Abstract
Background & Aims
Interactions between lymphocytes and intestinal epithelial cells (IECs) occur in the sub-epithelial space of the gastrointestinal tract. Normal human lamina propria lymphocytes (LPLs) induce differentiation of IECs. The absence of LPLs in mice, such as in RAG1−/− mice, results in defect in epithelial cell differentiation. We investigated the role of lympho–epithelial interactions in epithelial differentiation and barrier function.
Methods
We used adoptive transfer to determine if CD4+ T cells (CD4+CD62L+CD45RbHi and/or CD4+CD62L+CD45RbLo) could overcome permeability defect (quantified in Ussing chambers). Immunofluorescence staining was performed to determine expression of cleaved Notch-1, villin, and claudin-5 in colon samples from mice and humans. Caco-2 cells were infected with a lentivirus containing a specific Notch-1 or scrambled shRNA sequence. Tight junction assembly was analyzed by immunoblot and immunofluorescence analyses and transepithelial resistance was monitored.
Results
Expression of cleaved Notch-1, villin, or claudin-5 was not detected in RAG1−/− colonocytes; their loss correlated with increased intestinal permeability. Transfer of CD45RbHi and/or CD45RbLow cells into RAG1−/− mice induced expression of cleaved Notch, villin, and claudin-5 in colonocytes and significantly reduced the permeability of the distal colon. Loss of Notch-1 expression in Caco-2 cells correlated with decreased trans-epithelial resistance and dysregulated expression and localization of tight junction proteins. Levels of cleaved Notch-1 were increased in colonic epithelium of patients with Crohn’s disease.
Conclusion
LPLs promote mucosal barrier function, which is associated with activation of the Notch-1 signaling pathway. LPLs maintain intestinal homeostasis by inducing IEC differentiation, polarization, and barrier function.
Keywords: Inflammatory bowel disease, immune system
INTRODUCTION
The control of immune responses in the gut is critical for normal mucosal homeostasis in the host. Failure to control such responses has been proposed as one component in the development of inflammatory bowel disease (IBD) (1). The ability of the mucosal immune system to communicate with the overlying intestinal epithelium has been described in different models. We have reported that the crosstalk between lamina propria lymphocytes (LPLs) and intestinal epithelial cells (IECs) leads to IEC differentiation (2–4). This interaction results in increased epithelial differentiation as evidenced by expression of intestinal alkaline phosphatase (IAP), the transcription factors CDX2 and SOX9, and CEACAM5. The nature of this crosstalk is contact-dependent and not inflammation-related. These observations support our initial hypothesis that lymphocytes affect epithelial cell differentiation.
Our work showed that Crohn’s disease (CD) LPLs were more effective at inducing IEC differentiation than normal or Ulcerative colitis (UC) LPLs. Moreover, in vivo the colonic epithelium in CD patients displayed an increase in colonocyte differentiation (2). This accelerated pattern of differentiation was exclusively restricted to CD. Based on the early appearance of bi-clonal crypts in CD mucosa (indicating abnormal epithelial differentiation), we suggested that early changes in epithelial cell differentiation and migration could be involved in this process.
The epithelium is characterized by its rapid and constant renewal. The Notch signaling pathway regulates cell-fate decisions through close-range, cell-cell interactions (5). Notch is highly expressed in intestinal stem cells. Notch up-regulates the expression of the transcriptional repressor Hes-1, which in turns inhibits the expression of Math-1, a basic helix-loop-helix transcription factor. Math-1 expressing progenitor cells differentiate into secretory cells, while absorptive cells arise from progenitor cells that are Math-1 independent and express Hes-1 (6). Yang et al. proposed that Math1 expression is needed for IECs to make the first lineage-specifying commitment (7). Without Math1, cells remained in the progenitor stem cell pool and could only differentiate into enterocytes. These initial differentiation events are affected by the position of the epithelial cells along the crypt-villus axis and by their interactions with neighboring cells (8). The on/off switch regulating this pathway is not only responsible for the differentiation of one precise cell type but is also able to balance the pool of absorptive versus secretory cells. Notch-1 is critically important for such homeostasis and there are no human mutations known in this gene supporting its vital role. Indeed, there do not appear to be any known mutations of Notch 2 or 4 either, suggesting that this pathway is critical to normal homeostasis. Mice homozygous for loss-of-function alleles of Notch- 1 die between embryonic day 10 and 12, and no homozygous embryos have been recovered past embryonic day 12 (9, 10).
The purpose of this study was to understand the nature of the interactions between normal LPLs and IECs in order to ultimately characterize the defect occurring in IBD patients. We show that in RAG1−/− deficient mice there is a defect in barrier function associated with a defect with epithelial cell differentiation. Using an adoptive transfer model, these defects could be overcome by the presence of CD4+ T cells. The generation of Notch-1 knocked down cell lines confirmed a direct link between Notch-1 and barrier function. We also demonstrate that the activation of Notch-1 spreads throughout the epithelium in CD mucosa compared to normal or UC mucosa. Overall, our findings point toward a crucial role for LPLs in regulating epithelial cell differentiation and function.
METHODS
Mouse transfer model
C57BL/6 (WT) and RAG1 deficient mice, (Jackson Laboratories, Bar Harbor, ME), were bred and housed in the Mount Sinai School of Medicine SPF barrier facility. CD4+ T cells were isolated from WT spleens and lymph nodes using an anti-CD4 mAb conjugated to magnetic microbeads and the MACS Separation Columns kit according to the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA). The cells were stained with APC-conjugated anti-CD4, PE-conjugated anti-CD62L and FITC-conjugated anti-CD45Rb mAbs (eBioscience, San Diego, CA), and sorted at the Mount Sinai Flow Cytometry Core Facility (more than 95% pure). 3×105 CD4+CD62L+CD45RbHi T cells with or without 3×105 CD4+CD62L+CD45RbLo T cells, or 3×105 whole CD4+ T cells were adoptively transferred into RAG1−/− mice by intra-peritoneal injection. The efficiency of the transfer was assessed by measuring peripheral CD4+ T cells by Flow cytometry. Disease activity was monitored weekly on the basis of body weight, and the presence or absence of soft stool or diarrhea. The mice were sacrificed 3 or 6 weeks after T cell reconstitution.
All mouse procedures were approved by the institutional animal care and use committee.
Immunofluorescence staining of murine colonic tissues
Paraffin embedded colonic tissue sections were obtained and treated as previously described using anti-cleaved Notch-1 (Cell Signaling, Danvers, MA), anti-villin (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-claudin 5 (Invitrogen Carlsbad, CA) antibodies (3). The slides were examined with a Leica SP5-DM confocal microscope at 40X and 63X magnification.
