Abstract
Background & Aims
Caudal-related homeobox protein 2 (Cdx2) is an intestine-specific transcription factor that is important for intestinal development and intestine-specific gene expression. Cdx2 regulates intestinal cell–cell adhesion, proliferation, and the transcriptional activities of Wnt and β-catenin in cell culture systems. We generated transgenic mice that overexpress Cdx2 in the small intestinal and colonic epithelium to investigate the role of Cdx2 in differentiation and function of the intestinal epithelium.
Methods
We established 4 different lines of villin–Cdx2 transgenic mice. Intestines were collected from infant, 3-month old, and wild-type mice. Genes of interest and cell lineage markers were examined by PCR and immunohistochemistry.
Results
Villin–Cdx2 transgenic mice had complex phenotypes that were associated with transgene expression levels. The 2 lines that had the greatest levels of transgene expression had significant, pre-weaning failure to grow and death; these were the result of early epithelial maturation and alterations in nutrient digestion and absorption. Fat malabsorption was a prominent feature. Other effects associated with the transgene expression included loss of Paneth cell markers, increases in goblet cells, and migration of proliferating, EphB2-expressing cells to the crypt base. Loss of Paneth cell markers was associated with reduced nuclear localization of β-catenin but not homeotic posteriorization of the epithelium by Cdx2.
Conclusions
Overexpression of Cdx2 in the small intestine is associated with reduced post-natal growth, early epithelial maturation, alterations in crypt base organization, and changes in Paneth and goblet cell lineages. Cdx2 is a critical regulator not only of intestine-specific genes, but also processes that determine epithelial maturity and function,
Keywords: Cdx2, β-catenin, Paneth cells, crypt maturation, intestinal development, intestinal cancer, transcriptional regulation
INTRODUCTION
The intestinal epithelium is a continuously renewing system in which stem cells, located in monoclonal crypts, give rise to proliferating transit amplifying cells that differentiate into the mature intestinal epithelial cell types1. In the small intestine the absorptive enterocytes, mucus-producing goblet cells, and hormone secreting enteroendocrine cells differentiate as they migrate up from the crypt, while the antimicrobial protein producing Paneth cells reside in the bottom of the crypts where they may persist for 20 - 60 days. The transcriptional machinery orchestrating these complex processes of stem cell maintenance, cell proliferation, cell differentiation and lineage selection is beginning to be unraveled2, 3.
Several cell signaling pathways and transcription factors have been identified with important roles in intestinal epithelial development and maintenance1, 3. Wnt/β-catenin/TCF signals play a critical role in intestinal stem cell preservation2 as well as driving daughter cell proliferation in normal intestinal crypts4. More recently, it was determined that nuclear β-catenin/TCF activity is required both for Paneth cell differentiation and for crypt morphogenesis and maintenance5-10. Paneth cell differentiation appears to be quite sensitive to alterations in Wnt signaling levels, as modest increases or decreases in Wnt signaling activity can lead to significant changes in Paneth cell numbers without affecting crypt cell proliferation5. The precise contribution of Wnt/β-catenin to Paneth cell differentiation remains to be determined.
The homeodomain transcription factor Cdx2 is required for normal intestinal epithelial development11, 12. Genetic ablation studies of Cdx2 results in a small intestine epithelium that fails to develop normally. However Cdx2 also actively directs the correct temporal and spatial expression of a number of intestine-specific genes 13-16. The products from these genes participate in nutrient digestion and absorption in the small intestine13-16. Cdx2 also modulates a diverse set of cellular processes including cell proliferation, cell-cell adhesion, and the acquisition of a columnar cell morphology12, 17, 18. The complex molecular mechanisms by which Cdx2 regulates these important processes has been a focus of our research efforts.
To further elucidate the effects of Cdx2 in the intestine, we generated transgenic mice overexpressing Cdx2. These mice have a complex phenotype that includes premature intestinal maturation and fat malabsorption in the post-natal period. While there was no apparent effect upon cell proliferation in the crypts, there was premature intestinal crypt development and the disruption of Paneth cell differentiation, both associated with loss of detectable nuclear β-catenin. We conclude that Cdx2 is a critical regulator of intestinal epithelial maturation and function, crypt organization, and β-catenin localization and transcriptional activity.
MATERIALS AND METHODS
Generation of Villin-Cdx2 transgenic mice
Mouse Cdx2 cDNA with an N-terminal FLAG-tag19 was subcloned into a pCMV-Tag3c (Stratagene, La Jolla, CA) plasmid. The 12.4 Kb mouse Villin promoter was then subcloned before the cDNA to generate the final Villin-(FLAG)-Cdx2 construct. The Villin-Cdx2 DNA was linearized and injected into the male pronuclei of fertilized eggs and implanted into pseudopregnant females by the Transgenic and Chimeric Mouse Core Facility at the University of Pennsylvania. Founder animals were identified by PCR amplification of tail DNA. Four founders were obtained from two separate injections. Transgene founders were bred and offspring were analyzed for the transgene by PCR in this mixed genetic background.
Immunohistochemistry
Intestinal regions were isolated, rinsed in ice-cold PBS, fixed and embedded, then immunohistochemistry performed as described20. Please see Supplemental Methods for full list of primary antibodies and histochemical staining used in this study.
Western blot analysis
Nuclear proteins were isolated from the intestinal epithelium of 3 month old adult mice by an adaptation of a previously described method21. Western blot analysis was then performed using the Cdx2 monoclonal antibody (Biogenex, San Ramon, CA). For loading control, blots were stripped and reprobed with anti-YY1 (Santa Cruz Biotech., Santa Cruz, CA).
RNA detection by quantitative RT-PCR
Sections of the intestine were stored in RNAlater (Ambion, Austin, TX). Total RNA was isolated from the tissue with a RNeasy Midi kit (Qiagen, Valencia, CA). Please see Supplemental Methods for full list of conditions and primers used in this study.
