Abstract
Soft tissue fillers are rapidly gaining popularity for aesthetic improvements or repair of adipose tissue deficits. Several injectable biopolymers have been investigated for this purpose but often face rapid resorption or limited adipogenesis, and do not mimic the native adipose extracellular matrix (ECM). We have generated an injectable adipose matrix scaffold by efficiently removing both the cellular and lipid contents of human lipoaspirate. The decellularized material retained a complex composition of peptides and glycosaminoglycans found in native adipose ECM. This matrix can be further processed by solubilizing the extracted ECM to generate a thermally-responsive hydrogel that self-assembles upon subcutaneous injection. This hydrogel also supports the growth and survival of patient matched adipose - derived stem cells in vitro. The development of an injectable hydrogel from human lipoaspirate represents a minimally-invasive option for adipose tissue engineering in terms of both the collection of source material and delivery of the scaffold.
Keywords: Adipose tissue engineering, biomimetic material, thermally responsive material, hydrogel, glycosaminoglycan
1. Introduction
Adequate replacement of adipose tissue is often overlooked when restructuring soft tissues for aesthetic improvement or traumatic injury repair. In addition to its roles in energy storage and cushioning, adipose tissue also significantly contributes to bodily symmetry and aesthetics. Several researchers have investigated traditional biomaterials for adipogenic capability, but each one faces significant drawbacks, as it was not originally tailored for adipose tissue. Common synthetic polymers, such as poly(lactic-co-glycolic acid) (PLGA), have proven insufficient to cause natural regeneration of adipocytes and face some degree of fibrous encap sulation in animal models [1]. Natural biopolymers, such as collagen and hyaluronic acid, have also been molded into gels and cross-linked scaffolds. These materials improve biocompatibility but struggle to resist rapid resorption [2, 3]. Clinical trials of hyalauronic acid scaffolds have shown maintained shape and cellular infiltration, but the implants suffered from limited integration and an absence of mature adipocytes within the material [3].
In addition to an inability to adequately induce adipogenesis, these three dimensional scaffolds also require surgical implantation. To minimize the invasive delivery of materials for adipose regeneration, several natural and synthetic polymers with injectable functionality have been investigated for in vivo adipogenic potential. Alginate and fibrin have been extensively studied because they readily gel and their biocompatability is well known [4, 5]. These studies have shown positive cell survival and improved vascularization following implantation. However, acellular implants exhibited limited formation of new adipose tissue, and the presence of foreign body giant cells and a fibrous capsule [4, 6]. Recently, collagen and hyaluronic acid have emerged as popular soft tissue fillers and are the major components of several commercially available products. Collagen has a low incidence of allergic reaction but, in an injectable form, can be rapidly resorbed and encourages only limited adipogenesis [7, 8]. Hyaluronic acid has shown improved angiogenesis and adipogenesis; however, it too faces rapid resorption in vivo [9, 10]. Tan et al recently introduced a modified version of hyaluronic acid linked to poly-(N-isopropylacrylamide) that self-assembles at body temperature, but it has yet to be tested for adipogenic potential [11]. Despite the availability of several injectable materials, there has yet to be identified an engineered material that avoids immune complications and encourages new fat formation. Moreover, no injectable material has been designed to mimic the native adipose extracellular matrix (ECM).
Several clinicians have pursued autologous alternatives by using free fat transfer to augment soft tissues [12, 13]. These “lip otran sfer” treatments inject liposuctioned fat back into a patient through a cannula inserted into the subcutaneous space. This process has seen initial short-term success in small volume areas and a limited immune response [14]. However, mature adipocytes are poorly equipped to survive ischemic conditions which leads to rapid necrosis and resorption in many cases [15]. The lipoaspirate also exhibits variable mechanical properties and requires an 18 G needle to accommodate the viscous emulsion of adipose particulate [16]. Lipotransfer provides a material that contains many of the natural components of adipose tissue and consequently has promoted adequate integration with host tissue. However, the inability to control the composition or mechanics of lipoaspirate results in unpredictable implant contours and resorption.
Decellularization of tissues has recently emerged as a major player in the field of regenerative medicine and offers the possibility of prod ucing a scaffold that closely mimics the physical and chemical cues seen by cells in vivo [17, 18]. Materials produced in this manner often have positive angiogenic and chemoattractant properties [19-22]. A couple tissues have been decellularized for use in adipose regeneration studies with promising results, including skeletal muscle and placental tissue [23, 24]. However, these scaffolds do not directly match the composition of the native adipose ECM. While many tissues share similar ECM elements, it is becoming evident that each tissue has its own complex composition and concentration of chemical constituents [25], which are known to regulate numerous cell processes including attachment, survival, migration, proliferation, and differentiation [26-31]. It follows that the use of decellularized adipose tissue would provide the best matrix for adipose regeneration.
Recently, a couple groups have investigated the potential to generate an acellular material from human adipose tissue [32, 33]. While successful in removing a majority of the cellular content, these methods resulted in three-dimensional scaffolds. These products would necessitate surgical implantation and limit customization for varying dimensions in the subcutaneous space. Thus, there exists a need for an acellular, injectable material that will satisfy complex contours while also closely mimicking the complexity of natural adipose ECM. Processing of adipose ECM removed via liposuction could eliminate the necrosis and variability associated with current lipotransfer procedures. In this study, we set out to fabricate an injectable scaffold from decellularized human lipoaspirate that could be both collected and delivered in a minimally–invasive manner. Herein, we assess different decellularization and processing techniques, characterize the biochemical composition and mechanical properties of the resultant scaffold, and demonstrate cell compatibility.
2. Materials and methods
2.1 Collection of source material and cell isolation
Fresh human lipoaspirate was collected from female patients, ranging from 39-58 years of age with an average age of 43, undergoing elective liposuction surgery under local anesthesia at the La Jolla Plastic & Reconstructive Surgery Clinic (La Jolla, CA) with the approval of the UCSD Institutional Review Board. Adipose-derived mesenchymal stem cells (hASCs) were first isolated from the tissue according to established protocols [34, 35]. Briefly, the tissue was digested in 0.075% collagenase I (Worthington Biochemical Corp., Lakewood, NJ) for 20 minutes and the resulting suspension was centrifuged at 5000 x g. The hASC-rich pellet was resuspended in 160 mM ammonium chloride to lyse blood cells and again centrifuged at 5000 x g. The remaining cells were filtered and resuspended in Growth Medium (Dulbecco’s modified essential medium / Ham’s F12 (DMEM/ F12, Mediatech, Manassas, VA), 10% fetal bovine serum (FBS, Gemini Bio-Products, Sacramento, CA), and 100 I.U. penicillin/ 100 μg/ mL streptomycin) and cultured overnight on standard tissue culture plastic at 37°C and 5% CO2. After 24 hours, non-adherent cells were removed with two rinses in 1x phosphate-buffered saline (PBS) and the remaining cells were serially passaged as hASCs. Growth Medium was changed every 3-4 days. When cells reached 80% confluence they were washed with 1x PBS and released from the tissue culture surface using 0.25% Trypsin/ 2.21 mM EDTA (Mediatech, Manassas, VA). The cells were resuspended, counted, and plated in new flasks with fresh Growth Medium. The lipoaspirate not used for cell isolation was immediately stored at −80°C and kept frozen until further processing.
