Abstract
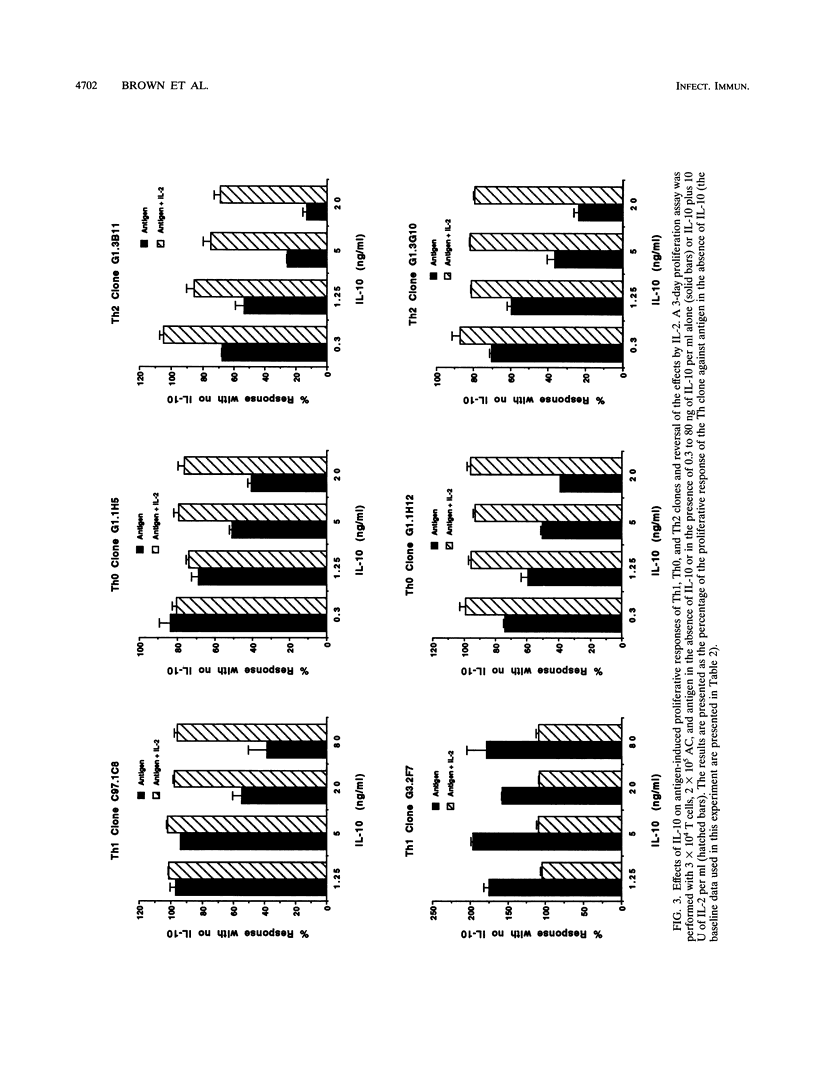
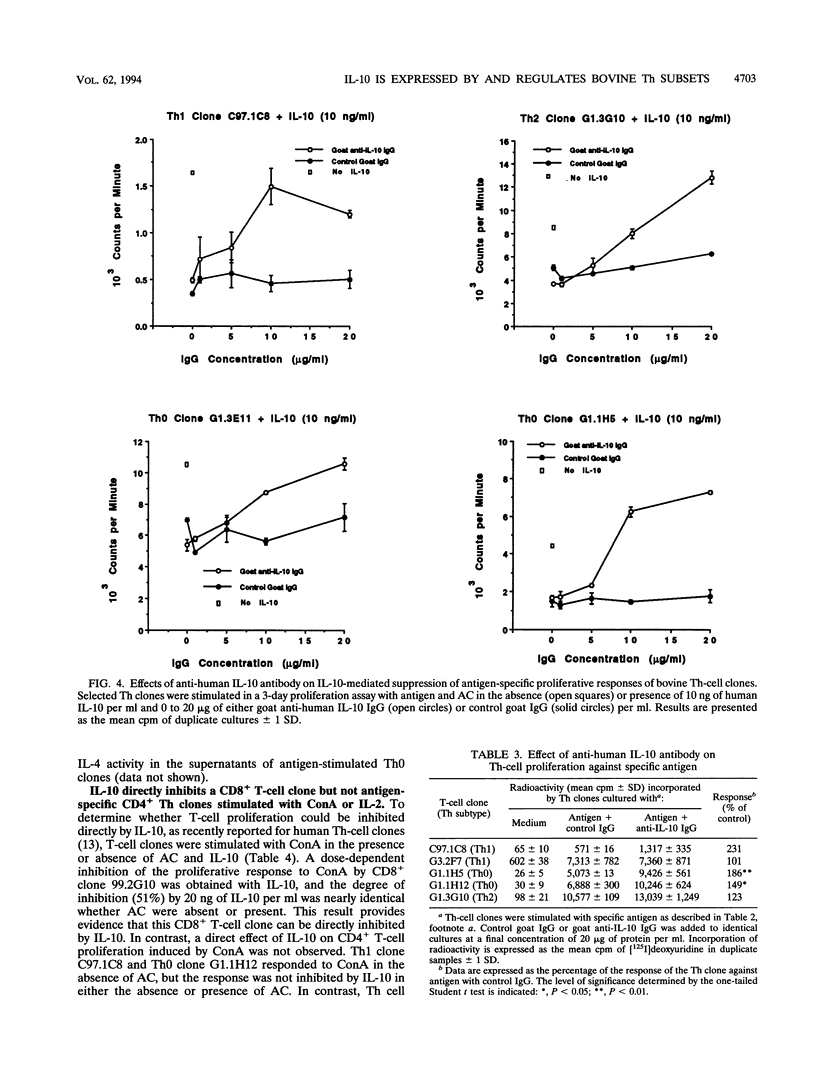
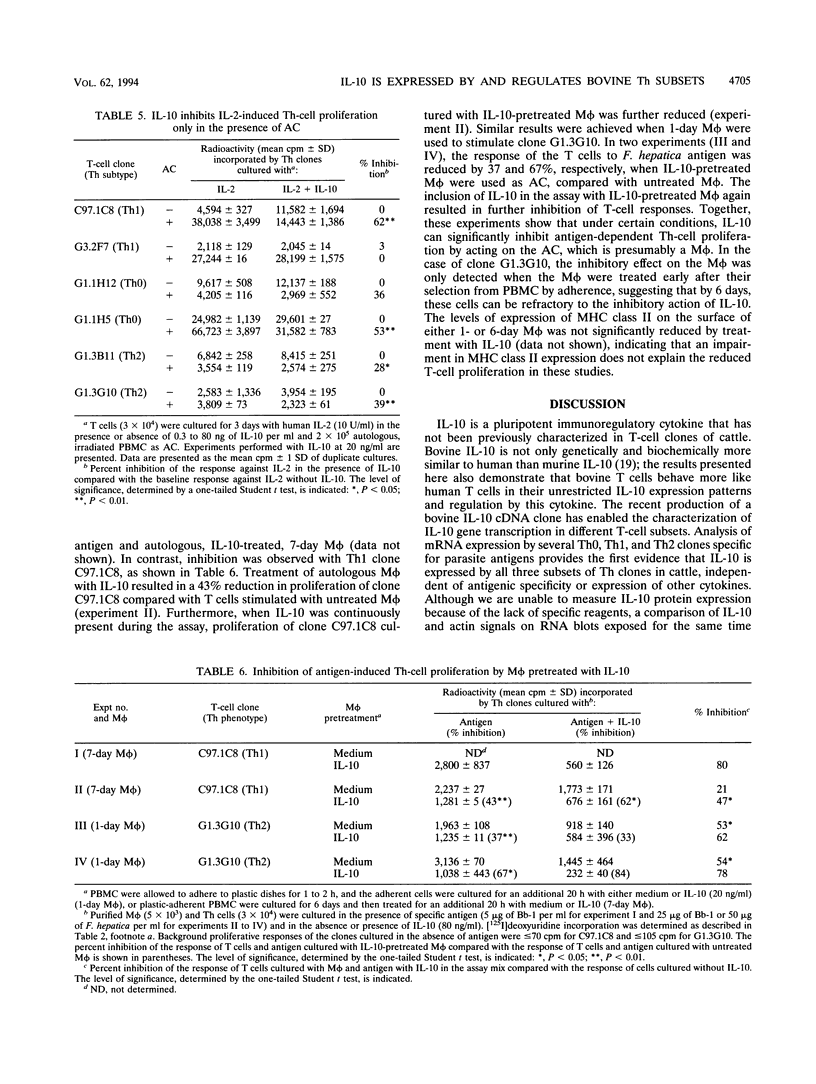
Murine interleukin-10 (IL-10) is produced by type 2 helper (Th2) cells and selectively inhibits cytokine synthesis by type 1 helper (Th1) cells, whereas human IL-10 is produced by and inhibits proliferation and cytokine synthesis by both Th1 and Th2 subsets. This study reports that bovine IL-10 mRNA is expressed by Th0, Th1, and Th2 clones of bovine T cells specific for either Babesia bovis or Fasciola hepatica but not by two CD8+ T-cell clones. The antigen-induced proliferative responses of all three subsets of CD4+ cells were inhibited by human IL-10, and low levels (10 U/ml) of exogenous human IL-2 restored the suppressed response. However, proliferation of one Th1 clone was never inhibited but was enhanced by IL-10. Human IL-10 also inhibited the expression of gamma interferon and IL-4 mRNA in Th0 clones. In the absence of accessory cells (AC), the responses of Th clones to concanavalin A or IL-2 were not inhibited by IL-10, whereas antigen-specific responses of Th1 and Th2 cells were reduced when IL-10-pretreated macrophages were used as AC. Together, our results with bovine T cells support the concept that IL-10 primarily affects AC function and does not directly inhibit CD4+ T cells and demonstrate that the immunoregulatory effects of IL-10 are not selectively directed at Th1 populations, as they are in mice.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell J. D., DeFranco A. L., Paul W. E., Schwartz R. H. Antigen presentation by resting B cells. Radiosensitivity of the antigen-presentation function and two distinct pathways of T cell activation. J Exp Med. 1984 Mar 1;159(3):881–905. doi: 10.1084/jem.159.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Davis W. C., Dobbelaere D. A., Rice-Ficht A. C. CD4+ T-cell clones obtained from cattle chronically infected with Fasciola hepatica and specific for adult worm antigen express both unrestricted and Th2 cytokine profiles. Infect Immun. 1994 Mar;62(3):818–827. doi: 10.1128/iai.62.3.818-827.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Grab D. J. Biological and biochemical characterization of bovine interleukin 2. Studies with cloned bovine T cells. J Immunol. 1985 Nov;135(5):3184–3190. [PubMed] [Google Scholar]
