Abstract
Problems in real-world functioning are pervasive in schizophrenia and much recent effort has been devoted to uncovering factors which contribute to poor functioning. The goal of this study was to examine the role of four such factors: social cognition (theory of mind), neurocognition, negative symptoms, and functional capacity (social competence). 178 individuals with schizophrenia or schizoaffective disorder completed measures of theory of mind, neurocognition, negative symptoms, social competence, and self-reported functioning. Path models sought to determine the relationships among these variables. Theory of mind as indexed by the Hinting Task partially mediated the relationship between neurocognition and social competence, and negative symptoms and social competence demonstrated significant direct paths with self-reported functioning. Study results suggest theory of mind serves as an important mediator in addition to previously investigated social cognitive domains of emotional and social perception. The current study also highlights the need to determine variables which mediate the relationship between functional capacity and real-world functioning.
Keywords: Schizophrenia, Real-world Functioning, Functional Capacity, Social Cognition, Neuropsychological Functioning, Negative Symptoms
1. Introduction
Deficits in such diverse areas of functioning as communicating with others, obtaining and maintaining employment, and general community functioning have been widely documented and are apparent throughout the schizophrenia spectrum (Addington et al., 2003; Walker, 1994; Wiersma et al., 2000). Interventions targeting functional impairment should be guided by research on the factors that contribute to problems in functioning. Several factors have been theoretically and empirically linked with functioning. First, functional attainment, or one's demonstrated ability to live independently in the real-world, is reliant on the skills necessary to achieve this independence, or functional capacity (Patterson & Mausbach, 2010). In addition, neurocognition, social cognition, and negative symptoms are also significantly associated with functioning in prior research. The empirical support for each of these constructs is outlined below.
Neurocognition, a constellation of cognitive abilities including processing speed, working memory, visual and verbal learning and memory, and executive functioning, has been reliably associated with functional impairment both concurrently and prospectively (e.g., Green et al., 2000). Research has shown that neurocognitive impairment is a well-established feature of schizophrenia (reviewed in Heinrichs and Zakzanis, 1998; Hoff and Kremen, 2002), with some proposing neurocognitive impairment plays a role in most of the disturbances observed in schizophrenia (Cornblatt et al., 2009). Although neurocognitive impairment may account for 20-60% of the variance in real-world outcomes (Green et al., 2000), 40-80% of the variance in functional outcome is unaccounted for by traditional neurocognitive measures. Clearly other relevant factors contribute to functional impairment in schizophrenia.
In line with this notion, independent research groups have suggested that attention must focus on identifying factors that mediate the relationship between neurocognition and functioning behaviors in order to enhance predictive value and identify further treatment targets (Green et al., 2000). Social cognition, “a domain of cognition that involves the perception, interpretation, and processing of social information” (Ostrom, 2984, p.176), clearly requires neurocognitive skills (e.g., reasoning, attention, basic perception) and has obvious links with social behavior. Thus, it is not surprising social cognition has been proposed as likely candidate for mediation. Specifically, it has been demonstrated that social cognition is distinct from neurocognition (e.g., Sergi et al., 2007), and that it is significantly associated with functional outcome (reviewed in Couture et al., 2006). In addition, several studies have investigated the hypothesis that social cognition serves as a mediator between neurocognition and functional outcome. For instance, Addington and colleagues (2006) found that social perception and social knowledge fully mediated the relationship between neurocognition and social problem solving, and partially mediated the relationship with social functioning in an early psychosis sample. Similarly, a second study also found support for the role of social perception as a mediator between early visual processing and functional outcome (Sergi et al., 2006), and social perception was also identified as a mediator in the relationship between neurocogniton and work skills in a third study (Vauth et al., 2004). In contrast, Nienow et al. (2006) found evidence that affect recognition performed as a moderator, rather than a mediator, in the relationship between attention/vigilance and social problem solving. Accordingly, it seems clear that social cognition, as measured by emotion or social perception, appears to play a crucial role in the relationship between neurocognition and domains of functional outcome.
Theory of Mind (ToM) is another aspect of social cognition that has not yet been evaluated as a mediator. ToM involves the ability to ascertain the mental states of others, and accordingly is likely to affect functioning behaviors to a great extent (Bora et al., 2006). Indeed, there is preliminary evidence to support a link between ToM and functional outcome (Couture et al., 2006), and several studies have found associations between ToM and neurocognition (e.g., Greig et al., 2004). Although ToM and emotion perception are both under the umbrella of social cognition, ToM and emotion perception are distinct constructs. For example, in individuals with traumatic brain injury, ToM and emotion perception performance could be dissociated (Henry et al., 2006; McDonald & Flanagan, 2004), and in studies of individuals with schizophrenia, ToM and emotion recognition have demonstrated no, or very little, association with one another (Bell et al., 2009; Brune, 2005). Therefore, the present study is an important extension of previous research by examining whether ToM also mediates the relationship between neurocognitive impairment and functional outcome.
A third factor found to mediate the relationship between neurocognition and functioning behaviors is functional capacity. There may be a discrepancy between which behaviors are performed in the real-world, versus which behaviors the individual is capable of performing (Harvey et al., 2007). This distinction between performance and capacity has given rise to investigation of several new performance-based measures assessing functional capacity. Social competence is one aspect of functional capacity which has been reliably measured with role play tasks such as the Maryland Assessment of Social Competence (MASC; Bellack et al., 2006). The MASC is able to differentiate between good and poor work outcomes within schizophrenia, and between patients with schizophrenia and healthy controls or those with bipolar disorder (Bellack et al., 2006). Similar to findings from real-world functioning measures, the MASC has also demonstrated relationships with neurocognition (Green et al., 2008). Other role-play tasks, such as the conversation probe (e.g., Penn et al., 1994), have evidenced significant relationships with the social cognitive domains of affect recognition, social perception, and ToM (Pinkham and Penn, 2006). Thus, there is evidence to suggest that the ability to understand the intentions of others (ToM) is associated with social competence, and ultimately, real-world functioning.