Permeability studies in mice
After sacrificing the mice, the colons were put into a 10mM mannitol-Krebs solution. The colon was divided into ascending, transverse, and distal segments. From each segment, a sample was taken for histological analysis of inflammation. Fresh tissues were mounted on a chamber slide connected to Voltage-clamp Ussing chambers (Physiological Instruments, San Diego, CA). A 10mM glucose-Krebs solution was added to the serosal side whereas a 10mM mannitol-Krebs solution was added to the luminal side. Each half-cell was continuously maintained at 37°C and oxygenated. Electrophysiological measurements were performed using a transepithelial voltage clamp. The resistance was measured after 30 min. A small molecule, Dextran-FITC (3kDa) (Sigma-Aldrich), was added to the luminal side, and permeability of the tissue was assessed by tracking the appearance of fluorescence in the serosal compartment every 30 min for 2 hours (flux across the mucosa).
Notch-1 knock-down in Caco-2 cells
Human 293T cells were transfected with 2.5 mg of a plasmid encoding vesicular stomatitis virus G protein, 7.5 mg of a plasmid encoding gag-pol, and 10 mg of either a retroviral pRS vector encoding Notch-1 shRNA (TCCTCGCAGTGCTTCCAGAGTGCCACCGA) (Origene, Rockville MD), a control pRS vector, or a plasmid encoding GFP using calcium phosphate. After 48h, supernatants were collected, filtered, and concentrated. The pellet was resuspended in 1 ml of medium for infection of Caco-2 cells (ATCC) seeded in 6 well cluster dishes. Viral supernatants containing 5mg/ml polybrene were added to the dishes, which were spun at 800×g for 1h. After two days, puromycin (1mg/ml, Invitrogen) was added to select for transformants.
Immunofluorescence staining of Caco-2 cells
Cells were fixed with methanol at 4°C for 10 min. Immunofluorescence detection was performed using anti-Notch-1 (Santa Cruz Biotechnology), anti-claudin 2, anti-claudin 5, anti-occludin, and anti-ZO-1 antibodies (Invitrogen). The slides were then incubated with the appropriate secondary antibody conjugated to Alexa-594 (Invitrogen) and treated as previously described (3). The slides were examined with a Leica SP5-DM confocal microscope at 63X magnification.
Measure of transepithelial resistance (TER) of Caco-2 monolayers
Cells were seeded at confluence on a 24 Transwell plate (Costar, Corning Inc., Corning, NY). The TER was measured after one week using ENDOHM-6 electrodes (World Precision Instruments, Inc. Sarasota, FL) connected to an Ohm-meter (World Precision Instruments, Inc.).
Western blot analysis of membrane protein extracts
Caco-2 cells were lysed using the manufacturer’s protocol to isolate membrane proteins (ProteoPrep Membrane extraction kit, Sigma-Aldrich). The protein content was quantified using the Bradford method (Biorad, Hercules, CA). Western blot analyses were performed as previously described using anti-claudin 2, anti-claudin 5, anti-claudin 8, anti-occludin, or anti-ZO-1 antibodies (Invitrogen) (2).
Immunohistochemical staining of colonic tissues
Biopsies from patients undergoing endoscopic examination at the Mount Sinai Medical Center were paraffin-embedded. Normal, CD and UC tissue sections were treated as previously described using an anti-cleaved Notch-1 antibody (Abcam, Cambridge, MA) (2). The slides were examined with a Zeiss Axioskop light microscope at 20X magnification.
Statistical analysis
All experiments were repeated at least 5 times and a representative result is shown for each experiment. Results are presented as the mean ± standard error. Statistical significance was determined by One-way Anova (when there were more than 2 groups), or t-test (2 groups only). A value of p < 0.05 was considered significant.
RESULTS
Cleaved Notch-1 is down-regulated in RAG1 deficient mice
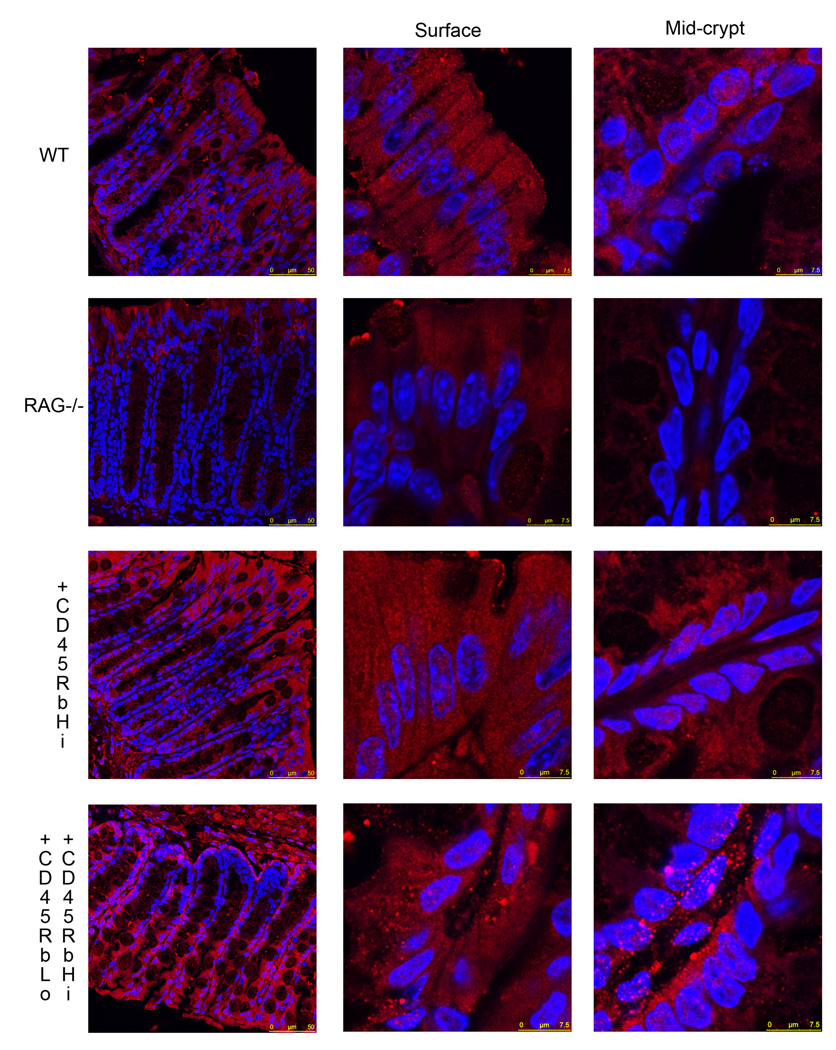
We have previously shown that RAG1 deficient mice exhibited impaired colonic IEC differentiation (2). Engagement of Notch receptors by Notch ligands induces proteolytic cleavage of Notch, regulating proliferation and differentiation of IECs toward an absorptive lineage. The role of Notch-1 signaling was analyzed ex vivo in colonic tissue sections by immunofluorescence using an antibody exclusively recognizing the cleaved fragment of Notch-1 in RAG1−/− mice (Fig 1). In the WT colonic mucosa, cleaved Notch-1 was expressed throughout the epithelium with both cytoplasmic and nuclear localization. RAG1 deficient mice demonstrated a marked decrease in nuclear staining in the crypt epithelium. Indeed, it seemed that the nuclear localization of the fluorescence signal rather than the total fluorescence was changed; this was confirmed when the quantification of the total fluorescence associated with the signal was assessed, it was not significant (19.36 ± 1.9 for WT mice vs. 15.9 ± 1.2 mean fluorescence/hpf for RAG deficient mice, ns).