Cell count studies
Paneth cell numbers were counted using Nomarski optics to visualize the Paneth cell granules, and β-catenin co-stain to identify cell edges. Three transgenic and two non-transgenic Line 1 3-month old littermates were used. Goblet cells were counted in FLAG-Cdx2 and Alcian Blue stained tissue sections. Mice were injected with BrdU (Zymed, San Francisco, CA) 1 hour before sacrifice, and BrdU(+) cells identified by immunofluorescence.
Serum Analysis
Serum was collected from decapitated pups in the first few days after birth. Samples pooled together from three to six pups were sent to AniLytics (Gaithersburg, MD) for analysis of lipid levels.
RESULTS
Development of transgenic mouse lines overexpressing Cdx2 in the mouse intestine
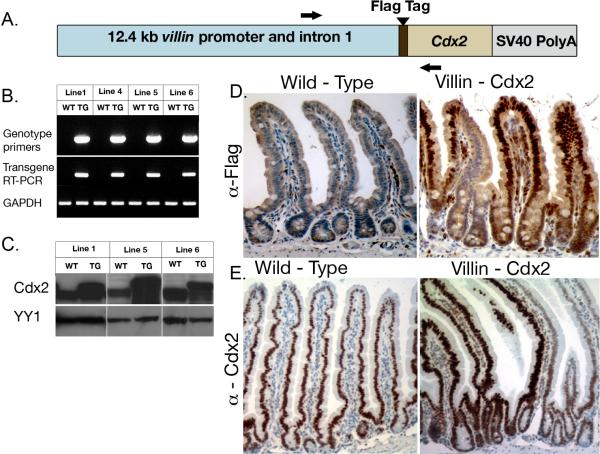
Transgenic mice were generated using the 12.4 kb mouse villin regulatory sequences (kindly provided by Dr. Deborah Gumucio, University of Michigan) to drive expression of a mouse Cdx2 cDNA with an N-terminal FLAG tag that differentiates transgenic from endogenous Cdx2 (Figure 1A)22. Four distinct founders expressing the transgene were obtained from two injections. All four lines expressed the transgene mRNA as detected by PCR, and levels of total intestinal Cdx2 protein were higher than wild-type littermates in 3 of the 4 lines (Figures 1B, 1C). Expression of the FLAG-Cdx2 transgene protein in the nucleus of cells in the base of the crypt was confirmed by immunohistochemistry, although the levels are reduced in the crypt compared to the villi (Figure 1D). In addition, immunohistochemical staining with the Cdx2 antibody confirms elevated Cdx2 levels throughout both the crypts and villi (Figure 1E).
Figure 1. Generation of Villin-Cdx2 transgenic mice.
A Flag-tagged mouse Cdx2 cDNA was expressed under the control of the 12.4 kb mouse Villin promoter. A. Schematic map of the transgene. Black Arrows sites for PCR primers. B. PCR detection of the transgene in genomic DNA and RNA in mouse intestine. C. Western blot detection of Cdx2 protein in intestinal epithelial nuclear extracts of transgenic and wild-type mice. YY1 served as loading control. D. Immunohistochemistry for the FLAG-Cdx2 protein in Line 1 mice jejunum. E. Immunohistochemistry for Cdx2 protein levels in the Line 5 mice duodenum.
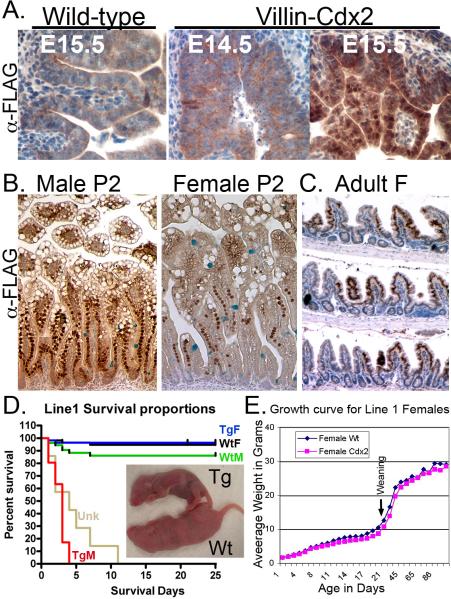
Transgene expression was first detected by embryonic day 15.5 (Figures 2A and Supplemental Figure S1A), similar to previous reports 22. Transgene expression is also detected in the colon (Supplemental Figure S1B). There were differences in transgene expression between the four lines. Unexpectedly, the adult transgene expression pattern is mosaic in all four lines (Figure 1D and E, Figure 2C, and Supplemental Figures S1C, D, E, S2D, and data not shown). Line 4 mice had the fewest intestinal crypt/villi segments expressing the transgene (15-25%) and were not extensively studied. In contrast, Lines 1 and 5 adults had the most significant levels of transgene expression (40% and 80%, respectively, Supplemental Figure S1E). Both demonstrated nearly identical phenotypes, and were the focus of our studies. Transgene expression levels in Line 6 mice lay between these two extremes and were used in several experiments (data not shown).
Figure 2. FLAG-Cdx2 transgene expression in Line 1 mice.
A. The FLAG-Cdx2 protein is first detected E15.5. B. Duodenal FLAG-Cdx2 transgene expression pattern in male and female Line 1 mice at two days post-partum (P2). C. Ileal FLAG-Cdx2 expression in 3-month old Line 1 female mice. D. Kaplan-Meier survival curve for Line 1 mice (214 mice from 16 litters); Wild-type male and female (WtM and WtF), transgenic male and female (TgM, TgF), and mice of unknown (Unk) genotype lost prior to genotyping. Survival curves are significantly different by Logrank testing p<0.0001 Inset: Male wild-type (Wt) and transgenic (Tg) littermates at P2. E. Growth curve for Line 1 female Wt and Tg mice n=4.