Finally, functioning behaviors have also been associated with negative symptoms, Clearly, features such as appropriate levels of motivation, the ability to experience rewards in the environment, and the expression of one's emotional state in an appropriate manner are necessary to navigate life challenges effectively. In support of this idea, negative symptoms have been significantly associated with functional outcome (e.g., Guaiana et al., 2007), neurocognition (e.g., Harvey et al., 2006), and ToM (e.g., Corcoran et al., 1995), which provides evidence of its suitability for inclusion in modern models of real-world functioning. In addition, a recent study (Leifker et al., 2009) found that negative symptoms served as a mediator between functional capacity and real-world functioning behaviors. These findings suggest that if individuals with schizophrenia possess the necessary skills to function well in the community, negative symptoms may be predictive of whether they actually engage in these behaviors in the real-world. Deficient skill level (i.e., functional capacity) may be impaired prior to illness onset and may thus affect the development of negative symptoms via lack of successful experiences and through the formation of dysfunctional beliefs and low self-efficacy (e.g., Beck et al., 2009). In contrast, one recent study found that negative symptoms are associated with neurocognitive ability and each independently predict functioning behaviors (Bowie et al., 2006), and another found that social competence mediates the relationship between negative symptoms and interpersonal functioning (Bowie et al., 2008). Thus, these findings are in line with conceptualizations of negative symptoms as illness factors which are more closely tied to the neurocognitive and biological markers of schizophrenia. Taken together, previous work clearly indicates negative symptoms are important in predicting real-world functioning, although it is unclear whether negative symptoms are best thought of as a proximal cause to functioning behaviors (i.e., reflecting poor drive and motivation to perform the behaviors they are capable of) or a more distal cause affecting the ability to correctly ascertain appropriate social and functional behaviors (i.e., causing impairment in social cognition and functional capacity).
Based on this prior research, path analysis was used to test a model of functioning behaviors in schizophrenia that includes relationships among neurocognition, social cognition (as measured by ToM), negative symptoms, functional capacity, and self-reported functioning in schizophrenia. To determine whether ToM may be an important focus for treatments that target functioning, it is important to ascertain whether ToM has a unique contribution to predicting functional behaviors in the context of other known predictors. Based on previous research, we hypothesized that ToM (as indexed by the Hinting Task) would mediate the relationships between neurocognition and social competence (as indexed by the MASC) and between neurocognition and functional behaviors. In addition, further examined two alternative hypotheses regarding negative symptoms generated from prior research: 1) that negative symptoms and neurocognition are correlated and serve as exogenous predictors relative to the other variables in the model (model 1), versus 2) that negative symptoms serve as a mediator between functional capacity and self-reported functioning (model 2). This is the only study to our knowledge to include measures of neurocognition, social cognition, negative symptoms, functional capacity, and self-reported functioning within the same model.
2. Methods and Materials
2.1 Participants
This study was approved by the Institutional Review Board of the University of California at San Diego, and all participants (or their legal guardians) provided written informed consent after a complete description of the study was provided. Participants were recruited from two ongoing treatment outcome studies and measures included in this report were obtained at the baseline assessment prior to randomization to treatments (N=178). Participants with schizophrenia or schizoaffective disorder over age 18 were recruited from outpatient treatment centers and residential settings in San Diego. Diagnosis was evaluated with the Structured Clinical Interview for DSM-IV (SCID; First et al., 1995), and supplemented by medical records and consultation with the treating psychiatrists, when available. Participants were excluded from the study if they had a disabling medical or psychiatric problem that would interfere with outpatient psychotherapy or had received cognitive-behavioral or social skills training interventions in the past 5 years. All participants had an IQ above 70 as estimated by the American National Adult Reading Test (ANART; Grober et al., 1991).
2.2 Measures
2.2.1 Symptoms
The Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987) was used to assess current levels of schizophrenia-related symptoms. Participants answer questions in a brief (30 minute) semi-structured interview, which allows the examiner to rate their current symptom level. The Negative Symptom subscale was used for the purposes of this study. Based on 10 taped interviews, the inter-rater reliability for negative symptoms was good (ICC=.83).
2.2.2. Theory of Mind (ToM)
A North American version of the Hinting Task (Greig et al., 2004) was used to assess theory of mind. This version is virtually identical to Corcoran et al.'s (1995) Hinting Task, with the exception that the language is more appropriate for American, as opposed to British, participants. For example, the original item reads: “Gordon goes to the supermarket with his mum. They arrive at the sweetie aisle. Gordon says, ‘Cor! Those treacle toffees look delicious!’” The revised item reads: “Gordon goes to the supermarket with his mother. They arrive at the cookie aisle. Gordon says, ‘Wow! Those Twinkies look delicious!’” The participant is then asked, “What does Gordon really mean when he says this?” A correct response (e.g., “he wants Twinkies”) at this stage receives a score of 2 and the next vignette is read aloud. If the participant does not answer correctly the examiner gives a prompt (e.g., “Gordon goes on to say: ‘I'm hungry Mom’”) and the participant has a second opportunity to answer the question. A correct response after the prompt receives a score of 1, while an incorrect response at this stage receives a score of 0. Therefore, the highest attainable score is 20 (2 points per 10 items). As part of a study of vocal prosody in our laboratory, test items were pre-recorded and presented in two standardized formats. Half of the items were recorded such that affective prosody emphasized the hint, whereas the remaining items were read with neutral vocal tone. Prosody and neutral items were counterbalanced across participants. The task was not administered twice. No differences in accuracy emerged across the two presentation styles (t181 = -.542, n.s.) and correlations with other study measures were similar for the two presentation styles; thus, the total score was used in the present analyses.