Figure 1.
Colonic tissues sections from C57/BL6 WT, RAG1 deficient mice, as well as RAG1 deficient mice who received either CD4+CD62L+CD45RbHi T cells or CD4+CD62L+CD45RbHi and CD4+CD62L+CD45RbLo T cells were immunostained using an anti-cleaved Notch-1 (red) antibody. The nuclei were stained with Hoechst 33342 (blue). All samples were analyzed with a Leica SP5-DM Confocal microscope at 40× and 63× magnification. These data are representative of 5 experiments.
These data are consistent with our previous findings of decreased IEC differentiation in the RAG1 deficient mice putatively due to the lack of lymphocytes.
Notch-1 activation is restored in RAG1−/− mice following transfer of CD4+ T cells
In order to define the mechanism leading to LPL-induced IEC differentiation, we elected to address the implications of the findings noted above using an adoptive transfer model where CD4+ T cells were transferred into RAG1−/− mice (11). 3×105 CD4+CD62L+CD45RbHi (naïve) with or without 3×105 CD4+CD62L+CD45RbLo (regulatory) T cells were adoptively transferred into RAG1 deficient mice. We monitored the efficiency of transfer by quantifying the presence of peripheral CD4+ T cells in the transferred mice. We sacrificed mice 3 (data not shown) or 6 weeks after transfer before any signs of weight loss in order to avoid effects due to the presence of active inflammation. Colonic tissues were collected and paraffin embedded. Each tissue was blindly graded by a pathologist for inflammation (12). 6 weeks after transfer, the average inflammation score was not significantly different (1.6 ± 0.67 for mice that received naïve T cells, and 0.78 ± 0.22 for mice receiving naïve and regulatory T cells). The effect on Notch-1 signaling was analyzed ex vivo in colonic tissue sections by immunofluorescence using an antibody exclusively recognizing the cleaved fragment of Notch-1 (Fig 2). The presence of CD4+CD62L+CD45RbHi with or without CD4+CD62L+CD45RbLo T cells in transferred RAG1 deficient mice restored IEC cleaved Notch-1 staining in both the cytoplasm and nucleus of both surface and crypt cells. The quantification of the fluorescence associated with the signal showed a significant increase in the mean fluorescence in the transferred mice compared to the RAG deficient mice (39 ± 3.3 for mice transferred with naïve T cells vs 15.9 ± 1.2 mean fluorescence/hpf for RAG deficient mice, p<0.001; and 42.3 ± 3.4 for mice transferred with naïve and regulatory T cells vs 15.9 ± 1.2 mean fluorescence/hpf for RAG deficient mice, p<0.001).
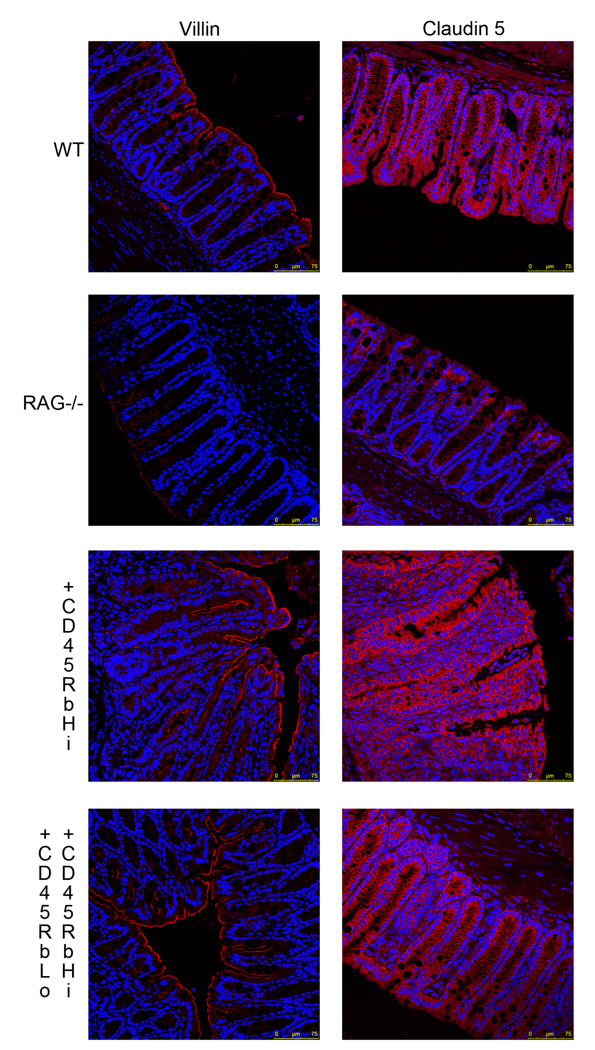
Figure 2.
Colonic tissues sections from C57/BL6 WT, RAG1 deficient mice, as well as RAG1 deficient mice who received either CD4+CD62L+CD45RbHi T cells or CD4+CD62L+CD45RbHi and CD4+CD62L+CD45RbLo T cells were immunostained using an anti-villin (left panel) (red) or anti-claudin 5 (right panel) antibody. The nuclei were stained with Hoechst 33342 (blue). All the samples were analyzed with a Leica SP5-DM Confocal microscope at 40× magnification. These data are representative of 5 experiments.