2.2 Decellularization and delipidization of human lipoaspirate
Frozen lipoaspirate was slowly warmed to room to temperature and washed in 1x PBS for 2 hours under constant stirring. The PBS was then strained and the washed adipose tissue was placed in either 1% sodium dodecyl sulfate (SDS) in DI water or 2.5 mM sodium deoxycholate in 1x PBS. Both of these detergents have been previously shown to be effective decellularization agents [36-38]. The tissue was stirred in detergent for 48 hours and subsequently thoroughly rinsed with DI water. Each group of decellularized tissue was then placed in 2.5 mM sodium deoxycholate in 1x PBS supplemented with 500 units of porcine lipase and 500 units of porcine colipase (both from Sigma-Aldrich, St. Louis, MO) to remove remaining lipids. This enzymatic digestion was continued until the tissue became visibly white, approximately 24-48 hours depending on the patient, or for a maximum of 72 hours if there was no change in color. Finally, the tissue was rinsed with DI water for 2 hours to remove excess detergents and frozen at −80°C overnight. Prior to freezing, representative samples were embedded in Tissue Tek OCT compound for histological analysis. Following the decellularization procedure, the frozen “adipose matrix” was then lyophilized and milled using a Wiley Mini Mill.
2.3 Evaluation of decellularization and delipidization
To examine the extent of decellularization of the adipose tissue, both fresh and decellularized samples that had been embedded in OCT were sectioned into 20 μm slices and stained with hematoxylin and eosin (H&E) for histological analysis. Decellularization was confirmed by staining slides with Hoechst 33342, a fluorescent nuclear stain. The tissue sections were fixed in acetone, rinsed, and stained in Hoechst dye at 0.1 μg/ mL for 10 minutes. The sections were then rinsed, mounted with Fluoromount (Sigma-Aldrich, St. Louis, MO), and imaged with a Carl Zeiss Observer D1. Decellularization was further quantified using a commercially available DNEasy kit (Qiagen, Valencia, CA). Samples of lyophilized adipose matrix were weighed and DNA was extracted according to manufacturer’s specifications. DNA content (μg/ mg dry weight ECM) was estimated from absorbance readings at 260 nm using a BioTek Synergy H4 microplate reader (Winooski, VT) and normalized to initial dry weight of the sample. As a control, lyophilized calf skin collagen (Sigma-Aldrich, St. Louis, MO) was included in the assay.
Lipid removal from the tissue was assessed by staining with Oil Red O dye (Sigma-Aldrich, St. Louis, MO), as previously described [39]. Sections of fresh tissue and decellularized tissue, both before and after lipase treatment, were fixed with 3.2% paraformaldehyde for 1 hour and rinsed in DI water and then 60% isopropanol. Oil Red O stain was prepared at 5 mg/ mL in 100% isopropanol and diluted 3:2 with DI water to make a working solution prior to use. Fixed tissue sections were stained in Oil Red O working solution for 15 minutes, rinsed in 60% isopropanol and then DI water, and mounted with 10% glycerol in 1x PBS. Images of the staining were taken using a Carl Zeiss Imager.
2.4 Solubilization and gelation of decellularized adipose matrix
Dry, milled adipose matrix was further processed using 0.1 M HCl and 3200 I.U. porcine pepsin (Sigma-Aldrich, St. Louis, MO), following a modified version of previously established protocols for different tissues [36, 40]. The pepsin was first solubilized in 0.1 M HCl and added to the adipose matrix at a ratio of 1 mg pepsin for every 10 mg lyophilized ECM. The adipose matrix was digested for 48 hours at room temperature under constant stirring. Subsequently, the pH was raised to 7.4 using 1 M NaOH and the matrix was diluted to 15 mg/ mL using 10x PBS so that the final solution contained 1x PBS. This digest was kept on ice until used for characterization assays or gelation studies in vitro or in vivo. To induce gelation in vitro, the solubilized, neutralized adipose matrix was warmed to 37°C in a humidified incubator with 5% CO2. in vitro gels were characterized using an AR-G2 rheometer (TA Instruments, New Castle, DE) with a 20 mm diameter parallel plate configuration. Gels produced from tissue decellularized with SDS and with sodium deoxycholate were tested at 37°C under a constant 2.5% strain at an oscillating angular frequency of 1 rad / s.
2.5 Characterization of adipose matrix
Peptide content of the solubilized adipose matrix was assessed using SDS-PAGE. Samples were run on a NuPAGE® Novex Bis-Tris gel (Invitrogen, Eugene, OR) at 12% w/ v in NuPAGE MOPS SDS running buffer (Invitrogen) and compared to rat tail collagen type I (2 mg/ mL; BD Biosciences, San Jose, CA). Samples were prepared under reducing conditions with NuPAGE LDS Sample Buffer (Invitrogen) and run in an XCell Surelock MiniCell (Invitrogen) at a constant 200 V. Peptide bands were visualized using Imperial Protein Stain (Pierce, Rockford, IL). Novex® Plus2 Pre-stained Standard (Invitrogen) was used as a protein ladder. Sulfated glycosaminoglycan content of the adipose matrix was quantified using a colorimetric Blyscan assay (Biocolor, Carrickfergus, United Kingdom) according to manufacturer’s instructions. Samples from different batches of adipose matrix were tested in triplicate and absorbance was recorded at 656 nm using a BioTek Synergy H4 microplate reader (Winooski, VT).
