Abstract
Exaggerated cardiovascular reactivity to stress confers risk for cardiovascular disease. Further, individual differences in stressor-evoked cardiovascular reactivity covary with the functionality of cortical and limbic brain areas, particularly within the cingulate cortex. What remains unclear, however, is how individual differences in personality traits interact with cingulate functionality in the prediction of stressor-evoked cardiovascular reactivity. Accordingly, we tested the associations between (i) a particular personality trait, Agreeableness, which is associated with emotional reactions to conflict, (ii) resting state functional connectivity within the cingulate cortex, and (iii) stressor-evoked blood pressure (BP) reactivity. Participants (N=39, 19 men, aged 20–37 yrs) completed a resting functional connectivity MRI protocol, followed by two standardized stressor tasks that engaged conflict processing and evoked BP reactivity. Agreeableness covaried positively with BP reactivity across individuals. Moreover, connectivity analyses demonstrated that a more positive functional connectivity between the posterior cingulate (BA31) and the perigenual anterior cingulate (BA32) covaried positively with Agreeableness and with BP reactivity. Finally, statistical mediation analyses demonstrated that BA31–BA32 connectivity mediated the covariation between Agreeableness and BP reactivity. Functional connectivity within the cingulate appears to link Agreeableness and a risk factor for cardiovascular disease, stressor-evoked BP reactivity.
Keywords: agreeableness, cingulate, personality, resting state connectivity, stressor-evoked cardiovascular reactivity
1. Introduction
1.1 Cardiovascular Disease and Stress Reactivity
Cardiovascular Disease (CVD) is the leading cause of death in industrialized nations, accounting for more than 630,000 deaths per year in the United States alone (Heron et al., 2008). CVD encompasses several clinical syndromes that develop slowly over the lifespan, including hypertension, atherosclerotic coronary heart disease, stroke, and heart failure (Thom et al., 2006). Several risk factors related to psychological stress are associated with a heightened risk for developing and dying from several syndromes related to CVD (Figueredo, 2009). One long-studied biobehavioral risk factor for CVD is an individual’s tendency to show large-magnitude or ‘exaggerated’ blood pressure (BP) reactions to stressful events (Lovallo and Gerin, 2003). If stress-related risk factors, such as exaggerated stressor-evoked BP reactions, are repeatedly expressed by some individuals over a long period of life, then they are likely to accelerate pathogenic processes that ultimately lead to CVD endpoints (e.g., infarction, stroke, etc.; (McEwen, 2006). In the laboratory, exaggerated stressor-evoked BP reactions can be induced by various behavioral tasks, and an individual’s cardiovascular reactivity to stress in the lab is predictive of the development of CVD markers, such as hypertension and a premature thickening of the intima-medial layer of the carotid arteries (Chida and Steptoe, 2010). There are two measures of BP that are typically used to in both clinical and laboratory research: diastolic BP (DBP), corresponding to the pressure before the cardiac cycle begins, and systolic BP (SBP), which corresponds to the peak pressure during the cardiac cycle as the ventricles contract. Within psychophysiological research, SBP reactivity tends to be more frequently associated with psychosocial measures and cardiovascular disease risk, although a limited number of psychosocial factors do sometimes associate with DBP reactivity (for review, see (Chida and Hamer, 2008).
Inter-individual variation in personality traits afford an opportunity to examine how stable individual differences can influence reactivity to psychological stressors, which may provide additional insight into stress-related risk for CVD. The five-factor model of personality is a widely adopted theoretical account of specific traits that characterizes reliable biobehavioral response tendencies and dispositions of individuals (McCrae and Costa, 1987; McCrae and John, 1992). In this model, the five factors are Neuroticism, Extraversion, Openness, Agreeableness, and Conscientiousness, each of which is measured via self-report questionnaires. The five-factor model has been widely supported (for review and background, see (Digman, 1990), and each of the five personality factors has been shown to associate with particular behaviors, including health outcomes (John et al., 2008). Relevant to the present study, these different personality traits are differentially related to individual differences in reactivity to emotionally-aversive, behaviorally-salient, or otherwise stressful stimuli (Robinson, 2007). More precisely, evidence suggests a link between Agreeableness and the processing of conflict engendered by discrepant person-environment fit (Suls et al., 1998; Tobin et al., 2000). Moreover, Agreeableness predicts variation in executive functioning, especially as elicited by tasks that engage the anterior cingulate cortex (ACC; Botvinick et al., 2001; Carter et al., 1998; Jensen-Campbell et al., 2002). Hence, individuals who score higher on Agreeableness scales perform faster on the Stroop Task, as well as make fewer errors and take fewer trials to complete the first category of the Wisconsin Card Sorting Task (Jensen-Campbell et al., 2002). These results suggest that Agreeableness may be associated with ACC functioning.
As discussed below, ACC functionality has been associated with individual differences in cardiovascular stress reactions. Therefore, if there is a relationship between individual differences in Agreeableness and cardiovascular reactivity to stressors – especially stressors that entail cognitive conflict – then this relationship may be partially mediated by the ACC.
