Abstract
NMDA glutamate receptors (NMDARs) and nicotinic acetylcholine receptors (nAChRs) are both involved in learning and synaptic plasticity. Increasing evidence suggests processes mediated by these receptors may interact to modulate learning; however, little is known about the neural substrates involved in these interactive processes. The present studies investigated the effects of nicotine on MK-801 hydrogen maleate (MK-801) and DL-2-Amino-5-phosphonovaleric acid (APV) induced disruption of contextual fear conditioning in male C57BL/6J mice, using direct drug infusion and selective nAChR antagonists to define the brain regions and the nAChR subtypes involved. Mice treated with MK-801 showed a deficit in contextual fear conditioning that was ameliorated by nicotine. Direct drug infusion demonstrated that the NMDAR antagonists disrupted hippocampal function and that nicotine acted in the dorsal hippocampus to ameliorate the deficit in learning. The high-affinity nAChR antagonist Dihydro-β-erythroidine hydrobromide (DhβE) blocked the effects of nicotine on MK-801-induced deficits while the α7 nAChR antagonist methyllycaconitine citrate salt hydrate (MLA) did not. These results suggest that NMDARs and nAChRs may mediate similar hippocampal processes involved in contextual fear conditioning. Furthermore, these results may have implications for developing effective therapeutics for the cognitive deficits associated with schizophrenia because a large subset of patients with schizophrenia exhibit cognitive deficits that may be related to NMDAR dysfunction and smoke at much higher rates than the healthy population, which may be an attempt to ameliorate cognitive deficits.
1. INTRODUCTION
Signaling through glutamate N-methyl-D-aspartic acid receptors (NMDARs) is critically involved in learning (Nakazawa et al., 2004) and synaptic plasticity (Malenka and Bear, 2004). Processes mediated by NMDARs support both short-term and long-term alterations in neural function that underlie changes in short- and long-term memory (For review see Lynch, 2004). The cholinergic system is also involved in learning and synaptic plasticity; cholinergic antagonists disrupt learning (Anagnostaras et al., 1995; Gale et al., 2001), and agonists such as nicotine enhance learning (Kenney and Gould, 2008; Levin et al., 2006) and synaptic plasticity as measured by long-term potentiation (LTP) (Buccafusco et al., 2005). Increasing evidence suggests that NMDAR-mediated and nicotinic acetylcholine receptor (nAChR)-mediated processes may interact during learning (Ciamei et al., 2001; Gould and Lewis, 2005; Levin et al., 1998) and changes in these processes may occur in mental illnesses that involve altered cognitive function (Gao et al., 2000; Harrison et al., 1991). Thus, understanding how NMDAR- and nAChR-mediated processes interact to alter learning may contribute to greater understanding of mental illnesses.
Schizophrenia is a psychiatric disorder characterized by positive symptoms (i.e., auditory hallucinations), negative symptoms (i.e., lack of affect), and cognitive deficits (i.e., difficulties with learning and concentration) (Gold and Harvey, 1993; Lewis and Lieberman, 2000). The cognitive deficits may be related to altered hippocampal function. The hippocampus is critically involved in learning, memory, and other cognitive processes (Eichenbaum et al., 2007; Squire, 1992) and patients with schizophrenia show physical abnormalities of the hippocampus that include altered neuronal size, organization, and shape (for review see Harrison, 2004). In addition to altered hippocampal structure, patients with schizophrenia have altered glutamate signaling (Eastwood et al., 1997; Eastwood et al., 1995; Harrison and Eastwood, 1998; Kerwin et al., 1990; Porter et al., 1997) and decreased expression of glutamate receptor genes in post-mortem hippocampal and parahippocampal tissue (Gao et al., 2000; Harrison et al., 1991). NMDARs are involved in synaptic plasticity (Herron et al., 1986; Muller and Lynch, 1990) and learning (Bannerman et al., 1995; Miserendino et al., 1990) (Fanselow and Kim, 1994; Fanselow et al., 1994; Gould et al., 2002; Stiedl et al., 2000) and thus changes in their function in the hippocampus may further contribute to cognitive deficits associated with schizophrenia. In fact, altering NMDAR function produces similar cognitive and neurochemical alterations (Duncan et al., 2006; Javitt et al., 1996; Kondziella et al., 2006; Krystal et al., 1994; Lisman et al., 2010; Luby et al., 1959; Newcomer et al., 1999; Umbricht et al., 2000).
In addition to changes in NMDAR signaling, schizophrenia is associated with altered nAChR function (Breese et al., 2000; Freedman et al., 1995). As mentioned, nAChRs are involved in cognitive processes (Kenney and Gould, 2008; Levin et al., 2006; Tinsley et al., 2004) and thus changes in nAChR function could contribute to cognitive deficits seen with schizophrenia. Interestingly, over 80% of patients with schizophrenia smoke compared to 30% of the normal population (de Leon et al., 1995; Leonard et al., 2001), and patients with schizophrenia who smoke usually use high nicotine content products and obtain more nicotine from cigarettes when compared to smokers without a mental illness (Olincy et al., 1997). It has been suggested that patients with schizophrenia use nicotine to self-medicate; thereby alleviating the cognitive symptoms associated with the disorder (Martin and Freedman, 2007; Olincy and Stevens, 2007; Olincy et al., 1997). It is unknown, however, how nicotine could ameliorate these cognitive deficits. Nicotine could decrease deficits directly associated with changes in nAChR function. In addition, if nAChR- and NMDAR-mediated processes interact, nicotine could reduce cognitive deficits associated with NMDAR dysfunction.
Multiple lines of evidence suggest that nicotine could act to ameliorate hippocampal-based learning deficits associated with changes in NMDAR function. Nicotine attenuated the effects of dizocilpine maleate (NMDAR antagonist also known as MK-801) on working and reference memory (Levin et al., 1998; Tsukada et al., 2005) and on signal detection errors (Rezvani and Levin, 2003). In addition, subthreshold doses of MK-801 blocked nicotine enhancement of memory consolidation (Ciamei et al., 2001), and co-administration of subthreshold doses of MK-801 and the nAChR antagonist mecamylmine disrupted contextual fear conditioning (Gould and Lewis, 2005). However, the neural basis for nicotine’s effects on NMDAR antagonist-induced deficits in learning is unknown.
It is the hypothesis of this study that nicotine will ameliorate deficits in contextual fear conditioning associated with NMDAR antagonism and that this is a hippocampally mediated effect. To test this, the effects of systemic nicotine on systemic NMDAR antagonist-induced deficits in fear conditioning were examined. This was followed by studies that examined if systemic nicotine would reverse deficits in fear conditioning produced by direct infusion of a NMDAR antagonist into the dorsal hippocampus and examined if direct infusion of nicotine into the dorsal hippocampus would reverse systemic NMDAR antagonist-induced deficits in fear conditioning. In addition, the nAChR subunits involved in the effects of nicotine on these NMDAR antagonist induced deficits are unknown. Neuronal nAChRs are part of a broad family of pentameric ion channels comprised of either an α (α2–10) or α and β (β2–4) subunits (Decker et al., 1995). Both α7 and β2-containing nAChRs are involved in cognitive effects of nicotine and may both be involved in schizophrenia (Arthur and Levin, 2002; Breese et al., 2000; Davis and Gould, 2007; Davis et al., 2007; Freedman et al., 1995). Furthermore, α7 and α4β2* (* denotes potential other subunits) nAChRs are found in the dorsal hippocampus (Alkondon and Albuquerque, 1993; Zarei et al., 1999), which suggests that either or possibly both of these nAChRs are involved in the effects of nicotine on NMDAR antagonist-induced deficits. Therefore, the effects of α7 and α4β2* nAChR antagonists on nicotine amelioration of NMDAR antagonist-induced deficits in fear conditioning were examined. Through examining the interactive effects of nAChR and NMDAR signaling on learning and the underlying substrates, these studies may further understanding of the neural substrates of learning and provide information useful in developing treatments for the cognitive deficits associated with schizophrenia as current therapeutics for schizophrenia commonly treat the positive symptoms with modest or no efficacy for treating the cognitive and negative symptoms (Goldberg et al., 2010; Keefe et al., 1999; Meltzer and McGurk, 1999).