2.2.3. Neurocognition
Several neurocognitive domains were assessed in the current study: (1) Speed of Processing assessed with Trail Making Test Part A (TMT; Reitan, 1979; Heaton et al., 1991), and Brief Assessment of Cognition in Schizophrenia (BACS; Keefe et al., 2004) Symbol Coding; (2) Working Memory was assessed with the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III; Wechsler, 1997a) Letter-Number Sequencing subtest and the Wechsler Memory Scale-Third Edition (WMS-III; Wechsler, 1997b) Spatial Span; (3) Verbal Learning was assessed with the Hopkins Verbal Learning Test-Revised (HVLT-R; Benedict et al., 1998) trials 1-3 total recall); (4) Visual Learning was assessed with the Brief Visuospatial Memory Test-Revised (BVMT-R; Benedict et al., 1996) trials 1-3 total recall; and (5) Executive Functioning was assessed with the Delis-Kaplan Executive Function System (D-KEFS; Delis et al., 2001) Card Sorting subtest (free sorting, correct number of sorts), 20 Questions subtest (initial abstract, number of questions asked), Word Context subtest (consecutively correct); and BACS letter fluency. Each domain score was calculated as the mean of the variables listed. First, age-corrected T-scores according to the published norms were used for each measure. Then, the Global Neurocognition Composite used in the model was calculated as the mean of the 5 domains. Participants needed scores on at least 3 of the 5 domains to have a Global score.
2.2.4. Social Competence
The Maryland Assessment of Social Competence (MASC; Bellack et al., 2006) was used to assess the functional capacity domain of social competence. The MASC is a structural behavioral assessment that measures the ability to resolve interpersonal problems through conversation. It is an abbreviated version of an empirically developed social problem solving procedure with established reliability and discriminant validity (Bellack et al., 1994; Sayers et al., 1995). The instrument takes 15-20 minutes to administer and consists of three 3-minute role play scenarios (1 conversation initiation, 2 assertiveness). Participant responses are videotaped for subsequent coding by blinded raters on dimensions of verbal content, nonverbal communication behavior, and an overall effectiveness score. Excellent inter-rater reliability was achieved (ICC=.86). For the purpose of the current analyses, the total score was used as has been done in previous studies (Dickinson et al., 2007).
2.2.5. Self-Reported Functioning
Self-reported functioning was assessed with the Independent Living Skills Survey (ILSS; Wallace et al., 2000). The ILSS is a 70-item self-report measure that assesses 10 domains of functioning: (1) Personal Hygiene, (2) Appearance, (3) Care of Personal Possessions, (4) Food Preparation, (5) Care of Personal Health and Safety, (6) Money Management, (7) Transportation, (8) Leisure, (9) Job Seeking, and, (10) Job Maintenance. Consistent with prior studies (Granholm et al., 2005; Mueser et al., 2010), a global composite of the average of the 10 functioning domains was used in the model. The ILSS was designed to have a simple and objective response scale involving yes or no responses to queries about the performance of specific behaviors (e.g., driving, riding the bus, talking with family or friends, washing clothing) during the past 30 days. The self-report version has demonstrated associations with reports of an informant of a similar magnitude to investigations of self-other agreement in other populations (e.g., Cui et al., 2005; McCrae and Costa, 1992). For the total ILSS, internal consistency is .79, stability over 6 months is .66, and inter-rater reliability (self-informant) is .44 (Wallace et al., 2000). The ILSS also has evidenced convergent validity with the Global Assessment Scale and is associated with employment status (Wallace et al., 2000), in addition to being sensitive to the effects of skills training and other treatments that target functioning (Granholm et al., 2005; Mueser et al., 2010; Wallace et al., 2000).
2.3. Statistical Analyses
Path analysis allows testing of a system of regression equations in which variables can serve as both “predictors” and “outcomes,” and multiple “outcomes” can be investigated in the same analysis. This procedure also allows effects to be parsed apart into their components (indirect, direct, and total effects), which is a significant advantage over multiple regression. For the current study we used path analysis with maximum likelihood estimation to assess the nature of the relationships among neurocognition, ToM, negative symptoms, social competence, and self-reported functioning. While it would be preferable to only estimate a portion of the paths in the model rather than linking each variable to every other variable, there is clear empirical evidence to support all paths between model variables (Brekke and Nakagami, 2010). For example, when inspecting the top panel of Figure 1, neurocognition has consistently demonstrated relationships with negative symptoms, ToM, social competence, and functioning. Thus, removal of any of these paths is inconsistent with prior research. Likewise, negative symptoms have been associated with ToM, social competence, and functioning (and so on with the other variables in the model). Because of this support in prior research, we first examined the saturated model that contains paths linking all variables in the model (top panel, Figure 1). It should be noted all models specified in this study are recursive. That is, all causal arrows specified in the model are unidirectional. The saturated model results in the χ2 statistic and corresponding degrees of freedom to be equal to zero. Post hoc model modifications were subsequently formed by testing nested models by eliminating paths suggested by model comparison statistics and theory, and examining model fit statistics, the statistical significance of each path in the model, and the amount of variance accounted for in each of the endogenous variables. This procedure can help ascertain whether a better fitting, more parsimonious model can be identified.
Figure 1.
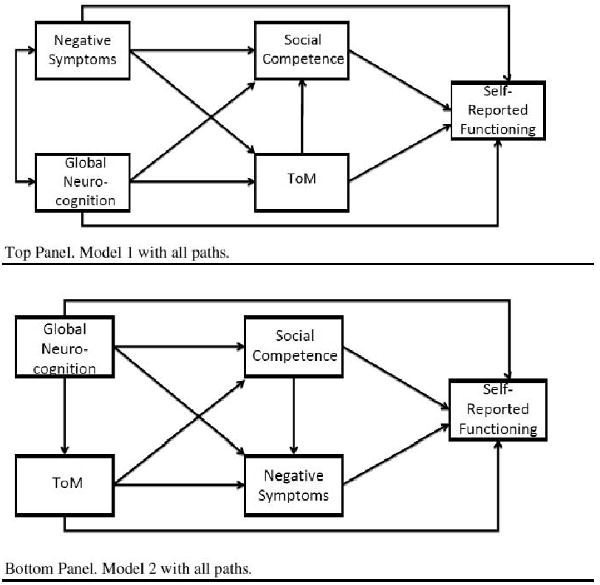
Saturated Models for Model 1 and Model 2.