1.2. The Central Autonomic Network
The brain is the central organ that controls peripheral physiological responses to stressful events that are thought to play a proximal role in mediating CVD risk (McEwen and Gianaros, 2009; McEwen, 2009). In the animal literature, the circuits that comprise a so-called ‘central autonomic network’, which control cardiovascular reactions to stress, have been well-described (for review, see Benarroch, 1997; Cechetto and Shoemaker, 2009). Only recently, however, have functional imaging studies begun to investigate the function of this network in humans. Across numerous studies, a consistent pattern of findings has emerged (for review, see Gianaros and Sheu, 2009), demonstrating positive associations between BP reactivity and task-related activity in the perigenual anterior cingulate cortex (pACC), dorsal anterior cingulate cortex (dACC), and insular cortices, as well as lateral prefrontal cortex, posterior cingulate cortex (pCC), cerebellar regions and the amygdala (Gianaros et al., 2009, 2005, 2007, 2005, 2008, 2009). These findings and others have led to the development of a neurobiological model that posits that central autonomic control areas integrate cognitive and emotional processes to calibrate autonomic responses in order to adaptively meet situational demands (Chida & Steptoe, 2010; Gianaros & Sheu, 2009). Moreover, the pACC has been viewed to specifically support appraisals of situations and, via reciprocal connections, signal with the insular cortices, amygdala, thalamus, and periaqueductal grey, and brainstem cell groups to generate, represent, and regulate stressor-evoked autonomic and cardiovascular reactions through feedforward and feedback processes (Gianaros et al., 2009). As such, the pACC provides an important interface between self-referential processing (such as appraisals) and regulating peripheral stress physiology. Similarly, the pCC is widely considered to be involved in monitoring the environment for threat, as well as appraising behaviorally-relevant situations and stimuli (Gusnard and Raichle, 2001; Maddock, 1999; Vogt and Laureys, 2005; Vogt et al., 2006). Unlike the pACC, however, the pCC does not have strong connections with brain systems that proximally govern autonomic nerve traffic to peripheral target organs (An et al., 1998); however, the pCC is densely networked with areas of rostral anterior cingulate (e.g., pACC; Vogt et al., 2006), which issues dense projections to downstream autonomic-cardiovascular control areas (Critchley et al., 2003; Critchley, 2005). Thus, it has been speculated that such projections may provide for a pathway linking pCC functionality to cardiovascular reactivity via ACC relays (Gianaros et al., 2009). In extension, examining resting levels of activity in brain regions associated with cardiovascular reactivity has proven to be helpful in predicting individual cardiovascular reactions to stress. For example, resting metabolic activity in the pACC, dACC, medial prefrontal and insular cortices has been shown to predict individual cardiovascular reactivity to stress (Gianaros et al., 2009).
1.3. The Default Mode Network
In recent years, the idea of measuring resting state brain activity, particularly resting functional connectivity, in neural circuitries has led to the conceptualization of resting state networks, such as the ‘default mode network’ (Buckner, Andrews-Hanna, & Schacter, 2008; Mazoyer et al., 2001; Raichle & Snyder, 2007). This particular network comprises several regions that are functionally connected, and that tend to show higher levels of metabolic activity in the “resting” state in functional imaging studies, i.e., the “default” mode. Across several studies, a distinct network of regions – including the pACC, pCC, and inferior parietal lobule – are consistently implicated in default mode functions. DMN activity is thought to support introspective processes, and correlates with stimulus independent thought (McKiernan et al., 2003, 2006) and daydreaming (Mason et al., 2007). Additionally, it has been proposed that the DMN may specialize in functions that are self-referential (Gusnard et al., 2001; Uddin et al., 2007). Key to the present study, several regions encompassed by the DMN, such as the pCC and pACC, are not only associated with cardiovascular reactivity (Gianaros et al., 2009), but are also implicated more broadly in the autonomic regulation of cardiovascular function (Goswami et al., in press; Wong, Massé, et al., 2007).
To date, however, no studies to our knowledge have examined potential relationships between the DMN resting state connectivity and stressor-evoked cardiovascular reactivity. However, based on the centrality of the cingulate cortex to both the DMN and the central autonomic network, DMN connectivity may be particularly useful in understanding the neurobiological factors that predict individual differences in cardiovascular reactivity. In particular, the pCC was one of the first components of the DMN identified to be more active in the resting state, and it is now considered to be a hub of default activity (Andreasen et al., 1995; Buckner et al., 2008). Moreover, several studies have demonstrated an association between pCC activity and cardiovascular reactions to psychological stress and autonomic adjustments to physical exertion (Gianaros et al., 2007, 2005, 2008; Wong, Massé, et al., 2007).
In addition to the open questions above, we are also unaware of any studies that have explicitly examined how personality factors may relate to individual differences in DMN connectivity, although a growing literature has begun to examine relationships between DMN function and self-referential processing and dispositional mindful awareness (Way et al., 2010). Given the associations between Agreeableness and performance on executive functioning tasks that entail conflict and engage the ACC, as well as between cingulate cortex functionality and cardiovascular reactivity, it is plausible that (1) Agreeableness would be associated with cardiovascular reactivity to cognitive stressors that also entail conflict processing and (2) functional connectivity between the pCC and pACC could mediate this association. Specifically, we hypothesized that individuals who score higher on a standard Agreeableness scale will exhibit greater cardiovascular reactivity to a stress task involving conflict. Second, we hypothesized that the resting functional connectivity within the DMN would mediate this relationship, such that individuals who score higher on Agreeableness would exhibit a stronger functional connectivity between the pCC and pACC, and this enhanced connectivity would predict SBP responses to a subsequent stressor.
2. Materials & methods
2.1. Participants
All experimental and recruitment procedures were approved by the University of Pittsburgh Institutional Review Board. Participants were 20 men (mean age SD: 24.8±5.1 years) and 20 women (mean age SD: 23.8±3.8 years) recruited from the Pittsburgh, PA metropolitan area. One male participant was excluded from data analysis due to excessive head motion in the scanner. Therefore, all results presented below are for the remaining 39 participants. All participants reported being right handed, and none had any of the following: (1) a history of cardiovascular disease (hypertension, stroke, myocardial infarction, congestive heart failure, atrial and ventricular arrhythmias); (2) previous cardiovascular surgery (including coronary bypass, carotid artery, or peripheral vascular surgery); (3) a history of cancer, chronic kidney or liver conditions, type I or II diabetes mellitus, or any pulmonary or respiratory disease; (4) any current or past self-reported psychiatric diagnosis of a substance abuse or mood disorder; (5) previous cerebrovascular trauma involving loss of consciousness; (6) pregnancy (verified by urine test in females); (7) previous neurosurgery or history of neurological conditions; (8) color blindness; (9) claustrophobia; (10) metallic implants; (11) a self-reported history of using any psychotropic, lipid-lowering, or cardiovascular medications.
2.2. Protocol
2.2.1. Overview
Participants were instructed to abstain from eating, exercising, and consuming caffeine and tobacco products for three hours prior to the testing session. Additionally, they were asked not to consume alcoholic beverages for 12 hours before testing. Upon arrival, participants completed informed consent and a screening interview followed by protocols to assess anthropometric measures, demographic information, and seated BP. For the MRI protocol, participants were fitted with a BP cuff matched to arm size, inserted into the scanner and asked to rest for approximately 20 minutes. Following this rest period, participants completed two stressor tasks described below. They were then removed from the scanner.