Line 1 also had a difference between the sexes as postnatal male mice extensively expressed the transgene, while Line 1 female expression was mosaic at birth (Figure 2C). Some of this mosaicism may be due to the polyclonal nature of the stem cells found in the intervillus region at this stage of development. The transgene increased total Cdx2 mRNA levels by 3.5-fold at postnatal day 3 in male Line 1 Villin-Cdx2 mice, and it remained nearly 2-fold elevated at three months of age in female Line 1 mice (Supplemental Figure S1F). There was a compensatory 50% reduction in endogenous Cdx2 mRNA levels but no effect on the homologue Cdx1's mRNA levels. In Line 5 both preweaning males and females had extensive expression (supplemental figure S2B and S2C). The adult expression was mosaic in both sexes at 3 months (Supplemental Figure S1D & S2D) and more extensive than Line 1 adult females (Supplemental figures S1C and S1D). This pattern is also noted in animals examined at later ages (data not shown). The expression pattern for the transgene is clonal in all adult lines; adjacent crypts, some expressing and some not are seen, but the entire crypt is expressing the transgene or not. However, since villi receive contributions from multiple adjacent crypts, we can observe mosaicism within the villi of adult mice (Figure 2C and Supplemental Figure S2D). Together, this pattern suggests the activation or silencing of the transgene occurs within the stem cells themselves. This pattern further suggests there may be a selective pressure against excessive Cdx2 expression in crypt stem cells.
Overexpression of Cdx2 is associated with reduced postnatal growth, and increased postnatal death
Line 1 transgenic pups were indistinguishable from wild-type littermates at birth, but male pups failed to grow and all died before weaning (Figure 2D). Growth of transgenic female pups was delayed until weaning, after which they caught up (Figure 2E). In Line 5 both females and males failed to gain weight normally, and died more frequently before weaning (Supplemental Figure S2E, F, and G). All the mice that failed to gain weight nurse normally, as milk is visible in their stomachs (Supplemental Figure S2E). However, surviving transgenic mice exhibited a weight gain post-weaning (Supplemental Figure S2G). Line 4 and Line 6 transgenic pups with reduced transgene expression were healthy and had no growth defects or premature deaths. However, when Line 6 mice were inbred, a quarter of the offspring similarly experienced reduced growth pre-weaning (both males and females) and died at increased rates before weaning (Supplemental Figure 3A and data not shown). These “poor growth” line 6 mice had higher levels of Cdx2 mRNA by qPCR and more intense and widely distributed transgene expression by FLAG immunohistochemistry (Supplemental Figures 3B and 3C). This suggests that high levels of Cdx2 expression are associated with neonatal failure to thrive, and poor weight gain.
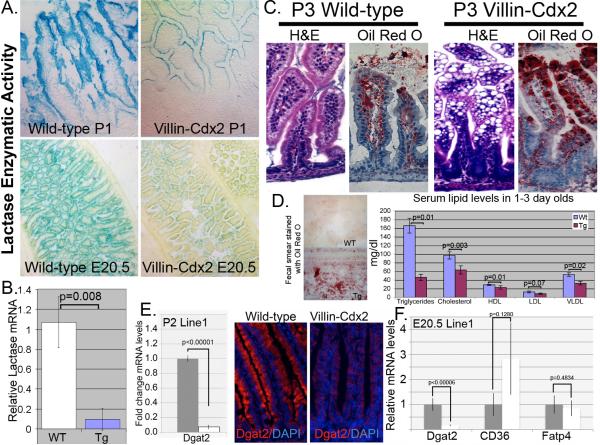
Lactose is the primary sugar in breast milk, whereas sucrose is a common sugar in adult diets. The intestinal enzymes that digest these sugars, Lactase-Phlorizin Hydrolase and Sucrase-Isomaltase (SI), are both transcriptional targets of Cdx2 although their expression patterns differ13, 14, 16. Lactase expression begins before birth and is lost at weaning. In contrast, SI expression increases at weaning. Histochemical assays on frozen tissue indicated that lactase activity in the Line 1 male Villin-Cdx2 mice was greatly reduced while significant aberrant SI activity was detected in both 1 day-old transgenic male mice and E20.5 embryos (Figure 3A and Supplemental Figure S4A), suggesting these changes were due to transgene expression and not stress from starvation. Quantitative RT-PCR confirmed a ten-fold reduction in lactase mRNA levels, whereas SI levels are increased four to ten-fold in the Line 1 male mice both at E20.5 and P1 (Figure 3B and Supplemental Figure S4B).
Figure 3. The Villin-Cdx2 transgene alters intestinal disaccharidase activity and fat absorption.
A. LPH enzymatic activity is detected histochemically in intestinal tissue from male P1 mice and E20.5 embryos. B. Quantitative RT-PCR determination of LPH mRNA levels in intestine of male Line 1 wild type (Wt) and transgenic (Tg) littermates. Statistically significant by Student's T-test, n=3. C. Hematoxylin and eosin staining and Oil Red O staining on duodenal sections from P3 male mice. D. Fecal smear on glass slide stained with Oil Red O and average serum lipid levels in pooled specimens from 1 to 3 day old Wt and Tg littermates. Blood from 3 to 6 pups was pooled for each serum sample. n=3 samples. E. Dgat2 mRNA and protein levels by qPCR and immunofluorescence in transgenic Line 1 male mice at post-partum day 2 (P2). n=3. F. mRNA levels for Dgat2, CD36, and Fatp4 by qPCR in Line 1 male mice at E20.5. n=3 for each.