Villin and claudin-5 expression is decreased in RAG1 deficient mice and restored following CD4+ T cell transfer
The above data suggested that RAG1 deficient mice had altered epithelial differentiation. Therefore, we analyzed the expression of villin, an apical cytoskeletal protein that contributes to the architecture of the epithelial brush border, and serves as a marker of IEC differentiation. Villin expression was analyzed ex vivo in colonic tissue sections by immunofluorescence (Fig 2). WT colonic mucosa stained positively throughout the surface epithelium. RAG1 deficient mice demonstrated a marked decrease (almost a lack of) in villin staining, which was confirmed by quantification of the fluorescence associated with the signal (31.9 ± 4.2 for WT mice vs. 13.3 ± 2.2 mean fluorescence/hpf for RAG deficient mice, p<0.05).
The epithelium creates a barrier between the luminal and subepithelial compartments. A tight paracellular space is critical to the formation of an intact barrier, mediated by TJ proteins. We assessed the expression of claudin-5, one of the TJ proteins by immunofluorescence (Fig 2). WT colonic mucosa stained positively throughout the epithelium. RAG1 deficient mice exhibited impaired claudin-5 staining, which was confirmed by quantification of the fluorescence associated with the signal (52.8 ± 5 for WT mice vs. 25.5 ± 1.9 mean fluorescence/hpf for RAG deficient mice, p<0.05).
These data are consistent with our previous findings of decreased IEC differentiation putatively due to the lack of lymphocytes in the RAG1 deficient mice.
Transfer of CD4+CD62L+CD45RbHi with or without CD4+CD62L+CD45RbLo T cells into RAG1 deficient mice restored the ability of colonic epithelial cells to express both villin and claudin-5. The quantification of the signal associated with villin expression did not show any significant difference upon transfer. However, the fluorescence associated with claudin-5 expression was significantly higher in mice that received naïve T cells (61.3 ± 6.4 for mice transferred with naïve T cells vs. 25.5 ± 1.9 mean fluorescence/hpf for RAG deficient mice, p<0.01). This correlates with the ability of CD4+ T cells to induce Notch-1 signaling and trigger IEC differentiation.
RAG1 deficient mice exhibit permeability defects
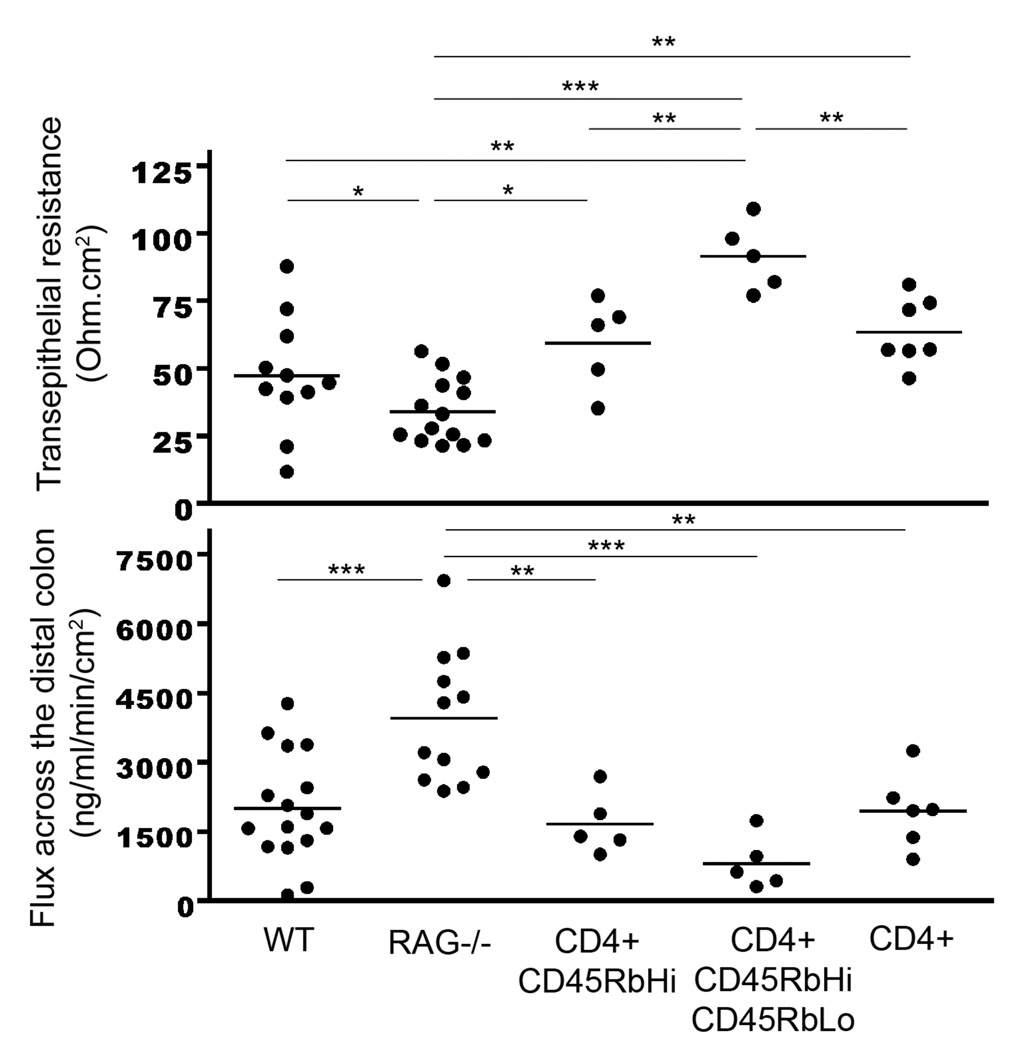
A defect in claudin-5 expression is likely to result in a defect in barrier function. Colonic tissues were divided into 3 segments (proximal, transverse and distal) and mounted onto a 0.3 cm2 chamber slide connected to Voltage-clamp Ussing chambers. Transepithelial resistance (TER) was measured after 30 min. A small molecule, Dextran-FITC (3kDa), was added to the luminal side, and its appearance in the serosal compartment was tracked every 30 min for 2 hours. While no differences were seen between WT and RAG1 deficient mice in the proximal and transverse colon (data not shown), there was a significant decrease in resistance in the RAG−/− distal colon (Fig 3) (47.2 ± 6.4 ohm.cm2 in WT vs. 34 ± 3.2 ohm.cm2 in RAG1 deficient mice, p<0.05). This decrease was associated with a significant increase in Dextran-FITC across the mucosa (2005 ± 294 ng of Dextran-FITC/ml/min/cm2 in WT vs. 3957 ± 415 ng of Dextran-FITC/ml/min/cm2 in RAG1 deficient mice, p<0.001).
Figure 3.