2. MATERIALS AND METHODS
2.1 Subjects
Male C57BL/6J mice (n= 7-14 per group) (Jackson Laboratory, Bar Harbor, ME) were housed four to a cage with food and water available ad libitum. Mice in the direct infusion studies were single housed following cannulation. All mice were tested between 8 and 12 weeks of age. The light–dark cycle was 12:12 h with lights on at 0700 h and all testing occurred between 0800 h and 1700 h. All procedures were approved by the Temple University Institutional Animal Care and Use Committee.
2.2 Apparatus
Training and context testing took place in four identical conditioning chambers (18 × 19 × 38 cm) with aluminum side walls, Plexiglas front and back walls, and grid floors. Each conditioning chamber was housed in a sound-attenuating cubicle (conditioning chambers and sound attenuating cubicles from MED Associates, St. Albans, VT, USA). Each cubicle contained a ventilation fan which provided air exchange and background noise (69 dB). A speaker mounted to the wall of each chamber produced an 85-dB white noise conditioned stimulus (CS). The grid floors of the conditioning chambers were connected to a shock scrambler and generator. The shock unconditioned stimuli (US) were 2 second 0.57 mA shocks. An IBM PC-compatible computer running MED-PC software interfaced with the conditioning chamber to control stimuli administration. The CS test took place in a different room with altered chambers that differed in size (20 × 23 × 19 cm) and construction from the conditioning chambers. The altered chambers, also in sound-attenuating cubicles, were additionally altered by covering the metal grid floor with flat plastic, the sound attenuating cubicles contained a dark background rather than a white background, and vanilla extract was added as an olfactory cue. Ventilation fans (70 dB) were located in the sides of the sound-attenuating cubicles. Speakers mounted to the outside walls of the altered context generated an 85 dB white noise CS during testing for the evaluation of the auditory CS-US association. All chambers were cleaned with 70% ethanol before and after each use.
2.3 Surgery
Mice were anesthetized using isoflurane gas (5% induction, 2% maintenance) and placed in a stereotaxic apparatus from David Kopf Instruments (Tujunga, CA). Bilateral stainless-steel guide cannulae (C232G, 22 gauge; Plastics One, Roanoke, VA) were placed into the appropriate location and fixed to the skull with dental cement. Dummy cannulae (C232DC; Plastics One) were inserted into the guide cannulae to prevent clogging. Coordinates determined from bregma using the mouse brain atlas (Paxinos and Franklin, 2001) were as follows: hippocampus: −1.7 mm posterior; ±1.5 mm mediolateral; −2.3 mm ventral; above control (cortex): −1.7 mm posterior; ±1.5 mm mediolateral; −1.3 mm ventral; below control (thalamus): −1.7 mm posterior; ± 1.5 mm mediolateral; −4.0 mm ventral. Ketoprofen (2.0 mg/kg) was administered subcutaneously for post-operative pain. Animals were allowed at least 5 days to recover before behavioral procedures began.
2.4 Drug and Administration
2.4.1 Systemic administration
Nicotine hydrogen tartrate (nicotine doses are reported in base weight), (+)-MK-801 hydrogen maleate, methyllycaconitine citrate salt hydrate (MLA), and dihydro-β-erythroidine hydrobromide (DhβE) (Sigma Co., St Louis, MO, USA) were dissolved in physiological saline. Mice received an injection of MK-801 (0 or 0.1 mg/kg, intraperitoneal (i.p.)) 15 min prior to training. The dose of MK-801 used was determined through a dose response curve analysis that demonstrated a dose that disrupted contextual fear conditioning while leaving cued fear conditioning largely intact (data not shown). MK-801 pretreatment time was based on previous studies (Gould and Lewis, 2005; Lewis and Gould, 2004). Mice received MLA (0, 10 or 20 mg/kg, subcutaneous (s.c.)) or DhβE (0 or 4 mg/kg, s.c.) 30 min prior to training. Higher doses of MLA may produce nonspecific effects that include antagonist activity at α4β2 nAChRs in addition to inhibiting α7 nAChRs (See Hashimoto et al., 2005). Mice received nicotine (0 or 0.18 mg/kg, i.p.) 5 min prior to training. Nicotine pretreatment time was based on a previous study by (Petersen et al., 1984). The dose of nicotine was determined by a dose response analysis (data not shown). The dose of nicotine used in the current studies does not enhance contextual fear conditioning (See figure 1) , which is consistent with previous work from our laboratory that showed that doses higher than 0.09 mg/kg nicotine do not enhance contextual fear conditioning in C57BL/6 mice (Gould and Higgins, 2003). All injections were at 10 ml/kg dose volume.
Figure 1. The interaction between systemic MK-801 and systemic nicotine on fear conditioning.
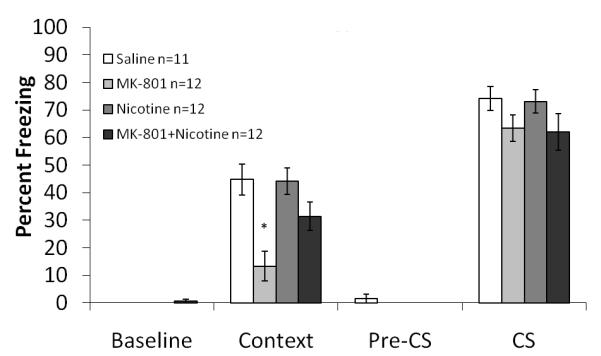
Mice treated with MK-801 froze significantly less to the context than mice treated with saline, nicotine, or both MK-801 and nicotine. There was no effect on freezing to the cue. Error bars represent ± the standard error of the mean. The * indicates significantly different than all other groups (p<0.05).
2.4.2 Direct Infusion
DL-2-Amino-5-phosphonovaleric acid (APV) and nicotine hydrogen tartrate (Sigma Co, St Louis, MO, USA) were dissolved in physiological saline. Mice received APV (0 or 0.25 μg/side) 10 min prior to training based on the work of Stiedl and colleagues (2000). The dose of APV was determined through a dose response analysis (data not shown). Mice received nicotine (0 or 0.75 μg/side) 5 min prior to training. Infusion pretreatment time was based on previous work (Gulick and Gould, 2009) and dose was based on preliminary dose response curve (data not shown). For direct infusions, mice were gently restrained and dummy cannulae were removed and replaced with 22-G infusion cannulae. Drugs were bilaterally infused at a rate of 0.50 μl/min. Infusion cannulae were attached to polyethylene tubing (PE50; Plastics One), which was attached to a 10 ml Hamilton (Reno, NV) syringe. Drug administration was controlled by a micro-infusion pump (KDS 100; KD Scientific, New Hope, PA). Injection cannulae were left in place for 1 min after infusion to allow for drug diffusion away from the cannulae.
2.5 General Experimental Procedure
Mice were fear conditioned as described in Gould and Higgins (2003). All drug administration occurred on training day. During training and testing, each mouse was scored as either freezing or active once every 10 seconds. Freezing was defined as an absence of visible movement except for respiration (Blanchard and Blanchard, 1969). For training, the mice were placed in conditioning chambers for 5.5 min. They received two co-terminating CS (30 sec, 85 dB white noise)-US (2 sec, 0.57 mA foot shock) presentations. Baseline freezing behavior was recorded during the first 120 seconds of the training session. At 120 sec, the first CS sounded for 30 sec. The US was presented during the last 2 sec of the CS. Mice received a second CS-US pairing identical to the first. The mice remained in the chamber 30 sec after the second CS-US presentation.