- Brown W. C., Logan K. S., Wagner G. G., Tetzlaff C. L. Cell-mediated immune responses to Babesia bovis merozoite antigens in cattle following infection with tick-derived or cultured parasites. Infect Immun. 1991 Jul;59(7):2418–2426. doi: 10.1128/iai.59.7.2418-2426.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Woods V. M., Dobbelaere D. A., Logan K. S. Heterogeneity in cytokine profiles of Babesia bovis-specific bovine CD4+ T cells clones activated in vitro. Infect Immun. 1993 Aug;61(8):3273–3281. doi: 10.1128/iai.61.8.3273-3281.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Zhao S., Rice-Ficht A. C., Logan K. S., Woods V. M. Bovine helper T cell clones recognize five distinct epitopes on Babesia bovis merozoite antigens. Infect Immun. 1992 Oct;60(10):4364–4372. doi: 10.1128/iai.60.10.4364-4372.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Zhao S., Woods V. M., Tripp C. A., Tetzlaff C. L., Heussler V. T., Dobbelaere D. A., Rice-Ficht A. C. Identification of two Th1 cell epitopes on the Babesia bovis-encoded 77-kilodalton merozoite protein (Bb-1) by use of truncated recombinant fusion proteins. Infect Immun. 1993 Jan;61(1):236–244. doi: 10.1128/iai.61.1.236-244.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. F., Zlotnik A. IL-10: a novel cytotoxic T cell differentiation factor. J Immunol. 1991 Jul 15;147(2):528–534. [PubMed] [Google Scholar]
- Defrance T., Vanbervliet B., Brière F., Durand I., Rousset F., Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992 Mar 1;175(3):671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen J. L., Neubauer M. G., Degen S. J., Seyfried C. E., Morris D. R. Regulation of protein synthesis in mitogen-activated bovine lymphocytes. Analysis of actin-specific and total mRNA accumulation and utilization. J Biol Chem. 1983 Oct 25;258(20):12153–12162. [PubMed] [Google Scholar]
- Del Prete G., De Carli M., Almerigogna F., Giudizi M. G., Biagiotti R., Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993 Jan 15;150(2):353–360. [PubMed] [Google Scholar]