Another finding of interest was the presence of extensive vacuoles in the villous enterocytes of nursing Line 1 and Line 5 pups of both sexes, and the inbred Line 6 mice (Figures 2B and 3C and Supplemental Figure S3C). These vacuoles are observed only in the nursing pups. They are not observed in adult mice fed normal chow. These vacuoles stain with Oil Red O, suggesting they are lipid-filled. Moreover, stool smears from nursing transgenic mice, but not wild-type littermates, stained positive for fat by Oil Red O (Figure 3D). These observations suggest the Villin-Cdx2 mouse pups are malabsorbing fats. In support of this, serum from Line 1 males and wild-type littermates in the first three days after birth was collected and pooled for lipid profiling. Triglyceride levels were similar at birth; however Villin-Cdx2 males had significantly lower triglyceride, total cholesterol, HDL, and VLDL on the subsequent days (Figure 3E and Supplemental Figure S43CB). We conclude that the reduced ability to process lactose sugar and absorb fat from breast milk caused the reduced growth and high pup death rates observed for the Villin-Cdx2 mice.
Several transporters and enzymes are critical for normal intestinal cell fat absorption, including CD36, Fatp4, Dgat2, and Mttp. Diglyceride acyltransferase (or O-acyltransferase), DGAT, catalyzes the formation of triglycerides from diacylglycerol and this reaction is considered the terminal end step in triglyceride synthesis 23. We investigated changes in the expression of these factors in our transgenic mice. By post-partum day 2, levels of all four factors are significantly reduced by qPCR and immunohistochemistry (Figures 3E and Supplemental Figures 5A, B, and C). Some of these effects persist into adults fed a normal lowfat mouse chow, as we can see significant reductions in MTTP protein levels in 3 month-old Line 1 female mice (Supplemental Figure S5D). Inhibition of Hedgehog signaling has been associated with a similar phenotype 24, however we did not observe significant changes in Ihh levels in the intestine of our transgenic mice (Supplemental Figure S5E). We thought it unlikely that Cdx2 expression globally reduced expression levels for these genes, and suspected some of the reductions might be due to a negative feedback in cells with lipid-filled vacuoles. qPCR for several of these factors at E20.5, prior to birth and nursing found that only Dgat2 mRNA levels were significantly reduced (Figure 3F). We conclude that the fat malabsorption phenotype induced by the Cdx2 transgene is due to significant reductions in Dgat2 expression in intestinal epithelial cells.
Cdx2 overexpression alters intestinal cell lineages
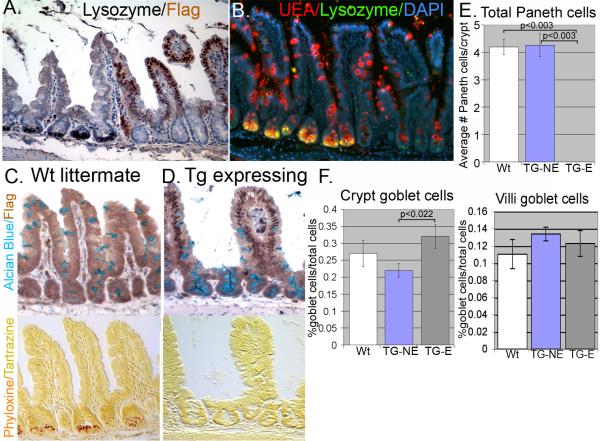
Another significant effect of the Cdx2 transgene was the disruption of the Paneth cell lineage. Adult mice from all four lines either had no detectable Paneth cells in the transgene-expressing epithelium (Lines 1, 4,or 5) (Figure 4 and data not shown) or severely diminished Paneth cell numbers (Line 6, Supplemental Figure S6). Moreover, when Line 6 mice are inbred, the poor-growing mice with increased transgene levels also have no detectable mature Paneth cells. Line 1, Line 4, Line 5, and inbred Line 6 mice, the Paneth cell marker lysozyme was essentially absent in regions that expressed the Cdx2 transgene (Figure 4A and Supplemental Figure S6). Staining with the lectin UEA confirmed the loss of mature Paneth cell granules in transgenic mice (Figures 4B). Goblet and Paneth cells are thought to be closely related and emerge from a common progenitor cell1, 25, 26. As a consequence of losing the Paneth cell lineage, we observed a small increase in goblet cell numbers in the transgene positive crypts but not the villi (Figure 4C and D). Formal counting of Paneth and goblet cells confirmed these observations (Figure 4E and 4F). Lastly, no differences were noted in enteroendocrine cell numbers based on Chromogranin A staining. (Supplemental Figure 7A, B, and C).
Figure 4. Overexpression of Cdx2 results in loss of Paneth cell markers.
A. Absence of Paneth cell marker lysozyme in FLAG-Cdx2 positive crypts. B. No Paneth cell granules are detected with co-staining of Lysozyme and UEA in many crypts. C. Immunohistochemistry for FLAG-Cdx2 costained with Alcian blue in wild-type littermate. Serial section is stained with phyloxine/tartrazine to identify Paneth cell granules. D. Same as in C except using serial sections from Villin-Cdx2 mice. E. Quantification of total Paneth and F. goblet cell numbers (separating crypt from villi goblet cell numbers) in 10 transgene-expressing (TG-E) crypts and 10 non-expressing (TG-NE) crypts from three month-old female mice (n=3 each). Statistically significant by Student's T-test.