Approximately twenty-four hours after training, mice were assessed for freezing to the context. To evaluate freezing to the context, mice were placed in the conditioning chamber and freezing behavior was recorded for 5 min. Approximately one hour after contextual fear conditioning testing, freezing to the CS was evaluated. Mice were placed in the altered chambers for 6 min. During the first 180 sec (Pre-CS or generalized freezing period) freezing in the absence of the CS was assessed to measure generalized freezing. During the final 180 sec (CS or cued freezing period), the CS was presented and freezing behavior to the CS was assessed.
2.6 Histology
Brains were placed in a 10% formalin solution (Fisher Scientific, Pittsburgh, PA) for at least 24 h before 60 μm thick coronal sections were sliced at −18° C. The sections were stained with cresyl violet and cannula placements were determined using a light microscope. Infusions have been estimated to diffuse to about 1 mm3 (Holt and Maren, 1999; Lewis and Gould, 2007a, b); data from animals with placements outside of the target area were excluded from analysis.
2.7 Statistical Analysis
To examine the interactive effects of nicotine and the NMDAR antagonists on fear conditioning, a 2×2 factorial analysis of variance (ANOVA) was performed with each drug acting as a between subjects factor. To further analyze the effects of each drug in these studies and to examine the effects of the nAChR antagonists on nicotine’s ameliorative effect, one way ANOVA was performed on baseline, context, pre-CS, and CS freezing. Tukey’s honestly significant difference or Games-Howell post hoc tests were performed depending on the homogeneity of the data set to examine the difference between groups. Any subjects with values more than 2.5 standard deviations away from the mean were deemed outliers and excluded from analysis, which totaled 9 throughout all experiments. Group sizes are reported in the figures. All statistical analyses were completed using SPSS Version 16.0.
3. RESULTS
Due to the multiple drug combinations tested, the groups will be identified by the active drug or drugs only (i.e., MK-801 rather than MK-801 and saline) whenever possible.
3.1 Systemic Nicotine Ameliorates Contextual Fear Conditioning Deficits Associated with Systemic MK-801
To determine if nicotine was able to ameliorate the deficits in contextual conditioning produced by MK-801, mice were administered systemic nicotine (0.5 mg/kg) and MK-801 (0.1mg /kg) prior to training of fear conditioning. A 2×2 ANOVA revealed a significant effect of MK-801 on contextual freezing: F (3, 40) =32.844, p<0.05, a significant effect of nicotine on contextual freezing: F (3, 40) = 5.814, p<0.05, and a significant interaction between the drugs: F (3, 40) = 4.933, p<0.05. Tukey’s post-hoc analysis revealed that mice receiving 0.1 mg/kg MK-801 froze significantly less than mice receiving nicotine, saline, or MK-801 and nicotine (p<0.05 for all comparisons) (Figure 1). There were no significant differences in baseline, generalized or cued freezing.
3.2 Systemic Administration of Nicotine Ameliorates the Contextual Fear Conditioning Deficits Associated with Direct Infusion of APV
Systemic administration of nicotine (0.5 mg/kg) was paired with direct infusion of the NMDAR antagonist APV (0.25 μg/side) into the hippocampus to examine if the hippocampus is involved in the interactive effects of nicotine and NMDAR antagonism on learning. A 2×2 ANOVA revealed a significant effect of APV on freezing to the context: F (3, 46) = 9.745, p<0.05, a significant effect of nicotine on contextual freezing: F (3, 46) = 4.849, p<0.05, but no significant interaction between the drugs (p=0.111). Post hoc analyses revealed that mice infused with APV into the dorsal hippocampus froze significantly less to the context than mice infused with saline, mice administered systemic nicotine, or mice infused with APV and administered systemic nicotine (p<0.05 for all comparisons) (Figure 2). There were no significant differences in baseline, generalized or cued freezing.
Figure 2. The interaction between direct infusion of APV with systemic administration of nicotine on fear conditioning.
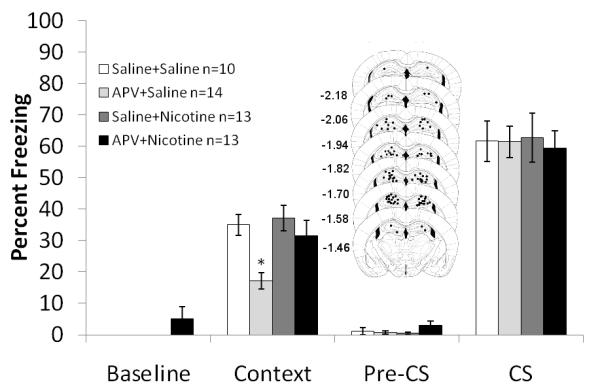
Mice treated with APV froze significantly less to the context than mice treated with saline, nicotine, or both APV and nicotine. There was no effect on freezing to the cue. Schematics of the drug infusion sites in the dorsal hippocampus are included. Circles represent the tip of the infusion tracts, and numbers represent distance in mm posterior to bregma. Error bars represent ± the standard error of the mean. The * indicates significantly different than all other groups (p<0.05).
3.3 The Hippocampus is involved in Nicotine’s Ameliorative Effect
To further elucidate the role of the hippocampus in the ameliorative effects of nicotine, direct infusion of nicotine (0.75 μg/side) in combination with systemic administration of MK-801 (0.1 mg/kg) was examined. A 2×2 ANOVA revealed a significant effect of MK-801 on freezing to the context: F (3, 35) = 12.734, p<0.05, a significant effect of nicotine on freezing to the context and the cue: F (2, 35) = 6.921, p<0.05 and F (3, 35) = 6.905, p<0.05, respectively, and a significant interaction between the drugs on freezing to the cue: F (3, 35) = 13.709, p<0.05 but not to the context (p=0.21). Post hoc analyses revealed that mice treated with MK-801 froze less to the context than mice treated with saline, mice directly infused with nicotine, or mice treated systemically with MK-801 and directly infused with nicotine (p<0.05 for all analyses). Post hoc analyses also revealed that mice administered systemic MK-801 froze less to the cue than mice treated with saline or those receiving systemic MK-801 and direct infusions of nicotine (p<0.05 for all comparisons) (Figure 3). There were no significant differences in baseline or generalized freezing.
Figure 3. The interaction between direct infusion of nicotine into the dorsal hippocampus with systemic administration of MK-801 on fear conditioning.
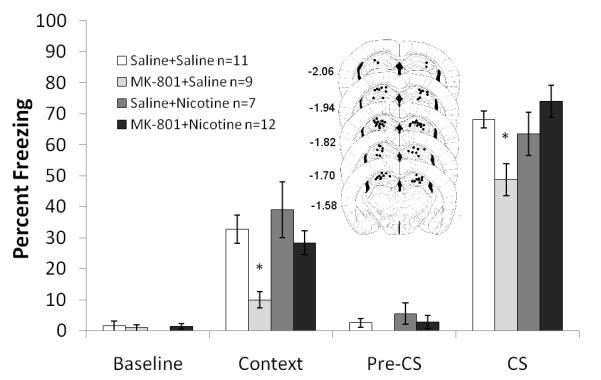
Mice treated with MK-801 froze less to the context and the cue than mice treated with saline, nicotine, or both MK-801 and nicotine. Schematics of the drug infusion sites in the dorsal hippocampus are included. Circles represent the tip of the infusion tracts, and numbers represent distance in mm posterior to bregma. Error bars represent ± the standard error of the mean. The * indicates significantly different than all other groups (p<0.05).
To rule out effects of the drug in areas outside of the hippocampus due to drug diffusion, nicotine (0.75 μg/side) was infused above the dorsal hippocampus into the cortex and below the hippocampus into the thalamus and MK-801 (0.1 mg/kg) was administered systemically. One way ANOVA revealed a significant effect of drug on freezing to the context: F (3, 26) = 5.564, p<0.05. There were no statistical differences between the above and below data for saline controls, therefore the data was pooled. Post-hoc analyses revealed that the saline treated group froze significantly more than all other groups (p<0.05 for all comparisons) (Figure 4). There were no significant differences in baseline, generalized or cued freezing.
Figure 4. The interaction between direct infusion of nicotine into areas above and below the hippocampus with systemic administration of MK-801 on fear conditioning.
