Abstract
We recently demonstrated that hypocretin/orexin (Hcrt) and nociceptin/orphanin FQ (N/OFQ) systems coordinately regulate nociception in a mouse model of stress-induced analgesia (SIA). However, the site of N/OFQ action on modulation of SIA was elusive, since N/OFQ was administered via intracerebroventricular (i.c.v.) injection acting on widely distributed N/OFQ receptors (NOP) in the brain. In the present study, we tested the hypothesis that N/OFQ modulates the SIA directly via the inhibition of the Hcrt neurons in the lateral hypothalamus. Using both fluorescent and electron microscopy, we found that N/OFQ-containing neurons are located in the lateral hypothalamus and the N/OFQ-containing fibers make direct contacts with the Hcrt neurons. Paw thermal nociceptive test revealed that the immobilization restraint of the rat increased the thermal pain threshold by 20.5±7.6%. Bilateral microinjection of N/OFQ (9 µg/side) into the rat perifornical area of the lateral hypothalamus, the brain area in which the Hcrt neurons are exclusively located, abolished the SIA. Activity of Hcrt neurons in the same animals was assessed using Fos immunohistochemistry. Percentage of Fos+/Hcrt neurons was lower in rats injected with N/OFQ than rats injected with saline, with the difference between groups stronger in the Hcrt neurons located medially to the fornix than in Hcrt neurons located laterally to the fornix. These results suggest that N/OFQ modulation of SIA is mediated by direct inhibition of Hcrt neuronal activity in the perifornical area. The uncovered peptidergic interaction circuitry may have broad implication in coordinated modulation by Hcrt and N/OFQ on other stress adaptive responses.
Keywords: Hypocretin/Orexin, stress, stress-induced analgesia, pain modulation, c-Fos expression, restraint stress
1. Introduction
Stress-induced analgesia (SIA) plays a role in neuroadaptation to threats and is an important component of defensive behavioral response to prepare for “fight or flight”. It is known that SIA is mediated and modulated by a number of neurotransmitter/neuromudulator systems, such as opioids, GABA, glutamate, monoamines, hormones of the hypothalamic–pituitary–adrenal axis, and endocannabinoids (Butler and Finn, 2009). Recent several lines of evidence suggest that two neuropeptide systems, Hcrt and N/OFQ, play important roles in the generation and modulation of SIA, respectively. Behavioral arousal and alertness are prerequisites of the stress response. The Hcrt system sets the baseline of arousal and vigilant state under normal conditions, and also plays a critical role in stress responses and behavioral defense under threat situations (Kayaba et al., 2003;Zhang and McDougall, 2006). The involvement of Hcrt in the generation of SIA was first shown by Watanabe and his colleagues (Watanabe et al., 2005). The magnitude of analgesia induced by the foot shock-associated stress was found to be decreased in the prepro-orexin knockout mice compared to wildtype mice. This result is consistent with our recent observations in orexin/ataxin-3 transgenic mice (orexin/ataxin-3) in which Hcrt neurons were genetically ablated by selective expression of a cytotoxic poly-Q-ataxin-3 protein in Hcrt neurons (Hara et al., 2001). Unlike the wildtype mice, the orexin/ataxin-3 mice did not exhibit the thermal pain threshold increase caused by restraint stress, while acute analgesia was induced by i.c.v. administration of Hcrt-1 (Xie et al., 2008).
Although SIA is a natural stress-induced neuroadaptation, exaggerated or prolonged SIA phenomenon has been regarded as a performance deficit (Blair et al., 1982;Amit and Galina, 1986). It has been suggested that SIA might serve as a measure of stress severity and a model of ‘depersonalization disorder’ in humans (Kenunen and Prakh'e IV, 2005;Kenunen et al., 2006). Thus, SIA must be critically regulated (Amit and Galina, 1988). SIA in rodents is partially depressed by the opioid antagonist naloxone, but is completely blocked by via i.c.v. administration of N/OFQ (Meunier et al., 1995;Reinscheid et al., 1995;Rizzi et al., 2001;Calo' et al., 2000). Furthermore, the studies employing targeted disruption of the N/OFQ or using a selective NOP antagonist indicate that endogenous N/OFQ plays a role in tonic inhibition of SIA and other stress responses (Koster et al., 1999;Rizzi et al.,2001). We recently explored the neuronal pathways that mediate the N/OFQ effect on SIA and showed that N/OFQ blocked SIA in wild-type mice. However, co-administration of Hcrt-1 via i.c.v. injection overcame the N/OFQ inhibition and restored the SIA (Xie et al., 2008). This study not only confirms that Hcrt neurotransmission is essential for the generation of SIA, but also for the first time reveals the presence of interaction between the Hcrt and N/OFQ systems (Xie et al., 2008).
However, since both N/OFQ and Hcrt were delivered via i.c.v. injection and the NOP receptors are widely distributed throughout the brain, we could not determine at which anatomical level the interaction between the two peptidergic systems occurred. As we have shown that N/OFQ could cause direct hyperpolarization, a decrease in input resistance, and blockade of the firing of action potentials of Hcrt neurons (Xie et al., 2008), we hypothesize that N/OFQ regulates SIA by directly inhibiting Hcrt neuronal activity. In the present study, we have tested this hypothesis by microinjecting N/OFQ locally into the perifornical area of the lateral hypothalamus where the Hcrt neurons are exclusively localized (Peyron et al., 1998), and measuring the extent of SIA using a plantar test and Hcrt neural activity using Fos immunohistochemistry. Double staining of Hcrt and Fos in the rat brain revealed activation of Hcrt neurons under restrained immobilization. Local microinjection of N/OFQ into the perifornical area of the lateral hypothalamus depressed the Fos expression in the Hcrt neurons and concomitantly abolished the SIA. These results support our hypothesis that N/OFQ can modulate stress-induced analgesia through direct inhibition of Hcrt neurons in the lateral hypothalamus. Preliminary results were communicated as an abstract form at the Society for Neuroscience Meeting (2009).