Note. ToM=Theory of mind.
The chi-square (χ2) goodness-of-fit statistic, which is one of the most commonly-used statistical tests within this framework, indicates the degree of consistency between the pattern of fixed and free parameters and the pattern of variances and covariances in the observed data. It tests the null hypothesis that the matrix estimated from the model parameters equals the observed data matrix. It should not be significant if there is good model fit. The Comparative Fit Index (CFI) and the Root Mean Squared Error of Approximation (RMSEA) were examined, given that they tend to be the least biased indices in small samples (Hu and Bentler, 1998). Both indices use conventional cut-offs (.90 for CFI, and .08-.05 for RMSEA) to indicate good model fit. The model parameters, which provide information about the relationships among the latent variables, were also interpreted. The standardized structural coefficients are the same as standardized regression weights, which facilitates interpretation. In addition, squared multiple correlations were obtained for each endogenous variable to acquire an estimate of the amount of variance explained by the other variables. Finally, the standardized coefficients for indirect effects were examined to evaluate meditational effects. Significant effects suggest mediation is present, and full mediation is indicated by the direct path no longer being significant.
As reviewed in the Introduction, it is unclear whether negative symptoms are best conceptualized as a correlate of neurocognition which influences other variables in the model, or a more proximal cause of functioning due to the inherent difficulties with drive and motivation that characterize negative symptoms. To further evaluate these possibilities, an alternative model was tested with negative symptoms serving as a mediator between social competence and self-reported functioning (bottom panel, Figure 1). A similar procedure was undertaken as with model 1. The best-fitting models were compared using the Akaike Information Criterion (AIC) and Consistent AIC (CAIC). The AIC and CAIC can be used to compare non-hierarchical (non-nested models). A lower AIC and CAIC indicates better model fit.
3. Results
The means and standard deviations for all variables in the path models and participant demographic characteristics are presented in Table 1. Pearson's correlations revealed all variables were significantly correlated with one another (see Table 2).
Table 1.
Means and Standard Deviations for Demographics and Study Measures.
| Mean | Standard Deviation | Minimum | Maximum | |
|---|---|---|---|---|
| Age | 45.9 | 10.9 | 18.5 | 78.3 |
| Years of Education | 12.2 | 1.8 | 8 | 20 |
| ILSS Composite | .7087 | .098 | .391 | .917 |
| Global Neurocognition Composite | 34.47 | 7.03 | 20.56 | 51.55 |
| MASC total score | 30.0 | 8.91 | 9.0 | 44.0 |
| Hinting Task total score | 13.2 | 4.5 | 1.0 | 20.0 |
| PANSS Negative Symptom | 15.7 | 6.0 | 7.0 | 36.0 |
| PANSS Positive Symptom | 19.2 | 6.4 | 7.0 | 35.0 |
| PANSS General Symptom | 33.9 | 9.9 | 16.0 | 62.0 |
| Percent | Number | |||
| Sex (% male) | 63.5 | 113 | ||
| Race | ||||
| Caucasian | 56.7 | 101 | ||
| African-American | 15.2 | 27 | ||
| Hispanic/Latino | 14.0 | 25 | ||
| Asian | 3.9 | 7 | ||
| Bi/Multi-racial | 5.1 | 9 | ||
| Other | 5.1 | 9 | ||
Note. ILSS=Independent Living Skills Survey; MASC=Maryland Assessment of Social Competence; PANSS=Positive and Negative Syndrome Scale, N=178.
Table 2.
Zero-order correlations among path model variables.
| ToM | Neg Sx | Social Comp | Self-Report Functioning | |
|---|---|---|---|---|
| Neurocognition | .516** | -.267** | .367** | .185* |
| ToM | -- | -.251** | .416** | .173* |
| Neg Sx | -- | -- | -.316** | -.214** |
| Social Comp | -- | -- | -- | .227** |
Note. Neurocognition=Global Neurocognition Composite; ToM=Theory of Mind; Neg Sx=Negative Symptoms; Social Comp=Social Competence; Real World Fxn = Real World Functioning
p<.05
p<.01.
The full model contains all possible paths and is presented in the top panel of Figure 1. Model fit statistics cannot be interpreted in a saturated model, but it is clear upon examining the statistical significance of each path in the model that there are a number of nonsignificant paths. Paths were eliminated in an iterative way to ensure their removal did not have a negative effect on model fit statistics. The paths between neurocognition and self-reported functioning, negative symptoms and ToM, and ToM and self-reported functioning were successively removed from the model. Removing any of these paths did not result in significantly poorer model fit. The final model, excluding these paths, is shown in the top panel of Figure 2. All remaining paths are statistically significant in the final model. The model has excellent fit statistics (χ2 (N=3) = 2.374, p=.498; CFI=1.00; RMSEA < .01). Neurocognition accounted for 17.3% of the variance in ToM; and neurocognition, ToM, and negative symptoms explained 23.9% of the variance in social competence. All variables in the model accounted for only 7.3% of the variance in self-reported functioning. Removing any other paths in the model results in significantly poorer model fit and unacceptable fit statistics.
Figure 2. Final Path Models after Post Hoc Trimming.