2.2.2. Stressor Tasks
Two tasks were used to evoke individual differences in BP reactivity– a titrated Stroop task and a modified multi-source interference task (Bush and Shin, 2006). Both tasks entail processing stimuli that evoke cognitive conflict as a source of psychological stress, as well as a feeling of lack of control, and have two alternating conditions that were repeated four times in an interleaved manner – a less-demanding condition in which stimuli are congruent, and a more-demanding condition in which stimuli are incongruent. During the incongruent condition of each task, performance is titrated, such that task accuracy remains at approximately 50% within and between individuals. Each task lasted 9 minutes and 20 seconds and the alternating conditions (congruent/incongruent) lasted 52 to 60 seconds. Each condition was preceded by a 10- to 17-second rest condition during which participants were shown a crosshair. Task order was counterbalanced across subjects, and the tasks were separated by a 10- to 12-minute recovery period, during which time subjects completed self-report items based on the first task (see below).
Task performance was averaged across the two stressor tasks. Task accuracy was computed by determining the percentage of trials that were correctly completed. Performance during the incongruent condition (55.4% correct, ± 6.8% SD) was lower compared to the congruent condition (89.8% correct, ± 3.9% SD), t (38) = 34.8, p < .001. Response times during the incongruent condition were significantly slower than during the congruent condition (465.8 ms, ±152.7ms), t (38) = 19.1, p < .001.
Following the rest period, and at the conclusion of the Stroop and MSIT tasks, participants reported subjective ratings of valence (1: very unhappy; 9: very happy), arousal (1: very calm; 9: very aroused), and perceived control (1: very little control; 9: very much in control) using a self-assessment manikin scale (Bradley and Lang, 1994).
2.2.3. Blood Pressure Acquisition
Participant BP was recorded in the MRI scanner from the nondominant (left) arm that was not used for task responding. Recordings were made during baseline (pre-stressor) and stressor periods using an oscillometric device (Multigas 9500 MedRac, Inc.) set to inflate every 2.5 minutes during the rest period and once during each condition of the stressor tasks described below. Resting BP was computed by averaging the final 3 measurements. Task-related BP was computed from the average of the demanding incongruent condition of the Stroop task and multi-source interference task. The incongruent condition minus resting BP difference score was used to compute BP reactivity following previous work (Gianaros et al., 2007, 2008). In the present sample, there was no difference in systolic BP (SBP) or diastolic BP (DBP) reactivity between men and women (t values: <= 1.6, p >= .11). Across individuals, SBP and DBP reactivity across conditions of the Stroop task and MSIT were correlated (SBP r = 0.70, p < 0.001; DBP r = 0.73, p < 0.001), indicating that the tasks were successful in evoking reliable individual differences in BP reactivity. Task-averaged SBP and DBP reactivity scores were used for subsequent analyses.
2.2.4. Personality Variables
In an initial visit to the lab, participants completed a packet of questionnaires, including the NEO-PI-R (Costa and McCrae, 1992). T-scores were calculated separately for the Five Factor scores (including Agreeableness) using means and standard deviations from Appendix B (form S for adults) in the NEO PI-R manual.
2.3 Neuroimaging Data
2.3.1. Acquisition Parameters
Neuroimaging data were acquired on a 3T Trio TIM whole-body scanner (Siemens), equipped with a 12-channel, phased-array head coil. Resting BOLD images were acquired over a 5-minute 6-second period with a gradient-echo echo-planar imaging sequence using the following parameters: field of view (FOV) = 205×205 mm; matrix size 64×64 mm; TR = 2 seconds; TE = 28 ms; flip angle = 90°. Thirty-nine sections (3mm thick, no gap) were obtained sequentially in an inferior-to-superior direction, yielding 150 BOLD images (the first 3 images were discarded to allow for magnetic equilibration). Anatomical images were collected over 7 minutes, 17 seconds using a T1-weighted 3D magnetization-prepared rapid gradient echo with the following parameters: FOV = 256×208 mm; matrix size = 256×208 mm; TR = 2100 ms; inversion time = 1100 ms; TE = 3.29 ms; flip angle = 8° (192 sections, 1mm thick, no gap).
2.3.2. Preprocessing and Analysis of Neuroimaging Data
Resting BOLD images were preprocessed using Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging). BOLD images were realigned to the first image of the series, co-registered to the magnetization-prepared rapid gradient echo image, normalized to the Montreal Neurological Institute 152 template, and smoothed with a 6-mm full-width at half-maximum isotropic Gaussian kernel. After preprocessing, a BOLD signal time series was extracted from a seed region in the pCC (BA31, x,y,z = −6, −40, 36; see Figure 1 inset, and Supplementary Figure S2 for a description of the seed region selection). A time-series was extracted from the mean BOLD signal of all the voxels in a 5-mm sphere surrounding the coordinates above. Each time-series extracted for each participant was mean centered, drift corrected, and inspected for outliers. Any values >3 SD of the series mean were replaced by averaging 2 surrounding values. The corrected time-series were then entered as regressors in individual GLM SPM8 design matrices. In addition to the time-series regressor, we extracted a cerebrospinal fluid (CSF) time-series from the fourth ventricle (x, y, z = 0, −43, −26) and included this series as a nuisance regressor in individual GLM matrices. For all individual-level connectivity analyses, low-frequency BOLD signal drift was high-pass filtered (128 sec cutoff), and high-frequency BOLD signal autocorrelations were corrected with a first-order autoregressive model. This routine produces a functional connectivity map for each participant identifying areas where BOLD signal changes cross-correlated with signal changes in the pCC seed. Given the centrality of the DMN model, particularly the pCC region as the hub of DMN activity, a model-driven approach such as seed-based connectivity is the preferred method for showing the regions with the strongest functional connectivity with the seed ROI (Cole et al., 2010).
Figure 1.