- Ding L., Linsley P. S., Huang L. Y., Germain R. N., Shevach E. M. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993 Aug 1;151(3):1224–1234. [PubMed] [Google Scholar]
- Ding L., Shevach E. M. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol. 1992 May 15;148(10):3133–3139. [PubMed] [Google Scholar]
- Ellis J. A., Davis W. C., MacHugh N. D., Emery D. L., Kaushal A., Morrison W. I. Differentiation antigens on bovine mononuclear phagocytes identified by monoclonal antibodies. Vet Immunol Immunopathol. 1988 Oct;19(3-4):325–340. doi: 10.1016/0165-2427(88)90118-3. [DOI] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Hash S. M., Brown W. C., Rice-Ficht A. C. Characterization of a cDNA encoding bovine interleukin 10: kinetics of expression in bovine lymphocytes. Gene. 1994 Feb 25;139(2):257–261. doi: 10.1016/0378-1119(94)90766-8. [DOI] [PubMed] [Google Scholar]
- Heussler V. T., Eichhorn M., Dobbelaere D. A. Cloning of a full-length cDNA encoding bovine interleukin 4 by the polymerase chain reaction. Gene. 1992 May 15;114(2):273–278. doi: 10.1016/0378-1119(92)90587-f. [DOI] [PubMed] [Google Scholar]
- Howard M., O'Garra A. Biological properties of interleukin 10. Immunol Today. 1992 Jun;13(6):198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- MacNeil I. A., Suda T., Moore K. W., Mosmann T. R., Zlotnik A. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol. 1990 Dec 15;145(12):4167–4173. [PubMed] [Google Scholar]
- Macatonia S. E., Doherty T. M., Knight S. C., O'Garra A. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J Immunol. 1993 May 1;150(9):3755–3765. [PubMed] [Google Scholar]
- Maggi E., Biswas P., Del Prete G., Parronchi P., Macchia D., Simonelli C., Emmi L., De Carli M., Tiri A., Ricci M. Accumulation of Th-2-like helper T cells in the conjunctiva of patients with vernal conjunctivitis. J Immunol. 1991 Feb 15;146(4):1169–1174. [PubMed] [Google Scholar]
- Maggi E., Parronchi P., Manetti R., Simonelli C., Piccinni M. P., Rugiu F. S., De Carli M., Ricci M., Romagnani S. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992 Apr 1;148(7):2142–2147. [PubMed] [Google Scholar]