2. Materials and methods
2.1. Experimental animals
Adult male Sprague-Dawley rats were used in this experiment. Food and water were provided ad libitum. Rats were kept on a 12:12 light-dark cycle (lights on at 7 AM) during the whole period of the study. Rats were treated in accordance with the protocol approved by the SRI International’s and AfaSci’s Institutional Animal Care and Use Committee in conformance with the PHS Guidelines on Care and Use of Animals in Research.
2.2. Double staining for N/OFQ- and Hcrt-immunoreactive neurons and fibers
Adult male Sprague-Dawley rats (body weight 250–280 g, n=5) were implanted with i.c.v. cannula into the lateral ventricle. Animals were allowed to recover for 5 days and then injected with colchicine through the implanted i.c.v. cannula (120 µg in 5 µl of sterile saline) 48 hours prior to transcardiac perfusion.
Rats were deeply anesthetized with sodium pentobarbital i.p. and transcardially perfused with approximately 50–75 ml of phosphate buffered saline (PBS). This was followed with approximately 400 ml of Zamboni’s fixative. After perfusion, brains were removed and postfixed in Zamboni’s fixative for 6 hours. Tissue was then transferred to a solution of 30% sucrose in PBS. Brains were stored at 4°C until sectioning. Brains were sectioned coronally on a microtome at 40 µm and stored in cryoprotectant (PBS, pH 7.4, 20% glycerol, and 30% ethylene glycol) at −20°C until use.
The immunohistochemistry technique employed in this study was based on the method described previously by Neal and his colleagues (Neal, Jr. et al., 1999). Floating rat brain sections were washed in PBS to remove cryoprotectant. Sections were then incubated for 60 minutes in a blocking solution (PBS, 1% Triton X-100, 5% normal donkey serum) and then transferred to a solution containing goat anti-orexin B antibody (Santa Cruz Biotechnology, 1:5000) and incubated at room temperature overnight. After primary antibody incubation, the sections were washed in PBS and incubated with Alexa Fluor® 488 donkey anti-goat IgG (1:500, Invitrogen, Carlsbad, CA) for 2 h at room temperature. Then, the sections were washed in PBS and incubated in rabbit anti-OFQ antibody (Abcam) diluted to 1:1000 at room temperature overnight. The sections were washed again in PBS and incubated in biotinylated donkey anti-rabbit immunoglobulin G (IgG; 1:500) for 2 hours at room temperature, followed by incubation with Alexa Fluor® 594 streptavidin conjugate (1:500, Invitrogen, Carlsbad, CA) for 2 hours. The tissue was rinsed in PBS, mounted on gelatin-coated slides, coverslipped using Fluoromount mounting media (Electron Microscopy Sciences, Hatfield, PA) and examined under fluorescent microscopy (Leica DM5000B).
2.3. Electron microscopy
Rat brain sections were first immunostained for N/OFQ with rabbit polyclonal antiserum against N/OFQ (1:1000, Neuromics, MN), incubated in donkey anti-rabbit IgG, ABC (Vectastain ABC Systems), and then visualized with a modified version of the nickel-diaminobenzidine (Ni-DAB) reaction as described (Winsky-Sommerer et al., 2004; Xie et al., 2008). The sections were further incubated in goat anti-Hcrt antisera (1:1000, Phoenix Pharmaceutical) for 24 h at 4°C, followed by secondary antibody (donkey anti-goat IgG diluted 1:50 in phosphate buffer, PB) and goat peroxidase-anti-peroxidase (PAP; 1:100 in PB). Ribbons of serial ultrathin sections were collected on Formvar-coated single slot grids and examined under the EM as previously described (Winsky-Sommerer et al., 2004; Xie et al., 2008).
2.4. In vivo plantar thermal pain test
Adult male Sprague-Dawley rats (body weight 350–400 g, n=11) were implanted with guide cannulae into perifornical area of the lateral hypothalamus. Animals were anesthetized (isoflurane 1–4%) and placed into a stereotaxic apparatus. A small hole was drilled through the skull to position a guide cannula at the following coordinates relative to bregma: AP –2.8mm; ML 1.4mm, DV 7.9mm. The cannula was affixed to the animal's skull with dental acrylic and closed with a removable style. At least one week was allowed for recovery from the surgery before beginning the experiment.
The animals were placed on the Plantar Test apparatus surface (University of California San Diego, CA) within Plexiglas cylinders, which allowed the animal to move about freely (Yeomans and Proudfit, 1994). After 15 min of acclimation to new environment, baseline pain thresholds were measured using the plantar test (Montagne-Clavel and Oliveras, 1996). Then, rats were lightly anesthetized with isoflurane (1%), and N/OFQ in 0.9% NaCl (9µg each side, 18µg total dose per rat; n=6) or saline (n=5) were injected bilaterally through implanted guide cannula into the perifornical area of the lateral hypothalamus in the volume of 1 µl using 35 gauge needle (outer diameter, 135 µm; inner diameter, 55 µm; tip length, 5.0 mm; Nanofil 35G Blunt Needle; World Precision Instruments, Sarasota, FL). Injection cannulae were attached to a 2 µl Hamilton microsyringe via PE-20 polyethylene tubing, and the solution was injected over a period of 15 s. After the completion of the microinjection, rats were immediately placed into a cone-shape plastic bag, and restrained for 30 min. Plantar tests on the same subjects were conducted in 30 min before and 0, 30 and 60 min after the release from 30 min restraint immobilization. An external source of radiant heat was placed under one of the tested hindpaws and activated. When the rat lifted the paw in response to the heat stimulation, the light beam was automatically turned off allowing measurement of the time between the start of the heat stimulation and the foot withdrawal. This time was defined as the withdrawal latency. A 20-second cut-off time of the withdrawal latency was imposed to prevent tissue damage in non-responding animals. However, the withdrawal latencies of all rats tested were less than the cut-off time. Nociception was measured on both right and left hindpaws and mean values from both hindpaws were used for analysis.
