diego, CA). In intracellular electrical and mechanical experiments means are compared by two-tailed paired Student’s t tests and Mann Whitney rank sum tests. A probability of <.05 was considered significant. For analysis of the picospritizing data, membrane hyperpolarization area following picospritzing was plotted as a function of mV.ms-1 until the membrane repolarized to control level. For force measurements, relaxation responses (10 min) were calculated as percents of the maximal inhibition following application of ß-NAD.
Drugs
ATP, ADP, AMP, adenosine, ß-NAD, nifedipine, PPADS, suramin, apamin, L-NNA, atropine, ω-conotoxin GVIA, and amphotericine B were purchased from Sigma-Aldrich (St. Louis, MO). ADPR and cADPR came from Biolog (Germany). MRS2179 and MRS2500 came from Tocris Bioscience (Ellisville, MO). Nifedipine, dissolved in ethanol at 10mmol/L, was added to the perfusion to make 1μmol/L. Other drugs were dissolved in de-ionized H2O and diluted in perfusion solutions.
Results
Neural release of purines
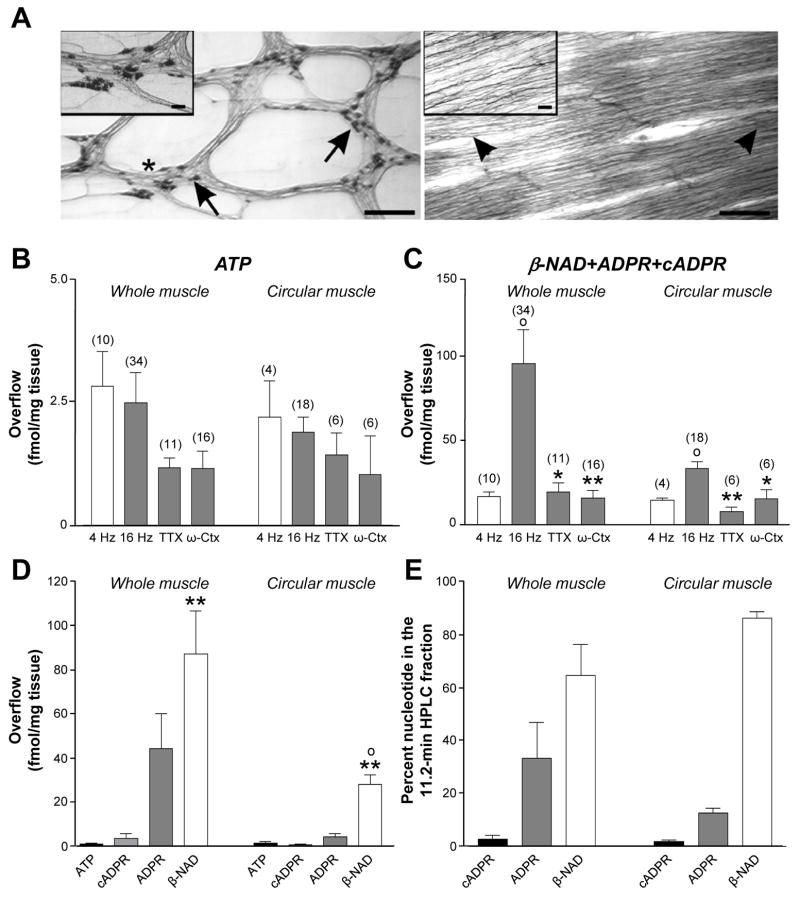
Stimulation of intrinsic nerves caused accumulation of ATP and ß-NAD and metabolites, ADP, AMP, ADO, ADPR and cADPR in tissue superfusates (Figs. 1-2, Tables 2S and 3S in Supplementary Materials). ADP is a product of ATP. AMP and ADO are products of ATP and ß-NAD, and cADPR and ADP-ribose are products of ß-NAD.26,27,28 Therefore, ATP and ß-NAD detected in superfusates are remnants of purines released less metabolic products. Fig. 1 shows overflow of purines from human colonic muscles. EFS evoked ß-NAD release at 4 Hz (Fig. 1A, C) and release increased at 16 Hz (Fig. 1C, P=.0227). In contrast, ATP did not increase significantly with stimulation frequency (Fig. 1B, P=.3325). ß-NAD was released from nerves, as it was reduced by 0.5μmol/L TTX (P=.0028), a blocker of neural Na+ channels, and 50nmol/L ω-conotoxin GVIA (P=.0469), an inhibitor of neuronal voltage-dependent, N-type Ca2+ channels (Fig. 1C). ATP release was not significantly reduced by TTX (P=.2360) or ω-conotoxin GVIA (P=.2994), Fig. 1B, suggesting that most ATP released during EFS may occur by TTX-insensitive mechanisms. EFS-evoked overflow of ADP, AMP, and ADO was not significantly reduced by TTX and ω-conotoxin GVIA (P>.05), but total purine release followed ß-NAD overflow (Table 2S, Supplementary Material).
Figure 1. Overflow of ATP and ß-NAD in human colonic muscle.

(A) Chromatograms of tissue superfusates collected during EFS (4 and 16 Hz) and with neural blockers at 16 Hz. EFS-evoked overflow of ß-NAD, but not ATP, increased with stimulation frequency and decreased with TTX (0.5μmol/L) or ω-conotoxin GVIA (50nmol/L); LU, luminescence units. Note that each chromatogram shows data from a different experimental tissue. (B and C) Averaged data are means±SEM; (o) denote significant differences from 4 Hz controls (P<.05); (*) denote significant differences from 16 Hz controls (*P<.05, **P<.01). Experiment number shown in parentheses. (D and E) HPLC fraction analysis demonstrated that ß-NAD is the primary purine nucleotide in ß-NAD+ADPR+cADPR peak. (E) Fraction analysis showed that the amount of ß-NAD exceeded ATP; (*) denote significant differences from ATP (**P<.01).