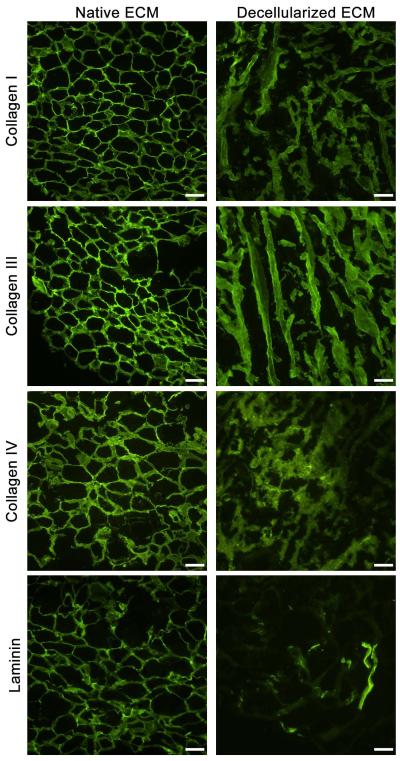
Immunofluorescent staining was used to identify specific proteins within the adipose matrix. Sections of both fresh lipoaspirate and adipose matrix were fixed with acetone and blocked with staining buffer (0.3% Triton X-100 and 2% goat serum in PBS). Samples were then stained with primary antibodies against collagen I, collagen III, collagen IV, and laminin (1:100 dilution, Abcam, San Francisco, CA). AlexaFluor 488 (1:200 dilution, Invitrogen) served as a secondary antibody. Both primary and secondary antibodies were individually omitted on control slides to confirm positive staining. Slides were mounted with Fluoromount (Sigma-Aldrich) and images were taken with a Carl Zeiss Observer D1.
Scanning electron microscopy was used to visualize the microstructure of adipose matrix gels. Gels were formed by warming solubilized adipose matrix to 37°C in a humidified in cubator with 5% CO2 overnight. Gels were immersed in 2.5% gluteraldehyde for 2 hours and then dehydrated in a series of 15-minute ethanol rinses (30-100%) according to previously published protocols [21, 25, 40]. The gels were then critical point dried using CO2 and coated with chromium using an Emitech K575X sputter coater. Scanning electron microscope images were taken using a Philips XL30 field emission SEM.
2.6 in vitro cytocompatibility assessment of adipose matrix
Solubilized adipose matrix was diluted to 5 mg/ mL using 0.1 M acetic acid and added to the bottom of wells of a 48-well tissue culture plate. The plate was kept at 4°C overnight to adsorb the matrix to the tissue culture plastic. Control wells were either left as normal tissue culture plastic or coated with 1 mg/ mL calf skin collagen solubilized in 0.1 M acetic acid. The leftover coatings were then aspirated and the wells were washed twice with 1x PBS. Passage 1 hASCs were seeded at 5 × 104 cells/ cm2 in Growth Medium. Media was changed every 2-3 days. After 1, 7 and 14 days, cells were stained with a fluorescent Live/ Dead Viability/ Cytotoxicity Kit (Invitrogen, Carlsbad, CA). A solution of 4 μM calcein and 2 μM ethidium homodimer (EthD-1) was prepared in PBS. The solution was added to the cells and allowed to incubate for 30-45 minutes at room temperature. The cells were subsequently rinsed twice with PBS and then observed under a fluorescent microscope to examine the viability of the cells.
Total DNA content was assessed at each time point as well using the Quant-IT PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA) to determine cellular proliferation. Briefly, the cells were rinsed twice in PBS and frozen at −20 °C for up to 1 week to aid cell lysis. Cellular DNA was then resuspended in 1x TE Buffer and incubated with a fluorescent PicoGreen Reagent for 30 minutes. Fluorescence was measured using a BioTek microplate reader with an excitation wavelength of 480 nm and emission wavelength of 520 nm. dsDNA was quantified by relating the sample absorbance to the absorbance measured for standards of known DNA concentration.
hASC morphology was visualized at each timepoint. Cells were washed with 1x PBS and fixed in 4% paraformaldehyde for 15 minutes. The cells were washed again and staining buffer (0.3% Triton X-100 and 1% bovine serum albumin in PBS) was added for 30 minutes to block non-specific binding. Cells were then incubated in AlexaFluor 488 Phalloidin (Invitrogen; 1:40 dilution in staining buffer) for 20 minutes to label F-actin and Hoechst 33342 (1 μg/ mL in water) for 10 minutes to label nuclei. Images of the cells were taken using a Zeiss Observer D1.
2.7 Subcutaneous injection and gelation of solubilized adipose matrix
All animal procedures were performed in accordance with the guidelines established by the Committee on Animal Research at the University of California, San Diego and the American Association for Accreditation of Laboratory Animal Care. Male athymic mice (nu/ nu) received an overdose of sodium pentobarbital and kept on heating pads. Solubilized and neutralized adipose matrix was drawn into a syringe using a 25 G needle. Six injections (100 μL each) were made subcutaneously into the dorsal region of the mouse. After 15 minutes, the injected material was excised and fresh frozen in TissueTek OCT compound. This tissue was then sectioned into 20 μm slices, stained with H&E for histological analysis, and examined using a Carl Zeiss Imager A1.
2.8 Statistical analysis
All data is presented as the mean ± standard deviation. Both the Blyscan and DNEasy assays were performed in triplicate and the results averaged. Significance was assessed using one-way analysis of variance (ANOVA) and post hoc analysis using either Dunnett’s test or Tukey’s test.
3. Results
3.1 Isolation of adipose ECM from human lipoaspirate
Fresh-frozen lipoaspirate was decellularized and delipidized within 4 days using a combined detergent and enzymatic digestion protocol. These methods were successfully repeated on samples from multiple patients, with the only variability arising in lipase digestion time (24-48 hours) due to initial lipid content. Average yield was 625 ± 96 mg of dry adipose ECM per 100 cc of lipoaspirate (n=8). We compared the use of either SDS or sodium deoxycholate, for decellularization , in combination with lipase and colipase for deplidization. Decellularization was confirmed by absence of nuclei with H&E and Hoechst 33342 in both the SDS and sodium deoxycholate groups (Fig. 1). While histological analysis demonstrated similar removal of cellular contents, a DNEasy kit revealed that SDS was more efficient in decellularizing the adipose ECM (Fig. 2), with significantly less DNA per mg of lyophilized ECM compared to the sodium deoxycholate group, and more closely approaching the collagen control.
Figure 1. Production of Decellularized and Delipidized Lipoaspirate.

Human lipoaspirate was processed to remove both cellular and lipid content. Raw lipoaspirate (A,D,G,J) was decellularized for 48 hours in SDS or sodium deoxycholate to produce a lipid filled, acellular matrix (B,E,H,K). Removal of lipids using lipase produced a white ECM, free of cellular and lipid content (C,F,I,L). H&E staining (D,E,F) and Hoechst staining (J,K,L) confirmed the absence of nuclei after processing. Oil red O staining (G,H,I) confirmed the removal of lipids. Scale bars = 100 μm.