Crypt organization and gene expression patterns are altered with expression of the Villin-Cdx2 transgene
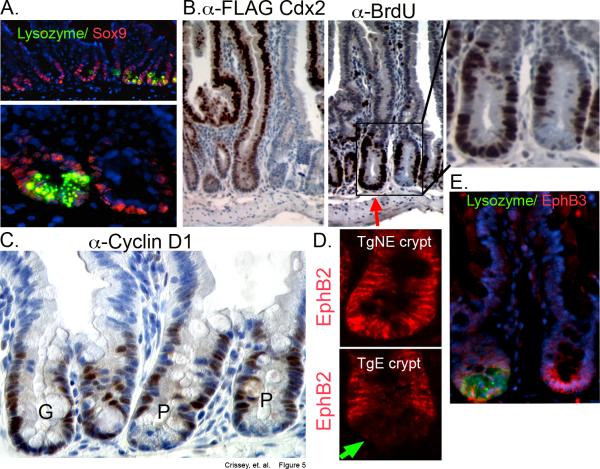
The transcription factor Sox-9 is a Wnt/β-catenin transcriptional target that is required for Paneth cell differentiation7. However, we found no difference in Sox-9 immunostaining between the transgene expressing and non-expressing crypts, nor in Sox-9 mRNA levels by qPCR (Figure 5A and Supplemental Figure 7D). As Sox-9 is also expressed in the transit-amplifying (TA) cell population8, we next examined the pattern of BrdU incorporating cells in the crypt base. We found that BrdU incorporating cells localize to the crypt base in transgene-expressing crypts (Figure 5B and Supplemental Figure 7E). This pattern is similar to the colon, in which Paneth cells are naturally absent. Careful counting of BrdU positive cells suggests there was a trend for increased cell proliferation with expression of the Cdx2 transgene, but this was not statistically significant (Supplemental Figure S7F). Cyclin D1, a Wnt-target gene27 and critical cell cycle protein for proliferating cells, is similarly affected. Overall levels do not appear different, though cyclin D1 expressing cell can now be detected in the crypt base formerly occupied by Paneth cells (Figure 5C).
Figure 5. Organization of the crypt compartment is disordered with Villin-Cdx2 transgene expression.
A. Immunofluorescence for Sox-9 and lysozyme. B. Serial sections were stained with the FLAG antibody or the anti-BrdU antibody, showing adjacent transgene-expressing and non-expressing crypts. Inset: Higher power magnification of adjacent crypts. C. Staining for cyclin D1. P: Paneth cells, G: goblet cells indicating transgene expressing crypt. D. Immunofluorescence for EphB2 in the crypt base. Transgene non-expressing (TgNE) and transgene expressing (TgE) crypts are represented. Green arrow indicates absence of signal from crypt with Paneth cell granules. E. EphB3 and lysozyme expression by immunofluorescence.
The organization of the intestinal crypt is thought to depend in part on the actions of Wnt/β-catenin regulated B-type Ephrin receptors. Localization of Paneth cells and TA daughter cells is disordered in EphB2 or EphB3 transgenic knock-out mice28. We assayed for Ephb2 and EphB3 expression in our Villin-Cdx2 mice, and observed that the EphB2 pattern was similar to that observed for BrdU and Sox-9. Crypts that did not express the transgene and retained Paneth cells had a normal EphB2 staining pattern (Figure 5D). In contrast, the EphB2 signal migrates to the base in the transgene-expressing crypts. EphB3 staining was not effected by the Villin-Cdx2 transgene, as EphB3 positive cells were retained at the base of the crypt regardless of whether lysozyme-positive Paneth cells were present (Figure 5E). Together this suggests the loss of fully differentiated Paneth cells was not due to their improper sorting and migration from the crypt base or loss of Sox-9 expression.
Disruption of Paneth cell differentiation is associated with loss of nuclear-localized β-catenin
A number of transcription factors and kinases are known to regulate Paneth and goblet cell differentiation in the small intestine, including Notch, Klf4, Math1(Atoh1), Spedf, and Lkb129-33. We therefore explored for changes in the levels of these factors in our Villin-Cdx2 transgenic mice in which Paneth cell development has been disrupted. Hes1, Hes5, and Hey1 are important Notch signaling effectors and target genes. The distribution of Hes1 protein followed BrdU and cyclin D1 to the crypt base in crypts lacking Paneth cell markers, however the overall mRNA levels were not significantly affected (Supplemental Figure S8A). Nor were the mRNA levels of Hes5 and Hey1. Klf4 protein levels appeared slightly increased in the intestinal epithelium of transgenic mice, however this was only observed in the villi (Supplemental Figure 8B). Moreover, mRNA levels by qPCR were not significantly changed, suggesting this may not be an important effect. Similarly, Math1, Lkb1, and Spdef levels were not significantly affected by expression of the transgene (Supplemental Figure S8C and D), suggesting they were not involved in the Cdx2-mediated disruption of the Paneth cell lineage.
Wnt/β-catenin signals promote a number of critical processes in the intestinal crypts2. Paneth cell differentiation appears to require the highest levels of Wnt/β-catenin signaling. Nuclear-localized β-catenin is typically seen only in Paneth cells6. Moreover, Paneth cell development is very sensitive to Wnt/β-catenin levels, as manipulation of β-catenin levels up or down caused Paneth cell numbers to increase or decrease, respectively5. This is observed in the absence of effects upon intestinal cell proliferation. Thus, of the intestinal processes regulated by Wnt signaling, Paneth cell differentiation appears to be uniquely dependent.
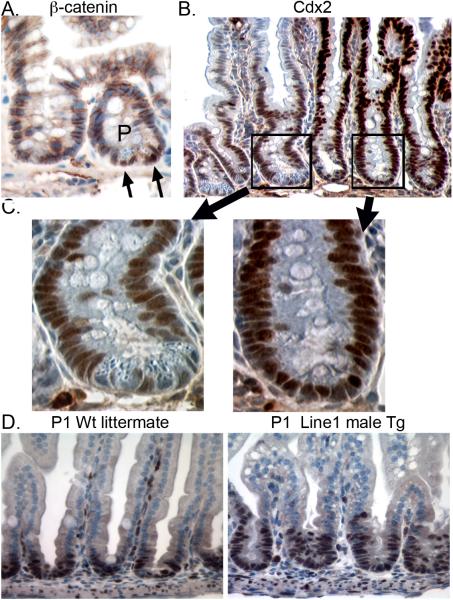
Given the observed loss of Paneth cell markers, we stained for β-catenin in our transgenic mice. We found that nuclear β-catenin was absent from transgene expressing crypts; it was detected only in non-expressing crypts that retained Paneth cells (Figure 6A). This suggests expression of the Cdx2 transgene can modulate nuclear β-catenin levels. In keeping with this, Cdx2 protein is undetectable in normal Paneth cells, but it is detected throughout the crypt base in transgene-expressing crypts lacking Paneth cells (Figure 6B and C). Together, these findings suggest Cdx2 overexpression can influence levels of nuclear β-catenin.