Assessment of changes in epithelial resistance (top panel) as a measure of passive transcellular and paracellular ion transport, and changes in paracellular permeability (lower panel) for small molecules. WT, non-reconstituted RAG−/− mice, RAG−/− mice reconstituted with CD4+CD62L+CD45RbHi T cells, RAG−/− mice reconstituted with CD4+CD62L+CD45RbHi and CD4+CD62L+CD45RbLo T cells or RAG−/− mice reconstituted with whole CD4+ T cells were sacrificed after 6 weeks. The distal colon was harvested and the resistance was measured along with paracellular permeability (assessed by flux measurements of Dextran-FITC from the mucosal to serosal compartment, measured by spectrofluorometry). Data represent the mean ± SEM of at least 5 mice/group. The symbols *, **, *** refer to statistical significance, respectively <0.05, <0.01, <0.001.
Thus, not only is the epithelium less differentiated in RAG1 deficient mice, but the barrier function is also impaired.
Barrier function is restored in RAG1 deficient mice who receive transferred CD4+ T cells
We monitored the barrier function of RAG1 deficient mice who received CD4+CD62L+CD45RbHi T cells with or without CD4+CD62L+CD45RbLo T cells or unfractionated CD4+ T cells after 3 (data not shown) and 6 weeks. When the mice received naïve T cells, the TER and flux were restored to a level similar to WT mice (54.3 ± 7.5 ohm.cm2 and 1660 ± 293 ng Dextran-FITC/ml/min/cm2 in RAG1 deficient mice repleted with naïve T cells vs. 47.2 ± 6.4 ohm.cm2 vs 2005 ± 294 ng Dextran-FITC/ml/min/cm2 in WT mice, p=ns, respectively). When the mice received naïve and regulatory T cells at a 1:1 ratio, the TER and flux were not only restored but also strengthened (91.5 ± 5.7 ohm.cm2 in RAG1 deficient mice repleted with naïve and regulatory T cells vs. 47.2 ± 6.4 ohm.cm2 in WT mice, p<0.01; and 811 ± 255 ng Dextran-FITC/ml/min/cm2 in RAG1 deficient mice repleted with naïve and regulatory T cells vs. 2005 ± 294 ng Dextran-FITC/ml/min/cm2 in WT mice, p=ns).
Co-transfer of naïve with regulatory CD4+ T cells at a 1:1 ratio has been documented to prevent the colitis associated with the adoptive transfer of naïve T cells (11). However, since this ratio is not physiologic, we transferred unfractionated whole CD4+ T cells. Under these conditions, the TER and flux were restored to a level similar to WT mice (respectively, 63.4 ± 4.7 ohm.cm2 and 1944 ± 326 ng Dextran-FITC/ml/min/cm2 in RAG1 deficient mice repleted with whole CD4+ T cells vs. 47.2 ± 6.4 ohm.cm2 and 2005 ± 294 ng Dextran-FITC/ml/min/cm2 in WT mice, p=ns). Thus all three-transfer conditions (naïve, naïve and regulatory, and whole CD4+ T cells) significantly restored resistance and flux when compared to non-transferred RAG1 deficient mice. Altogether these data support a critical role for T cells in the induction of a fully differentiated and functional epithelium.
The Notch-1 signaling pathway is involved in barrier function
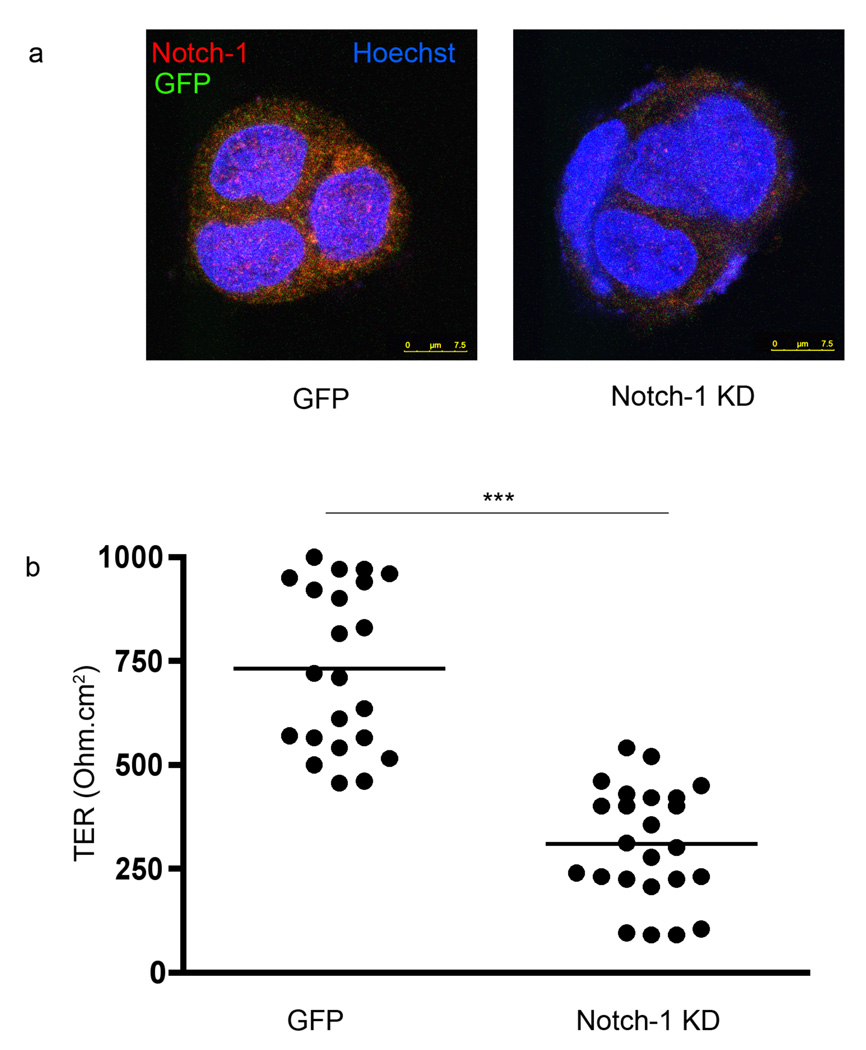
Thus far, the defect in barrier function and the decrease in epithelial cleaved Notch-1 receptor in mice lacking lymphocytes were reversed when the mice received adoptively transferred CD4+ T cells. We have previously shown that LPLs modulate the expression of the transcription factors CDX2 and SOX9, key regulators of IEC differentiation and maturation (2, 3, 13–16). The association between CDX2 and the Notch signaling pathway is well described (17–19). However, a direct link between the Notch signaling pathway and functional TJs does not exist. We therefore decided to knock down Notch-1 in the Caco-2 cell line using an shRNA approach. The Caco-2 cell line exhibits a highly differentiated enterocyte phenotype. The IEC line was infected by a lentivirus containing a GFP encoding plasmid and a puromycin selection cassette with or without Notch-1 shRNA. The efficiency of infection was visualized by fluorescence microscopy, detecting the GFP signal associated with the virus containing the GFP encoding plasmid. Only the clones surviving puromycin selection were maintained in culture. Using this technology, we successfully knocked down Notch-1 as seen in Fig 4a. The GFP and the Notch-1 Knock-Down (KD) Caco-2 lines were seeded on chamber slides, and immunostained for Notch-1. In contrast with the Notch-1 KD Caco-2 cells (right panel), the GFP Caco-2 cells (left panel) exhibited significant red fluorescence consistent with the expression of Notch-1. In order to capture an optimized Notch-1 signal, we used a fixation method (methanol) that resulted in limited detection of GFP (green signal).