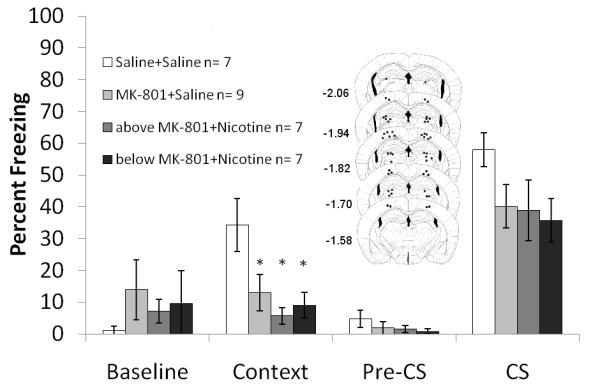
Mice treated with saline froze significantly more to the context than mice treated with MK-801 or both MK-801 and nicotine. Schematics of the drug infusion sites in the dorsal hippocampus are included. Circles represent the tip of the infusion tracts, and numbers represent distance in mm posterior to bregma. Error bars represent ± the standard error of the mean. The * indicates significantly different from saline (p<0.05).
3.4 Nicotine acts through High Affinity nAChRs to Ameliorate NMDAR Antagonist-Inducted Deficits
To determine which nAChR subtypes were involved in the ameliorative effects of nicotine on NMDAR antagonist-induced deficits in contextual conditioning, mice were administered different nAChR subtype antagonists with nicotine and MK-801. One way ANOVA analysis of the high affinity nAChR antagonist DhβE (4 mg/kg) in combination with MK-801 (0.1 mg/kg) and nicotine (0.5 mg/kg) revealed a significant drug effect on contextual freezing: F (3, 26) = 21.660, p<0.05. Post-hoc analysis revealed that contextual fear conditioning after administration of DhβE along with MK-801 and nicotine was not significantly different from contextual fear conditioning after administration of MK-801 (p>0.5) but was significantly different from saline and MK-801 in combination with nicotine (p<0.05 for both comparisons). These results show that the high affinity nAChR antagonist DhβE was able to block the ameliorating effect of nicotine on the MK-801-induced deficit (Figure 5). There were no significant differences in baseline, generalized or cued freezing.
Figure 5. The interaction between DhβE and MK-801 and nicotine on fear conditioning.
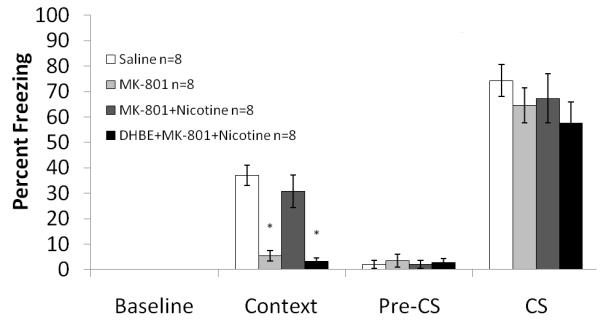
The high affinity nAChR antagonist DhβE was able to block the ameliorating effect of nicotine on the MK-801-induced deficit. Error bars represent ± the standard error of the mean. The * indicates significantly different from saline (p<0.05).
One way ANOVA on the effects of the α7 nAChR antagonist MLA (10 and 20 mg/kg) in combination with MK-801 (0.1 mg/kg) and nicotine (0.5 mg/kg) on fear conditioning revealed a significant effect of drug combination on baseline freezing, freezing to the context, and cued freezing for MLA: F (4, 34) =28.607; F (4, 34) =24.572; and F (4, 34) =10.227, respectively, p<0.05 for all comparisons. Post-hoc analyses revealed that mice that were administered 10 mg/kg MLA in combination with MK-801 and nicotine froze to a similar extent to those that received only saline or MK-801 and nicotine (p >0.05 for all comparisons). For the combination of 20 mg/kg MLA with MK-801 and nicotine, post hoc analyses revealed freezing that was significantly lower than the saline, the MK-801 and nicotine, and the 10 mg/kg MLA with MK-801 and nicotine groups for freezing to the context (p<0.05 for all comparisons). Furthermore, freezing to the cue was significantly less in 20 mg/kg MLA with MK-801 and nicotine group than all other groups (p<0.05 for all comparisons), while baseline freezing was significantly greater than all other groups (p<0.05 for all comparisons) (Figure 6). There were no significant differences in generalized freezing. The lower dose of MLA did not block nicotine’s amelioration of MK-801 induced deficits while the higher dose may have produced nonspecific effects that may be unrelated to α7 nAChR-mediated processes (See Hashimoto et al., 2005). The effects of the nAChR antagonists alone on fear conditioning were examined; the doses tested were 4 mg/kg for DhβE and 10 and 20 mg/kg for MLA. One way ANOVA with drug as between subjects effect revealed a significant effect of drug on freezing to the context: F (3, 27) = 12.252, p<0.05. Post hoc analyses revealed that that the group administered 20 mg/kg MLA froze significantly less to the context than all other groups (p<0.05 for all analyses) (Figure 7). There were no significant differences in baseline, generalized or cued freezing.
Figure 6. The interaction between MLA and MK-801 and nicotine on fear conditioning.
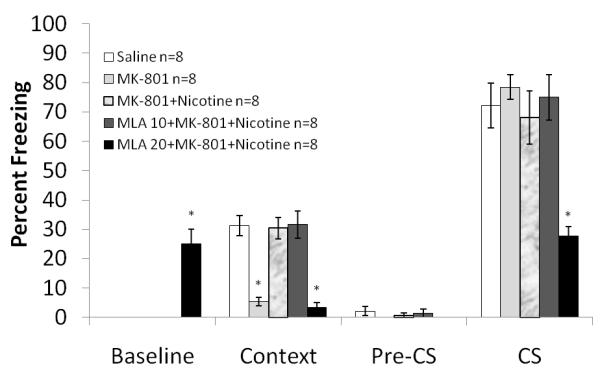
The low affinity receptor nAChR antagonist MLA (10 mg/kg) was not able to block the ameliorating effect of nicotine. With 20 mg/kg MLA, a deficit in freezing to the context and the cue was observed. Furthermore, increased baseline freezing was also present in this group. Error bars represent ± the standard error of the mean. The * indicates significantly different from saline (p<0.05).
Figure 7. The effects of nAChRs antagonists on fear conditioning.
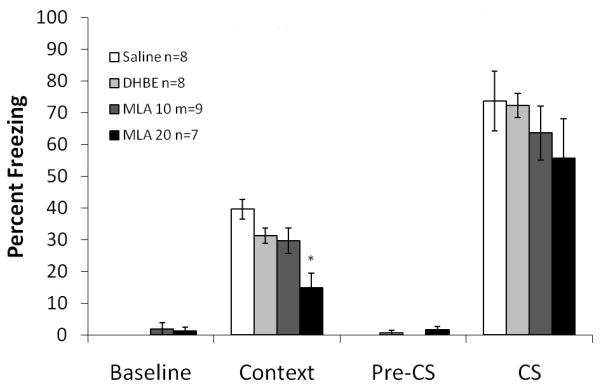
20 mg/kg MLA caused a deficit in freezing to the context, DhβE and 10 mg/kg MLA did not. Error bars represent ± the standard error of the mean. The * indicates significantly different from saline (p<0.05).
Finally, to examine if combined administration of nicotine and nAChR antagonist produced unexpected results, a third study examined the effects on fear conditioning of administering DhβE (4 mg/kg) or MLA (20 mg/kg) in combination with nicotine. A significant effect of drug on baseline freezing was shown: F (2, 21) = 8.178, p<0.05 Post hoc analyses revealed that mice receiving the combination of 20 mg/kg MLA and nicotine (0.18 mg/kg) froze marginally more than the other groups during the baseline period (p=0.056). There was also a significant effect of drug on freezing to the context: F (2, 21) = 4.729, p<0.05. Post hoc analyses showed that mice receiving the combination of 20 mg/kg MLA and nicotine froze less than the other groups to the context (p<0.05 for all comparisons) (Figure 8). There were no significant differences in generalized or cued freezing.