Figure 6. The Villin-Cdx2 transgene alters β-catenin nuclear localization and induces premature crypt development.
A. Nuclear-localized β-catenin is observed in Paneth (P) cells at the crypt base (Black arrows) but not in the adjacent transgene expressing crypt. B. Cdx2 expression is absent in Paneth cells. (P) Paneth cells; (G) goblet cells. C. Higher power view of crypts stained for Cdx2 demonstrating no Cdx2 staining in Paneth cells. D. Ki-67 staining in P1 wild-type (P1 Wt) and transgenic (Tg) pups.
Wnt signaling is also known to be required for crypt morphogenesis during development and crypt maintenance in adults9, 10. In the developing intestine, Wnt signaling is found primarily in villi until birth34. The Cdx2 transgene is also well expressed in the villi during these stages (Figure 2A and B and Supplemental Figure S2B and C) By P3 Wnt signaling has migrated to the intervillus area, from which crypts develop. While there were no obvious effects of the Cdx2 transgene on adult crypt morphology, we noted crypt development was accelerated by the Cdx2 transgene, as crypt development was apparent by the second day of life in the Villin-Cdx2 mice (Figure 6D), nearly two weeks earlier than occurs normally35. Early crypt development was not observed in Line 6 mice. However, when Line 6 mice were inbred, early crypt maturation was observed in the poor growth pups (Supplemental Figure S3C), suggesting it is also a response to high levels of Cdx2 expression. This suggests that the Cdx2 overexpression induces premature crypt development, possibly by modulating Wnt/β-catenin activity during development.
Villin-Cdx2 transgene does not induce a posterior homeotic transformation in the small intestine crypts
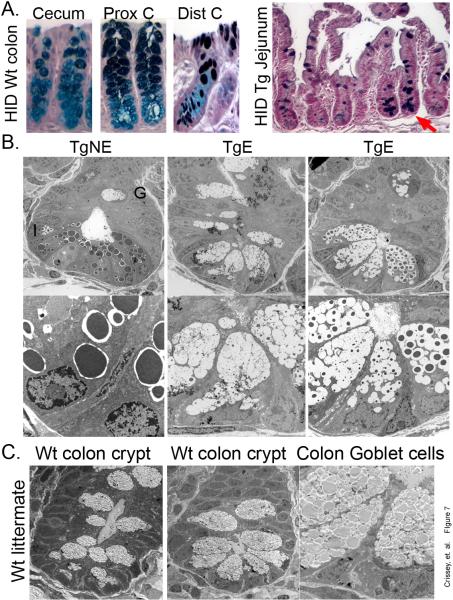
The absence of mature Paneth cells, localization of cyclin D1, BrdU+, and EphB2-expressing cells to the crypt base, all suggest Cdx2 overexpression may be inducing a posterior homeotic shift by transforming small intestinal crypts into colonic ones. Colonic mucins are less sulfated than small intestinal mucins26. This difference can be demonstrated using a high iron diamine (HID) staining for sulfated mucins. In the colonic cecum, little staining is observed (Figure 7A). Some staining is detected in the proximal colon, but only in differentiated goblet cells on the surface; goblet cells in the colonic crypt base lack sulfated mucins. In contrast, all goblet cells in the small intestine stain positive for sulfated mucins, including those that replace Paneth cells at the crypt base. This suggests the transgene-induced goblet cells retain their small intestine identity.
Figure 7. Villin-Cdx2 transgene expression does not induce a posterior homeotic transformation.
A. High iron diamine (HID) staining of wild-type (Wt) littermate mouse colon for sulfated mucins. (C) cecum; (PC) proximal colon. HID staining of the small intestine from a Villin-Cdx2 mouse illustrating positive goblet calls at base of crypts lacking Paneth cells (Red arrow). B. Transmission electron micrographs (TEM) of crypts from transgenic mouse ileum. A transgene non-expressing (TgNE) crypt with normal Paneth cells, an immature goblet cell (G), and an intermediate cell (I) are indicated. Two transgene-expressing crypts without normal Paneth cells demonstrate large intermediate cell types. C. TEM study of proximal colon crypts for comparison from Wt littermate.
Transmission electron microscopy (TEM) further supports this observation. We studied cells at the base of small intestine crypts in our Villin-Cdx2 mice. We observed that Paneth cells were replaced by cells with features suggestive of intermediate or granular goblet cells (Figure 7B)25, 26. These cell had granules of different sizes in the maturing goblet cell vacuoles, with some of these crypt goblet cells having larger granules than others. These “intermediate” cells had larger mucin-filled vacuoles than seen in the classic intermediate cells found in the crypts with Paneth cells. Moreover, these granular goblet cells were significantly different from the goblet cells observed in the colon (Figure 7C). No granules were observed in the colon goblet cells. Together these findings suggest the Villin-Cdx2 transgene is not inducing a posterior homeotic shift in the small intestine epithelium. Rather, it appears to inhibit a late stage in Paneth cell differentiation, forcing the cells down the default goblet cell pathway. We conclude that overexpression of Cdx2 in the small intestine epithelium accelerates its maturation, alters crypt organization and influences cell lineage selection by direct modification of these processes and not by global posteriorization of the epithelial identity.
DISCUSSION
It has been established that the homeodomain transcription factor Cdx2 plays a critical role in regulating intestinal epithelial differentiation and function12. Our transgenic mice unexpectedly display a complex phenotype suggesting Cdx2 plays important roles in other intestinal cell processes.