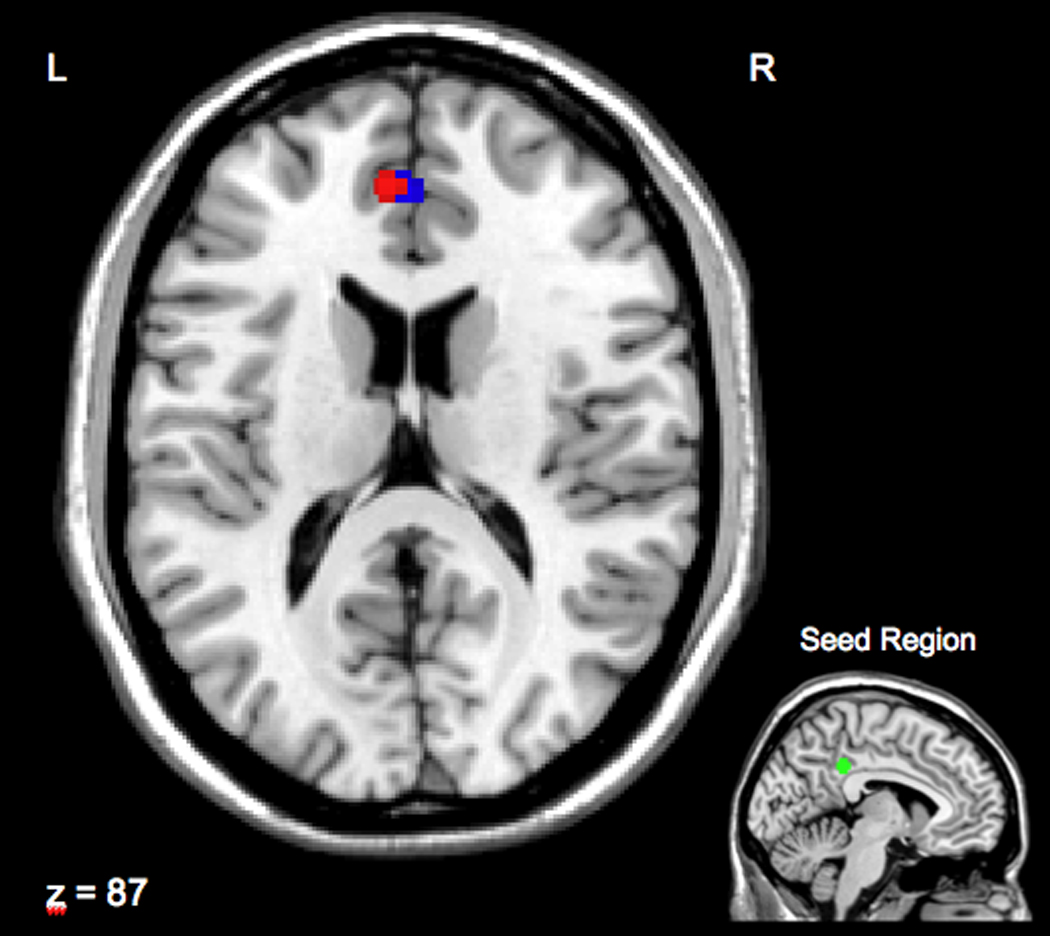
Displayed in red is the region of the perigenual cingulate cortex that was more strongly correlated with posterior cingulate cortex in individuals scoring higher in Agreeableness (Brodmann area 32; Talairach coordinates for peak activation voxel: X = −6, Y = 44, Z = 16. The cluster in blue is the region correlated with posterior cingulate cortex that was positively related to systolic blood pressure reactivity (Brodmann area 32; Talairach coordinates for peak activation voxel: X = −2, Y = 44, Z = 16). The seed region on which connectivity analyses are based is displayed in the lower right inset (X = −6, Y = −40, Z = 36). For discussion of seed selection, see supplementary figure S2.
Second-level analyses were performed using SPM8. First-level functional connectivity maps were submitted to a regression model and Agreeableness scores were entered as a regression vector corresponding to a covariate of interest. The regression was then repeated with SBP reactivity as the covariate of interest. For whole-brain analyses, a significance threshold of p < .005 (uncorrected) and k = 20 was utilized for exploratory purposes. In addition to whole-brain analyses, a region-of-interest analysis was performed on the pACC. The region of interest was created by two 10-mm spheres (one on the right, one on the left) centered on ±5 41 21 (x, y, z) based on previous research (Margulies et al., 2007). Because we did not have explicit hypotheses for a laterality effect, the spheres were created bilaterally. To correct for multiple voxel-wise comparisons, and to determine an appropriate threshold, Monte Carlo simulations were performed using Alphasim (Madison, WI). For the region-of-interest, a threshold of p < .005 (uncorrected) and k = 21 was utilized to maintain a corrected false-positive detection threshold of p < .05 for the search volume. It should be emphasized that these connectivity maps are not indicative of inhibitory, excitatory, or causal (effective) associations between the pCC and the regions that were associated with the pCC seed.
2.3.3. Region-of-Interest Mediation Analysis
To test for mediation, eigenvalues were extracted for the cluster in the pACC associated with Agreeableness scores, and for the similar region in the pACC associated with SBP reactivity (each time with a p = .005, 21 contiguous voxel threshold). Consistent with previous research using the same procedure, cluster activity was then regressed on the opposite variable – i.e., the Agreeableness cluster regressed on SBP reactivity scores; SBP cluster regressed on Agreeableness scores (Eisenberger et al., 2007). As is discussed by Eisenberger et al. (2007), the probability of a cluster being associated with both variables is p < .00025 (uncorrected) and is therefore more conservative than p < .001 (uncorrected), typical of fMRI studies. Mediation was then tested using a standard distribution of products method (MacKinnon and Fairchild, 2009).
3. Results
3.1. Task Ratings and BP Reactivity
Relative to the resting condition, participants reported feeling less happy, more aroused, and less in control when they performed the two stressor tasks (t values all ≥3.7, p ≤ 0.001). Additionally, SBP and DBP both were higher during task performance relative to the rest period (t values all ≥3.0, p ≤ 0.006). Agreeableness was neither related to changes in subjective reports of arousal (r (36) = .08, p > .05), nor changes in valence (r (36) = −0.27, p > .05) after controlling for resting values. Similarly, Agreeableness and changes in feelings of control were unrelated (r (36) = −0.16, p > .05). There were no significant correlations between subjective ratings of valence, control, nor arousal or any of the other personality variables (neuroticism, openness, conscientiousness, extraversion; null findings available upon request).