Figure 8. The effects of MLA or DhβE with nicotine on fear conditioning.
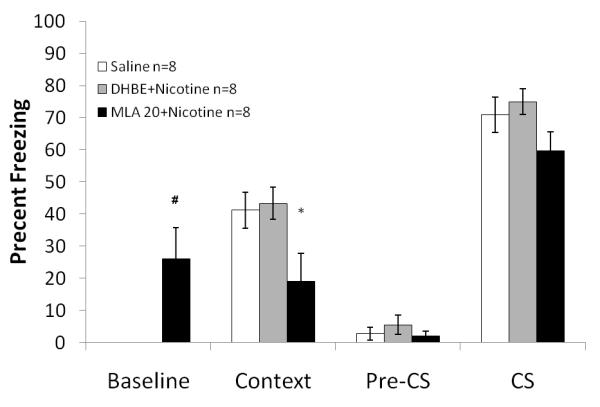
Mice treated with 20 mg/kg MLA and nicotine showed increased baseline freezing but significantly less contextual and cued freezing compared to all other groups. Error bars represent ± the standard error of the mean. The * indicates significantly different from saline (p<0.05). The # indicates marginal significance compared to saline (p=0.056).
4. DISCUSSION
The present results provide evidence for a functional interaction between NMDARs and nAChRs during learning. Nicotine reversed NMDAR antagonist-induced learning deficits. This effect was hippocampally mediated as demonstrated by using direct infusions of nicotine into the dorsal hippocampus paired with systemic administration of the NMDAR antagonist MK-801 and by using direct infusions of the NMDAR antagonist APV into the dorsal hippocampus paired with systemic administration of nicotine. In addition to identifying the dorsal hippocampus as a site where nAChR and NMDAR signaling may interact to modulate learning, the study also found that the effects of nicotine on NMDAR-induced learning deficits were mediated by high-affinity nAChRs, a broad family of receptors that include the α4β2 nAChRs (Ramirez-Latorre et al., 1998). These findings have implications for both understanding the neurobiology of learning and memory, as they suggest that nAChRs and NMDARs have common downstream targets involved in synaptic plasticity, and understanding mental illnesses that involve changes in cognition and NMDAR and/or nAChR function, such as schizophrenia and Alzheimer’s disease (Konradi and Heckers, 2003; Woodruff-Pak and Gould, 2002).
Nicotine binding to α4β2 nAChRs could ameliorate NMDAR antagonist-associated deficits through either pre or postsynaptic mechanisms. Presynaptically, nAChR activation stimulates glutamate release (Gray et al., 1996; McGehee et al., 1995). In addition, Aramakis and Metherate (1998) demonstrated that nicotine acts at presynaptic nAChRs in rat auditory cortex to increase glutamate release onto postsynaptic NMDARs. Thus, nicotine may ameliorate NMDAR antagonist-associated deficits in learning by increasing synaptic levels of glutamate.
It is also possible that nicotine acts postsynaptically to ameliorate the effects of NMDAR antagonists on learning. The NMDAR, unlike most receptors, is both ligand and voltage gated (Nowak et al., 1984). Therefore, binding of glutamate to an NMDAR is not enough to gate calcium ion influx; the membrane needs concurrent depolarization to remove the magnesium block in the ion pore. This depolarization along with glutamate binding will trigger an influx of calcium through NMDAR ion channels (Rathouz et al., 1996; Zarei et al., 1999). Glutamate binding to α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptors (AMPARs) is thought to mediate the necessary depolarization for NMDAR activation (Herron et al., 1986). However, there is behavioral evidence that nAChRs may act in an analogous manner to AMPARs (Gould and Lewis, 2005). Specifically, in the hippocampus, postsynaptic currents were present in pyramidal cells after antagonism of NMDARs and AMPARs, and these residual currents were completely inhibited by broad spectrum nAChR antagonism by d-tubocurarine and partially inhibited by α7 specific antagonists MLA and α-bungarotoxin (Hefft et al., 1999) indicating that some current may be mediated by non-α7 type receptors. If nAChRs are contributing to the depolarization necessary for NMDAR activation in the hippocampus, these receptors should be located postsynaptically in the hippocampus, which has been reported(Alkondon et al., 2003). Thus, nAChRs could contribute to activation of NMDAR signaling by providing the postsynaptic depolarization necessary for NMDAR activation.
Nicotine could also ameliorate the NMDAR antagonist-induced deficit in learning by directly activating cell signaling cascades activated by NMDARs. Both nAChRs and NMDARs gate calcium influx (Fisher and Dani, 2000; Krupp et al., 1999; Zhang et al., 1998). It is possible that nicotine ameliorates NMDAR antagonist-induced deficits in contextual fear conditioning by gating a sufficient amount of calcium to directly activate learning-related cell signaling cascades (Dajas-Bailador and Wonnacott, 2004; Gould, 2006; Tinsley et al., 2004). For example, nicotine has been shown to induce cell signaling cascades involving PKA activation and CREB phosphorylation (Chiamulera et al., 2008; Hu et al., 2002; Welsby et al., 2006). Nicotine may also modulate learning through activation of the extracellular regulated kinase 1/2 (ERK1/2) cascade, a member of the mitogen activated protein kinase family of proteins that is involved in synaptic plasticity and hippocampus-dependent learning (Kelleher et al., 2004; Nakayama et al., 2001; Sweatt, 2001; Tomizawa and Casida, 2002; Welsby et al., 2006). In support, inhibition of ERK1/2 blocked the nicotine enhancement of contextual fear conditioning (Raybuck and Gould, 2007). Clearly, more work is needed to understand how nAChRs and NMDARs interact to alter learning and synaptic plasticity.
An alternative explanation for the data is that the NMDAR antagonists produce state dependent deficits and nicotine ameliorates these deficits. However, while possible, there are reasons this explanation may not fully account for our findings. It has been shown that MK-801 can produce state dependent deficits in a discriminative goal-tracking task when nicotine serves as a CS (Murray et al., 2010). However, rats in this study received both MK-801 and nicotine and still showed deficits. If nicotine was rescuing state dependent deficits, then no deficits should have been seen when both nicotine and MK-801 were administered. In addition, it is not clear that MK-801 produces state dependent deficits in contextual fear conditioning. In the present study, mice administered 0.1 mg/kg MK-801 on training day showed deficits in contextual fear conditioning. If the MK-801 deficit in contextual fear conditioning was state dependent, then no deficit should occur if nicotine is administered before both training and testing. However, using similar fear conditioning procedures to those used in the current studies, Lu and Wehner (1997) showed that 0.1mg/kg MK-801 administered prior to training and testing caused a deficit in contextual fear conditioning. In addition, Bast and colleagues (2003) argued that because the MK-801 induced deficit after dorsal hippocampal infusions was confined to the contextual portion of the task and cued conditioning remained intact, similar to our results, state dependent effects are not driving the results in contextual fear conditioning . Finally, state dependent effects of MK-801 on passive avoidance and extinction of conditioned fear were not seen (Baker and Azorlosa, 1996; Kim and McGaugh, 1992; Nakagawa and Iwasaki, 1996).