Cdx2 overexpression promotes premature maturation of the intestinal epithelium and fat malabsorption in newborn mice
The reduced post-natal growth and early demise was observed in those lines with the highest level of transgene expression. We suspect the early loss of lactase expression coupled with the fat malabsorption contribute to this phenotype by limiting the nutrition of the nursing pups. The ability of the surviving mice to “catch up” in weight once they are weaned supports this conclusion. Mechanistically, diminished Dgat2 expression is responsible for the defect, though how Cdx2 reduces Dgat2 levels is unclear. Little is known about the mechanisms regulating Dgat2 gene expression. It is interesting to speculate that the fat malabsorption effect may persist into adulthood, though we have not yet challenged our mice with a high-fat diet. This is an objective for future studies, where we will seek to identify the transcription factor networks modulated by Cdx2 that reduce Dgat2 gene expression and intestinal fat absorption.
Cdx2 levels alter crypt maturation and organization
Other striking changes in the crypt compartment of Villin-Cdx2 mice include premature crypt development and disruption of the Paneth cell lineage. We have previously published that Cdx2 expression can inhibit β-catenin/TCF transcriptional activity19-21. Given the importance for Wnt/β-catenin signaling in driving both crypt and Paneth cell development, we suspect that Cdx2 overexpression may be altering Wnt/β-catenin activity in the crypt compartment, and have studies underway to explore this possibility. While we think it more likely that a single mechanism is responsible for both effects, it is possible that Paneth cell loss and early crypt development may be due to two different processes. Disruption of the Paneth cell lineage also led to the migration of proliferating cells to the crypt base. As these cells also express cyclin D1, Hes1, and Sox9, it is not unexpected to find these markers migrate to the crypt base as well. Given these changes, it is interesting to speculate what effect this has upon the stem cell niche. It appears from our studies that differentiated Paneth cells are not required to establish this niche. We have several experiments underway to investigate for changes in the stem cell niche with Cdx2 overexpression.
The Villin-Cdx2 phenotype suggests broad modulation of small intestine transcription factor activity
While Cdx2 is well known to regulate intestine-specific expression of genes, the correct temporal regulation of LPH was thought to depend on other factors including GATA-4 and HNF-1α/β14, 36. We suspect the premature loss of LPH expression and induction of SI is due to Cdx2 inducing these factors to adopt a mature pattern of expression. We have begun looking for evidence for this in our mice. Moreover, while Cdx2 is known to regulate several aspects of nutrient digestion and absorption, this report is the first to suggest a critical role for Cdx2 in regulating fat absorption. Histologically, the fat malabsorption appears to be a block in lipid transport out of the intestinal epithelial cells, and resembles human conditions like Abetalipoproteinemia or chylomicron retention disease37. It is presently unclear if there is any mechanistic similarity between this mouse model and these human conditions.
Cdx2 regulates a wider variety of intestinal epithelium functions than previously suspected
Our findings, published here and elsewhere12, 17-19, 38, support a model in which Cdx2 actively influences a greater variety of processes than previously considered including cell morphology and polarity, cell lineage decisions, temporal gene expression, and the stem cell niche. We suspect some of these effects are mediated by Cdx2 modulation of other transcription factor activities including GATA-4, HNF-α/β, and Wnt/β-catenin/TCF. This is a focus of ongoing studies.
In summary, intestinal Cdx2 overexpression gives rise to mice with a prominent small intestinal phenotype associated with failure to thrive before weaning, likely due to an accelerated maturation of the epithelium and fat malabsorption. Moreover, Cdx2 overexpression resulted in significant alterations to the small intestine crypt compartment, including the disruption of Paneth cell differentiation, and the migration of transit-amplifying daughter cells to the crypt base. This was not due to a homeotic posteriorization, but more likely is the result of effects upon Wnt/β-catenin signaling and other transcription factor activity. We conclude that the intestine-specific homeodomain transcription factor Cdx2 is a critical regulator of intestinal epithelial maturation and function, crypt organization, and β-catenin localization and transcriptional activity.
Supplementary Material
ACKNOWLEDGEMENTS
We thank William Hockheimer and Abena Kwaa for their technical expertise. This work was supported by NIDDK Grants DK068366 and DK085551 (J. Lynch), an NCI Program Project P01 DE12467, and Core Facilities of the Center for Molecular Studies in Digestive and Liver Disease at the University of Pennsylvania (P30-DK50306).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest to disclose for all authors
REFERENCES
- 1.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–59. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 2.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–60. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 3.Scoville DH, Sato T, He XC, et al. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849–64. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 4.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Andreu P, Peignon G, Slomianny C, et al. A genetic study of the role of the Wnt/beta-catenin signalling in Paneth cell differentiation. Dev Biol. 2008;324:288–96. doi: 10.1016/j.ydbio.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 6.van Es JH, Jay P, Gregorieff A, et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7:381–6. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 7.Mori-Akiyama Y, van den Born M, van Es JH, et al. SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology. 2007;133:539–46. doi: 10.1053/j.gastro.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Formeister EJ, Sionas AL, Lorance DK, et al. Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1108–18. doi: 10.1152/ajpgi.00004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korinek V, Barker N, Moerer P, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nature Genetics. 1998;19:379–83. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 10.Fevr T, Robine S, Louvard D, et al. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27:7551–9. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao N, White P, Kaestner KH. Establishment of Intestinal Identity and Epithelial-Mesenchymal Signaling by Cdx2. Dev Cell. 2009;16:588–99. doi: 10.1016/j.devcel.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo RJ, Suh ER, Lynch JP. The Role of Cdx Proteins in Intestinal Development and Cancer. Cancer Biol Ther. 2004;3:593–601. doi: 10.4161/cbt.3.7.913. [DOI] [PubMed] [Google Scholar]