3.2. Agreeableness and Cardiovascular Reactivity
Relationships between Agreeableness and reactivity were computed separately for SBP and DBP via hierarchical regression by regressing the reactivity score on resting blood pressure (SBP or DBP) in the first step, and Agreeableness in the second step. Results are displayed in Table 1. Agreeableness predicted SBP reactivity to the stressor tasks, Fchange (1, 37) = 5.13, R2change = 0.11, p < .05, but did not predict DBP reactivity, Fchange (1, 37) = 1.85, p = .18, R2change = .05.
Table 1.
Agreeableness predicts systolic blood pressure (SBP) reactivity, but not diastolic blood pressure (DBP) reactivity to conflict tasks after controlling for sex, age and resting SBP (or DBP, respectively).
| Step | Variable | β | df | Fchange | R2change | |
|---|---|---|---|---|---|---|
| SBP | 1 | Sex, Age, Resting SBP | .23, −.08, .28 | 3,35 | 1.92 | .14 |
| 2 | Agreeableness | .33 | 1,34 | 4.17 | .09* | |
| DBP | 1 | Sex, Age, Resting DBP | .20, −.13, −.04 | 3,35 | 0.69 | .06 |
| 2 | Agreeableness | .20 | 1,34 | 1.39 | .04 | |
p < .05
There was no difference in Agreeableness scores between male and female participants, t (37) = .38, p = .71. To examine the potential moderating effect of sex on the relationship between Agreeableness and cardiovascular reactivity, an interaction term was created by taking the product of each participant’s centered Agreeableness score and the contrast coded sex variable. The interaction term was entered in a second step after controlling for resting blood pressure, Agreeableness, and sex. The interaction term was non-significant, indicating that sex did not moderate the relationship between Agreeableness and SBP (Fchange (1, 34) = 0.17, p = .90), nor the relationship between Agreeableness and DBP (Fchange (1, 34) = 0.35, p = .56).
3.3. Agreeableness and Resting Functional Connectivity
To investigate the relationship between Agreeableness and functional connectivity, Agreeableness scores were entered as a predictor in a multiple regression model in a whole-brain analysis. Results are displayed in Table 2. Several regions were found to covary with pCC activity and Agreeableness, such that higher Agreeableness scores were related to stronger covariation between the region and pCC activity. These regions included bilateral cuneus/precuneus, left middle occipital, middle temporal, and supramarginal gyri, as well as the left pACC (BA32). Additionally, there were significantly stronger associations between pCC activity and the right lingual gyri and anterior cingulate (extending into BA10 of the medial prefrontal cortex) in people who scored higher in Agreeableness. There were no regions that showed inverse relationships between connectivity and Agreeableness (i.e. lower Agreeableness scores predicting stronger connectivity between the region and the pCC).
Table 2.
Brain Regions That Are Jointly Associated With Greater Positive Functional Connectivity With the Posterior Cingulate and Agreeableness. Next to each left (L) and right (R) region and approximate Brodmann area (BA) are the MNI coordinates (for the peak voxel in each cluster), where x = right (+) to left (−); y = anterior (+) to posterior (−); z = superior (+) to inferior (−). The clusters in the tables are driven from a whole brain exploration study. T-values for voxels of peak activation and their corresponding cluster extents were derived from a mixed-effects analysis employing an uncorrected height threshold of p < .005 and extent cluster threshold of k = 20 voxels.
| Side | Region | Brodmann | MNI Coordinates | Number of voxels | t value | ||
|---|---|---|---|---|---|---|---|
| Area | X | Y | Z | in region | |||
| L | Precuneus | 7 | −12 | −76 | 44 | 231 | 4.09 |
| L | Cuneus | 18 | −10 | −76 | 12 | 114 | 3.94 |
| R | Cuneus | 18 | 4 | −94 | 10 | 185 | 3.93 |
| R | Lingual Gyrus | 19 | 32 | −68 | −6 | 37 | 3.83 |
| L | Corpus Callosum | −18 | 28 | 18 | 25 | 3.68 | |
| L | Middle Occipital Gyrus | 37 | −46 | −70 | −12 | 41 | 3.61 |
| L | Cerebellum | −4 | −52 | −26 | 29 | 3.55 | |
| R | Anterior Cingulate Cortex | 10 | 16 | 36 | −4 | 30 | 3.34 |
| L | Middle Temporal Gyrus | 39 | −46 | −74 | 20 | 82 | 3.34 |
| L | Supramarginal Gyrus | 40 | −52 | −54 | 36 | 23 | 3.32 |
| L | Anterior Cingulate Cortex | 32 | −6 | 44 | 16 | 21 | 3.19 |
| R | Precuneus | 7 | 14 | −68 | 7 | 28 | 3.04 |
Consistent with our hypotheses, the connectivity between a cluster in the pACC (BA32; t (37)=3.33, P<0.005, k = 24, x,y,z = −6, 44, 16) and pCC was proportional to self-reported levels of Agreeableness. As discussed above, activity in this region is consistently found to be associated with cardiovascular reactivity (Gianaros et al., 2008, 2009), as well as tasks that involve cognitive conflict (Botvinick et al., 2001; Carter et al., 1998).
3.4. Functional Connectivity and SBP Reactivity
The analysis was then repeated, this time using SBP reactivity as the predictor in the model1. (Results are displayed in Supplementary Table 1). Numerous regions showed a positive correlation between SBP reactivity and connectivity with the pCC, including regions that have been previously shown to have relationships with cardiovascular reactivity such as the left insula (BA 13), and right ACC (BA 32). Moreover, activity in the left pACC overlapped with the same pACC region identified in the analyses involving Agreeableness discussed above (BA32; t (37)=3.56, p < 0.005, k = 34, x,y,z = −2,44,16).