Figure 2. Quantification of Remaining DNA.
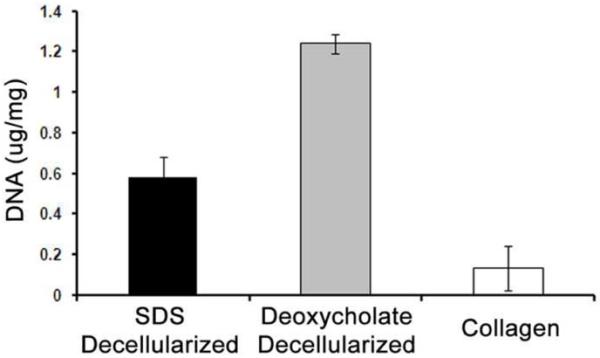
A DNEasy assay quantified the remaining nuclear content after decellularization and delipidization of the lipoaspirate. * p < 0.0001.
After decellularization, removal of lipids was achieved through the addition of lipase and colipase for 24-48 hours, producing a white ECM compared to the characteristic yellow tint of adipose tissue. As seen in Figure 1, Oil Red O staining of tissue sections revealed substantial levels of oils within fresh tissue, however treatment with lipase effectively removed lipids within the decellularized ECM, as evidenced by an absence of red staining. Decellularized tissue that was not treated with lipase only slightly reduced lipid levels compared to fresh lipoaspirate, even after 1 week of processing.
3.2 in vitro characterization and gelation of adipose matrix
Following decellularization and delipidization, the isolated adipose ECM was lyophilized, milled into a fine powder (Fig. 3A), and then solubilized with pepsin to generate a liquid injectable form of adipose matrix (Fig. 3B). The presence of lipids in the matrix prevented complete lyophilization and efficient solubilization. Groups that did not employ lipase and colipase during the decellularization process remained oily after lyophilization and could not be milled nor fully solubilized, resulting in a highly particulate digest that could not be pushed through a 25 G needle. These groups also exhibited inconsistent gelation in vitro and in vivo. However, groups that were delipidized produced a dry matrix following lyophilization that could be easily milled into a fine powder. SDS-PAGE analysis of digested adipose matrix revealed multiple peptides and low molecular weight peptide fragments. Peptide bands characteristic of collagen were present within the digest, in addition to multiple peptides below 39 kDa (Fig. 4). Specifically, collagens I, III, and IV were all present in immunofluorescent stains of adipose tissue both before and after processing (Fig. 5). Collagens I and III were more prevalent, however this could be the result of cross-reactivity of the antibody between isoforms. Laminin was also expressed at both time points, however to a lesser extent after decellularization (Fig 5). Control slides showed negligible background staining when primary or secondary antibodies were omitted (Supplementary Fig 1). Glycosaminoglycan analysis estimated an average of 2.18 ± 0.32 μg of sulfated GAG per mg dry adipose ECM, with no significant difference between tissue decellularized with SDS versus sodium deoxycholate.
Figure 3. Solubilization and Gelation of Adipose Matrix.

Decellularized and delipidized lipoaspirate produced a dry, white powder (A) that was solubilized using pepsin and HCl (B). This solubilized ECM could be induced to self-assemble (C) when placed under physiologic conditions (37°C and 5% CO2).
Figure 4. SDS-PAGE Analysis of Peptide Content.
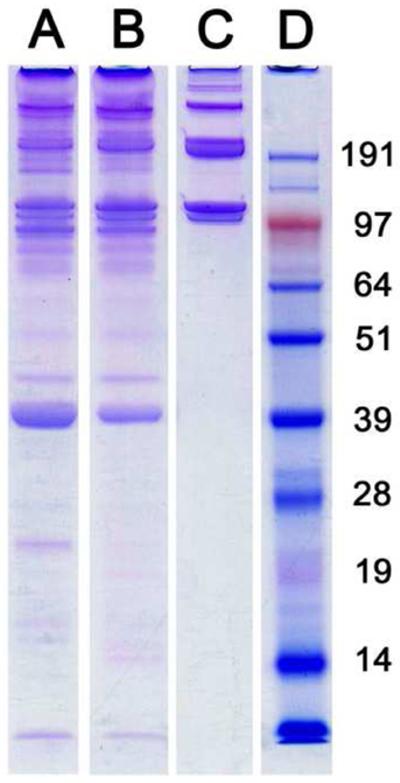
As compared to a collagen control (C), gel electrophoresis revealed collagen as well as multiple lower molecular weight peptides present within adipose matrix that had been decellularized using SDS (A) or sodium deoxycholate (B). Protein ladder was run in lane D with peptide weights in kDa.
Figure 5. Immunofluorescent Staining of Adipose Matrix.
Fluorescent antibody staining of both fresh human lipoaspirate and adipose matrix decellularized with SDS showed retention of collagens I, III, and IV. Laminin was also present in both cases, but there was some loss of content as a result of the decellularization. Scale bar = 100 μm.
Upon adjusting the pH and temperature of the liquid adipose matrix to physiologic conditions (pH 7.4, 37 °C), the solution self-assembled into a gel (Fig. 3C). SEM analysis revealed the gels were nanofibrous scaffolds with an average fiber diameter of 100 nm and interconnecting pores (Fig. 6). Storage moduli were determined at 1 rad/ s and ranged from 5-9 Pa for tissue processed with SDS and from 7-18 Pa for tissue processed with sod ium deoxycholate.
Figure 6. Scanning Electron Microscopy of Adipose Matrix.
SEM images of adipose matrix gels revealed a porous structure composed of intermeshed fibers with a diameter of approximately 100 nm. Scale bars = 2 μm (A) and 500 nm (B).
3.3 Adipose matrix coatings support hASC culture in vitro
To investigate the ability of the adipose matrix to support cell adhesion and survival, patient - matched hASCs were cultured either on adipose matrix coated tissue culture plates or collagen coated plates, and maintained in growth media. On adipose matrix coated plates, hASCs readily adhered to the surface, displaying a healthy, fibroblast-like phenotype within 24 hours (Fig. 7) [41, 42]. Live/ Dead staining revealed negligible cell death on the adipose ECM after 14 days (Fig. 7 A -C). This level of viability was consistent regardless of the surface coating. Furthermore, DNA quantification indicated that cellular growth was not hindered by the adipose ECM (Fig. 7E). hASC proliferation continued for 2 weeks on the adipose ECM and was not significantly different from normal proliferation on uncoated or collagen coated surfaces.