- 13.Fang R, Santiago NA, Olds LC, et al. The homeodomain protein Cdx2 regulates lactase gene promoter activity during enterocyte differentiation. Gastroenterology. 2000;118:115–27. doi: 10.1016/s0016-5085(00)70420-3. [DOI] [PubMed] [Google Scholar]
- 14.Boudreau F, Zhu Y, Traber PG. Sucrase-isomaltase gene transcription requires the hepatocyte nuclear factor-1 (HNF-1) regulatory element and is regulated by the ratio of HNF-1 alpha to HNF-1 beta. J Biol Chem. 2001;276:32122–8. doi: 10.1074/jbc.M102002200. [DOI] [PubMed] [Google Scholar]
- 15.Dalmasso G, Nguyen HT, Yan Y, et al. Butyrate transcriptionally enhances peptide transporter PepT1 expression and activity. PLoS One. 2008;3:e2476. doi: 10.1371/journal.pone.0002476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchelmore C, Troelsen JT, Spodsberg N, et al. Interaction between the homeodomain proteins Cdx2 and HNF1alpha mediates expression of the lactase-phlorizin hydrolase gene. Biochem J. 2000;346(Pt 2):529–35. [PMC free article] [PubMed] [Google Scholar]
- 17.Ezaki T, Guo RJ, Li H, et al. The homeodomain transcription factors Cdx1 and Cdx2 induce E-cadherin adhesion activity by reducing beta- and p120-catenin tyrosine phosphorylation. Am J Physiol Gastrointest Liver Physiol. 2007;293:G54–65. doi: 10.1152/ajpgi.00533.2006. [DOI] [PubMed] [Google Scholar]
- 18.Funakoshi S, Ezaki T, Kong J, et al. Repression of the Desmocollin 2 gene in colorectal cancer cells is relieved by the homeodomain transcription factors Cdx1 and Cdx2. Molecular Cancer Research. 2008;6:1478–1490. doi: 10.1158/1541-7786.MCR-07-2161. [DOI] [PubMed] [Google Scholar]
- 19.Guo R, Funakoshi S, Lee HH, et al. The intestine-specific transcription factor Cdx2 inhibits beta-catenin/TCF transcriptional activity by disrupting the beta-catenin/ TCF protein complex. Carcinogenesis. 2010;31:159–66. doi: 10.1093/carcin/bgp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crissey MA, Guo RJ, Fogt F, et al. The homeodomain transcription factor Cdx1 does not behave as an oncogene in normal mouse intestine. Neoplasia. 2008;10:8–19. doi: 10.1593/neo.07703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo RJ, Huang E, Ezaki T, et al. Cdx1 inhibits human colon cancer cell proliferation by reducing β-catenin/TCF transcriptional activity. J Biol Chem. 2004;279:36865–75. doi: 10.1074/jbc.M405213200. [DOI] [PubMed] [Google Scholar]
- 22.Madison BB, Dunbar L, Qiao XT, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–83. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 23.Iqbal J, Hussain MM. Intestinal lipid absorption. Am J Physiol Endocrinol Metab. 2009;296:E1183–94. doi: 10.1152/ajpendo.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang LC, Nassir F, Liu ZY, et al. Disruption of hedgehog signaling reveals a novel role in intestinal morphogenesis and intestinal-specific lipid metabolism in mice. Gastroenterology. 2002;122:469–82. doi: 10.1053/gast.2002.31102. [DOI] [PubMed] [Google Scholar]
- 25.Garabedian EM, Roberts LJ, McNevin MS, et al. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. Journal of Biological Chemistry. 1997;272:23729–40. doi: 10.1074/jbc.272.38.23729. [DOI] [PubMed] [Google Scholar]
- 26.Mahoney ZX, Stappenbeck TS, Miner JH. Laminin alpha 5 influences the architecture of the mouse small intestine mucosa. J Cell Sci. 2008;121:2493–502. doi: 10.1242/jcs.025528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 28.Batlle E, Henderson JT, Beghtel H, et al. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–63. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- 29.Yang Q, Bermingham NA, Finegold MJ, et al. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–8. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 30.Katz JP, Perreault N, Goldstein BG, et al. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–28. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shorning BY, Zabkiewicz J, McCarthy A, et al. Lkb1 deficiency alters goblet and paneth cell differentiation in the small intestine. PLoS One. 2009;4:e4264. doi: 10.1371/journal.pone.0004264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Es JH, van Gijn ME, Riccio O, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–63. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 33.Gregorieff A, Stange DE, Kujala P, et al. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology. 2009;137:1333–45. e1–3. doi: 10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 34.Kim BM, Mao J, Taketo MM, et al. Phases of canonical Wnt signaling during the development of mouse intestinal epithelium. Gastroenterology. 2007;133:529–38. doi: 10.1053/j.gastro.2007.04.072. [DOI] [PubMed] [Google Scholar]
- 35.Bry L, Falk P, Huttner K, et al. Paneth cell differentiation in the developing intestine of normal and transgenic mice.. Proceedings of the National Academy of Sciences of the United States of America; 1994. pp. 10335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boudreau F, Rings EH, van Wering HM, et al. Hepatocyte nuclear factor-1 alpha, GATA-4, and caudal related homeodomain protein Cdx2 interact functionally to modulate intestinal gene transcription. Implication for the developmental regulation of the sucrase-isomaltase gene. J Biol Chem. 2002;277:31909–17. doi: 10.1074/jbc.M204622200. [DOI] [PubMed] [Google Scholar]
- 37.Tarugi P, Averna M, Di Leo E, et al. Molecular diagnosis of hypobetalipoproteinemia: an ENID review. Atherosclerosis. 2007;195:e19–27. doi: 10.1016/j.atherosclerosis.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Funakoshi S, Kong J, Crissey MA, et al. Cdx2 promotes E-cadherin function and cell-cell adhesion in colon cancer cells by enhancing E-cadherin trafficking to the cell membrane. Am J Physiol Gastrointest Liver Physiol. 2010 doi: 10.1152/ajpgi.00297.2010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.