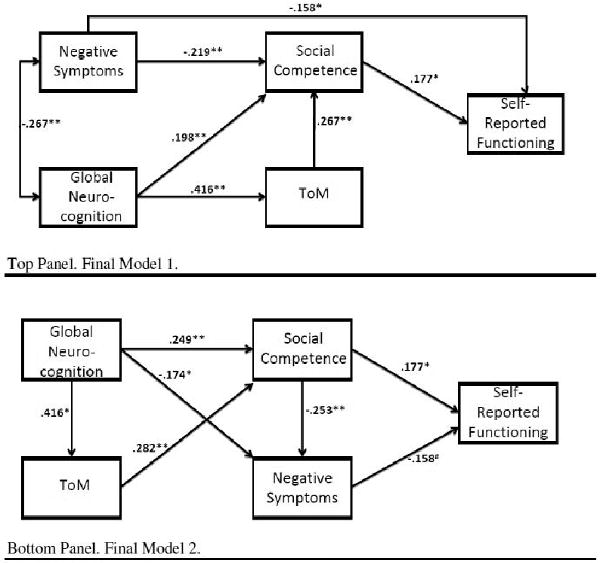
Note. ToM=Theory of mind; # p<.10; * p<.05; ** p<.01; N=178.
In addition, all of the indirect effects in the model were significant, including negative symptoms on self-reported functioning (standardized coefficient for indirect effect=-.039, p=.013), neurocognition on social competence (standardized coefficient for indirect effect=.111, p=.011), neurocognition on self-reported functioning (standardized coefficient for indirect effect=.055, p=.012), and ToM on self-reported functioning (standardized coefficient for indirect effect=.047, p=.022). Of note, this indicates that social competence partially mediates the relationship between negative symptoms and self-reported functioning, and that ToM partially mediates the relationship between neurocognition and social competence. Results also suggest that social competence fully mediates the relationship of neurocognition or ToM with self-reported functioning.
Following the procedures outlined above, the saturated model depicted in the bottom panel of Figure 1 was trimmed by successively removing the paths between ToM and negative symptoms, ToM and self-reported functioning, and neurocognition and self-reported functioning. Removal of any additional paths resulted in significantly poorer model fit, despite the marginally significant path between negative symptoms and self-reported functioning. The final model (bottom panel, Figure 2) had excellent fit statistics (χ2 (N=3) = 1.591, p=.662; CFI=1.00; RMSEA < .01). Neurocognition and social competence explained 12.6% of the variance in negative symptoms, and neurocognition and ToM explained 20.0% of the variance in social competence. Again, all of the indirect effects were significant, including negative symptoms on self-reported functioning (standardized coefficient for indirect effect=-.093, p<.01), neurocognition on social competence (standardized coefficient for indirect effect=.117, p<.01), neurocognition on self-reported functioning (standardized coefficient for indirect effect=.107, p<.01), and ToM on self-reported functioning (standardized coefficient for indirect effect=.061, p<.01). In this case, social cognition serves as a partial mediator between neurocognition and social competence, negative symptoms and social competence fully mediate the relationship between neurocognition or social cognition and self-reported functioning, and negative symptoms partially mediate the relationship between social competence and self-reported functioning. The AIC for model 1 tested above was 26.37 and the CAIC was 76.55, whereas the AIC for model 2 was 25.59 and the CAIC was 75.77.
4. Discussion
The current study aimed to further evaluate the role of social cognition as a mediator between neurocognition and functioning in schizophrenia. By using ToM (Hinting Task) as a measure of social cognition, this study extended the work of previous studies that used social or emotional perception to assess these relationships. As noted previously, ToM is a distinct social cognitive ability, and thus it was unclear if it would operate similarly to social and emotion perception. Moreover, negative symptoms and functional capacity (MASC), both of which have been found to have significant relationships with the constructs of interest, were also incorporated into the model. Path analyses suggested that ToM as indexed by the Hinting Task partially mediated the relationship between neurocognition and a more proximal domain of functioning, social competence, as indexed by the MASC. Negative symptoms and social competence were the only variables in the model that demonstrated significant direct paths to self-reported functioning.
In line with previous studies (Addington et al., 2006; Brekke et al., 2005; Meyer and Kurtz, 2009), social cognition appears to serve a meditational role between neurocognition and social competence. This study extended previous work by providing evidence that ToM (as indexed by the Hinting Task) also mediates the neurocognition-functioning relationship in addition to previously investigated domains of emotional or social perception. These findings support current broad-based social cognitive interventions that target multiple social cognitive domains (e.g., Roberts and Penn, 2009). Neurocognition, ToM, and negative symptoms were all significant predictors of social competence (MASC) in model 1, which is consistent with prior research (e.g., Bora et al., 2006; Couture et al., 2006; Green et al., 2000). Furthermore, the significant relationships often observed between neurocognition and negative symptoms (e.g., Harvey et al., 2006) and neurocognition and social cognition (e.g., Sergi et al., 2007) were replicated. In addition, previous work demonstrating that functional capacity (in this case, the MASC) serves a meditational role between neurocognition and functioning (e.g., Bowie et al., 2006) was further supported. Thus, this study provides additional verification that negative symptoms, neurocognition, and ToM are all relevant factors in predicting functioning in people with schizophrenia, or at the least, the capacity to function well in one's environment. Given that the individual must first possess the skills necessary to function before they will be able to pursue real-world functional goals, these findings suggest neurocognition, ToM, and negative symptoms are all relevant factors to target in treatments aiming to improve community functioning in individuals with schizophrenia.