Figure 2. ATP and ß-NAD released in monkey whole and circular muscle.
(A) NADPH-diaphorase staining of LM and CM of monkey colon. Left panel shows NADPH-diaphorase+ neurons (arrows) in myenteric plexus LM preparation. Inset is magnification showing neurons within MG (*). Right panel shows that CM is free of ganglia. Nerve fibers parallel to CM remain (arrow heads). Inset is magnification showing nerve fibers. Scale bars are 500μm in both panels and 50μm in insets. (B and C) ATP and ß-NAD released from monkey WM and CM (4 and 16 Hz) and with TTX (0.5 μmol/L) and ω-conotoxin-GVIA (50 nmol/L). Averaged data (fmol/mg tissue) are means±SEM; (o) denote significant differences from 4 Hz controls (P<.05); (*) denote significant differences from 16 Hz controls (*P<.05, **P<0.01). (D and E) HPLC fraction analysis demonstrates that ß-NAD is the primary nucleotide in ß-NAD+ADPR+cADPR peak in WM and CM. ß-NAD was greater in WM than CM. (o) denote significant difference from WM (P<.05). ß-NAD exceeded ATP in WM and CM (**P<0.01).
In human colon ß-NAD, ADPR and cADPR contributed 82%, 17.4% and 0.6% of the eADPR peak, respectively (Fig. 1D). Therefore, ß-NAD was the dominant purine nucleotide in ß-NAD+ADPR+cADPR peaks. ß-NAD release exceeded ATP by about 15 fold (P=.0072) (Fig.1E).
ß-NAD released in intact muscles might originate from enteric ganglia rather than motor nerve terminals. Thus, we measured purine release from pure CMs with LM and MG removed. Fig. 2 shows overflow of ß-NAD and ATP and metabolites from monkey colonic WM (containing MG) and pure CM, with nerve processes but no cell bodies (Fig. 2A). ATP release was not increased between 4 Hz and 16 Hz (Fig. 2B, WM, P=.7831; CM, P=.6706), and was not significantly reduced by TTX (WM, P=.2487; CM, P=.4649) or ω-conotoxin GVIA (WM, P=.1688; CM, P=.2371). In contrast ß-NAD release increased with stimulus frequency (Fig. 2C, WM, P=.0398; CM, P=.0300). Release of ß-NAD from CM was 2- to 3-fold lower than from WM at 16 Hz (P=.0289), but no difference was observed at 4 Hz (P=.6117). Thus, at lower stimulation frequencies, ß-NAD may be released exclusively from nerve endings, but at 16 Hz, ß-NAD may also be released in ganglia. ß-NAD release (16 Hz) was inhibited by TTX (WM P=.0374, CM P=.0024) and ω-conotoxin GVIA (WM, P=.0092, CM, P=.0340), indicating that ß-NAD was released from enteric motor nerves.
In monkey WM, ß-NAD, ADPR and cADPR contributed ~64%, 33% and 3% of the eADPR peak, respectively (Fig. 2D). In CM, ß-NAD, ADPR and cADPR contributed 86%, 12.5% and 1.5% of the eADPR peak, respectively. Therefore, ß-NAD is the dominant nucleotide in ß-NAD+ADPR+cADPR peaks. ß-NAD exceeded the mass of ATP by 25-fold in WM (P=.0094) and 15-fold in CM (P=.0043, Fig. 2E).
Purinergic component of inhibitory junction potentials (IJPs)
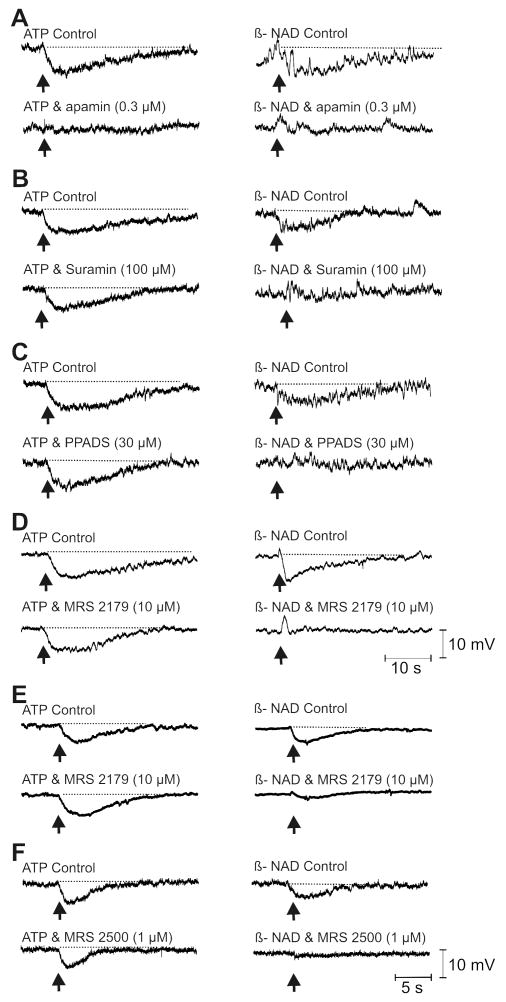
Functional responses of colonic muscles were tested by measuring membrane potentials and contractions (Supplementary materials; Figs. 1S, 2S). CM cells of monkey proximal colon and human colon had resting membrane potentials averaging -51.2±1.1 mV (n=39) and -43.0±0.6 (n=41), respectively. The effects of antagonists, including suramin (100μmol/L), PPADS (30μmol/L), MRS2179 (10μmol/L), MRS2500 (1 μmol/L) and apamin (0.3μmol/L) were tested on the purinergic component of IJPs (Fig. 3). Apamin reduced IJPs in monkey colon (by 70±3.9%; n=19, P<.00001), suramin (by 51±1.7%; P<.00005, n=10), PPADS (by 61±4.8%; P<.00001, n=15), and MRS2179, selective P2Y1 antagonist,29,30 (by 85±6.4%; P<.00001, n=14), Fig. 3A. Apamin tended to reduce IJPs of human colon (by 16±4.7%; n=19; but did not reach significance; P>.05), Fig. 3B. Human IJPs were reduced by suramin (by 60±6.0%; P<.0001, n=5), PPADS (by 81±1.6%; P<.0005, n=5), MRS2179 (by 83±2.1%; P<.00001, n=15), and MRS2500 (by 95±1.3%; P<0.0000000001, n=10), Fig. 3B. Therefore, non-nitrergic inhibitory responses in human colon were mediated via P2Y1 receptors, as previously reported10 and these responses have similar pharmacology in monkey colon.