FluoroGold (1%, 1 µl) was injected through the same guide cannula to verify the injection site after the last measurement of plantar test and the rats were scarified by an overdose of pentobarbital and perfused with PBS followed by 4% paraformaldehyde in PBS. Brains were removed and processed for Fos/Hcrt immunohistochemical staining.
2.5. Double staining of Hcrt and Fos
Brains were fixed for 8 h in formalin, equilibrated in PBS with 30% sucrose, and stored at 4°C. Five series of coronal sections encompassing the area between 0 mm and 5 mm from bregma were cut at 40 µm thickness on a freezing microtome. Brain sections were treated with 1% H2O2 for 15 min to quench endogenous peroxidases and then incubated overnight in rabbit-anti-cFos antisera (1:15,000; Calbiochem, EMD, Millipore) at room temperature. Tissue was then rinsed in PBS, incubated in biotinylated donkey anti-rabbit IgG (1:500; Jackson ImmunoResearch) for 2 h at room temperature, incubated with peroxidase-conjugated avidin–biotin complex (ABC; Vector Laboratories) for 1 h, and followed by the incubation in 0.05% diaminobenzidine tetrahydrochloride and 0.01% H2O2 with 1% NiSO4 to produce a black reaction product in cell nuclei. The sections were then incubated in goat anti-orexin-B antiserum (1:5,000; Santa Cruz Biotechnology). Tissue was rinsed in PBS, incubated in biotinylated donkey anti-goat IgG (1:500; Jackson ImmunoResearch) for 1 h at room temperature, incubated with biotin-conjugated alkaline phosphatase (ABCAP) for 2 h (Vector Laboratories), washed again, and reacted in a working solution of Vector Red substrate (Vector Red AP Substrate Kit I; Vector Laboratories) to produce a red reaction product. All tissue was mounted on gelatin-coated slides and coverslipped. Dilutions of antibodies was done in 5% donkey serum (Jackson ImmunoResearch), PBS, and 1% Triton X-100.
2.6. Cell counts
Brain sections were examined under light microscopy (Leica DM5000B) equipped with a CCD video camera operating with a computer-based, anatomical-mapping and image-analysis system (StereoInvestigator, Microbrightfield). A single examiner, blind to treatment conditions, performed cell counts. Fos was counted in Hcrt cells located medially or laterally with respect to the fornix (f, Figs. 3 and 4). The regions for cell counting were selected by outlining them bilaterally at low power magnification (×5 objective). The selected regions were then scanned at high magnification (×20 objective) for the presence of Hcrt-single (Fos−/Hcrt+) and double-labeled (Fos+/Hcrt+) neurons. Neurons were considered to be Fos+ if they contain black Ni+-DAB reaction product that was darker than a visually established threshold. Hcrt cells were counted in brain sections located at −2.8 mm from bregma. The counts were averaged and used for further statistical analysis.
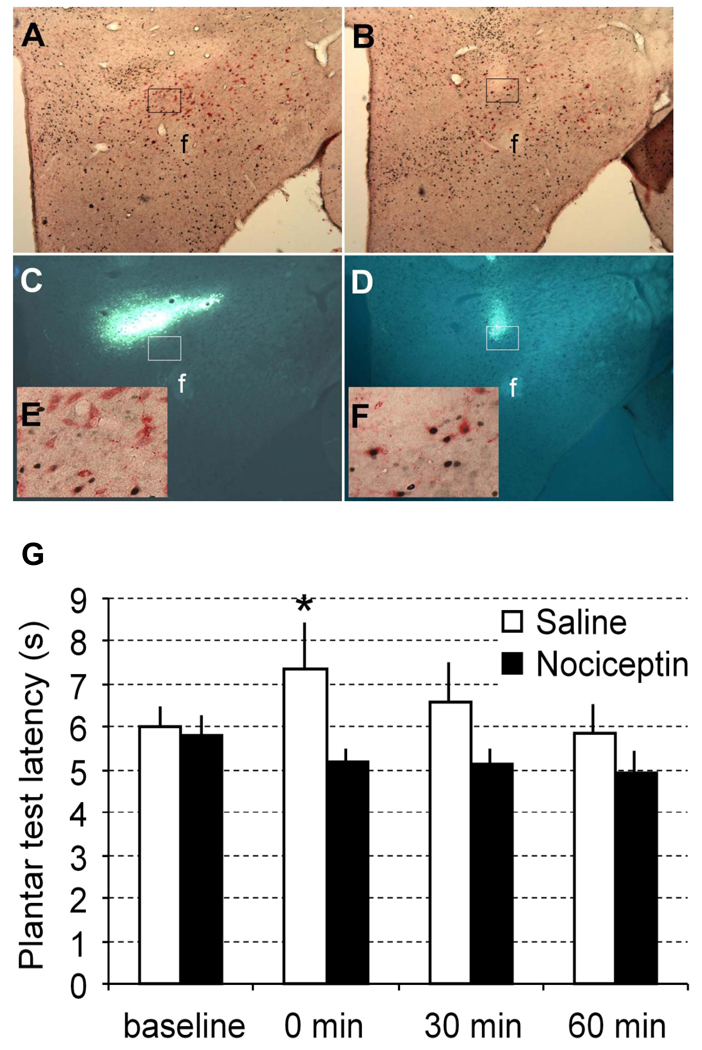
Fig. 3.
Microinjection of N/OFQ into the rat lateral hypothalamus abolishes stress-induced analgesia. Distribution of Hcrt- and Fos-immunoreactive cells in a representative brain section of a rat injected with N/OFQ (A) or with saline (B). Location of the injection site in the same brain section as A indicated by Fluorogold fluorescence (C). Note that the location of the injection site is dorsal to the distribution of Hcrt neurons. Location of the injection site in the same brain section as B indicated by Fluorogold fluorescence (D). The insert in (E) showing high magnification of the brain area marked by a rectangle in A and C. The insert in (F) showing high magnification of the brain area marked by a rectangle in B and D. Hcrt cells are labeled in red, and Fos nuclei are labeled in black. f, fornix. (G) Plantar test in rats before and after restraint stress. The plantar test was repeated in four measurements. The first baseline measurement was performed 30 min before the restraint, the second measure was done immediately after the release from the restraint (0 min), the third and fourth measures were made at 30 min and 60 min after the release from the restraint, respectively. *P<0.05 between N/OFQ and saline groups at the same timepoint.