Figure 7. In vitro Culture of hASCs on 2D Adipose Matrix.
Live/ Dead analysis after 14 days in culture revealed negligible cell death of hASCs (Green = alive, red = dead) seeded on normal tissue culture plastic (A), calf skin collagen (B), or decellularized adipose matrix (C). Cells growing on the adipose matrix also exhibited a healthy fibroblast-like phenotype (D, Green = F-actin, blue = nuclei). PicoGreen analysis at various timepoints indicates that the adipose ECM promoted normal proliferation over 2 weeks in culture (E). Each group increased significantly between time points but no significant difference was found between groups at each timepoint. * p<0.0001 for Day 7 values for each group compared to Day 1 values. † p<0.0001 for Day 14 values for each group compared to Day 7 values. Scale bars = 100 μm.
3.4 Gelation of adipose matrix in vivo
Liquid adipose matrix was injected subcutaneously in mice to investigate in vivo self-assembly (Fig. 8A). Solubilized adipose matrix formed a compact, white bolus when injected subcutaneously using a 25G needle (Fig. 8B). Within 15 minutes, the bolus had solidified into gel that maintained its shape when excised (Fig. 8C). Immediately following injection, the bolus could be pinched or molded to create elongated structures prior to gelation. H&E analysis of excised tissue showed an acellular, porous matrix in close contact with subcutaneous adipose tissue (Fig. 8D).
Figure 8. in vivo Gelation of Solubilized Adipose Matrix.
Solubilized adipose matrix was injected subcutaneously into nude mice using a 25G needle (A). The solubilized ECM formed a solid bolus beneath the skin within 15 minutes (B). Gels held their shape when excised (C) and were analyzed with H&E (D). This staining showed an acellular matrix (m) in close contact with native fat (f). Scale bar = 50 μm.
4. Discussion
While several three dimensional scaffolds have been proposed for adipose tissue regeneration, injectable fillers offer unique characteristics that are specifically advantageous for application in adipose tissue. Because adipose regeneration is typically associated with enhancement or contouring of natural features to improve aesthetics, the minimally-invasive delivery of an injectable material is desirable to reduce scarring at the surgical site. Furthermore, the collection of source material from liposuction, as opposed to surgical excision of whole fat pads, compliments this minimally-invasive approach by limiting donor site damage. Injectable materials also allow for contouring of complex features within the face, a common area of desired adipose regeneration. Solid scaffolds cannot offer this level of customization. Consequently, the ideal scaffold for adipose tissue engineering would allow for injectable delivery, match the chemical complexity of the native microenvironment, and promote natural regeneration of the tissue as it is resorbed.
We present here the production of decellularized and delipidized adipose ECM from human lipoaspirate using a combined detergent and enzymatic method. Our results indicate that decellularized lipoaspirate retains a complex composition of proteins, peptides, and glycosaminoglycans (GAGs). Immunofluorescent staining indicated the preservation of multiple collagen isoforms, a major component of native adipose ECM. Despite a slight reduction in content compared to native tissue, laminin was also expressed within the decellularized adipose ECM. Adipose ECM has been previously reported to contain many of the components of basement membrane, including collagens I, IV, and VI, laminin, and fibronectin [43, 44]. Excessive oils within the lipoaspirate prevented accurate calculation of the GAG content of native adipose tissue using a Blyscan assay. However, there are reports of multiple GAGs and proteoglycans present in the secretome of mouse 3T3-L1 adipocytes, such as perlecan, mimecan, and decorin [43, 45, 46]. We likewise found native GAGs retained within the adipose matrix material. Currently, a wide range of values have been reported in literature for GAGs retained within solubilized versions of decellularized tissues. Singelyn et al reported 23.2 ± 4.63 μg GAG per mg solubilized myocardial ECM, but Stern et al were unable to detect any GAGs within their solubilized skeletal muscle ECM [36, 47]. Clearly there exists extensive variability in ECM composition among tissue types and decellularization protocols. While this decellularization protocol likely causes a reduction in protein and GAG concentration compared to native tissue, this assortment of native biochemical cues mimics the microenvironment of adipose tissue, unlike existing soft-tissue fillers, and could provide adipose specific cues for cell migration, survival, and differentiation. Sulfated GAGs are recognized for their ability to sequester growth factors and subsequently present them to cells [48-50], and thus their presence within the matrix provides a possible avenue for bioactive molecule delivery both in vitro and in vivo. In addition, PAGE analysis of the injectable adipose matrix confirmed the presence of peptides with a molecular weight at 16 kDa and below, which have been previously shown with other decellularized matrices to have chemoattractant potential [19].
We investigated SDS and sodium deoxycholate to decellularize the lipoaspirate as they have previously been shown to effectively decellularize multiple tissues [17]. When applied to fresh tissue, these ionic detergents disrupt the cell and nuclear membranes and entrap the freed nuclear contents into micelles, which are then washed away [17, 51]. Through gross and histological observation, it appeared that both SDS and sodium deoxycholate adequately removed all cellular debris. However, by quantifying the extent of decellularization with DNEasy, SDS proved to have a significantly lower amount of contaminating DNA. There remains debate over what level of DNA qualifies the material as “decellularized.” Gilbert et al suggest that there may exist a threshold DNA concentration below which no immune response will be triggered [52]. It is possible that the detergents also degrade the structure of DNA and other nuclear proteins to an extent that they are no longer recognized as foreign antigens. In fact, many commercially available acellular matrices have been found to contain some degree of cellular contaminants despite their successful use in clinical treatment [52]. Apart from decellularization efficiency, the two detergents appeared to perform at a similar degree. They both produced similar gel electrophoresis bands and GAG content, indicating that neither detergent had a more pronounced deleterious effect on the ECM. Both methods also produced gels that showed a similar range of storage moduli, which align with previously published reports for the mod ulus of self-assembling collagen gels [53, 54].