Figure 3. Purinergic component of IJPs.
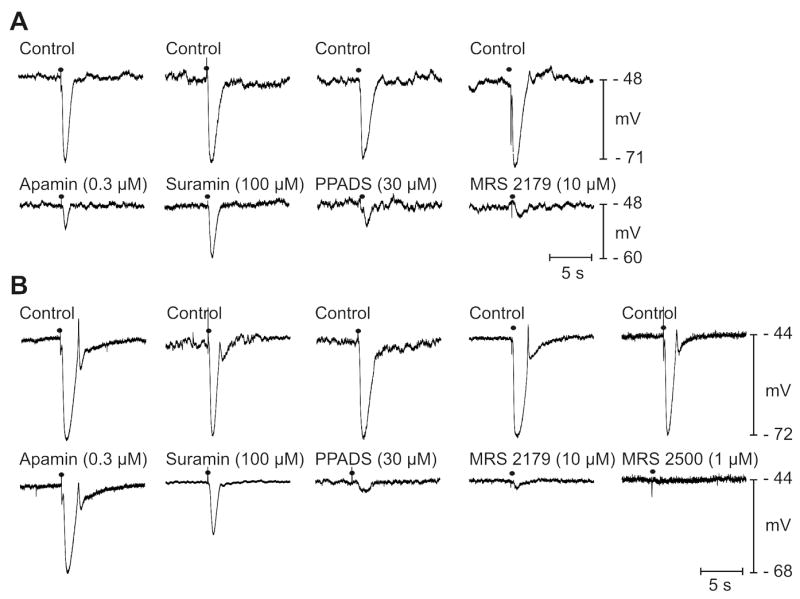
IJPs generated by EFS (0.5 ms; 1 pulse; solid circles) in monkey (A) and human colonic muscles (B) in the presence of atropine (1 μmol/L), L-NNA (100 μmol/L) and nifedipine (1 μmol/L). Purinergic component of IJP in monkey colon was reduced by apamin, suramin, PPADS, and MRS2179. In human colon apamin only partially reduced IJPs, but surmain, PPADS, MRS2179 and MRS2500 significantly reduced IJPs.
Antagonists of purinergic inhibitory responses also inhibited responses to exogenous transmitters. With atropine (1 μmol/L) and L-NNA (100 μmol/L) present, picospritzing of ATP onto monkey colonic muscles caused hyperpolarization averaging 12±0.9 mV (n=21). Apamin (0.3 μmol/L) inhibited this response by 97±2.4% (n=5, P<.05, Fig. 4A, left). Responses to exogenous ATP were not inhibited significantly by suramin (100 μmol/L, reduced by 5±5.2% of control, P>.05, n=5), PPADS (30 μmol/L, by 12±9.6%, P>.05, n=6), or MRS2179 (10 μmol/L, by 23±12%, n=5, P>.05) (Fig. 4B-D, left). Picospritzing ATP onto human muscles under the same conditions caused hyperpolarization averaging 10.9±0.7 mV (n=11). Apamin did not reduce responses to ATP significantly, and ATP responses were not reduced by MRS2179 (10 μmol/L, by 9±2.1%, n=5, P>.05, Fig. 4E, left) or MRS2500 (1 μmol/L, by 12.3±7.3%, n=3, P>.05). Thus, IJPs and hyperpolarizations to ATP displayed different pharmacological profiles, suggesting responses to the endogenous neurotransmitter and exogenous ATP are mediated by different receptors.
Figure 4. Membrane responses to exogenous purines.
Picospritzed ATP (10mmol/L, left panels) and ß-NAD (50mmol/L, right panels) hyperpolarized monkey (A-D) and human muscles (E, F). Responses to ß-NAD, but not to ATP, were inhibited by purine receptor antagonists (as labeled). In monkey muscles, apamin inhibited both ATP and ß-NAD induced hyperpolarization (A, left and right panels). In some muscles ß-NAD caused transient depolarization after MRS2179 (D, right). In human muscles MRS2179 and MRS2500 did not affect ATP response (E,F left), but significantly reduced ß-NAD response (E, F right)). Scale bars in D&F apply to traces in A-D and E-F, respectively.
Picospritzing ß-NAD caused hyperpolarization of monkey colon averaging 7.9±0.8 mV (n=23). Responses to ß-NAD were reduced by apamin (0.3 μmol/L) by 98±2.4% (n=6, P<.05), suramin (100 μmol/L) by 95±3.8% (n=5, P<.05), PPADS (30 μmol/L) by 88±3.2% (n=6, P<.05) and MRS2179 (10 μmol/L) by 98±1.3% (n=6, P<.005), Fig. 4A-D, right. ß-NAD hyperpolarized human colon by 6.8±0.5 mV (n=13). ß-NAD solutions are acidic, but picospritzing of acidified Krebs solution (pH 3.6) had no effect on resting membrane potential. Apamin did not affect the hyperpolarization responses to ß-NAD (n=2). However, ß-NAD responses were reduced by MRS2179 (10 μmol/L) by 68±6.5% (n=5, P<.05, Fig. 4E, right) and by MRS2500 (1 μmol/L) by 97±1.7% (n=3, P<.05, Fig. 4F, right). Thus, ß-NAD mimicked the pharmacology of the purine inhibitory neurotransmitter.