3.5. Functional Overlap and Mediation
To investigate if the neural activity in the region of pACC mediated the relationship between Agreeableness and SBP reactivity, eigenvalues were extracted for the cluster in the pACC associated with Agreeableness (Figure 1). These values were then regressed on SBP reactivity, which was a significant predictor of activity in the cluster (b = .02, t = 2.82, p < .01). Values were then extracted from the SBP cluster that overlapped with the Agreeableness cluster, and regressed on Agreeableness scores. Agreeableness scores were a significant predictor of SBP cluster activity (b = .01, t = 2.56, p < .05). Using the distribution of products method, it was found that activity—reflecting connectivity strength with the pCC—in this region significantly mediated the relationship between Agreeableness and SBP reactivity (ZαZβ = 7.22, p < .05).2
4. Discussion
The first primary finding of this study was that Agreeableness predicted cardiovascular reactivity, such that individuals who scored higher on Agreeableness were more reactive to subsequent mental stressor tasks. Second, the relationship between Agreeableness and reactivity could be explained utilizing resting functional connectivity between regions commonly involved in the DMN. Specifically, several regions previously implicated in the neurobiology of cardiovascular reactivity – including the insula and ACC – demonstrated greater positive functional connectivity with the pCC in individuals who showed greater cardiovascular reactions to stress. This is the first study to our knowledge to examine how resting functional connectivity specifically in the DMN is associated with cardiovascular reactivity to stress. Third, Agreeableness was related to stronger functional connectivity between the pCC and several regions, including the inferior frontal gyrus, middle frontal gyrus, several occipital and parietal regions, and, most relevant to this study, the pACC. Although several studies have investigated the relationship between default mode connectivity and self-referential processing, this is the first study of which we are aware that has found that a major dimension of personality correlates with default connectivity. Finally, we found that the connectivity between the pCC and pACC statistically mediated the relationship between Agreeableness and enhanced cardiovascular reactivity to stress.
There is a long tradition in health psychology examining the personality correlates of cardiovascular reactivity, but the majority of studies have found the best predictors of reactivity to be hostility, aggression and Type-A behavior (Chida and Hamer, 2008). However, these variables tend to be predictive of cardiovascular reactivity only in tasks in which social stressors are used to provoke the negative emotion, anger. In the present study, we utilized cognitive stressors engendering psychological conflict (MSIT and Stroop tasks), and as such did not expect these measures to be as useful for predicting cardiovascular reactivity because they lack a social dimension. Hence, an important new direction in this line of research will be to utilize social stressors that can be implemented in an fMRI environment. Previous studies have examined the relationship between Agreeableness and psychological and physiological responses to various types of stimuli, including viewing emotional faces (Haas et al., 2007), diary reports of the experience of quarrelsome behavior (Moskowitz and Cote, 1995; Suls et al., 1998), and viewing emotionally-evocative photographs (Tobin et al., 2000). Although the study by Tobin and colleagues utilized a psychophysiological measurement, facial electromyography, the present study is the first to report a relationship between Agreeableness and cardiovascular reactivity while in a stressful situation. Although the methodology is distinct, the results are consistent with the previous work: participants scoring high in Agreeableness were the most reactive (or, alternately, “sensitive”) to the conflict engendered by incongruent trials in a cognitive conflict task. We acknowledge that the relationship between Agreeableness and reactivity is a novel finding, and as such, replication of this finding should be demonstrated to ensure this is not a sample-specific finding. However, our finding that Agreeableness was predictive of SBP reactivity (but not DBP) is in line with previous studies that have shown SBP reactivity to be particularly sensitive to psychosocial measures (Chida and Hamer, 2008).
Furthermore, the present study explains a potential neural mediator that could predict this relationship – resting or default brain connectivity. The finding in the present study that resting pACC activity mediated the relationship between Agreeableness and cardiovascular reactivity thus complements findings that have examined the neural correlates of social evaluation threat. A series of studies performed by Wager et al. (Wager, Ast, et al., 2009; Wager, Waugh, et al., 2009) found that the relationship between social evaluative threat and heart rate reactivity was mediated by activity in the pACC. Taken together, these studies would suggest that the pACC may act as an important hub between health-relevant psychological and social constructs (such as being evaluated by others, or in the case of Agreeableness, sensitivity to conflict) and cardiovascular-autonomic reactions to stress.
Recently, a growing body of literature has focused on individual differences in resting state functional connectivity and how they may relate to cognition. Many of these studies have relied on clinical populations (for review, see (Greicius et al., 2007). In the present study, using healthy adults, we have shown how this resting connectivity is related to both a dispositional factor as well as psychophysiological reactivity in subsequent stressor tasks. Recent research has shown the DMN to be reliable within subjects over time (Shehzad et al., 2009) and resting state functional connectivity predicts BOLD activity during tasks (Mennes et al., 2010), thereby suggesting that resting state scans could be a useful tool for understanding trait-like individual differences in brain function. Although we focused our study on the resting state “default mode” network, there are additional functional networks, such as the Salience network (Seeley et al., 2007; Sridharan et al., 2008) and Central Executive network (Corbetta et al., 2002) that have been found in the human brain. The central goal of the present study was to examine how resting state activity could be associated with a personality variable and how this connectivity may help explain cardiovascular reactivity on a subsequent task. Given that prior research has found individual differences in reactivity to be associated with activity in the pCC (Gianaros et al., 2005), and our hypotheses predicted activity in the pACC, we maintained that the DMN (which encompasses both regions) was the most appropriate choice for our resting data. However, future research should focus on alternate networks and in particular utilize effective connectivity analyses to better understand how resting state data may inform our understanding of pathways from personality to physiological responses to stress.
Previous work has found that resting metabolic activity, as measured by resting cerebral blood flow (rCBF), predicts BP responses to subsequent stressors (Gianaros et al., 2009c). Specifically, heightened rCBF in regions commonly involved in cardiovascular reactivity – dorsal and perigenual anterior cingulate, insular and medial prefrontal cortices – was associated with greater BP responses to stress tasks. Here, it was hypothesized that these individual differences may be related to dispositional variations between subjects, but this hypothesis was not explicitly tested. In addition to quantifying resting cerebral blood flow, Gianaros et al. (2009) used resting state BOLD activity to map a network of regions, including the pACC, which may partly characterize a circuitry supporting stressor-evoked BP responses. The present work builds upon these findings by examining the default mode network connectivity – a network of interconnected regions comprising a major functional network in the brain – and how it is associated to cardiovascular-autonomic responses to stress.