Adipose tissue was, unsurprisingly, adept at trapping lipids within its ECM, resulting in multiple complications during processing into an injectable scaffold. While detergents could sufficiently eliminate free lipids surrounding the tissue, a large proportion of oily residue remained trapped on and within the adipose matrix. These sequestered lipids inhibited consistent lyophilization, milling, and solubilization of the adipose matrix. To eliminate lipid s from the decellularized adipose matrix, we have produced a method inspired by the body’s natural lipid metabolism mechanism [55]. Lipase is a naturally occurring esterase produced in the pancreas to digest dietary fats within the small intestine. It specifically targets the ester bond of triglycerides, separating the compound into glycerol and fatty acids, which are readily emulsified by bile salts, such as sodium deoxycholate [56]. Lipase is also actively involved in the breakdown of triglycerides from adipose stores for energy homeostasis [57]. SDS has, however, been shown to cooperatively bind with lipase and irreversibly inhibit its activity [58]. This finding was confirmed in our research and necessitated that sodium deoxycholate be used during lipase digestion, regardless of the initial decellularization detergent (data not shown). Additionally, Labourdenne et al demonstrated that bile salts can partially inhibit lipase activity, but this inhibition can be overcome by the addition of colipase [59]. They reported that colipase increased lipase activity by 10-15 fold. In our investigation, we found that exposing the adipose matrix to lipase in excess of 72 hours resulted in significant protein degradation and an inability to self-assemble following solubilization (data not shown). For this reason, we incorporated colipase to keep enzymatic digestion times to a minimum.
Detergent-based decellularization method s have received criticism for their potential to degrade the extracellular matrix during processing. To avoid the use of detergents, several groups have investigated the direct injection of lipoaspirate via “lipotransfer” operations or the injection of homogenized lipoaspirate emulsifications [12-14, 16, 60]. However, none of these studies attempted to remove cells or lipids from the injected material. While autologous lipid injection should not initiate a foreign antigen response initially, apoptotic cells within the implant could serve as nucleation sites for calcification [61]. Implant calcification has also been associated with the presence of cell membrane phospholipids [62]. Additionally, emulsions of lipids or cellular contents would create heterogeneity within an injectable material, yielding unpredictable material behavior in vivo and limited contouring capability. The sequelae of cellular and lipid remnants in an injected soft tissue filler argue in favor of decellularization despite the possible degradation of proteins. Our results indicate that decellularized adipose matrix retains much of the protein complexity of native tissue alongside the complete removal of lipids from the material. This removal of both cellular and lipid content reduces concerns surrounding implant immune rejection and calcification.
We have demonstrated here that human lipoaspirate can be effectively decellularized and subsequently solubilized to produce a self-assembling subcutaneous filler. While not every component of native adipose ECM was fully retained, this adipose matrix is comprised of a com plex arrangement of natural proteins and polysaccharides that more closely mimics the in vivo microenvironment than currently approved fillers such as collagen and hyaluronic acid. Furthermore, this material could be used as a delivery vehicle for incorporating adipose derived stem cells in a regenerative treatment. It has been postulated that the success of lipotransfer treatments can be attributed to the presence of a small population of resident hASCs within the injected material [13]. Using solubilized adipose matrix as a delivery vehicle, these cells could be delivered in a concentrated and more consistent manner.
Patient-matched hASCs readily proliferated on 2D adipose matrix coatings and showed positive viability. These systems could allow for the investigation of the influence of multiple physical and biochemical parameters on hASC differentiation. Several groups have reported control over adipogenesis using various chemical additives and paracrine signals [63-65]. However, there has been growing literature indicating that the surrounding microenvironment has a significant impact on stem cell fate as well. Here we demonstrate proof-of-concept for generating a scaffold derived from human lipoaspirate. Analysis of degradation, immune response, and long-term in vivo function will be critical to fully assess the benefit of this new biomaterial. Furthermore, differentiation studies using hASCs are required to examine the adipogenic potential of this adipose matrix. These experiments are essential to assess whether the adipose matrix is as an efficient scaffold for adipose tissue engineering, providing more utility than currently available soft tissue fillers. Decellularized adipose matrix could provide the biochemical cues seen by hASCs in vivo, yet allow the specific control over extraneous conditions offered by an in vitro setting. Thus, this material has the potential to be both an injectable scaffold for adipose tissue engineering, and a platform for discovering the controlling mechanisms behind adipogenesis.
5. Conclusion
This work demonstrates the feasibility of human lipoaspirate as a minimally-invasive option for adipose tissue engineering, from collection of source material to delivery of the scaffold. While other injectable soft tissue fillers have been investigated, acellular adipose matrix provides a closer approximation to the biochemical compositional complexity of native adipose ECM. The removal of both lipids and cellular contents produces an implant with limited immune concerns, even if the lipoaspirate originates from an allogeneic source. Its gelation at body temperature permits small needle delivery, which would facilitate fine contouring of complex voids. Thus, decellularized lipoaspirate produces a potentially autologous soft tissue filler capable of thermally-responsive gelation and minimally-invasive delivery.
Supplementary Material
Supplementary Figure 1: ECM Antibody Controls for Immunofluorescent Staining. Omission of secondary antibodies revealed negligible background fluorescence for each of the antibodies used, whether on fresh human lipoaspirate or on decellularized adipose matrix (A-H). Omission of primary antibodies also showed exiguous non-specific binding of the fluorescent secondary antibody (I-J). Scale bar = 100 μm.