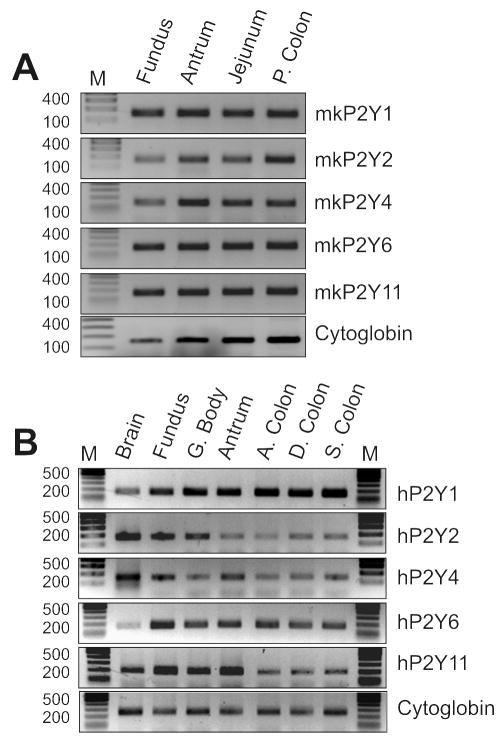
Monkey and human colonic muscles expressed P2Y receptor transcripts, including P2Y1 receptors (Fig. 5).
Figure 5. Expression of P2Y receptors in monkey and human tissues.
(A) Expression of P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11 receptor transcripts in the tunica muscularis of the monkey fundus, antrum, jejunum and proximal colon. (B) Expression of P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11 receptor transcripts in human brain, fundus, gastric body (G. Body), antrum, ascending colon (A. Colon), distal colon (D. Colon) and sigmoid colon (S. Colon). C ytoglobin was used as a house keeping gene and M represents base pair marker.
Effects of β-NAD and ATP on SMC conductance
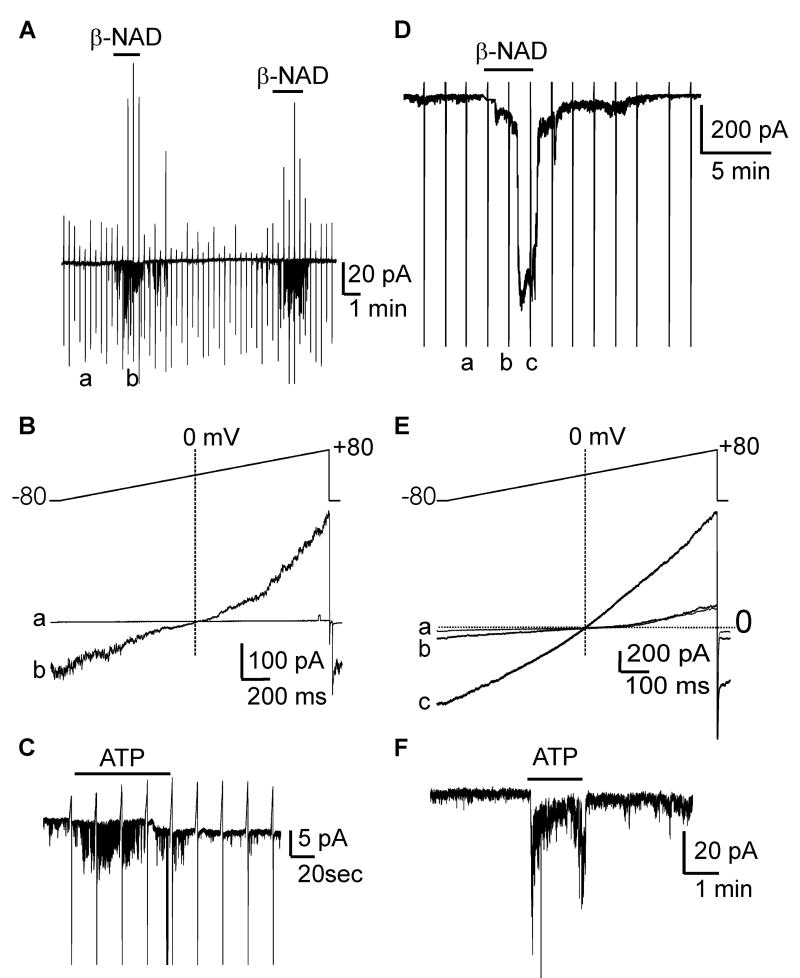
ß-NAD induced hyperpolarization in human and monkey colonic muscles, which might be accomplished by activation of K+ channels or inhibition of a tonic inward current in colonic SMCs. The effects of ß-NAD on isolated SMC were tested with cell-attached patch clamp recording. Monkey colonic SMC were held at -80 mV and depolarized by ramping potential to +80 mV. Control single channel openings at -80 mV were negligible, but ß-NAD (1mmol/L) increased channel openings (-53±17 pA, n=5, P<.05, Fig. 6A). These effects returned to control level upon washout and were repeatable. Currents activated by ß-NAD reversed at 0 mV (Fig. 6B), suggesting that non-selective cation channels, but not Cl- channels (ECl ≈ -30 mV) or K+ channels (EK ≈ -80 mV) were responsible. ATP (1 mmol/L) on cell-attached patches also activated non-selective cation channels at -80 mV (n=2, Fig. 6C).
Figure 6. ß-NAD and ATP activate inward currents in SMCs of monkey (A-C) and human (D-F).