For most of the current experiments, the NMDAR antagonist-induced deficits in fear conditioning were limited to the contextual association; however, in one experiment a deficit was seen in cued fear conditioning. This replicates previous findings suggesting that contextual fear conditioning may be more sensitive to NMDAR inhibition than auditory cued fear conditioning (Bast et al., 2003; Goeldner et al., 2009; Gould et al., 2002). The deficit in cued fear conditioning seen with systemic MK-801 administration could have been due to effects of MK-801 in the amygdala. The amygdala is critically involved in both cued and contextual fear conditioning (Phillips and LeDoux, 1992) and contains NMDARs (Maren et al., 1996). In support, direct infusion of APV into the amygdala disrupted acoustic fear potentiated startle and fear conditioning (Fanselow and Kim, 1994; Fanselow et al., 1994; Kim et al., 1991; Laurent and Westbrook, 2009; Maren et al., 1996; Miserendino et al., 1990; Savonenko et al., 2003). It is also possible that the observed deficit in cued fear conditioning was due to the effects of MK-801 in other brain regions, including the hippocampus. While studies have shown that the hippocampus is not necessary for cued fear conditioning (Phillips and LeDoux, 1992), increasing evidence suggests that disrupting hippocampal function can, in some cases, alter cued fear conditioning (Bardgett et al., 2003; Bast et al., 2001; Yin et al., 2002; Zhang et al., 2001). Because the hippocampus sends efferent projections to the amygdala (Ishikawa and Nakamura, 2006; Maren and Fanselow, 1995; Pitkanen et al., 2000), changes in hippocampal function via systemic NMDA antagonism could disrupt non-contextual memory formation.
MK-801 has been shown to have inhibitory effects at nAChRs (Amador and Dani, 1991; Ramoa et al., 1990). While MK-801 effects on nAChRs are seen with higher doses than those used in the present study, it is possible that the interactive effects of MK-801 and nicotine on learning were due to both drugs acting on nAChRs. However, the current study also found that nicotine reversed learning deficits associated with infusion of APV into the dorsal hippocampus. A search of the literature did not find evidence for APV having effects on nAChRs. Therefore, the current results are interpreted as nicotine reversing learning deficits associated with disruption of NMDAR function.
Finally, our finding that high-affinity nAChRs in the hippocampus are critical for the ameliorative effects of nicotine on NMDAR antagonist-induced deficits in contextual fear conditioning may contribute to further understanding schizophrenia. Several the theories of schizophrenia propose that changes in the glutamate system contribute to the disorder (Bickel and Javitt, 2009; Lindsley et al., 2006; Olney et al., 1999; Weiner and Arad, 2009). In support of these theories, lower levels of Glu1 and Glu2 AMPAR mRNA (Eastwood et al., 1997; Eastwood et al., 1995; Harrison et al., 1991; Kerwin et al., 1990) and decreased NR1 NMDAR mRNA as well as increases in NR2b NMDAR mRNA (Gao et al., 2000) expression are associated with schizophrenia. Also, decreased NMDAR binding was found in hippocampi from patients with schizophrenia (Beneyto et al., 2007). In addition, schizophrenia is also associated with changes in hippocampal nAChR number and function (de Leon et al., 1995; Leonard et al., 2001), and patients with schizophrenia smoke at high rates (80%) (Durany et al., 2000; Leonard et al., 2001; Olincy et al., 1997). It has been proposed that this high rate of smoking may reflect self-medication (Martin and Freedman, 2007; Olincy and Stevens, 2007; Olincy et al., 1997), which could be an attempt to counter NMDAR and/or nAChR dysfunction. This fits with the current results that nicotine ameliorated deficits in learning associated with NMDAR inhibition.
The potential role of the α7 nAChR in schizophrenia has received much attention (Breese et al., 2000; Freedman et al., 1995); however, the present findings suggest that high-affinity nAChRs could also be involved. This supports research showing that changes in high-affinity receptors in patients with schizophrenia may be associated with cognitive deficits (Breese et al., 2000) and research suggesting that targeting high-affinity nAChR may be effective in the treatment of the cognitive deficits associated with schizophrenia (Radek et al., 2010). A better understanding of the nature of the interaction between high-affinity nAChRs and NMDARs will increase understanding of the neurobiology of learning and cognitive dysfunction observed in patients with schizophrenia. This should aid in the development of pharmacological interventions that provide more than modest relief for the cognitive deficits associated with schizophrenia.
5. ACKNOWLEDGEMENTS
The work was funded with grant support from the National Institute on Drug Abuse (DA01749, DA246787, T.G.) and the National Cancer Institute/National Institute on Drug Abuse Transdiciplinary Tobacco Use Research Center Grant (P5084718 PI: Caryn Lerman). We thank Derek Wilkinson for his comments on earlier versions of this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. REFERENCES
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther. 1993;265:1455–1473. [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX. NMDA and AMPA receptors contribute to the nicotinic cholinergic excitation of CA1 interneurons in the rat hippocampus. J Neurophysiol. 2003;90:1613–1625. doi: 10.1152/jn.00214.2003. [DOI] [PubMed] [Google Scholar]
- Amador M, Dani JA. MK-801 inhibition of nicotinic acetylcholine receptor channels. Synapse. 1991;7:207–215. doi: 10.1002/syn.890070305. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS. Scopolamine selectively disrupts the acquisition of contextual fear conditioning in rats. Neurobiol Learn Mem. 1995;64:191–194. doi: 10.1006/nlme.1995.0001. [DOI] [PubMed] [Google Scholar]
- Aramakis VB, Metherate R. Nicotine selectively enhances NMDA receptor-mediated synaptic transmission during postnatal development in sensory neocortex. J Neurosci. 1998;18:8485–8495. doi: 10.1523/JNEUROSCI.18-20-08485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur D, Levin ED. Chronic inhibition of alpha4beta2 nicotinic receptors in the ventral hippocampus of rats: impacts on memory and nicotine response. Psychopharmacology (Berl) 2002;160:140–145. doi: 10.1007/s00213-001-0961-6. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RG. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378:182–186. doi: 10.1038/378182a0. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Boeckman R, Krochmal D, Fernando H, Ahrens R, Csernansky JG. NMDA receptor blockade and hippocampal neuronal loss impair fear conditioning and position habit reversal in C57Bl/6 mice. Brain Res Bull. 2003;60:131–142. doi: 10.1016/s0361-9230(03)00023-6. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. The ventral hippocampus and fear conditioning in rats. Different anterograde amnesias of fear after tetrodotoxin inactivation and infusion of the GABA(A) agonist muscimol. Exp Brain Res. 2001;139:39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. Dorsal hippocampus and classical fear conditioning to tone and context in rats: effects of local NMDA-receptor blockade and stimulation. Hippocampus. 2003;13:657–675. doi: 10.1002/hipo.10115. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- Bickel S, Javitt DC. Neurophysiological and neurochemical animal models of schizophrenia: focus on glutamate. Behav Brain Res. 2009;204:352–362. doi: 10.1016/j.bbr.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, Marks MJ, Collins AC, Leonard S. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Letchworth SR, Bencherif M, Lippiello PM. Long-lasting cognitive improvement with nicotinic receptor agonists: mechanisms of pharmacokinetic-pharmacodynamic discordance. Trends Pharmacol Sci. 2005;26:352–360. doi: 10.1016/j.tips.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Di Chio M, Tedesco V, Cantu C, Formaggio E, Fumagalli G. Nicotine-induced phosphorylation of phosphorylated cyclic AMP response element-binding protein (pCREB) in hippocampal neurons is potentiated by agrin. Neurosci Lett. 2008;442:234–238. doi: 10.1016/j.neulet.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Ciamei A, Aversano M, Cestari V, Castellano C. Effects of MK-801 and nicotine combinations on memory consolidation in CD1 mice. Psychopharmacology (Berl) 2001;154:126–130. doi: 10.1007/s002130000584. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador F, Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci. 2004;25:317–324. doi: 10.1016/j.tips.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. beta2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2007;190:343–352. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon J, Dadvand M, Canuso C, White AO, Stanilla JK, Simpson GM. Schizophrenia and smoking: an epidemiological survey in a state hospital. Am J Psychiatry. 1995;152:453–455. doi: 10.1176/ajp.152.3.453. [DOI] [PubMed] [Google Scholar]