Limitations
Several limitations to this study are acknowledged. First, we utilized a seed-based analysis examining regions that are functionally correlated with activity in the pCC. It is mentionable that numerous other functional networks exist in the brain, and future research should investigate the potential role of these networks in stress and stressor-evoked cardiovascular reactivity. For instance, work by Seeley and colleagues have identified a resting state network centered on the dorsal anterior cingulate (possibly extending into the pACC) that has been implicated in generating autonomic reactions and monitoring for salient stimuli (Seeley et al., 2007). Investigating the relationships between these networks and stressor-evoked cardiovascular reactivity will be an important step in understanding the functional architecture of the central autonomic network. Second, there is not yet a well-established relationship between Agreeableness and diseases associated with heightened cardiovascular reactivity, such as coronary heart disease. There are several potential explanations for this lack of relationship. As discussed above, individuals who are high in Agreeableness find situations involving interpersonal conflict to be more aversive (marked by negative affect) than individuals lower in Agreeableness. Suls et al. (1998) have shown that Agreeable persons report fewer conflict problems suggesting that they may self-select environmental conditions and situations where conflict will be low to minimize stressful or conflictual encounters. In the present study, the heightened reactivity of individuals who are more Agreeable may be a reflection of the conflict task itself. Finally, this is the first study of which we are aware that demonstrates a relationship between Agreeableness and cardiovascular reactivity to a mental stressor task. This could be due to several factors including some special characteristics of our sample, a chance correlation between the two variables, or a third (e.g., genetic) variable that accounts for systematic covariation between Agreeableness and stressor-evoked cardiovascular reactivity. However, the pACC was hypothesized a priori to be involved in cardiovascular reactivity, and the findings are therefore in line with the hypothesis that pCC-pACC connectivity would mediate the relationship between Agreeableness and SBP reactivity. Regardless, it will be important for this finding to be replicated to strengthen any conclusions to be drawn between Agreeableness and conflict sensitivity.
5. Conclusions
The present study examined the relationship between a personality variable – Agreeableness, DMN connectivity at rest, and cardiovascular reactions to standardized stressors. We found that individuals who were more Agreeable were more reactive to the stressor tasks, and this was partly explained by heightened functional connectivity between the posterior and perigenual cingulate cortices. Overall, these results suggest that Agreeableness may be associated with conflict sensitivity, and that default mode network activity may hold promise for explaining individual differences in stressor-evoked cardiovascular reactivity, a predictor of future cardiovascular health.
Research Highlights
More Agreeable people have higher blood pressure reactions to stress.
Agreeableness correlates with resting cingulate connectivity.
Resting cingulate connectivity mediates between Agreeableness and reactivity.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants K01-MH070616, R01-HL089850, T32-HL00756, and a Commonwealth Universal Research Enhancement Grant from the Pennsylvania Department of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Due to the high degree of multicollinearity, resting SBP was not included as a covariate in the model. However, if resting SBP is included the pACC region of activation is still present, albeit at a reduced threshold (T = 2.97, k = 6, alpha = .12). This reduction in activity is likely due to the shared variance between resting SBP and the reactivity score (r = .30, p = .07).
On the suggestion of an anonymous reviewer, analyses were performed using a seed region in the pACC (x,y,z = −4, 44, 16). Two regions in the pCC showed stronger connectivity with the pACC in individuals exhibiting higher SBP reactivity. Eigenvariates in both pCC regions predicted Agreeableness (rs > 0.35, ps < 0.05), further corroborating the finding that pCC - pACC connectivity mediates the relationship between Agreeableness and SBP reactivity.
References
- An X, Bandler R, Ongür D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J. Comp. Neurol. 1998;401:455–479. [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LL, Hichwa RD. II. PET studies of memory: novel versus practiced free recall of word lists. Neuroimage. 1995;2:296–305. doi: 10.1006/nimg.1995.1037. [DOI] [PubMed] [Google Scholar]
- Benarroch E. Central Autonomic Network: Functional Organization and Clinical Correlations. 1st ed. Wiley-Blackwell; 1997. [Google Scholar]
- Botvinick M, Braver T, Barch D, Carter C, Cohen J. Conflict monitoring and cognitive control. Psychol. Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Buckner R, Andrews-Hanna J, Schacter D. The Brain's Default Network: Anatomy, Function, and Relevance to Disease. Ann. N.Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Bush G, Shin L. The Multi-Source Interference Task: an fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. Nat Protoc. 2006;1:308–313. doi: 10.1038/nprot.2006.48. [DOI] [PubMed] [Google Scholar]
- Carter C, Braver T, Barch D, Botvinick M, Noll D, Cohen J. Anterior Cingulate Cortex, Error Detection, and the Online Monitoring of Performance. Science. 1998;280:747. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Shoemaker JK. Functional neuroanatomy of autonomic regulation. NeuroImage. 2009;47:795–803. doi: 10.1016/j.neuroimage.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychol Bull. 2008;134:829–885. doi: 10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater Cardiovascular Responses to Laboratory Mental Stress Are Associated With Poor Subsequent Cardiovascular Risk Status: A Meta-Analysis of Prospective Evidence. Hypertension. 2010;55:1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and Pitfalls in the Analysis and Interpretation of Resting-State FMRI Data. Front Syst Neurosci. 2010;4:1–15. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural Systems for Visual Orienting and Their Relationships to Spatial Working Memory. J. Cogn. Neurosci. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Costa P, McCrae RR. NEO PI-R professional manual. Odessa, FL: Psychological Assessment Resources, Inc; 1992. [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J. Comp. Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar B, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- Digman JM. Personality Structure: Emergence of the Five-Factor Model. Annu. Rev. Psychol. 1990;41:417–440. [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35:1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueredo VM. The Time Has Come for Physicians to Take Notice: The Impact of Psychosocial Stressors on the Heart. Am. J. Med. 2009;122:704–712. doi: 10.1016/j.amjmed.