(A) In cell-attached patches (pipette CaPSS; bath HK), channel openings were negligible. ß-NAD (1mmol/L) increased openings at -80 mV. (B) Stepping from -80 to +80 mV showed ß-NAD-activated currents reversed at 0 mV in monkey SMC. a (control) and b (ß-NAD) show expanded traces from panel A during ramp depolarization (-80 mV to +80 mV). (C) ATP (1mmol/L) increased channel activity at -80 mV in monkey SMC. (D) Perforated whole-cell conditions (pipette Cs-TEA, bath CaPSS), ß-NAD (5mmol/L) activated inward current at -80 mV. ß-NAD activated-currents reversed upon washout. (E) a, b, and c show expanded traces from panel D during ramp depolarization. ß-NAD activated-currents reversed at 0 mV, demonstrating non-selective cation conductance was activated by ß-NAD. Dotted lines in B and E denote 0 mV and 0 pA. (F) ATP (1 mmol/L) activated inward currents at -80 mV.
ß-NAD was also tested on human colonic SMC using permeabilized patch, whole-cell recording. Contamination from K+ and Cl- currents were eliminated with Cs-TEA pipette solution with ECl set to -40 mV. Under these conditions, ß-NAD (5mmol/L) activated inward current, averaging -424±0.4 pA (-80mV; n=7, P<.01 control net inward current was -37±4 pA) (Fig. 6D). The currents activated reversed at about 0 mV (Fig. 6E). Therefore, ß-NAD-activated currents were not due to K+ and Cl- conductances, but appeared to be due to activation of non-selective cation channels. Activation of non-selective cation channels would generate net inward current and depolarize SMC. ATP also activated non-selective cation current at -80 mV (-99±47pA, n=4, Fig. 6F).
Discussion
This study demonstrates several new features of enteric inhibitory motor neurotransmission in human and non-human primate colonic muscles. Stimulation of intrinsic neurons caused release of ATP and ß-NAD, and ß-NAD was the dominant purine released. ß-NAD was previously suggested as an enteric inhibitory neurotransmitter in murine colonic muscles,9 but it has never been considered as a candidate for neurotransmission in human GI smooth muscles. The characteristics of ß-NAD release suggest it is more likely to be a neurotransmitter than ATP. For example, release of ß-NAD, but not ATP, was frequency-dependent and blocked by neurotoxins. Localized ß-NAD application mimicked purinergic responses to enteric inhibitory neurotransmission; antagonists that successfully blocked neural responses also blocked responses to exogenous ß-NAD. This was not true for ATP, because MRS2179 and MRS2500 effectively blocked purinergic IJPs but failed to block responses to ATP. These data suggest that ß-NAD could be the primary purinergic enteric inhibitory neurotransmitter in human and non-human primate colonic muscles. Purinergic neurotransmission may be the dominant form of enteric inhibitory control in human colon as brief stimuli failed to elicit nitrergic responses (Supplementary Data), as commonly elicited in laboratory animals, and block of NO synthesis failed to reveal tonic nitrergic inhibition, as commonly observed in animal models.31
ATP and ß-NAD were released from human and monkey colonic muscles spontaneously and during nerve stimulation. At low frequency stimulation, ß-NAD appeared to be released from motor nerve terminals, but ganglionic release may also occur at higher frequencies. ATP release was equivalent in muscles with and without ganglia, and ATP release was not significantly reduced by neurotoxins. EFS-evoked overflow of the ATP metabolite, ADP, however, was significantly reduced by ω-conotoxin GVIA in WM, suggesting that a portion of ATP might have originated in ganglia but was degraded to ADP. ß-NAD release exceeded ATP by 15-25 fold, suggesting that higher concentrations of ß-NAD might be achieved near post-junctional receptors. Taken together, these data suggest that ß-NAD may serve as an inhibitory neurotransmitter.
Tissue superfusates contained not only ß-NAD and ATP but also their metabolites ADP, AMP, ADPR, cADPR, and adenosine. Thus, mechanisms for terminating actions of purines are available in colon muscles. As in murine colon9 ß-NAD is the primary purine in ß-NAD+ADPR+cADPR peaks in human and monkey colons. Unlike murine colon, where ADPR comprised only ~6% of the ß-NAD+ADPR+cADPR mixture, ADPR contributes 12%-33% in the human and monkey colons. Future studies should determine whether ADPR originates only from degradation of β-NAD, or whether ADPR is also released from nerves. If the latter is true, ADPR may also elicit responses in post-junctional cells and be a co-transmitter.
Comparisons of postjunctional responses to neurotransmitters released during EFS with responses to exogenous, putative transmitters, ATP and ß-NAD, shed new light on the nature of enteric inhibitory neurotransmission in the human colon. In agreement with previous studies7,9,10,12,32 electrical and mechanical responses of human and monkey colons to EFS demonstrated P2Y receptor- and NO-mediated components (Supplementary Data). In monkey and human muscles the primary component of IJPs, a non-nitrergic, fast hyperpolarization, was significantly reduced by the nonselective P2Y receptor antagonists PPADS and suramin and by the P2Y1 inhibitors MRS2179 and MRS2500.29, 33 IJPs in the human colon were less sensitive to 0.3μmol/L apamin than monkey responses, and this finding is consistent with previous reports demonstrating relative insensitivity of human colonic10,32 and intestinal34 muscles to apamin. Therefore, our data are in agreement with previous studies6,10 showing that IJPs in human colon are mediated by P2Y1 receptors through a pathway that involves, in part, activation of apamin-sensitive channels. The major K+ conductance activated during purinergic IJPs in the human is unknown. Of major importance in this study is the finding that ß-NAD also elicited hyperpolarization via P2Y1 receptors, but ATP responses were not blocked by MRS2179 and MRS2500. These data, coupled with findings on neurotransmitter release, suggest that ß-NAD is a better candidate for the purine inhibitory neurotransmitter than ATP. There are, however, additional explanations (as below) why the P2Y1 receptor antagonists were effective in blocking neurogenic responses but incapable of blocking responses to exogenous ATP. Thus, we cannot completely rule out a role for ATP as a neurotransmitter.