- Moore K. W., O'Garra A., de Waal Malefyt R., Vieira P., Mosmann T. R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- Parronchi P., Macchia D., Piccinni M. P., Biswas P., Simonelli C., Maggi E., Ricci M., Ansari A. A., Romagnani S. Allergen- and bacterial antigen-specific T-cell clones established from atopic donors show a different profile of cytokine production. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4538–4542. doi: 10.1073/pnas.88.10.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R., Spies A. G., Nissen M. S., Buck C. D., Weinberg A. D., Barr P. J., Magnuson N. S., Magnuson J. A. Molecular cloning of a functional bovine interleukin 2 cDNA. Proc Natl Acad Sci U S A. 1986 May;83(10):3228–3232. doi: 10.1073/pnas.83.10.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset F., Garcia E., Defrance T., Péronne C., Vezzio N., Hsu D. H., Kastelein R., Moore K. W., Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H., de Waal Malefyt R. Functional characterization of human IL-10. Int Arch Allergy Immunol. 1992;99(1):8–15. doi: 10.1159/000236329. [DOI] [PubMed] [Google Scholar]
- Taga K., Tosato G. IL-10 inhibits human T cell proliferation and IL-2 production. J Immunol. 1992 Feb 15;148(4):1143–1148. [PubMed] [Google Scholar]
- Thompson-Snipes L., Dhar V., Bond M. W., Mosmann T. R., Moore K. W., Rennick D. M. Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J Exp Med. 1991 Feb 1;173(2):507–510. doi: 10.1084/jem.173.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira P., de Waal-Malefyt R., Dang M. N., Johnson K. E., Kastelein R., Fiorentino D. F., deVries J. E., Roncarolo M. G., Mosmann T. R., Moore K. W. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A. D., Magnuson N. S., Reeves R., Wyatt C. R., Magnuson J. A. Evidence for two discrete phases of IL-2 production in bovine lymphocytes. J Immunol. 1988 Aug 15;141(4):1174–1179. [PubMed] [Google Scholar]
- Yssel H., De Waal Malefyt R., Roncarolo M. G., Abrams J. S., Lahesmaa R., Spits H., de Vries J. E. IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol. 1992 Oct 1;149(7):2378–2384. [PubMed] [Google Scholar]
- Zlotnik A., Moore K. W. Interleukin 10. Cytokine. 1991 Sep;3(5):366–371. doi: 10.1016/1043-4666(91)90039-g. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Yssel H., de Vries J. E. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993 Jun 1;150(11):4754–4765. [PubMed] [Google Scholar]