Remarkably, only a small proportion of the variance in self-reported functioning (7.3%) was accounted for by all variables in the model. While this is not entirely discrepant from other similar studies using path analysis or structural equation modeling (e.g., variance accounted for ranges from 10-25%; Brekke et al., 2005; Sergi et al., 2006; Vauth et al., 2004), it is lower than expected and is substantially less than estimates of 25-50% of the variance explained by neurocognition alone in other studies (Green et al., 2000). Although statistically, ToM, as indexed by the Hinting Task, did mediate the relationship between neurocognition and functioning, it only accounted for a small amount of variance in self-reported functioning in the model. The most salient difference between the other similar studies and the current one is that they used informant and/or interview-based assessments of real-world functioning, while this study relied on self-report. The ILSS does assess social and leisure activities (e.g., talking to friends, going to the movies) and thus should overlap to some degree with the types of behaviors assessed in the MASC. However, the ILSS does not exclusively focus on interpersonal or social functioning, and thus its relationship with our measure of functional capacity, social competence, may have been somewhat attenuated. It is possible these methodological differences can explain current findings, and it is not unusual for studies to vary widely, with some reporting no relationship between one of the variables of interest and functional outcome (e.g., Bellack et al., 1994), to others supporting strong relationships (e.g., Dickinson and Coursey, 2002). A host of possible explanations for this could be entertained, such as differences in sample characteristics (e.g., illness severity, demographics), or differences in the measures used across studies.
A larger proportion (24%) of the variance in functional capacity was explained by negative symptoms, neurocognition, and ToM; neurocognition and ToM (as assessed with the Hinting Task) alone explained 20% of the variance in social competence (MASC). This is consistent with the notion that performance of real-world behaviors is multi-determined and a more distal outcome further downstream from neurocognition and social cognition then is functional capacity. Performance-based assessments might be more strongly linked to neurocognition and social cognition because they assess whether individuals are capable of performing certain behaviors not whether they do perform them in the real world (McKibbin et al., 2004; Patterson and Mausbach, 2010). Actual performance of behaviors in the real world is not always strongly related to performance-based capacity measures (Cohen et al., 2006; Dickerson et al., 2000); presumably because real world behavior is influenced by factors outside the individual's control, such as level of social support, financial means, personal resources (e.g., having an automobile), etc. (Brekke et al., 2005). Interestingly, the current study tested an alternative model (model 2) with negative symptoms identified as one of the factors contributing to self-reported functioning beyond the influence of functional capacity alone. The results from the path models were not conclusive. Although model 2 fit the data equally well and demonstrated a slight advantage when examining the AIC and CAIC statistics, there does not appear to be a substantive statistical difference between the two models and the direct path of negative symptoms to functioning only approached significance in this model. Theoretically, it seems intuitive that deficits in motivation, persistence and goal-directed behavior (as is captured by negative symptoms) would be more likely to serve as a mediator between functional capacity and real-world functioning. Clearly, more research is needed to determine the role of negative symptoms in the context of other known predictors of functioning behaviors.
Limitations of the current study include the use of a self-report measure of real-world functioning behaviors, and this may have contributed to the relatively lower amount of variance accounted for in functioning. This hypothesis is consistent with previous work, which has found varying degrees of relationships between functional capacity and real-world functioning depending on whether the functioning measure used was informant-based (Twamley et al., 2002) or reliant on self-report (McKibbin et al., 2004). In line with suggestions by other investigators, it may be best to use multiple measures of functioning behaviors to capture the complexity of functioning in schizophrenia (Bowie et al., 2006). This criticism can also apply to the domains of social cognition and social competence given that each was also assessed with only one measure. It should be noted that interpretation of study conclusions is limited by the fact that only one measure (i.e., the Hinting Task and the MASC, respectively) was used to index ToM and functional capacity domains. Findings may differ for other ToM tasks or other measures of functional capacity.
Within the domain of social cognition, it should be noted that the Hinting Task does require participants to use verbal and working memory skills to respond correctly to items, and thus may have increased the magnitude of the relationship between neurocognition and the Hinting Task. Although neurocognition was indexed as a composite of several abilities, recent research has suggested a single factor fits the data best (Dickinson et al., 2006; Keefe et al., 2006), and it was necessary to reduce the number of parameters estimated in the model (given that our models already required at least 150 participants). It should also be noted that while path analysis has statistical procedures to detect mediation and causal relationships, because the data are cross-sectional in nature, conclusions about explaining or causing outcomes across time are necessarily speculative. In addition, the participants in the current study were those who volunteered for a treatment trial. While few exclusionary criteria were applied, it can still be argued that these individuals were willing to engage in a year long intervention designed to improve functioning, and thus may not be representative of all individuals with schizophrenia.
The current results suggest that neurocognition and social cognition are viable targets for treatment for individuals who perform poorly on measures of functional capacity. It is also clear that negative symptoms and social competence are inter-related. However, results also suggest that improving functional capacity will not necessarily result in improved community functioning. Future research may benefit from identifying variables that impede individuals from functioning at their optimal capacity level. That is, those variables which affect the relationship between functional capacity and functioning behaviors. Enhancing understanding of other factors that hinder functional success is vital for treatments to translate into more positive outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shannon M. Couture, Email: scouture@psyc.umd.edu.
Eric L. Granholm, Email: egranholm@ucsd.edu.
Scott C. Fish, Email: scott.c.fish@gmail.com.