Acknowledgements
The authors would like to thank Ryan Anderson and the CalIT2 Nano3 facility for use of the scanning electron microscope, and Dr. Paul Chasan and the Chasan clinic for providing source material. Funding for this project was provided in part by the NIH Director’s New Innovator Award Program, part of the NIH Roadmap for Medical Research, through grant number 1-DP2-OD004309-01. D.A.Y. would like to thank the NSF for a Graduate Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patrick C, Jr, Chauvin P, Hobley J, Reece G. Preadipocyte seeded PLGA scaffolds for adipose tissue engineering. Tissue Engineering. 1999;5:139–51. doi: 10.1089/ten.1999.5.139. [DOI] [PubMed] [Google Scholar]
- 2.von Heimburg D, Zachariah S, Kühling H, Heschel I, Schoof H, Hafemann B, et al. Human preadipocytes seeded on freeze-dried collagen scaffolds investigated in vitro and in vivo. Biomaterials. 2001;22:429–38. doi: 10.1016/s0142-9612(00)00186-1. [DOI] [PubMed] [Google Scholar]
- 3.Stillaert FB, Di Bartolo C, Hunt JA, Rhodes NP, Tognana E, Monstrey S, et al. Human clinical experience with adipose precursor cells seeded on hyaluronic acid-based spongy scaffolds. Biomaterials. 2008;29:3953–9. doi: 10.1016/j.biomaterials.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Marler JJ, Guha A, Rowley J, Koka R, Mooney D, Upton J, et al. Soft-tissue augmentation with injectable alginate and syngeneic fibroblasts. Plastic and reconstructive surgery. 2000;105:2049–58. doi: 10.1097/00006534-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Torio-Padron N, Baerlecken N, Momeni A, Stark GB, Borges J. Engineering of adipose tissue by injection of human Preadipocytes in fibrin. Aesthetic plastic surgery. 2007;31:285–93. doi: 10.1007/s00266-006-0221-6. [DOI] [PubMed] [Google Scholar]
- 6.Halberstadt C, Austin C, Rowley J, Culberson C, Loebsack A, Wyatt S, et al. A hydrogel material for plastic and reconstructive applications injected into the subcutaneous space of a sheep. Tissue Engineering. 2002;8:309–19. doi: 10.1089/107632702753725067. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann C, Schuller-Petrovic S, Soyer HP, Kerl H. Adverse reactions after cosmetic lip augmentation with permanent biologically inert implant materials. J Am Acad Dermatol. 1999;40:100–2. doi: 10.1016/s0190-9622(99)70536-0. [DOI] [PubMed] [Google Scholar]
- 8.Lemperle G, Morhenn V, Charrier U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic plastic surgery. 2003;27:354–66. doi: 10.1007/s00266-003-3022-1. discussion 67. [DOI] [PubMed] [Google Scholar]
- 9.Okabe K, Yamada Y, Ito K, Kohgo T, Yoshimi R, Ueda M. Injectable soft-tissue augmentation by tissue engineering and regenerative medicine with human mesenchymal stromal cells, platelet-rich plasma and hyaluronic acid scaffolds. Cytotherapy. 2009;11:307–16. doi: 10.1080/14653240902824773. [DOI] [PubMed] [Google Scholar]
- 10.Hemmrich K, Van de Sijpe K, Rhodes NP, Hunt JA, Di Bartolo C, Pallua N, et al. Autologous in vivo adipose tissue engineering in hyaluronan-based gels--a pilot study. J Surg Res. 2008;144:82–8. doi: 10.1016/j.jss.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Tan H, Ramirez C, Miljkovic N, Li H, Rubin J, Marra K. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials. 2009;30:6844–53. doi: 10.1016/j.biomaterials.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen G, Treherne A. Treatment of facial lipoatrophy via autologous fat transfer. J Drugs Dermatol. 2009;8:486–9. [PubMed] [Google Scholar]
- 13.Meier J, Glasgold R, Glasgold M. Autologous Fat Grafting: Long-term Evidence of Its Efficacy in Midfacial Rejuvenation. Archives of Facial Plastic Surgery. 2009;11:24. doi: 10.1001/archfacial.2008.518. [DOI] [PubMed] [Google Scholar]
- 14.Kanchwala SK, Holloway L, Bucky LP. Reliable soft tissue augmentation: a clinical comparison of injectable soft-tissue fillers for facial-volume augmentation. Annals of plastic surgery. 2005;55:30–5. doi: 10.1097/01.sap.0000168292.69753.73. discussion 5. [DOI] [PubMed] [Google Scholar]
- 15.Patrick CW. Tissue engineering strategies for adipose tissue repair. Anat Rec. 2001;263:361–6. doi: 10.1002/ar.1113. [DOI] [PubMed] [Google Scholar]
- 16.Toledo LS, Mauad R. Fat injection: a 20-year revision. Clin Plast Surg. 2006;33:47–53. vi. doi: 10.1016/j.cps.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomaterialia. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Li F, Li W, Johnson S, Ingram D, Yoder M, Badylak S. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium. 2004;11:199–206. doi: 10.1080/10623320490512390. [DOI] [PubMed] [Google Scholar]
- 20.Reing JE, Zhang L, Myers-Irvin J, Cordero KE, Freytes DO, Heber-Katz E, et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Engineering Part A. 2009;15:605–14. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- 21.Ott HC, Matthiesen TS, Goh S-K, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 22.Brennan EP, Tang X-H, Stewart-Akers AM, Gudas LJ, Badylak SF. Chemoattractant activity of degradation products of fetal and adult skin extracellular matrix for keratinocyte progenitor cells. J Tissue Eng Regen Med. 2008;2:491–8. doi: 10.1002/term.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abberton KM, Bortolotto SK, Woods AA, Findlay M, Morrison WA, Thompson EW, et al. Myogel, a novel, basement membrane-rich, extracellular matrix derived from skeletal muscle, is highly adipogenic in vivo and in vitro. Cells Tissues Organs (Print) 2008;188:347–58. doi: 10.1159/000121575. [DOI] [PubMed] [Google Scholar]
- 24.Flynn L, Semple JL, Woodhouse KA. Decellularized placental matrices for adipose tissue engineering. J Biomed Mater Res. 2006;79:359–69. doi: 10.1002/jbm.a.30762. [DOI] [PubMed] [Google Scholar]
- 25.Uriel S, Labay E, Francis-Sedlak M, Moya M, Weichselbaum R, Ervin N, et al. Extraction and Assembly of Tissue-Derived Gels for Cell Culture and Tissue Engineering. Tissue engineering Part C, Methods. 2008 doi: 10.1089/ten.tec.2008.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leor J, Amsalem Y, Cohen S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol Ther. 2005;105:151–63. doi: 10.1016/j.pharmthera.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–93. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 28.Uriel S, Labay E, Francis-Sedlak M, Moya ML, Weichselbaum RR, Ervin N, et al. Extraction and Assembly of Tissue-Derived Gels for Cell Culture and Tissue Engineering. Tissue Eng Part C Methods. 2008 doi: 10.1089/ten.tec.2008.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 30.Macfelda K, Kapeller B, Wilbacher I, Losert UM. Behavior of cardiomyocytes and skeletal muscle cells on different extracellular matrix components--relevance for cardiac tissue engineering. Artif Organs. 2007;31:4–12. doi: 10.1111/j.1525-1594.2007.00334.x. [DOI] [PubMed] [Google Scholar]
- 31.Brown L. Cardiac extracellular matrix: a dynamic entity. Am J Physiol Heart Circ Physiol. 2005;289:H973–4. doi: 10.1152/ajpheart.00443.2005. [DOI] [PubMed] [Google Scholar]
- 32.Choi JS, Yang H-J, Kim BS, Kim JD, Lee S-H, Lee EK, et al. Fabrication of Porous Extracellular Matrix (ECM) scaffolds from Human adipose Tissue. Tissue engineering Part C, Methods. 2009 doi: 10.1089/ten.TEC.2009.0276. [DOI] [PubMed] [Google Scholar]