IJPs may be mediated by different post-junctional receptors, possibly expressed by different cell types, than hyperpolarization responses to exogenous substances. Utilization of specific receptors may result from compartmentalization of P2Y1 receptors in restricted receptive fields close to sites of neurotransmitter release and broader expression of purinergic receptors in cells not closely associated with active zones of transmitter release. In fact tunica muscularis expresses multiple P2Y receptors, including P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11, but specific junctional and/or extrajunctional distribution could not be delineated because the antibodies we tested performed poorly in immunohistochemistry. ß-NAD might be a more exclusive agonist for P2Y1 receptors than ATP, and therefore responses to exogenous ß-NAD were readily blocked by MRS2179 and MRS2500. In contrast, responses to exogenous ATP appear to be mediated by receptors other than P2Y1 receptors, and therefore insensitive to the antagonists. ATP, if released from nerves, might generate responses via a specialized, junctional population of P2Y1 receptors, and our data do not rule out this possibility.
ß-NAD- and ATP-induced hyperpolarizations of human and monkey colonic muscles were modest in comparison to IJPs. Multiple factors may be involved in this difference, including degradation of purines, insufficient penetration of ß-NAD and ATP to appropriate receptors, or simultaneous activation of opposing conductances. Picospritzing drugs close to recording sites, instead of superfusing drugs, was meant to simulate rapid direct application and avoid some of the factors that might dampen responses. ß-NAD and ATP in picospritz pipettes were likely comparable to concentrations of neurotransmitters achieved near nerve terminals following exocytosis, since synaptic vesicles have been estimated to contain 5-1000 mmol/L neurotransmitters.35,36 Close examination of electrical responses to ß-NAD in monkey colonic muscles (Fig. 4) after P2Y antagonists revealed transient depolarizations in response to ß-NAD. Equivalent observations were made in contractile responses of human muscles to ß-NAD where transient contractions often occurred before relaxation (Fig. 2S). We considered the possibility that ß-NAD might activate opposing conductances in different cells, and tested the effects of this compound on isolated SMC. The endogenous purine neurotransmitter and exogenous ß-NAD caused hyperpolarization. In monkey cells, hyperpolarization resulted, in part, from activation of apamin-sensitive (SK) channels. Thus, if the effects of ß-NAD were mediated by SMC, one would expect activation of SK channels in these cells. In fact, the dominant effect of both ATP and ß-NAD was activation of non-selective cation channels in SMC. These observations support the idea that purinergic neurotransmission is targeted toward specific cells in GI muscles, and SMC are unlikely to mediate purinergic IJPs.
There are two additional cells in close proximity to nerve terminals and coupled to SMCs via gap junctions in GI muscles: i) interstitial cells of Cajal (ICC)37, and ii) fibroblast-like cells (FLC).38 It has not been possible to identify either ICC or FLC in dispersions of human or monkey GI muscles. Previous studies on mice, however, suggest that ICC are unnecessary for purinergic responses39, but FLC express SK3 channels abundantly.40, 41,42,43 Thus, FLC, not SMC, may mediate purinergic IJPs in colonic muscles. Our data demonstrate that when ß-NAD and ATP are spritzed or bath applied to colonic muscles, activation of opposing conductances tends to antagonize hyperpolarization responses.
The present study demonstrates the complexity of the enteric purinergic signaling and suggests that the β-NAD system can be a new target for treating motility disorders of the large intestine.
Supplementary Material
Acknowledgments
A program project grant from the NIDDK: P01 DK41315 supported this study. We are grateful to Charles River Laboratories, Preclinical Services for monkey colonic muscles and Renown, St. Mary’s and Samsung hospitals for human specimens.
Grant Support: This work was supported by National Institutes of Health Program Project Grant P01 DK41315 (to S.M.W., S.D.K., K.M.S., and V.N.M-Y).
Abbreviations
- ADP
adenosine 5’-diphosphate
- ADO
adenosine
- ADPR
adenosine 5’-diphospho-ribose
- ATP
adenosine 5’-triphosphate
- cADPR
cyclic adenosine 5’-diphospho-ribose
- CM
circular muscle
- EFS
electrical field stimulation
- GI
gastrointestinal
- HEPES
N-2-hydroxyethyl piperazine-N’-ethanesulphonic acid
- HPLC
high-pressure liquid chromatography
- IJP
inhibitory junction potential
- LM
longitudinal muscle
- L-NNA
NG-nitro-L-agrginine
- MG
myenteric ganglia
- MRS2179
2’-deoxy-N6-methyladenosine 3’,5’-bisphosphate
- MRS2500
(1R,2S,4S,5S)-4-[2-Iodo-6-(methylamino)-9H-purin-9-yl]- 2-(phosphonooxy)bicyclo[3.1.0]hexane-1-methanol dihydrogen phosphate ester tetraammonium salt
- ß-NAD
ß-nicotinamide adenine dinucleotide
- PPADS
pyridoxal-phosphate-6-azophenyl-2’,4’-disulfonate
- Tris
tris hydroxymethyl aminomethane
Footnotes
Author contributions: S.J.H. and L.D. contributed equally to this study; S.M.W., S.D.K., K.M.S., and V.N.M-Y designed research; S.J.H., L.D., L.Dw., P-L.R., S.D.K and V.N.M-Y performed research; S.M.W, S.D.K, K.M.S and V.N.M-Y wrote the paper.