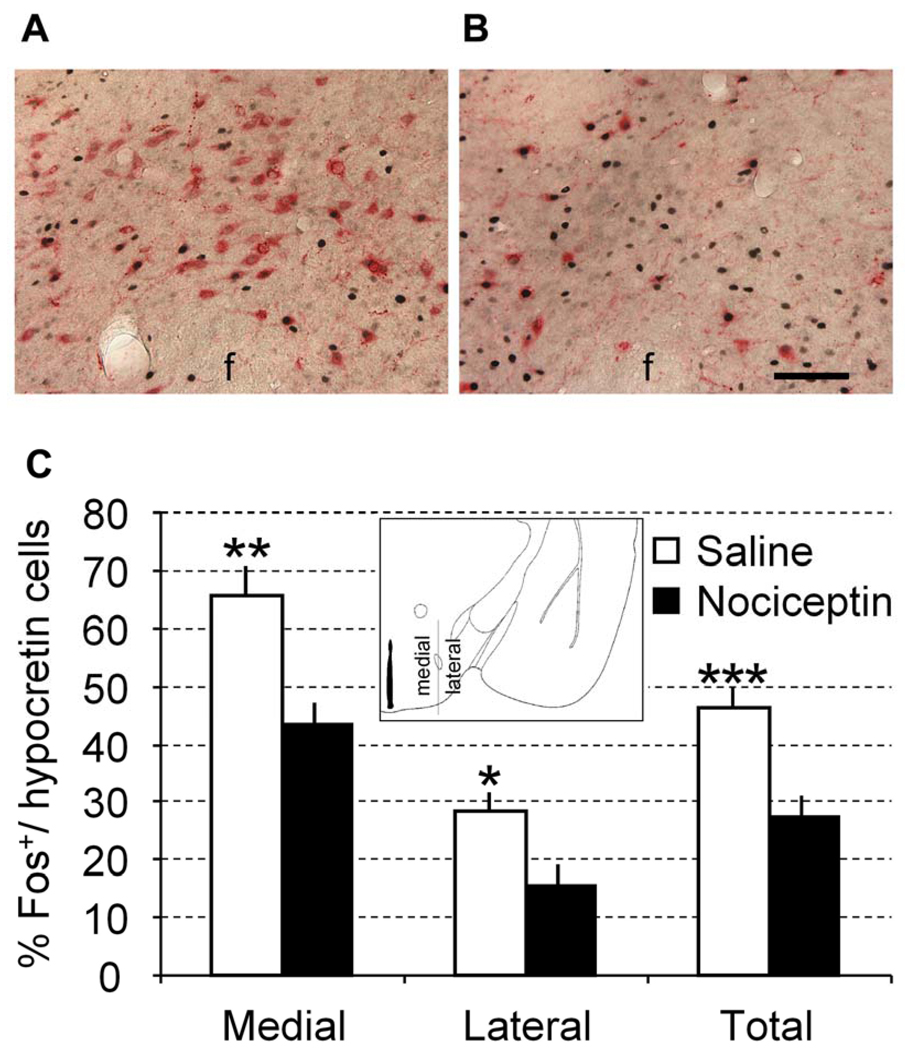
Fig. 4.
Local microinjection of N/OFQ depresses Fos expression in Hcrt neurons in the rats. Representative images show Hcrt-immunoreactive cells (red color) expressing Fos (black color) in the rats injected with N/OFQ (A) or with saline (B). Percentage of Fos+Hcrt neurons is shown in rats injected with N/OFQ or saline (C). The counts were analyzed separately for the Hcrt neurons located medially and laterally to the fornix (f), as schematically shown in the insert. Total counts include Hcrt neurons located on both sides of the fornix. Paired comparisons versus corresponding saline group, Mann Whitney U test: *p < 0.05; **p <. 0.02; ***p < 0.01. Scale bar represents 100 µm.
2.7. Statistical methods
Data were presented as means ± SEM and analyzed by using two-way ANOVA and the Student–Newman–Keuls posthoc test in behavioral pain test. Counts of Fos-immunoreactive nuclei in Hcrt cells were expressed as % from the total number of HCRT cells and compared by using Mann–Whitney tests. Differences were considered significant at P < 0.05.