References
- Addington J, Saeedi H, Addington D. Influence of social perception and social knowledge on cognitive and social functioning in early psychosis. Br J Psychiatry. 2006;189(4):373–378. doi: 10.1192/bjp.bp.105.021022. [DOI] [PubMed] [Google Scholar]
- Addington J, Young J, Addington D. Social outcome in early psychosis. Psychol Med. 2003;33:1119–1124. doi: 10.1017/s0033291703007815. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rector NA, Stolar NM, Grant PM. Schizophrenia: Cognitive theory, research, and therapy. Guilford Press; New York: 2009. [Google Scholar]
- Bell M, Tsang HWH, Greig TC, Bryson GJ. Neurocognition, social cognition, perceived social discomfort, and vocational outcomes in schizophrenia. Schizophr Bull. 2009;35(4):738–747. doi: 10.1093/schbul/sbm169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellack A, Brown C, Thomas-Lohrman S. Psychometric characteristics of role-play assessments of social skill in schizophrenia. Behav Therapy. 2006;37:339–352. doi: 10.1016/j.beth.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Bellack A, Sayers M, Mueser KT, Bennett M. Evaluation of social problem solving in schizophrenia. J Abnorm Psychol. 1994;103:371–378. doi: 10.1037//0021-843x.103.2.371. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Schretlen D, Groninger L, Dobraski M. Revision of the Brief Visuospatial Memory Test: Studies of normal performance, reliability, and validity. Psychol Assessment. 1996;8:145–153. [Google Scholar]
- Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychologist. 1998;12:43–55. [Google Scholar]
- Bora E, Eryavuz A, Kayahan B, Sungu G, Veznedaroglu B. Social functioning, theory of mind and neurocognition in outpatients with schizophrenia; mental state decoding may be a better predictor of social functioning than mental state reasoning. Psychiatry Res. 2006;145:95–103. doi: 10.1016/j.psychres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, Harvey PD. Predicting schizophrenia patients' real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63:505–511. doi: 10.1016/j.biopsych.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD. Determinants of real-world functional performance in schizophrenia subjects: Correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163:419–425. doi: 10.1176/appi.ajp.163.3.418. [DOI] [PubMed] [Google Scholar]
- Brekke JS, Kay DD, Kee KS, Green MF. Biosocial pathways to functional outcome in schizophrenia. Schizophr Res. 2005;80:213–225. doi: 10.1016/j.schres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Brekke JS, Nakagami E. The relevance of neurocognition and social cognition for outcome and recovery in schizophrenia. In: Roder V, Medalia A, editors. Neurocognition and Social Cognition in Schizophrenia Patients: Basic Concepts and Treatment. Karger; Basel: 2010. pp. 23–36. [Google Scholar]
- Brune M. Emotion recognition, ‘theory of mind,’ and social behavior in schizophrenia. Psychiatry Res. 2005;33(2-3):135–147. doi: 10.1016/j.psychres.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Forbes CB, Mann MC, Blanchard JJ. Specific cognitive deficits and differential domains of social functioning impairment in schizophrenia. Schizophr Res. 2006;81:227–238. doi: 10.1016/j.schres.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Corcoran R, Mercer G, Frith CD. Schizophrenia, symptomatology and social inference: Investigating “theory of mind” in people with schizophrenia. Schizophr Res. 1995;17:5–13. doi: 10.1016/0920-9964(95)00024-g. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Green MF, Walker EF, Mittal VA. Schizophrenia: Etiology and neurocognition. In: Blaney PH, Millon T, editors. Oxford Textbook of Psychopathology. Oxford University Press; New York: 2009. pp. 298–332. [Google Scholar]
- Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: A review. Schizophr Bull. 2006;32(S1):S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Lorenz FO, Conger RD, Melby JN, Bryant CM. Observer, self-, and partner reports of hostile behaviors in romantic relationships. J Marriage Family. 2005;67:1169–1181. [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) The Psychological Corporation; San Antonio, Texas: 2001. [Google Scholar]
- Dickerson F, Parente F, Ringel N. The relationship among three measures of social functioning in outpatients with schizophrenia. J Clin Psychol. 2000;58(12):1509–1519. doi: 10.1002/1097-4679(200012)56:12<1509::AID-3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Bellack AS, Gold JM. Social/communication skills, cognition, and vocational functioning in schizophrenia. Schizophr Bull. 2007;33(5):1213–1220. doi: 10.1093/schbul/sbl067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Coursey RD. Independence and overlap among neurocognitive correlates of community functioning in schizophrenia. Schizophr Res. 2002;56:161–170. doi: 10.1016/s0920-9964(01)00229-8. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ragland JD, Calkins ME, Gold JM, Gur RC. A comparison of cognitive structure in schizophrenia patients and healthy controls using confirmatory factor analysis. Schizophr Res. 2006;85:20–29. doi: 10.1016/j.schres.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green MF, Neuchterlein KH, Kern RS, Baade LE, Fenton WS, Gold JM, et al. Functional co-primary measures for clinical trials in schizophrenia: Results from the MATRICS psychometric and standardization study. Am J Psychiatry. 2008;165:221–228. doi: 10.1176/appi.ajp.2007.07010089. [DOI] [PubMed] [Google Scholar]
- Greig TC, Bryson G, Bell MD. Theory of mind performance in schizophrenia: Diagnostic, symtpom, and neuropsychological correlates. J Nerv Ment Dis. 2004;192:12–18. doi: 10.1097/01.nmd.0000105995.67947.fc. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Guaiana G, Tyson P, Roberts K, Mortimer A. Negative symptoms and not cognition predict social functioning among patients with schizophrenia. Schweizer Archiv für Neurologie und Psychiatrie. 2007;158:25–31. [Google Scholar]
- Harvey PD, Koren D, Reichenberg A, Bowie CR. Negative symptoms and cognitive deficits: What is the nature of their relationship? Schizophr Bull. 2006;32(2):250–258. doi: 10.1093/schbul/sbj011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Velligan DI, Bellack AS. Performance-based measures of functional skills: Usefulness in clinical treatment studies. Schizophr Bull. 2007;33(5):1138–1148. doi: 10.1093/schbul/sbm040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Matthews CG. Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Psychological Assessment Resources; Odessa, FL: 1991. [Google Scholar]