Disclosures: The authors declare no conflict of interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol. 1999;61:117–42. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- 2.Bassotti G, Villanacci V. Slow transit constipation: a functional disorder becomes an enteric neuropathy. World J Gastroenterol. 2006;12:4609–4613. doi: 10.3748/wjg.v12.i29.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camilleri M, Cowen T, Koch TR. Enteric neurodegeneration in ageing. Neurogastroenterol Motil. 2008;20:418–429. doi: 10.1111/j.1365-2982.2008.01134.x. [DOI] [PubMed] [Google Scholar]
- 4.Lomax AE, Sharkey KA, Furness JB. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol Motil. 2010;22:7–18. doi: 10.1111/j.1365-2982.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- 5.Wood JD. The enteric purinergic P2Y1 receptor. Curr Opin Pharmacol. 2006;6:564–570. doi: 10.1016/j.coph.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Gallego d, Gil V, Aleu J, Auli M, Clave P, Jimenez M. Purinergic and nitrergic junction potential in the human colon. Am J Physiol Gastrointest Liver Physiol. 2008;295:G522–G533. doi: 10.1152/ajpgi.00510.2007. [DOI] [PubMed] [Google Scholar]
- 7.Grasa L, Gil V, gallego d, Martin MT, jimenez m. P2Y(1) receptors mediate inhibitory neuromuscular transmission in the rat colon. Br J Pharmacol. 2009;158:1641–1652. doi: 10.1111/j.1476-5381.2009.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnstock G. The journey to establish purinergic signalling in the gut. Neurogastroenterol Motil. 2008;20(Suppl 1):8–19. doi: 10.1111/j.1365-2982.2008.01107.x. [DOI] [PubMed] [Google Scholar]
- 9.Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. beta-Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. PNAS. 2007;104:16359–16364. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallego D, Hernandez P, Clave P, Jimenez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G584–G594. doi: 10.1152/ajpgi.00474.2005. [DOI] [PubMed] [Google Scholar]
- 11.De Man JG, De Winter BY, Seerden TC, De Schepper HU, Herman AG, Pelckmans PA. Functional evidence that ATP or a related purine is an inhibitory NANC neurotransmitter in the mouse jejunum: study on the identity of P2X and P2Y purinoceptors involved. Br J Pharmacol. 2003;140:1108–1116. doi: 10.1038/sj.bjp.0705536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serio R, Alessandro M, Zizzo MG, Tamburello MP, Mule F. Neurotransmitters involved in the fast inhibitory junction potentials in mouse distal colon. Eur J Pharmacol. 2003;460:183–190. doi: 10.1016/s0014-2999(02)02923-0. [DOI] [PubMed] [Google Scholar]
- 13.Auli M, Martinez E, Gallego D, Opazo A, Espin F, Marti-Gallostra M, Jimenez M, Clave P. Effects of excitatory and inhibitory neurotransmission on motor patterns of human sigmoid colon in vitro. Br J Pharmacol. 2008;155:1043–1055. doi: 10.1038/bjp.2008.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallego D, Vanden Berghe P, Farre R, Tack J, Jimenez M. P2Y1 receptors mediate inhibitory neuromuscular transmission and enteric neuronal activation in small intestine. Neurogastroenterol Motil. 2008;20:159–168. doi: 10.1111/j.1365-2982.2007.01004.x. [DOI] [PubMed] [Google Scholar]
- 15.Smyth LM, Bobalova J, Mendoza MG, Lew C, Mutafova-Yambolieva VN. Release of {beta}-Nicotinamide Adenine Dinucleotide upon Stimulation of Postganglionic Nerve Terminals in Blood Vessels and Urinary Bladder. J Biol Chem. 2004;279:48893–48903. doi: 10.1074/jbc.M407266200. [DOI] [PubMed] [Google Scholar]
- 16.Breen LT, Smyth LM, Yamboliev IA, Mutafova-Yambolieva VN. {beta}-NAD is a novel nucleotide released on stimulation of nerve terminals in human urinary bladder detrusor muscle. Am J Physiol Renal Physiol. 2006;290:F486–F495. doi: 10.1152/ajprenal.00314.2005. [DOI] [PubMed] [Google Scholar]
- 17.Smyth LM, Breen LT, Mutafova-Yambolieva VN. Nicotinamide adenine dinucleotide (NAD) is released from sympathetic nerve terminals via a botulinum neurotoxin A mediated mechanism in canine mesenteric artery. Am J Physiol Heart Circ Physiol. 2006;290:H1818–H1825. doi: 10.1152/ajpheart.01062.2005. [DOI] [PubMed] [Google Scholar]
- 18.Yamboliev IA, Smyth LM, Durnin L, Dai Y, Mutafova-Yambolieva VN. Storage and secretion of beta-NAD, ATP and dopamine in NGF-differentiated rat pheochromocytoma PC12 cells. Eur J Neurosci. 2009;30:756–768. doi: 10.1111/j.1460-9568.2009.06869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bobalova J, Mutafova-Yambolieva VN. Co-release of endogenous ATP and noradrenaline from guinea-pig mesenteric veins exceeds co-release from mesenteric arteries. Clin Exp Pharmacol Physiol. 2001;28:397–401. doi: 10.1046/j.1440-1681.2001.03460.x. [DOI] [PubMed] [Google Scholar]
- 20.Levitt B, Head RJ, Westfall DP. High-pressure liquid chromatographic-fluorometric detection of adenosine and adenine nucleotides: application to endogenous content and electrically induced release of adenyl purines in guinea pig vas deferens. Anal Biochem. 1984;137:93–100. doi: 10.1016/0003-2697(84)90352-x. [DOI] [PubMed] [Google Scholar]
- 21.Bobalova J, Bobal P, Mutafova-Yambolieva VN. High-Performance Liquid Chromatographic Technique for Detection of a Fluorescent Analogue of ADP-Ribose in Isolated Blood Vessel Preparations. Analytical Biochemistry. 2002;305:269–276. doi: 10.1006/abio.2002.5667. [DOI] [PubMed] [Google Scholar]