3. Results
3.1. Neuroanatomical connection between Hcrt- and N/OFQ-immunoreactive neurons
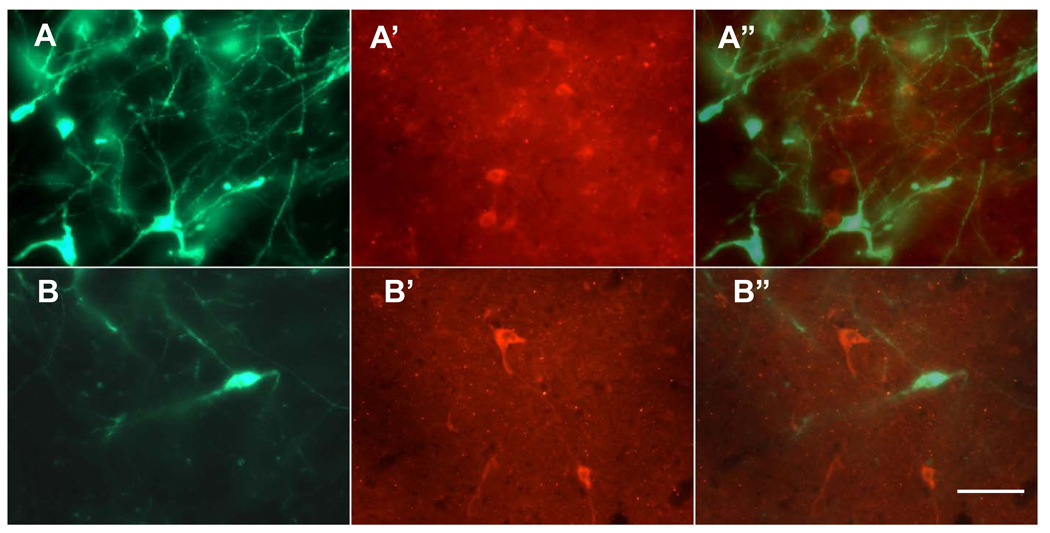
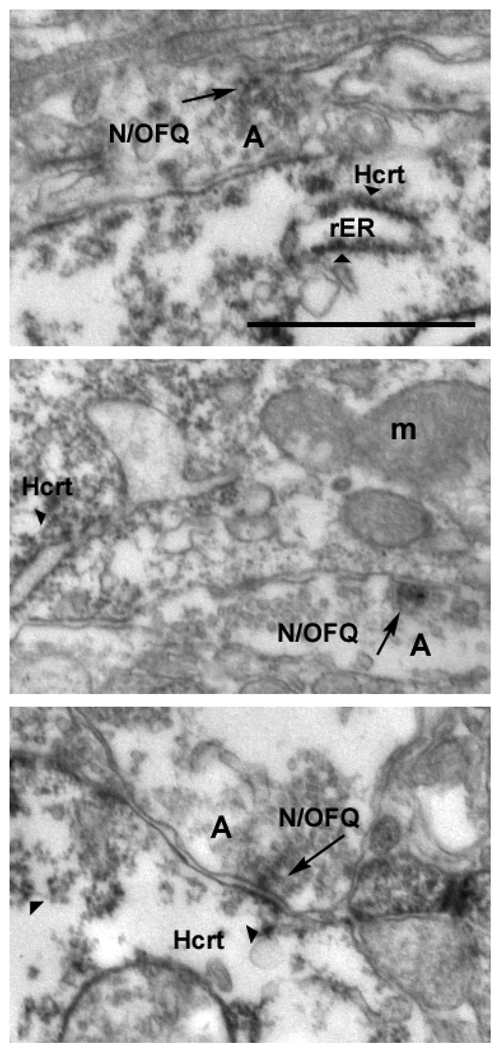
To demonstrate a direct modulation of N/OFQ on the Hcrt neurons in the rat brain, we started with identifying the sources of N/OFQ input to the Hcrt neurons. Although Hcrt neurons do not constitute a nucleus, they are exclusively located in the lateral hypothalamus. In contrast, N/OFQ-containing neurons and fibers are widely distributed throughout the brain of rodents (Neal, Jr. et al., 1999;Anton et al., 1996). We observed N/OFQ-immunoreactive cells in the lateral hypothalamic area and in the area dorsal to the core of distribution of Hcrt-immunoreactive cells. Both neuropeptides are not co-localized in the same neurons, raising a possibility that Hcrt and N/OFQ neurons can be connected and form a local circuit (Fig. 1). Therefore, we investigated whether these two peptidergic systems are synaptically connected using an electron microscopic (EM) analysis. Figure 2 presents three examples of N/OFQ-immunolabeled axon terminals evidently in synaptic contacts with Hcrt-immunolabeled dendrites of Hcrt neurons from different rat brains.
Fig. 1.
Hcrt-immunoreactive cells (green color) and N/OFQ-immunoreactive cells (red color) are located in the perifornical area (A and A’) and in the area dorsal to perifornical area (B and B’). A” shows merged images of A and A’, and B” shows merged images of B and B’.
Fig. 2.
Electron micrograph showing an asymmetrical synaptic membrane specialization (black arrow) between the N/OFQ bouton-like structure and the Hcrt immunolabeled dendrite in the lateral hypothalamus. Annotations to the photographs indicate the different cellular elements: A to indicate axon terminals, arrowheads to indicate Hcrt IHC postsynaptically, rER to point to rough endoplasmaic reticulum, and m to indicate mitochondria. Scale bar represents 1 µm.
3.2. Local microinjection of N/OFQ into the perifornical area of the lateral hypothalamus blocks stress-induced analgesia
To investigate whether local microinjection of N/OFQ into the lateral hypothalamic area alters nociception and modulates SIA, focal thermal nociceptive pain threshold in rats was assessed using the plantar test. Nociception threshold indicated by the paw withdrawal latency was measured at 30 min before and 0, 30 and 60 min after release from the restraint immobilization. Immediately after the release from the restraint (0 min), the paw withdrawal latency was increased by 20.5±7.6% compared to baseline in control group (with saline microinjection, n = 5), indicating the generation of SIA. The SIA gradually waned and returned to the baseline in 60 min (Fig. 3). Direct microinjection of N/OFQ (9µg/side per rat) locally into both sides of the perifornical area in the lateral hypothalamus at the beginning of the restraint completely abolished the SIA phenomenon, whereas the SIA persisted in the saline microinjection (n = 6 for N/OFQ group and n = 5 for saline group; two-way ANOVA, factor group, DF=1, F=7.3, P < 0.02). There were significant differences in the paw withdrawal at time point of 0 (P<0.02), but not at 30 and 60 min after the end of immobilization in rats injected with N/OFQ compared to the same timepoint in saline control (Fig. 3). There were no significant differences in the paw withdrawal latency between N/OFQ treated and saline control rats under unrestraint conditions (data not shown). To assess the extent of the diffusion, we injected Fluorogold solution through the implanted guide cannula at the end of the experiment. Histological analysis indicated that Fluorogold was localized mainly within the perifornical area, although some traces of Fluorogold were seen along the track of injection needle (Fig. 3A–D).