- Decker MW, Brioni JD, Bannon AW, Arneric SP. Diversity of neuronal nicotinic acetylcholine receptors: lessons from behavior and implications for CNS therapeutics. Life Sci. 1995;56:545–570. doi: 10.1016/0024-3205(94)00488-e. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Lieberman JA, Koller BH. Typical and atypical antipsychotic drug effects on locomotor hyperactivity and deficits in sensorimotor gating in a genetic model of NMDA receptor hypofunction. Pharmacol Biochem Behav. 2006;85:481–491. doi: 10.1016/j.pbb.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durany N, Zochling R, Boissl KW, Paulus W, Ransmayr G, Tatschner T, Danielczyk W, Jellinger K, Deckert J, Riederer P. Human post-mortem striatal alpha4beta2 nicotinic acetylcholine receptor density in schizophrenia and Parkinson’s syndrome. Neurosci Lett. 2000;287:109–112. doi: 10.1016/s0304-3940(00)01144-7. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Kerwin RW, Harrison PJ. Immunoautoradiographic evidence for a loss of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate-preferring non-N-methyl-D-aspartate glutamate receptors within the medial temporal lobe in schizophrenia. Biol Psychiatry. 1997;41:636–643. doi: 10.1016/S0006-3223(96)00220-X. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, McDonald B, Burnet PW, Beckwith JP, Kerwin RW, Harrison PJ. Decreased expression of mRNAs encoding non-NMDA glutamate receptors GluR1 and GluR2 in medial temporal lobe neurons in schizophrenia. Brain Res Mol Brain Res. 1995;29:211–223. doi: 10.1016/0169-328x(94)00247-c. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Kim JJ. Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D,L-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behav Neurosci. 1994;108:210–212. doi: 10.1037//0735-7044.108.1.210. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Kim JJ, Yipp J, De Oca B. Differential effects of the N-methyl-D-aspartate antagonist DL-2-amino-5-phosphonovalerate on acquisition of fear of auditory and contextual cues. Behav Neurosci. 1994;108:235–240. doi: 10.1037//0735-7044.108.2.235. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Dani JA. Nicotinic receptors on hippocampal cultures can increase synaptic glutamate currents while decreasing the NMDA-receptor component. Neuropharmacology. 2000;39:2756–2769. doi: 10.1016/s0028-3908(00)00102-7. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Fanselow MS. Cholinergic modulation of pavlovian fear conditioning: effects of intrahippocampal scopolamine infusion. Hippocampus. 2001;11:371–376. doi: 10.1002/hipo.1051. [DOI] [PubMed] [Google Scholar]
- Gao XM, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- Goeldner C, Reiss D, Wichmann J, Kieffer BL, Ouagazzal AM. Activation of nociceptin opioid peptide (NOP) receptor impairs contextual fear learning in mice through glutamatergic mechanisms. Neurobiol Learn Mem. 2009;91:393–401. doi: 10.1016/j.nlm.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Harvey PD. Cognitive deficits in schizophrenia. Psychiatr Clin North Am. 1993;16:295–312. [PubMed] [Google Scholar]
- Goldberg TE, Keefe RS, Goldman RS, Robinson DG, Harvey PD. Circumstances under which practice does not make perfect: a review of the practice effect literature in schizophrenia and its relevance to clinical treatment studies. Neuropsychopharmacology. 2010;35:1053–1062. doi: 10.1038/npp.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning: implications for addiction. Mol Neurobiol. 2006;34:93–107. doi: 10.1385/MN:34:2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Higgins JS. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Lewis MC. Coantagonism of glutamate receptors and nicotinic acetylcholinergic receptors disrupts fear conditioning and latent inhibition of fear conditioning. Learn Mem. 2005;12:389–398. doi: 10.1101/lm.89105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, McCarthy MM, Keith RA. MK-801 disrupts acquisition of contextual fear conditioning but enhances memory consolidation of cued fear conditioning. Behav Pharmacol. 2002;13:287–294. doi: 10.1097/00008877-200207000-00005. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. The hippocampus and cingulate cortex differentially mediate the effects of nicotine on learning versus on ethanol-induced learning deficits through different effects at nicotinic receptors. Neuropsychopharmacology. 2009;34:2167–2179. doi: 10.1038/npp.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Eastwood SL. Preferential involvement of excitatory neurons in medial temporal lobe in schizophrenia. Lancet. 1998;352:1669–1673. doi: 10.1016/S0140-6736(98)03341-8. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, McLaughlin D, Kerwin RW. Decreased hippocampal expression of a glutamate receptor gene in schizophrenia. Lancet. 1991;337:450–452. doi: 10.1016/0140-6736(91)93392-m. [DOI] [PubMed] [Google Scholar]
- Hefft S, Hulo S, Bertrand D, Muller D. Synaptic transmission at nicotinic acetylcholine receptors in rat hippocampal organotypic cultures and slices. J Physiol. 1999;515(Pt 3):769–776. doi: 10.1111/j.1469-7793.1999.769ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron CE, Lester RA, Coan EJ, Collingridge GL. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature. 1986;322:265–268. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- Holt W, Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. J Neurosci. 1999;19:9054–9062. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Liu QS, Chang KT, Berg DK. Nicotinic regulation of CREB activation in hippocampal neurons by glutamatergic and nonglutamatergic pathways. Mol Cell Neurosci. 2002;21:616–625. doi: 10.1006/mcne.2002.1202. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Ventral hippocampal neurons project axons simultaneously to the medial prefrontal cortex and amygdala in the rat. J Neurophysiol. 2006;96:2134–2138. doi: 10.1152/jn.00069.2006. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A. 1996;93:11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RS, Silva SG, Perkins DO, Lieberman JA. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr Bull. 1999;25:201–222. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin R, Patel S, Meldrum B. Quantitative autoradiographic analysis of glutamate binding sites in the hippocampal formation in normal and schizophrenic brain post mortem. Neuroscience. 1990;39:25–32. doi: 10.1016/0306-4522(90)90219-t. [DOI] [PubMed] [Google Scholar]
- Kim JJ, DeCola JP, Landeira-Fernandez J, Fanselow MS. N-methyl-D-aspartate receptor antagonist APV blocks acquisition but not expression of fear conditioning. Behav Neurosci. 1991;105:126–133. doi: 10.1037//0735-7044.105.1.126. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Brenner E, Eyjolfsson EM, Markinhuhta KR, Carlsson ML, Sonnewald U. Glial-neuronal interactions are impaired in the schizophrenia model of repeated MK801 exposure. Neuropsychopharmacology. 2006;31:1880–1887. doi: 10.1038/sj.npp.1300993. [DOI] [PubMed] [Google Scholar]
- Konradi C, Heckers S. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol Ther. 2003;97:153–179. doi: 10.1016/s0163-7258(02)00328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL. Interactions of calmodulin and alpha-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors. J Neurosci. 1999;19:1165–1178. doi: 10.1523/JNEUROSCI.19-04-01165.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr., Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Infusion of the NMDA receptor antagonist, DL-APV, into the basolateral amygdala disrupts learning to fear a novel and a familiar context as well as relearning to fear an extinguished context. Learn Mem. 2009;16:96–105. doi: 10.1101/lm.1218709. [DOI] [PubMed] [Google Scholar]