- 33.Flynn LE. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials. 2010;31:4715–24. doi: 10.1016/j.biomaterials.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 34.Zuk P, Zhu M, Mizuno H, Huang J, Futrell J, Katz A, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Engineering. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 35.Bernacki S, Wall M, Loboa E. Isolation of human mesenchymal stem cells from bone and adipose tissue. Methods in cell biology. 2008;86:257. doi: 10.1016/S0091-679X(08)00011-3. [DOI] [PubMed] [Google Scholar]
- 36.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–16. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hudson TW, Liu SY, Schmidt CE. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Engineering. 2004;10:1346–58. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 38.Cheng H-W, Tsui Y-K, Cheung KMC, Chan D, Chan BP. Decellularization of chondrocyte-encap sulated collagen microspheres: a three-dimensional model to study the effects of acellular matrix on stem cell fate. Tissue engineering Part C, Methods. 2009;15:697–706. doi: 10.1089/ten.TEC.2008.0635. [DOI] [PubMed] [Google Scholar]
- 39.Koopman R, Schaart G, Hesselink MK. Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem Cell Biol. 2001;116:63–8. doi: 10.1007/s004180100297. [DOI] [PubMed] [Google Scholar]
- 40.Freytes DO, Martin J, Velankar SS, Lee AS, Badylak SF. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29:1630–7. doi: 10.1016/j.biomaterials.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Zuk P, Zhu M, Ashjian P, De Ugarte D, Huang J, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Molecular biology of the cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gimble J, Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–9. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- 43.Mariman ECM, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 2010;67:1277–92. doi: 10.1007/s00018-010-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang P, Mariman E, Keijer J, Bouwman F, Noben J-P, Robben J, et al. Profiling of the secreted proteins during 3T3-L1 adipocyte differentiation leads to the identification of novel adipokines. Cell Mol Life Sci. 2004;61:2405–17. doi: 10.1007/s00018-004-4256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lim J-M, Sherling D, Teo CF, Hausman DB, Lin D, Wells L. Defining the regulated secreted proteome of rodent adipocytes upon the induction of insulin resistance. J Proteome Res. 2008;7:1251–63. doi: 10.1021/pr7006945. [DOI] [PubMed] [Google Scholar]
- 46.Roelofsen H, Dijkstra M, Weening D, de Vries MP, Hoek A, Vonk RJ. Comparison of isotope-labeled amino acid incorporation rates (CILAIR) provides a quantitative method to study tissue secretomes. Mol Cell Proteomics. 2009;8:316–24. doi: 10.1074/mcp.M800254-MCP200. [DOI] [PubMed] [Google Scholar]
- 47.Stern MM, Myers RL, Hammam N, Stern KA, Eberli D, Kritchevsky SB, et al. The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials. 2009;30:2393–9. doi: 10.1016/j.biomaterials.2008.12.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mullen LM, Best SM, Brooks RA, Ghose S, Gwynne JH, Wardale J, et al. Binding and Release Characteristics of Insulin-Like Growth Factor-1 from a Collagen-Glycosaminoglycan Scaffold. Tissue engineering Part C, Methods. 2010 doi: 10.1089/ten.tec.2009.0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Doran MR, Markway BD, Aird IA, Rowlands AS, George PA, Nielsen LK, et al. Surface-bound stem cell factor and the promotion of hematopoietic cell expansion. Biomaterials. 2009;30:4047–52. doi: 10.1016/j.biomaterials.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 50.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–8. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 51.Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta. 2004;1666:105–17. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Gilbert TW, Freund JM, Badylak SF. Quantification of DNA in biologic scaffold materials. J Surg Res. 2009;152:135–9. doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raub CB, Putnam AJ, Tromberg BJ, George SC. Predicting bulk mechanical properties of cellularized collagen gels using multiphoton microscopy. Acta biomaterialia. 2010 doi: 10.1016/j.actbio.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miron-Mendoza M, Seemann J, Grinnell F. The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials. 2010;31:6425–35. doi: 10.1016/j.biomaterials.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Tilbeurgh H, Bezzine S, Cambillau C, Verger R, Carrière F. Colipase: structure and interaction with pancreatic lipase. Biochim Biophys Acta. 1999;1441:173–84. doi: 10.1016/s1388-1981(99)00149-3. [DOI] [PubMed] [Google Scholar]
- 56.Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009;48:275–97. doi: 10.1016/j.plipres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–6. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 58.Borgstrom B, Donner J. Interactions of pancreatic lipase with bile salts and dodecyl sulfate. The Journal of Lipid Research. 1976;17:491. [PubMed] [Google Scholar]
- 59.Labourdenne S, Brass O, Ivanova M, Cagna A, Verger R. Effects of colipase and bile salts on the catalytic activity of human pancreatic lipase. A study using the oil drop tensiometer. Biochemistry. 1997;36:3423–9. doi: 10.1021/bi961331k. [DOI] [PubMed] [Google Scholar]
- 60.Choi JS, Yang H-J, Kim BS, Kim JD, Kim JY, Yoo B, et al. Human extracellular matrix (ECM) powders for injectable cell delivery and adipose tissue engineering. J Control Release. 2009;139:2–7. doi: 10.1016/j.jconrel.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 61.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circulation Research. 2000;87:1055–62. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 62.Hirsch D, Drader J, Thomas TJ, Schoen FJ, Levy JT, Levy RJ. Inhibition of calcification of glutaraldehyde pretreated porcine aortic valve cusps with sodium dodecyl sulfate: preincubation and controlled release studies. J Biomed Mater Res. 1993;27:1477–84. doi: 10.1002/jbm.820271203. [DOI] [PubMed] [Google Scholar]
- 63.Hebert TL, Wu X, Yu G, Goh BC, Halvorsen Y-DC, Wang Z, et al. Culture effects of epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) on cryopreserved human adipose-derived stromal/ stem cell proliferation and adipogenesis. J Tissue Eng Regen Med. 2009;3:553–61. doi: 10.1002/term.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hemmrich K, von Heimburg D, Cierpka K, Haydarlioglu S, Pallua N. Optimization of the differentiation of human preadipocytes in vitro. Differentiation. 2005;73:28–35. doi: 10.1111/j.1432-0436.2005.07301003.x. [DOI] [PubMed] [Google Scholar]
- 65.van Harmelen V, Skurk T, Hauner H. Primary culture and differentiation of human adipocyte precursor cells. Methods Mol Med. 2005;107:125–35. doi: 10.1385/1-59259-861-7:125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: ECM Antibody Controls for Immunofluorescent Staining. Omission of secondary antibodies revealed negligible background fluorescence for each of the antibodies used, whether on fresh human lipoaspirate or on decellularized adipose matrix (A-H). Omission of primary antibodies also showed exiguous non-specific binding of the fluorescent secondary antibody (I-J). Scale bar = 100 μm.