3.3. Local microinjection of N/OFQ into the perifornical area depresses Fos expression in Hcrt neurons
To evaluate the proposed direct modulation of N/OFQ on Hcrt neurons, we further measured stress-induced Fos expression in Hcrt neurons, and assessed N/OFQ effects on the Fos expression in Hcrt neurons, using Fos/Hcrt double immunostaining. After completing the behavioral pain test, the rat brains were removed and processed for Fos/Hcrt immunohistochemical staining (Fig. 4A and B). The counts per brain section for Fos+/Hcrt+ and Fos−/Hcrt+ cells are shown in Table 1. Percentage of Fos+/Hcrt neurons in the restraint immobilization group injected with N/OFQ was 27.7±3.7% (n = 6), significantly lower than that saline injection group (46.7±3.1%; n = 5, P< 0.01). Interestingly, the differences between N/OFQ treatment and control groups were greater in the Fos+/Hcrt neurons located medially to the fornix (22.3%, P<0.02) than in Hcrt neurons located laterally to the fornix (13.2%, P<0.05, Fig. 4C).
Table 1.
Average numbers of Fos-positive and Fos-negative Hcrt neurons located medially and laterally to the fornis (see schematic drawing of the counting areas in Fig. 4).
| Fos+/Hcrt+ | Fos−/Hcrt+ | |||||
|---|---|---|---|---|---|---|
| Medial | Lateral | Total | Medial | Lateral | Total | |
| N/OFQ | 45.7±4.5* | 19.2±2.4** | 64.8±4.4** | 61.3±9.2* | 121.0±16.3 | 182.3±22.9* |
| Saline | 76.2±14.3 | 34.4±3.4 | 110.8±13.8 | 36.0±3.6 | 87.3±8.1 | 123.3±6.4 |
Note: Counts are shown per section (−2.8 mm from bregma). Differences between N/OFQ and saline groups are statistically significant by one way ANOVA (DF=11, F=17.981, P<0.001) followed by Student–Newman–Keuls tests on the data.
P<0.05
P<0.01.
4. Discussion
The Hcrt system is well recognized as a central neurotransmitter system in the maintenance of wakefulness, in addition to its roles in feeding, metabolism and reward. Defects in this system result in the sleep disorder narcolepsy in both humans and animal models (for review see Kilduff, 2005;Sakurai, 2005). Several lines of evidence have suggested that the Hcrts play a role in nociceptive processing. Localization of Hcrt fibers in the hypothalamus, thalamus, and periaqueductal gray is consistent with a role in sensory processing (Date et al., 1999). The presence of robust projections from the hypothalamus to lamina I of the spinal cord (van den Pol, 1999), an area associated with nociceptive transmission, strongly implicates Hcrt in the modulation of nociceptive pathways.
Recent studies including those from our laboratory suggest that Hcrt is critical to the generation of SIA (Watanabe et al., 2005;Xie et al., 2008). The SIA was originally thought to be mediated by at least two distinct neuronal mechanisms: opioid- (naloxone-reversible) and non-opioid-mediated pain inhibitory pathways (naloxone-irreversible), depending on the severity of stress. While SIA is partially antagonized by naloxone, it is completely blocked by supraspinal administration of N/OFQ (Calo' et al., 2000; Rizzi et al., 2006). The neural substrates modulated by N/OFQ that could account for antagonism of nonopioid analgesia are currently unclear. Based on our recent original observations in mice (Xie et al., 2008), we hypothesize that N/OFQ modulation of the SIA could occur directly via the inhibition of the Hcrt neurons in the lateral hypothalamus. To test this hypothesis, several criteria must be met to establish that these two peptidergic systems have direct interplay in coordinated regulation of SIA.
First, using immunochemistry methods, we observed that N/OFQ-containing neurons are localized in the LHA but not colocalized with Hcrt neurons (Fig. 1). Furthermore, electron microscopic techniques were employed to establish synaptic contacts between N/OFQ fibers and Hcrt neurons in rats (Fig. 2). This observation is consistent with the findings in other species, e.g. the mouse (Xie et al., 2008). It provides neuroanatomical evidence that the two peptidergic systems have physical contacts at the synaptic level.
Second, microinjection of N/OFQ into the LHA significantly reduced the percentage of Fos+/Hcrt cells in N/OFQ-treated animals compared to saline control, suggesting decreased Hcrt neuronal activity (Fig. 4). Percentage of Fos+/Hcrt neurons is increased by a variety of stressors including electric foot shock, cold exposure (e.g., forced swimming) and restraint immobilization both in mice and rats (Sakamoto et al., 2004;Winsky-Sommerer et al., 2004;Watanabe et al., 2005; Xie et al., 2008). Under our experimental conditions, restraint immobilization resulted in 46.7% of Fos+/Hcrt cells in the LHA in saline control rats while the percentage of Fos+/Hcrt cells was significantly lower (27.7%) in N/OFQ treatment group (Fig. 4).
Several recent studies suggest that Hcrt neurons could be functionally separated into two groups in which the Hcrt neurons located medially to the fornix and in the perifornical area. The Hcrt neurons in the fornix are mainly involved in arousal and waking; whereas those located laterally to the fornix are primarily involved in reward processing (Harris and Aston-Jones, 2006). Significant correlation between Fos expression and sleep-wake behavior was observed in Hcrt neurons in the medial and perifornical region, but not in the lateral region (Estabrooke et al., 2001). In contrast, correlation between Fos expression and reward-seeking behavior was observed only in the subpopulation of Hcrt neurons located laterally to the fornix (Harris et al., 2005). Stress-induced activation of Fos expression was observed in Hcrt neurons located medially to the fornix and in the perifornical region, but not in Hcrt neurons located laterally to the fornix (Harris et al., 2005). Consistent with these observations, in the present study we also found that the difference in Fos expression in Hcrt neurons between rats injected with saline and N/OFQ was higher in the medial than lateral area (Fig. 4). This result indicates that Hcrt neurons located medially to the fornix are more likely to be involved in the SIA regulation than the neurons located laterally to the fornix.