- 22.Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong ID, Koh SD, Bayguinov O, Sanders KM. Small conductance Ca2+-activated K+ channels are regulated by Ca2+-calmodulin-dependent protein kinase II in murine colonic myocytes. J Physiol. 2000;524(Pt 2):331–337. doi: 10.1111/j.1469-7793.2000.t01-1-00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward SM, Xue C, Shuttleworth CW, Bredt DS, Snyder SH, Sanders KM. NADPH diaphorase and nitric oxide synthase colocalization in enteric neurons of canine proximal colon. Am J Physiol. 1992;263:G277–G284. doi: 10.1152/ajpgi.1992.263.2.G277. [DOI] [PubMed] [Google Scholar]
- 25.Young HM, Furness JB, Shuttleworth CW, Bredt DS, Snyder SH. Co-localization of nitric oxide synthase immunoreactivity and NADPH diaphorase staining in neurons of the guinea-pig intestine. Histochemistry. 1992;97:375–378. doi: 10.1007/BF00270041. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann H. Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog Neurobiol. 1996;49:589–618. doi: 10.1016/0301-0082(96)00026-3. [DOI] [PubMed] [Google Scholar]
- 27.Graeff RM, Franco L, De Flora A, Lee HC. Cyclic GMP-dependent and -independent effects on the synthesis of the calcium messengers cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate. J Biol Chem. 1998;273:118–125. doi: 10.1074/jbc.273.1.118. [DOI] [PubMed] [Google Scholar]
- 28.Lee HC. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu Rev Pharmacol Toxicol. 2001;41:317–345. doi: 10.1146/annurev.pharmtox.41.1.317. [DOI] [PubMed] [Google Scholar]
- 29.Camaioni E, Boyer JL, Mohanram A, Harden TK, Jacobson KA. Deoxyadenosine bisphosphate derivatives as potent antagonists at P2Y1 receptors. J Med Chem. 1998;41:183–190. doi: 10.1021/jm970433l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu HZ, Gao N, Zhu MX, Liu S, Ren J, Gao C, Xia Y, Wood JD. Slow excitatory synaptic transmission mediated by P2Y1 receptors in the guinea-pig enteric nervous system. J Physiol. 2003;550:493–504. doi: 10.1113/jphysiol.2003.041731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyster DJ, Bywater RA, Taylor GS. Neurogenic control of myoelectric complexes in the mouse isolated colon. Gastroenterology. 1995;108:1371–1378. doi: 10.1016/0016-5085(95)90684-3. [DOI] [PubMed] [Google Scholar]
- 32.Keef KD, Du C, Ward SM, McGregor B, Sanders KM. Enteric inhibitory neural regulation of human colonic circular muscle: role of nitric oxide. Gastroenterology. 1993;105:1009–1016. doi: 10.1016/0016-5085(93)90943-7. [DOI] [PubMed] [Google Scholar]
- 33.Cattaneo M, Lecchi A, Ohno M, Joshi BV, Besada P, Tchilibon S, Lombardi R, Bischofberger N, Harden TK, Jacobson KA. Antiaggregatory activity in human platelets of potent antagonists of the P2Y 1 receptor. Biochem Pharmacol. 2004;68:1995–2002. doi: 10.1016/j.bcp.2004.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue L, Farrugia G, Sarr MG, Szurszewski JH. ATP is a mediator of the fast inhibitory junction potential in human jejunal circular smooth muscle. Am J Physiol. 1999;276:G1373–G1379. doi: 10.1152/ajpgi.1999.276.6.G1373. [DOI] [PubMed] [Google Scholar]
- 35.Van der Kloot W. Loading and recycling of synaptic vesicles in the Torpedo electric organ and the vertebrate neuromuscular junction. Prog Neurobiol. 2003;71:269–303. doi: 10.1016/j.pneurobio.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Borycz JA, Borycz J, Kubow A, Kostyleva R, Meinertzhagen IA. Histamine compartments of the Drosophila brain with an estimate of the quantum content at the photoreceptor synapse. J Neurophysiol. 2005;93:1611–1619. doi: 10.1152/jn.00894.2004. [DOI] [PubMed] [Google Scholar]
- 37.Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- 38.Horiguchi K, Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/W(nu) mouse small intestine. J Auton Nerv Syst. 2000;80:142–147. doi: 10.1016/s0165-1838(00)00089-8. [DOI] [PubMed] [Google Scholar]
- 39.Sergeant GP, Large RJ, Beckett EA, McGeough CM, Ward SM, Horowitz B. Microarray comparison of normal and W/Wv mice in the gastric fundus indicates a supersensitive phenotype. Physiol Genomics. 2002;11:1–9. doi: 10.1152/physiolgenomics.00052.2002. [DOI] [PubMed] [Google Scholar]
- 40.Klemm MF, Lang RJ. Distribution of Ca2+-activated K+ channel (SK2 and SK3) immunoreactivity in intestinal smooth muscles of the guinea-pig. Clin Exp Pharmacol Physiol. 2002;29:18–25. doi: 10.1046/j.1440-1681.2002.03601.x. [DOI] [PubMed] [Google Scholar]
- 41.Vanderwinden JM, Rumessen JJ, de Kerchove dA, Jr, Gillard K, Panthier JJ, de Laet MH, Schiffmann SN. Kit-negative fibroblast-like cells expressing SK3, a Ca2+-activated K+ channel, in the gut musculature in health and disease. Cell Tissue Res. 2002;310:349–358. doi: 10.1007/s00441-002-0638-4. [DOI] [PubMed] [Google Scholar]
- 42.Fujita A, Takeuchi T, Jun H, Hata F. Localization of Ca2+-activated K+ channel, SK3, in fibroblast-like cells forming gap junctions with smooth muscle cells in the mouse small intestine. J Pharmacol Sci. 2003;92:35–42. doi: 10.1254/jphs.92.35. [DOI] [PubMed] [Google Scholar]
- 43.Iino S, Nojyo Y. Immunohistochemical demonstration of c-Kit-negative fibroblast-like cells in murine gastrointestinal musculature. Arch Histol Cytol. 2009;72:107–115. doi: 10.1679/aohc.72.107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.