- Henry JD, Phillips LH, Crawford JR, Ietswaart M, Summers F. Theory of mind following traumatic brain injury: The role of emotion recognition and executive dysfunction. Neuropsychologia. 2006;44:1623–1628. doi: 10.1016/j.neuropsychologia.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Hoff AL, Kremen WS. Is there a cognitive phenotype for schizophrenia: The nature and course of the disturbance in cognition? Curr Opin Psychiatry. 2002;15(1):43–48. [Google Scholar]
- Hu L, Bentler PM. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychol Methods. 1998;3(4):424–453. [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Bilder RM, Harvey PD, Davis SM, Palmer BW, Gold JM, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31:2033–2046. doi: 10.1038/sj.npp.1301072. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The Brief Assessment of Cognition in Schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Leifker FR, Bowie CR, Harvey PD. Determinants of everyday outcomes in schizophrenia: The influences of cognitive impairment, functional capacity, and symptoms. Schizophr Res. 2009;115:82–87. doi: 10.1016/j.schres.2009.09.004. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. Discriminant validity of the NEO-PIR facet scales. Ed Psychol Measurement. 1992;52:229–237. [Google Scholar]
- McDonald S, Flanagan S. Social perception deficits after traumatic brain injury: Interaction between emotion recognition, mentalizing ability, and social communication. Neuropsychology. 2004;18(3):572–579. doi: 10.1037/0894-4105.18.3.572. [DOI] [PubMed] [Google Scholar]
- McKibbin CL, Brekke JS, Sires D, Jeste DV, Patterson TL. Direct assessment of functional abilities: Relevance to persons with schizophrenia. Schizophr Res. 2004;72:53–67. doi: 10.1016/j.schres.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Meyer MB, Kurtz MM. Elementary neurocognitive function, facial affect recognition and social-skills in schizophrenia. Schizophr Res. 2009;110:73–179. doi: 10.1016/j.schres.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser KT, Pratt SI, Bartels SJ, Swain K, Forester B, Cather C, Feldman J. Randomized trial of social rehabilitation and integrated health care for older people with severe mental illness. J Consult Clin Psychol. 2010;78(4):561–573. doi: 10.1037/a0019629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienow TM, Docherty NM, Cohen AS, Dinzeo TJ. Attentional dysfunction, social perception, and social competence: What is the nature of the relationship? J Abnorm Psychol. 2006;115(3):408–417. doi: 10.1037/0021-843X.115.3.408. [DOI] [PubMed] [Google Scholar]
- Ostrom TM. The sovereignty of social cognition. In: Wyer RS, Srull TK, editors. Handbook of social cognition. Vol. 1. Lawrence Erlbaum Associates; Hillsdale, NJ: 1984. pp. 1–37. [Google Scholar]
- Patterson TL, Mausbach BT. Measurement of functional capacity: A new approach to understanding functional difficulties and real-world behavioral adaptation in those with mental illness. Ann Rev Clin Psychol. 2010;6:139–154. doi: 10.1146/annurev.clinpsy.121208.131339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn DL, Hope DA, Spaulding W, Kucera J. Social anxiety in schizophrenia. Schizophr Res. 1994;11:277–284. doi: 10.1016/0920-9964(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Penn DL. Neurocognitive and social cognitive predictors of interpersonal skill in schizophrenia. Psychiatry Res. 2006;143:167–178. doi: 10.1016/j.psychres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Manual for administration of neuropsychological test batteries for adults and children. Reitan Neuropsychological Laboratory; Tucson, AZ: 1979. [Google Scholar]
- Roberts D, Penn DL. Social cognition and interaction training (SCIT) for outpatients with schizophrenia: A preliminary study. Psychiatry Res. 2009;166:141–147. doi: 10.1016/j.psychres.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Sayers MD, Bellack AS, Wade JH, Bennett ME. An empirical method for assessing social problem solving in schizophrenia. Behav Mod. 1995;19:267–289. doi: 10.1177/01454455950193001. [DOI] [PubMed] [Google Scholar]
- Sergi MJ, Rassovsky Y, Nuechterlein KH, Green MF. Social perception as a mediator of the influence of early visual processing on functional status in schizophrenia. Am J Psychiatry. 2006;163:448–454. doi: 10.1176/appi.ajp.163.3.448. [DOI] [PubMed] [Google Scholar]
- Sergi MJ, Rassovsky Y, Widmark C, Reist C, Erhart S, Braff DL, Marder SR, Green MF. Social cognition in schizophrenia: Relationships with neurocognition and negative symptoms. Schizophr Res. 2007;90:316–324. doi: 10.1016/j.schres.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Doshi RR, Nayak GV, Palmer BW, Golshan S, Heaton RK, et al. Generalized cognitive impairments, ability to perform everyday tasks, and level of independence in community living situations of older patients with psychosis. Am J Psychiatry. 2002;159:2013–2020. doi: 10.1176/appi.ajp.159.12.2013. [DOI] [PubMed] [Google Scholar]
- Vauth R, Rusch N, Wirtz M, Corrigan PW. Does social cognition influence the relation between neurocognitive deficits and vocational functioning in schizophrenia? Psychiatry Res. 2004;128:155–165. doi: 10.1016/j.psychres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Walker EF. Developmentally moderated expressions of the neuropathology underlying schizophrenia. Schizophr Bull. 1994;20:453–480. doi: 10.1093/schbul/20.3.453. [DOI] [PubMed] [Google Scholar]
- Wallace CJ, Liberman RP, Tauber R, Wallace J. The independent Living Skills Survey: a comprehensive measure of the community functioning of severely and persistently mentally ill individuals. Schizophr Bull. 2000;26(3):631–666. doi: 10.1093/oxfordjournals.schbul.a033483. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale–third edition. Psychological Corporation; San Antonio, TX: 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale–third edition. Psychological Corporation; San Antonio, TX: 1997b. [Google Scholar]
- Wiersma D, Wanderling J, Dragomirecka E, Ganev K, Harrison G, An Der Heiden W, et al. Social disability in schizophrenia: its development and prediction over 15 years in incidence cohorts in six European centres. Psychol Med. 2000;30(5):1155–1167. doi: 10.1017/s0033291799002627. [DOI] [PubMed] [Google Scholar]