Although there are no specific antibodies against NOP are available at present, the NOP-mediated functional responses were supported by direct cellular inhibitory effects of N/OFQ on the Hcrt neurons. Using electrophysiological techniques, N/OFQ has been shown to consistently produce potent and long-lasting inhibitory actions via activating G protein-coupled inwardly rectifying K+ (GIRK) channels on all types of neurons studied in different regions of the rodent brain (Heinricher, 2003;Chiou et al., 2004). We recently showed that N/OFQ could directly modulate Hcrt neuronal activity via both pre- and post-synaptic mechanisms (Xie et al., 2008).
Finally, a link between N/OFQ and Hcrt at the whole organism level must be demonstrated. We showed that local microinjection of N/OFQ into the perifornical area in the lateral hypothalamus prevented SIA in rats (Fig. 3). This N/OFQ action is consistent with its effects in mice through i.c.v. administration (Calo' et al., 2000;Rizzi et al., 2006;Xie et al., 2008). The effective dose of N/OFQ in this study is apparently higher than the dose of N/OFQ used in other studies using microinjections to other brain areas in rats (Economidou et al., 2008;Chen et al., 2008;Rodi et al., 2008;Yang et al., 2003). The difference in the dose required could be explained by the different neruobehavior endpoints, and/or by the location of the injection areas. Although N/OFQ was injected locally into the perifornical area in our study, there is a possibility that it could diffuse into some adjacent brain regions. However, injected Fluorogold solution through the implanted guide cannula at the end of the experiment and histological analysis showed that Fluorogold was localized mainly within the lateral hypothalamus (Figure 3).
During stress, activation of the hypothalamic-pituitary-adrenal axis (HPA) axis is initiated by corticotropin-releasing factor (CRF), which is synthesized in the paraventricular hypothalamic nucleus (PVN). CRF-immunoreactive terminals make direct synaptic contacts with Hcrt-expressing neurons, and numerous Hcrt neurons express the CRF-R1/2 receptors (Winsky-Sommerer et al., 2004). Furthermore, CRF excites a subpopulation of Hcrt cells through CRF-R1 receptors, as indicated by an increase in Fos expression in the Hcrt neurons in response to acute stress (e.g., foot shock or restraint) (Winsky-Sommerer et al., 2004). Based on these observations, it is likely that upon stress CRF can directly activate Hcrt neurons. Presumably, the consequent increase in release of Hcrt activates pain inhibitory pathway and contributes to the generation of SIA.
It has been suggested that endogenous N/OFQ release may increase during acute stress in some brain areas, such as the basal forebrain (Devine et al., 2003). The basal forebrain neurons project to the lateral hypothalamus (Tomimoto et al., 1987) in which Hcrt neurons are exclusively located (De Lecea et al., 1998; Peyron et al., 1998). Locally distributed N/OFQ-containing neurons are other possible source of N/OFQ in the lateral hypothalamus (Neal, Jr. et al., 1999). Indeed, we found N/OFQ-immunoreactive cells in the vicinity of Hcrt cells and the N/OFQ fibers form synaptic contacts with Hcrt neuron dendrites (Figure 1 and 2). The N/OFQ released during acute stress could directly interact with Hcrt neurotransmission, but in a negative manner to modulate the magnitude and duration of the SIA. The opposite modulation by CRF and N/OFQ on the Hcrt neuronal activity would provide a fine tuning on SIA and possibly on other stress adaptive responses and beyond (Johnson, et al., 2010).
Research Highlights
Stress-induced analgesia (SIA) plays a role in neuroadaptation to threats and is a defensive behavioral response to prepare for “fight or flight”.
The neuropeptide hypocretin/orexin (Hcrt) is essential in the generation of SIA.
The neuropeptide nociceptin/orphanin FQ (N/OFQ) system negatively modulate the SIA.
These two peptidergic systems have synaptic contacts and can interact directly in the lateral hypothalamus to fine tune the SIA and other stress adaptive responses.
Acknowledgments
This study was supported by grants from the National Institute of Mental Health (R01 MH078194 and R43MH076309) and the National Institute of Neurological Disorders and Stroke (R43 NS065555).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amit Z, Galina ZH. Stress-induced analgesia: adaptive pain suppression. Physiol Rev. 1986;66:1091–1120. doi: 10.1152/physrev.1986.66.4.1091. [DOI] [PubMed] [Google Scholar]
- Amit Z, Galina ZH. Stress induced analgesia plays an adaptive role in the organization of behavioral responding. Brain Res.Bull. 1988;21:955–958. doi: 10.1016/0361-9230(88)90033-0. [DOI] [PubMed] [Google Scholar]
- Anton B, Fein J, To T, Li X, Silberstein L, Evans CJ. Immunohistochemical localization of ORL-1 in the central nervous system of the rat. J.Comp Neurol. 1996 Apr 29;368:229–251. doi: 10.1002/(SICI)1096-9861(19960429)368:2<229::AID-CNE5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Blair R, Galina ZH, Holmes LJ, Amit Z. Stress-induced analgesia: A performance deficit or a change in pain responsiveness? Behav.Neural Biol. 1982;34:152–158. doi: 10.1016/s0163-1047(82)91538-2. [DOI] [PubMed] [Google Scholar]