Figure 4.
a. GFP (control shRNA) and Notch-1 KD Caco-2 lines were immunostained using an anti-Notch-1 (red) antibody. The presence of GFP (green) was observed at a wavelength of 488nm. The nuclei were staining with Hoechst 33342 (blue). All the samples were analyzed with a Leica SP5-DM Confocal microscope at 63× magnification. These data are representative of 5 experiments. b. Transepithelial resistance (TER) in GFP and Notch-1 KD Caco-2 cell monolayers seeded on Transwells for one week. TER was measured using an Ohm-meter. The symbol *** reflects a statistical significance of p<0.001.
We controlled that Notch-1 KD had an effect on IAP activity, a well-described marker of differentiation (data not shown) (2). Indeed, there was a dramatic decrease in IAP activity when Notch-1 was knocked down (100 ± 0.0% in the GFP line vs. 74.4 ± 4.2% in the Notch-1 KD line, p<0.001) compared to the GFP control.
The lines were seeded and maintained for a week in Transwell cultures and the TER was monitored using an Ohm-meter (Fig 4b). There was a significant decrease in the TER when Notch-1 was knocked down (732 ± 42 Ohm.cm2 in the GFP line vs. 309 ± 28 Ohm.cm2 in the Notch-1 KD line, p<0.001).
Thus, the accumulated evidence supports the concept that the Notch-1 signaling pathway is critical for IEC differentiation and function.
Notch-1 is involved in TJ architecture
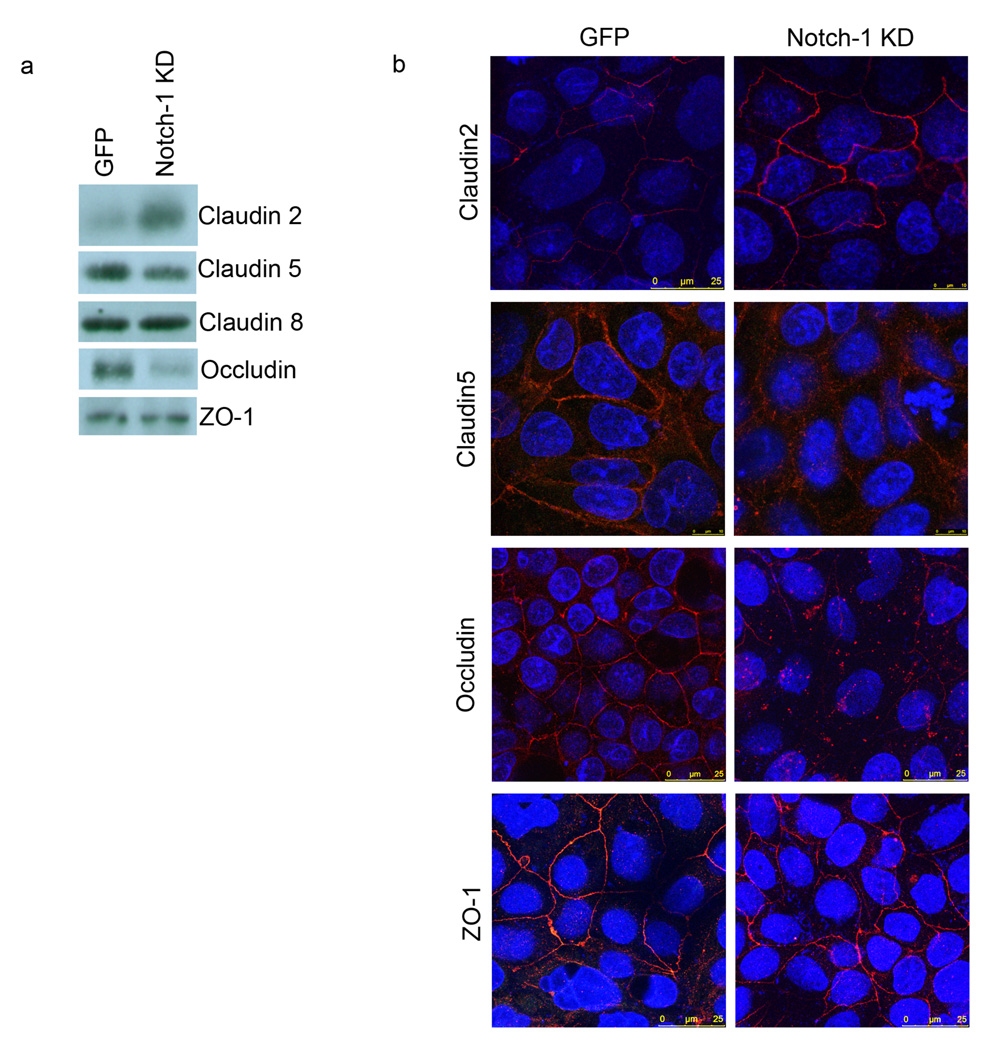
Depending on the nature and the site of the inflammation, occludin, ZO-1 and different claudin proteins have been implicated in the pathogenesis of IBD (20–22). In order to define the mechanism that associates Notch-1 signaling and barrier function, membrane proteins from GFP and Notch-1 KD Caco-2 lines were extracted and subjected to Western blot analysis (Fig 5a). The absence of a Notch-1 signaling pathway dramatically increased claudin-2 expression. Conversely, occludin expression was markely decreased. The effect on claudin-5 was quite mild, and claudin-8 and ZO-1 remained unchanged.
Figure 5.
a. Tight junction protein expression in GFP and Notch-1 KD Caco-2 cell membranes. Immunoblotting for claudin-2, claudin-5, claudin-8, occludin, and ZO-1 in membrane protein extracts obtained from GFP and Notch-1 KD Caco-2 cells. b. GFP and Notch-1 KD Caco-2 lines were immunostained using anti-claudin-2, claudin-5, occludin, and ZO-1 antibodies (red). The nuclei were stained with Hoechst 33342 (blue). All the samples were analyzed with a Leica SP5-DM Confocal microscope at 63× magnification. These data are representative of 5 experiments.