- Leonard S, Adler LE, Benhammou K, Berger R, Breese CR, Drebing C, Gault J, Lee MJ, Logel J, Olincy A, Ross RG, Stevens K, Sullivan B, Vianzon R, Virnich DE, Waldo M, Walton K, Freedman R. Smoking and mental illness. Pharmacol Biochem Behav. 2001;70:561–570. doi: 10.1016/s0091-3057(01)00677-3. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bettegowda C, Weaver T, Christopher NC. Nicotine-dizocilpine interactions and working and reference memory performance of rats in the radial-arm maze. Pharmacol Biochem Behav. 1998;61:335–340. doi: 10.1016/s0091-3057(98)00109-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Lieberman JA. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Gould TJ. Latent inhibition of cued fear conditioning: an NMDA receptor-dependent process that can be established in the presence of anisomycin. Eur J Neurosci. 2004;20:818–826. doi: 10.1111/j.1460-9568.2004.03531.x. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Gould TJ. Reversible inactivation of the entorhinal cortex disrupts the establishment and expression of latent inhibition of cued fear conditioning in C57BL/6 mice. Hippocampus. 2007a;17:462–470. doi: 10.1002/hipo.20284. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Gould TJ. Signal transduction mechanisms within the entorhinal cortex that support latent inhibition of cued fear conditioning. Neurobiol Learn Mem. 2007b;88:359–368. doi: 10.1016/j.nlm.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Lindsley CW, Shipe WD, Wolkenberg SE, Theberge CR, Williams DL, Jr., Sur C, Kinney GG. Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr Top Med Chem. 2006;6:771–785. doi: 10.2174/156802606777057599. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Pi HJ, Zhang Y, Otmakhova NA. A thalamo-hippocampal-ventral tegmental area loop may produce the positive feedback that underlies the psychotic break in schizophrenia. Biol Psychiatry. 2010;68:17–24. doi: 10.1016/j.biopsych.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. Study of a new schizophrenomimetic drug; sernyl. AMA Arch Neurol Psychiatry. 1959;81:363–369. doi: 10.1001/archneurpsyc.1959.02340150095011. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Stote DL, Fanselow MS. N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behav Neurosci. 1996;110:1365–1374. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LF, Freedman R. Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int Rev Neurobiol. 2007;78:225–246. doi: 10.1016/S0074-7742(06)78008-4. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Muller D, Lynch G. Synaptic modulation of N-methyl-D-aspartate receptor mediated responses in hippocampus. Synapse. 1990;5:94–103. doi: 10.1002/syn.890050203. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Numakawa T, Ikeuchi T, Hatanaka H. Nicotine-induced phosphorylation of extracellular signal-regulated protein kinase and CREB in PC12h cells. J Neurochem. 2001;79:489–498. doi: 10.1046/j.1471-4159.2001.00602.x. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5:361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, Craft S, Olney JW. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Olincy A, Stevens KE. Treating schizophrenia symptoms with an alpha7 nicotinic agonist, from mice to men. Biochem Pharmacol. 2007;74:1192–1201. doi: 10.1016/j.bcp.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olincy A, Young DA, Freedman R. Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry. 1997;42:1–5. doi: 10.1016/S0006-3223(96)00302-2. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- Petersen DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos. 1984;12:725–731. [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Porter RH, Eastwood SL, Harrison PJ. Distribution of kainate receptor subunit mRNAs in human hippocampus, neocortex and cerebellum, and bilateral reduction of hippocampal GluR6 and KA2 transcripts in schizophrenia. Brain Res. 1997;751:217–231. doi: 10.1016/s0006-8993(96)01404-7. [DOI] [PubMed] [Google Scholar]
- Radek RJ, Kohlhaas KL, Rueter LE, Mohler EG. Treating the cognitive deficits of schizophrenia with alpha4beta2 neuronal nicotinic receptor agonists. Curr Pharm Des. 2010;16:309–322. doi: 10.2174/138161210790170166. [DOI] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Crabtree G, Turner J, Role L. Molecular Composition and Biophysical Characteristics of Nicotinic Receptors. In: Arneric S, Brioni J, editors. Neuronal Nicotinic Receptors: Pharmacology and Therapeutic Opportunities. Wiley-Liss, Inc.; New York: 1998. pp. 43–64. [Google Scholar]
- Ramoa AS, Alkondon M, Aracava Y, Irons J, Lunt GG, Deshpande SS, Wonnacott S, Aronstam RS, Albuquerque EX. The anticonvulsant MK-801 interacts with peripheral and central nicotinic acetylcholine receptor ion channels. J Pharmacol Exp Ther. 1990;254:71–82. [PubMed] [Google Scholar]
- Rathouz MM, Vijayaraghavan S, Berg DK. Elevation of intracellular calcium levels in neurons by nicotinic acetylcholine receptors. Mol Neurobiol. 1996;12:117–131. doi: 10.1007/BF02740649. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. Extracellular signal-regulated kinase 1/2 involvement in the enhancement of contextual fear conditioning by nicotine. Behav Neurosci. 2007;121:1119–1124. doi: 10.1037/0735-7044.121.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Nicotinic-glutamatergic interactions and attentional performance on an operant visual signal detection task in female rats. Eur J Pharmacol. 2003;465:83–90. doi: 10.1016/s0014-2999(03)01439-0. [DOI] [PubMed] [Google Scholar]
- Savonenko A, Werka T, Nikolaev E, Zielinski K, Kaczmarek L. Complex effects of NMDA receptor antagonist APV in the basolateral amygdala on acquisition of two-way avoidance reaction and long-term fear memory. Learn Mem. 2003;10:293–303. doi: 10.1101/lm.58803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Birkenfeld K, Palve M, Spiess J. Impairment of conditioned contextual fear of C57BL/6J mice by intracerebral injections of the NMDA receptor antagonist APV. Behav Brain Res. 2000;116:157–168. doi: 10.1016/s0166-4328(00)00269-2. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Tinsley MR, Quinn JJ, Fanselow MS. The role of muscarinic and nicotinic cholinergic neurotransmission in aversive conditioning: comparing pavlovian fear conditioning and inhibitory avoidance. Learn Mem. 2004;11:35–42. doi: 10.1101/lm.70204. [DOI] [PubMed] [Google Scholar]
- Tomizawa M, Casida JE. Desnitro-imidacloprid activates the extracellular signal-regulated kinase cascade via the nicotinic receptor and intracellular calcium mobilization in N1E-115 cells. Toxicol Appl Pharmacol. 2002;184:180–186. doi: 10.1006/taap.2002.9503. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Miyasato K, Nishiyama S, Fukumoto D, Kakiuchi T, Domino EF. Nicotine normalizes increased prefrontal cortical dopamine D1 receptor binding and decreased working memory performance produced by repeated pretreatment with MK-801: a PET study in conscious monkeys. Neuropsychopharmacology. 2005;30:2144–2153. doi: 10.1038/sj.npp.1300745. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- Weiner I, Arad M. Using the pharmacology of latent inhibition to model domains of pathology in schizophrenia and their treatment. Behav Brain Res. 2009;204:369–386. doi: 10.1016/j.bbr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Welsby P, Rowan M, Anwyl R. Nicotinic receptor-mediated enhancement of long-term potentiation involves activation of metabotropic glutamate receptors and ryanodine-sensitive calcium stores in the dentate gyrus. Eur J Neurosci. 2006;24:3109–3118. doi: 10.1111/j.1460-9568.2006.05187.x. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Gould TJ. Neuronal nicotinic acetylcholine receptors: involvement in Alzheimer’s disease and schizophrenia. Behav Cogn Neurosci Rev. 2002;1:5–20. doi: 10.1177/1534582302001001002. [DOI] [PubMed] [Google Scholar]
- Yin H, Bardgett ME, Csernansky JG. Kainic acid lesions disrupt fear-mediated memory processing. Neurobiol Learn Mem. 2002;77:389–401. doi: 10.1006/nlme.2001.4037. [DOI] [PubMed] [Google Scholar]
- Zarei MM, Radcliffe KA, Chen D, Patrick JW, Dani JA. Distributions of nicotinic acetylcholine receptor alpha7 and beta2 subunits on cultured hippocampal neurons. Neuroscience. 1999;88:755–764. doi: 10.1016/s0306-4522(98)00246-2. [DOI] [PubMed] [Google Scholar]
- Zhang S, Ehlers MD, Bernhardt JP, Su CT, Huganir RL. Calmodulin mediates calcium-dependent inactivation of N-methyl-D-aspartate receptors. Neuron. 1998;21:443–453. doi: 10.1016/s0896-6273(00)80553-x. [DOI] [PubMed] [Google Scholar]
- Zhang WN, Bast T, Feldon J. The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after infusion of N-methyl-D-aspartate or its noncompetitive antagonist MK-801 into the ventral hippocampus. Behav Brain Res. 2001;126:159–174. doi: 10.1016/s0166-4328(01)00256-x. [DOI] [PubMed] [Google Scholar]