- Butler RK, Finn DP. Stress-induced analgesia. Prog.Neurobiol. 2009;88:184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Calo' G, Guerrini R, Bigoni R, Rizzi A, Marzola G, Okawa H, Bianchi C, Lambert DG, Salvadori S, Regoli D. Characterization of [Nphe(1)]nociceptin(1–13)NH(2), a new selective nociceptin receptor antagonist. Br.J.Pharmacol. 2000;129:1183–1193. doi: 10.1038/sj.bjp.0703169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LY, Huang JX, Yu LC. Involvement of ORL1 receptor and ERK kinase in the orphanin FQ-induced nociception in the nucleus accumbens of rats. Regul.Pept. 2008 Nov 29;151:43–47. doi: 10.1016/j.regpep.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Chiou LC, Fan SH, Chuang KC, Liao YY, Lee SZ. Pharmacological characterization of nociceptin/orphanin FQ receptors, a novel opioid receptor family, in the midbrain periaqueductal gray. Ann.N.Y.Acad.Sci. 2004;1025:398–403. doi: 10.1196/annals.1316.049. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc.Natl.Acad.Sci.U.S.A. 1999 Jan 19;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc.Natl.Acad.Sci.U.S.A. 1998 Jan 6;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine DP, Hoversten MT, Ueda Y, Akil H. Nociceptin/orphanin FQ content is decreased in forebrain neurones during acute stress. J.Neuroendocrinol. 2003;15:69–74. doi: 10.1046/j.1365-2826.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, Fedeli A, Martin-Fardon R, Massi M, Ciccocioppo R, Heilig M. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol.Psychiatry. 2008 Aug 1;64:211–218. doi: 10.1016/j.biopsych.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J.Neurosci. 2001 Mar 1;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006 Aug 10; doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005 Sep 22;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Heinricher MM. Orphanin FQ/nociceptin: from neural circuitry to behavior. Life Sci. 2003;73:813–822. doi: 10.1016/s0024-3205(03)00412-0. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Träskman-Bendz L, Goddard AW, Brundin L, Shekhar A. A key role for orexin in panic anxiety. Nat Med. 2010;16(1):111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am.J.Physiol Regul.Integr.Comp Physiol. 2003;285:R581–R593. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- Kenunen OG, Prakh'e IV. Is stress-induced analgesia a measure of stress severity? Ross.Fiziol.Zh.Im I.M.Sechenova. 2005;91:277–285. [PubMed] [Google Scholar]
- Kenunen OG, Prakh'e IV, Kozlovskii VL. Changes in anxiety levels are followed by changes in behavioral strategy in mice subjected to stress and in the extent of stress-induced analgesia. Neurosci.Behav.Physiol. 2006;36:151–156. doi: 10.1007/s11055-005-0173-3. [DOI] [PubMed] [Google Scholar]
- Kilduff TS. Hypocretin/orexin: maintenance of wakefulness and a multiplicity of other roles. Sleep Med.Rev. 2005;9:227–230. doi: 10.1016/j.smrv.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Koster A, Montkowski A, Schulz S, Stube EM, Knaudt K, Jenck F, Moreau JL, Nothacker HP, Civelli O, Reinscheid RK. Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc.Natl.Acad.Sci.U.S.A. 1999;96:10444–10449. doi: 10.1073/pnas.96.18.10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Montagne-Clavel J, Oliveras JL. The "plantar test" apparatus (Ugo Basile Biological Apparatus), a controlled infrared noxious radiant heat stimulus for precise withdrawal latency measurement in the rat, as a tool for humans? Somatosens.Mot.Res. 1996;13:215–223. doi: 10.3109/08990229609052577. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J.Comp Neurol. 1999;406:503–547. [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, De Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J.Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Marzola G, Bigoni R, Guerrini R, Salvadori S, Mogil JS, Regoli D, Calo G. Endogenous nociceptin signaling and stress-induced analgesia. Neuroreport. 2001;12:3009–3013. doi: 10.1097/00001756-200110080-00006. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Nazzaro C, Marzola GG, Zucchini S, Trapella C, Guerrini R, Zeilhofer HU, Regoli D, Calo' G. Endogenous nociceptin/orphanin FQ signalling produces opposite spinal antinociceptive and supraspinal pronociceptive effects in the mouse formalin test: pharmacological and genetic evidences. Pain. 2006;124:100–108. doi: 10.1016/j.pain.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Rodi D, Zucchini S, Simonato M, Cifani C, Massi M, Polidori C. Functional antagonism between nociceptin/orphanin FQ (N/OFQ) and corticotropin-releasing factor (CRF) in the rat brain: evidence for involvement of the bed nucleus of the stria terminalis. Psychopharmacology (Berl) 2008;196:523–531. doi: 10.1007/s00213-007-0985-7. [DOI] [PubMed] [Google Scholar]
- Sakamoto F, Yamada S, Ueta Y. Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons. Regul.Pept. 2004 May 15;118:183–191. doi: 10.1016/j.regpep.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Sakurai T. Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med.Rev. 2005;9:231–241. doi: 10.1016/j.smrv.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Tomimoto H, Kamo H, Kameyama M, McGeer PL, Kimura H. Descending projections of the basal forebrain in the rat demonstrated by the anterograde neural tracer Phaseolus vulgaris leucoagglutinin (PHA-L) Brain Res. 1987 Nov 10;425:248–255. doi: 10.1016/0006-8993(87)90507-5. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J.Neurosci. 1999 Apr 15;19:3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Kuwaki T, Yanagisawa M, Fukuda Y, Shimoyama M. Persistent pain and stress activate pain-inhibitory orexin pathways. Neuroreport. 2005 Jan 19;16:5–8. doi: 10.1097/00001756-200501190-00002. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, De Lecea L. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J.Neurosci. 2004 Dec 15;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wisor JP, Hara J, Crowder TL, LeWinter R, Khroyan TV, Yamanaka A, Diano S, Horvath TL, Sakurai T, Toll L, Kilduff TS. Hypocretin/orexin and nociceptin/orphanin FQ coordinately regulate analgesia in a mouse model of stress-induced analgesia. J.Clin.Invest. 2008;118:2471–2481. doi: 10.1172/JCI35115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZL, Gao YJ, Wu GC, Zhang YQ. The rostral ventromedial medulla mediates the facilitatory effect of microinjected orphanin FQ in the periaqueductal gray on spinal nociceptive transmission in rats. Neuropharmacology. 2003;45:612–622. doi: 10.1016/s0028-3908(03)00234-x. [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK. Characterization of the foot withdrawal response to noxious radiant heat in the rat. Pain. 1994;59:85–94. doi: 10.1016/0304-3959(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Zhang C, McDougall JJ. Stimulation of sensory neuropeptide release by nociceptin/orphanin FQ leads to hyperaemia in acutely inflamed rat knees. Br.J.Pharmacol. 2006;148:938–946. doi: 10.1038/sj.bjp.0706804. [DOI] [PMC free article] [PubMed] [Google Scholar]