These data were correlated by immunofluorescence staining (Fig 5b). We confirmed that claudin-2 was localized to the cell junction and was up-regulated when Notch-1 was knocked down. Once again, the opposite pattern was observed for occludin. No change was noted in ZO-1 expression or localization, while there was a rearrangement of claudin-5 localization (becoming more cytoplasmic). Several studies have suggested that the stochiometry of the TJ complex is critical for optimal function. Therefore, Notch-1 KD mediated disruption of this stochiometry may account for the abnormal barrier function seen. Altogether, these findings provide evidence for the involvement of the Notch-1 signaling pathway not only in barrier function but also in the architecture of the epithelium.
The activation of Notch-1 spreads throughout the CD epithelium
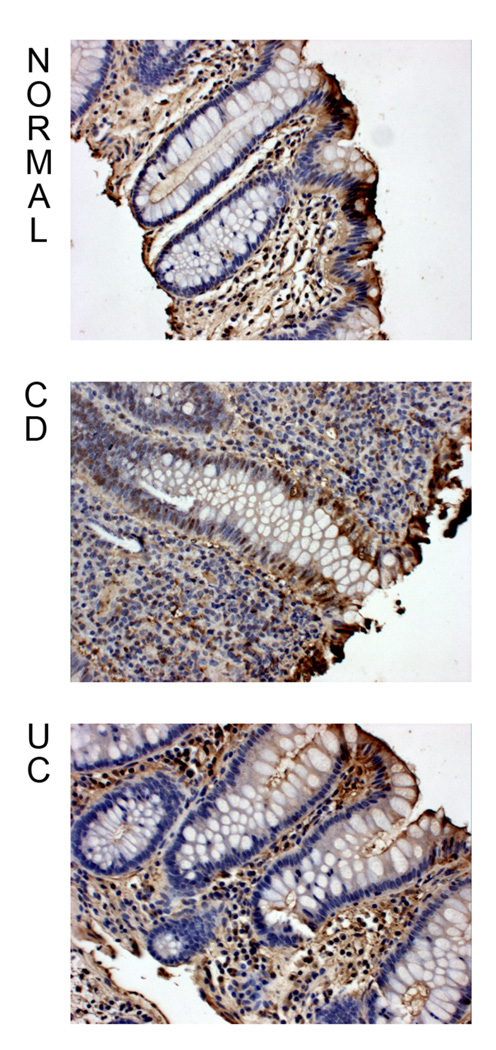
We have previously shown that there is a T cell-driven IEC dysregulation in IBD (2). Therefore, we decided to investigate the role of the Notch signaling pathway in the physiopathology of the disease. We stained colonic tissue sections with an antibody that exclusively recognizes the cleaved fragment of Notch-1 (Fig 6). Normal and UC mucosal epithelium revealed the same pattern of cleaved Notch-1 staining, i.e. cytoplasmic and nuclear expression in surface epithelium, and sporadic nuclear expression in the crypt epithelium. However, in contrast, CD mucosa exhibited a marked increase in nuclear staining in the crypt epithelium.
Figure 6.
Nor, CD, and UC colonic tissue sections were immunostained using an anti-cleaved Notch-1 antibody. The slides were counter-stained with Mayer’s Hematoxylin solution, and examined with a Zeiss Axioskop light microscope at 20X magnification. These data are representative of 5 experiments.
Thus, the Notch pathway appears to be much more activated in epithelial cells in CD mucosa than Normal or UC mucosa. Crypt cells, which are undifferentiated in the normal state, were the most dysregulated cell type in CD.
DISCUSSION
There is growing evidence suggesting that the immune system plays an important role in modulating intestinal permeability. We have previously demonstrated that interactions between IECs and lymphocytes lead to lymphocyte activation (23). In this manuscript, we asked whether lymphocytes could affect epithelial barrier function and whether this effect was altered in lymphopenic mice.
Using the in vivo adoptive transfer model of colitis, we elucidated a critical role of LP cells on barrier function in a non-inflammatory setting. After transfer of naïve and/or regulatory T cells, we restored barrier function in RAG1−/−mice to a level comparable (or even better in presence of regulatory T cells) to WT mice with a normal distribution of the TJ proteins. Thus, LPLs appear to directly regulate epithelial homeostasis.
Boirivant et al. recently showed that an increase in mucosal permeability as the result of a transient inflammatory response led to the induction of regulatory T cells and a state of resistance to the induction of TNBS colitis (24). These findings provided some proof that limited exposure of the mucosal immune system to the microflora is an important mechanism for tolerance induction. Together with our data, the evidence points toward a role for T cell-IEC interactions in barrier integrity. The immune imbalance seen in IBD suggests a different role for specific subsets of T cells in epithelial homeostasis. Understanding the nature of the interactions between these two cell types is crucial, and might aid in our understanding of how epithelial barriers work and how this knowledge can be exploited for disease therapy, drug discovery and drug targeting (25).
In this study, we provide a mechanistic insight into the nature of these interactions. We showed that the interaction between the Notch receptor and its ligand leads to the formation of TJs. This has not been previously described as a functional outcome of the Notch signaling pathway. Notch-1 is expressed in both colonic crypt and lamina propria cells, whereas Notch-2 is only present in the colonic lamina propria (26). With regard to ligand expression, Jagged-1 is only present in colonic crypts whereas Jagged-2 is expressed in both colonic crypt and lamina propria cells. Based upon these previous observations and our current work, Jagged-2 on LP T cells might be involved in triggering the activation of the Notch signaling pathway in IECs. Studies to address this possibility are currently underway in our lab.
The Notch signaling pathway not only constitutes a critical component of fetal development, but it has also been implicated in many disease processes, especially in colorectal carcinomas (26–29). While the Notch pathway has been suggested to be involved in IBD pathogenesis only recently have Gersemann et al. published an exhaustive study on differences in goblet cells in CD versus UC (30). The authors showed that the number of goblet cells was decreased in both forms of IBD, but that only active CD mucosa exhibited an increase in Math1, KLF4, and to a lesser extent in Hes1. These data suggest that the regulation of the Notch signaling pathway is quite different depending on the nature of the inflammatory stimulus. Even though CD and UC exhibit overlapping patterns of inflammation as well as a decrease in goblet cells, the underlying mechanism accounting for these observations is distinct. In our study, we showed that cleaved Notch-1, which is the upstream signal regulating Hes1 expression, is up-regulated in CD epithelia. Interestingly, in this study, we describe some striking differences in the distal colon of RAG1 deficient compared to WT mice. These differences were not as dramatic in other parts of the colon. Thus, there is a level of the complexity of the interplay between the flora, the epithelium and the underlying immune system that is site specific. One could argue that a differential efficiency of gut homing for different T cell subsets according to resident flora might have a potential role in the underlying mechanism that drives IEC differentiation in a site-dependent manner (31). Differences in disease site need to be strongly considered as we attempt to dissect the pathogenesis of IBD.