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Gianaros P, Hariri A, Sheu L, Muldoon M, Sutton-Tyrrell K, Manuck S. Preclinical Atherosclerosis Covaries with Individual Differences in Reactivity and Functional Connectivity of the Amygdala. Biol. Psychiatry. 2009;65:1–8. doi: 10.1016/j.biopsych.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Derbyshire SWG, May JC, Siegle GJ, Gamalo MA, Jennings JR. Anterior cingulate activity correlates with blood pressure during stress. Psychophysiology. 2005;42:627–635. doi: 10.1111/j.1469-8986.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Derbyshire SWG, Matthews KA. Heightened functional neural activation to psychological stress covaries with exaggerated blood pressure reactivity. Hypertension. 2007;49:134–140. doi: 10.1161/01.HYP.0000250984.14992.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, May JC, Siegle GJ, Jennings JR. Is there a functional neural correlate of individual differences in cardiovascular reactivity? Psychosom Med. 2005;67:31–39. doi: 10.1097/01.psy.0000151487.05506.dc. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J. Neurosci. 2008;28:990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros P, Sheu L. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: Emerging evidence for a brain-body pathway to coronary heart disease risk. NeuroImage. 2009;47:922–936. doi: 10.1016/j.neuroimage.2009.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros P, Sheu L, Remo A, Christie I, Crtichley H, Wang J. Heightened Resting Neural Activity Predicts Exaggerated Stressor-Evoked Blood Pressure Reactivity. Hypertension. 2009;53:819–825. doi: 10.1161/HYPERTENSIONAHA.108.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami R, Frances MF, Shoemaker JK. Representation of somatosensory inputs within the cortical autonomic network. Neuroimage. doi: 10.1016/j.neuroimage.2010.09.050. (in press) [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biol. Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D, Raichle M. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Haas B, Omura K, Constable R, Canli T. Is automatic emotion regulation associated with agreeableness? A perspective using a social neuroscience approach. Psychol. Sci. 2007;18:130–132. doi: 10.1111/j.1467-9280.2007.01861.x. [DOI] [PubMed] [Google Scholar]
- Heron MP, Hoyert DL, Xu J, Scott C, Tejada-Vera B. Deaths: Preliminary data for 2006. Natl. Vit. Stat. Rep. 2008;56:1–52. [Google Scholar]
- Jensen-Campbell L, Rosselli M, Workman K, Santisi M, Rios J, Bojan D. Agreeableness, conscientiousness, and effortful control processes. J. Res. Pers. 2002;36:476–489. [Google Scholar]
- John O, Naumann L, Soto C. Handbook of personality: Theory and research. New York, NY: Guilford Press; 2008. Paradigm Shift to the Integrative Big-Five Trait Taxonomy: History, Measurement, and Conceptual Issues; pp. 114–158. [Google Scholar]
- Lovallo WR, Gerin W. Psychophysiological Reactivity: Mechanisms and Pathways to Cardiovascular Disease. Psychosom Med. 2003;65:36–45. doi: 10.1097/01.psy.0000033128.44101.c1. [DOI] [PubMed] [Google Scholar]
- MacKinnon D, Fairchild A. Current Directions in Mediation Analysis. Curr. Dir. Psychol. Sci. 2009;18:16–22. doi: 10.1111/j.1467-8721.2009.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock R. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering Minds: The Default Network and Stimulus-Independent Thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houdé O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res. Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. Validation of the five-factor model of personality across instruments and observers. J Pers Soc Psychol. 1987;52:81–90. doi: 10.1037//0022-3514.52.1.81. [DOI] [PubMed] [Google Scholar]
- McCrae RR, John OP. An introduction to the five-factor model and its applications. J Pers. 1992;60:175–215. doi: 10.1111/j.1467-6494.1992.tb00970.x. [DOI] [PubMed] [Google Scholar]
- McEwen B, Gianaros P. Central role of the brain in stress and adaptation: links to socioeconomic status, health and disease. Ann. N.Y. Acad. Sci. 2009;1(34) doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci. 2006;8:367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcewen B. The brain is the central organ of stress and adaptation. NeuroImage. 2009;47:911–913. doi: 10.1016/j.neuroimage.2009.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, D'Angelo BR, Kaufman JN, Binder JR. Interrupting the "stream of consciousness": an fMRI investigation. Neuroimage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Zuo X, Di Martino A, Biswal BB, Castellanos FX, Milham MP. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage. 2010;50:1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz D, Cote S. Do interpersonal traits predict affect: A comparison of three models. J. Pers. Soc. Psychol. 1995;69:915–924. [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. NeuroImage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Robinson MD. Personality, Affective Processing, and Self-Regulation: Toward Process-Based Views of Extraversion, Neuroticism, and Agreeableness. Soc. Personal. Psychol. Compass. 2007;1:223–235. [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AMC, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP. The resting brain: unconstrained yet reliable. Cereb. Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U.S.A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suls J, Martin R, David J. Person-environment fit and its limits: Agreeableness, neuroticism, and emotional reactivity to interpersonal conflict. Pers. Soc. Psychol. Bull. 1998;24:88. [Google Scholar]
- Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O'Donnell C, Kittner S, et al. Heart disease and stroke statistics–2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- Tobin RM, Graziano WG, Vanman EJ, Tassinary LG. Personality, emotional experience, and efforts to control emotions. J Pers Soc Psychol. 2000;79:656–669. [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends. Cogn. Sci. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog. Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29:452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T, Ast V, Hughes B, Davidson M, Lindquist M, Ochsner K. Brain mediators of cardiovascular responses to social threat, Part II: Prefrontal-subcortical pathways and relationship with anxiety. NeuroImage. 2009;47:836–851. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T, Waugh C, Lindquist M, Noll D, Fredrickson B, Taylor S. Brain mediators of cardiovascular responses to social threat. NeuroImage. 2009;47:821–835. doi: 10.1016/j.neuroimage.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM, Creswell JD, Eisenberger NI, Lieberman MD. Dispositional mindfulness and depressive symptomatology: correlations with limbic and self-referential neural activity during rest. Emotion. 2010;10:12–24. doi: 10.1037/a0018312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SW, Massé N, Kimmerly DS, Menon RS, Shoemaker JK. Ventral medial prefrontal cortex and cardiovagal control in conscious humans. Neuroimage. 2007;35:698–708. doi: 10.1016/j.neuroimage.2006.12.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