In this report, we focused only on T cell induced-epithelial barrier function. Certainly, other cellular components within the LP might be involved in maintaining epithelial function in health including B cells, DCs and IELs. DCs and IELs express TJ proteins (32, 33), and DCs and B cells express Jagged-2, which has been implicated in NK cell activation and plasma cell malignancy respectively (34, 35). In the RAG1 deficient mice where there are defects in TER in the distal colon, DCs and the NK cells are present. While these cells might not be fully functional because of the absence of lymphoid populations, we cannot attribute the defect seen in barrier function of the distal colon to these cell subsets. In addition, Kernéis et al. documented the ability of Peyer’s patch B lymphocytes to induce the differentiation of murine IECs and Caco-2 cells into M cells (36), and showed that this was cell contact dependant. This observation supports the hypothesis that different lymphocyte populations can affect epithelial cell differentiation and barrier function (35).
These findings, in conjunction with our own, support the hypothesis that lymphoepithelial crosstalk goes both ways: promoting regulatory T cell activation in addition to enhancing epithelial differentiation and barrier integrity. Disruption of this crosstalk has implications for a number of immune mediated intestinal disorders.
Acknowledgments
Grant support:
This work was supported by the Crohn’s and Colitis Foundation of America (2442) and NIH grants (AI044236, AI084952, DK072201, DK086605). Confocal laser scanning microscopy was performed at the MSSM-Microscopy Shared Resource Facility, supported with funding from NIH-NCI shared resources grant (5R24 CA095823-04), NSF Major Research Instrumentation grant (DBI-9724504) and NIH shared instrumentation grant (1 S10 RR0 9145-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution:
S.D. did all experiments, and collected and analyzed all data with assistance from L.M. K.M.R. and A.P.M. assisted in the development of the mouse colitis model. K.M.R. assisted in the membrane protein extraction and analysis. M.C.B. assisted in the Ussing chamber experiments. J.C.U. assisted in the Notch-1 knock-down. S.D. and L.M. obtained funding and wrote the manuscript.
No conflict of interest to disclose.
BIBLIOGRAPHY
- 1.Hershberg RM, Mayer LF. Antigen processing and presentation by intestinal epithelial cells - polarity and complexity. Immunol Today. 2000 Mar;21(3):123–128. doi: 10.1016/s0167-5699(99)01575-3. [DOI] [PubMed] [Google Scholar]
- 2.Dahan S, Roda G, Pinn D, Roth-Walter F, Kamalu O, Martin AP, et al. Epithelial: lamina propria lymphocyte interactions promote epithelial cell differentiation. Gastroenterology. 2008 Jan;134(1):192–203. doi: 10.1053/j.gastro.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roda G, Dahan S, Mezzanotte L, Caponi A, Roth-Walter F, Pinn D, et al. Defect in CEACAM family member expression in Crohn's disease IECs is regulated by the transcription factor SOX9. Inflamm Bowel Dis. 2009 Jul 27;15(12):1775–1783. doi: 10.1002/ibd.21023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahan S, Roth-Walter F, Martin AP, Arnaboldi P, Mayer L. Lymphoepithelial interactions: a new paradigm. Ann N Y Acad Sci. 2009 May;1165:323–326. doi: 10.1111/j.1749-6632.2009.04061.x. [DOI] [PubMed] [Google Scholar]
- 5.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009 Apr 17;137(2):216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroder N, Gossler A. Expression of Notch pathway components in fetal and adult mouse small intestine. Gene Expr Patterns. 2002 Dec;2(3–4):247–250. doi: 10.1016/s1567-133x(02)00060-1. [DOI] [PubMed] [Google Scholar]
- 7.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001 Dec 7;294(5549):2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 8.Hermiston ML, Green RP, Gordon JI. Chimeric-transgenic mice represent a powerful tool for studying how the proliferation and differentiation programs of intestinal epithelial cell lineages are regulated. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8866–8870. doi: 10.1073/pnas.90.19.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conlon RA, Reaume AG, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995 May;121(5):1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- 10.Huppert SS, Le A, Schroeter EH, Mumm JS, Saxena MT, Milner LA, et al. Embryonic lethality in mice homozygous for a processing-deficient allele of Notch1. Nature. 2000 Jun 22;405(6789):966–970. doi: 10.1038/35016111. [DOI] [PubMed] [Google Scholar]
- 11.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993 Nov;5(11):1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 12.Totsuka T, Kanai T, Nemoto Y, Makita S, Okamoto R, Tsuchiya K, et al. IL-7 Is essential for the development and the persistence of chronic colitis. J Immunol. 2007 Apr 15;178(8):4737–4748. doi: 10.4049/jimmunol.178.8.4737. [DOI] [PubMed] [Google Scholar]
- 13.Blache P, van de Wetering M, Duluc I, Domon C, Berta P, Freund JN, et al. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol. 2004 Jul 5;166(1):37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Escaffit F, Pare F, Gauthier R, Rivard N, Boudreau F, Beaulieu JF. Cdx2 modulates proliferation in normal human intestinal epithelial crypt cells. Biochem Biophys Res Commun. 2006 Mar 31;342(1):66–72. doi: 10.1016/j.bbrc.2006.01.128. [DOI] [PubMed] [Google Scholar]
- 15.Alkhoury F, Malo MS, Mozumder M, Mostafa G, Hodin RA. Differential regulation of intestinal alkaline phosphatase gene expression by Cdx1 and Cdx2. Am J Physiol Gastrointest Liver Physiol. 2005 Aug;289(2):G285–G290. doi: 10.1152/ajpgi.00037.2005. [DOI] [PubMed] [Google Scholar]
- 16.Houde M, Laprise P, Jean D, Blais M, Asselin C, Rivard N. Intestinal epithelial cell differentiation involves activation of p38 mitogen-activated protein kinase that regulates the homeobox transcription factor CDX2. J Biol Chem. 2001 Jun 15;276(24):21885–21894. doi: 10.1074/jbc.M100236200. [DOI] [PubMed] [Google Scholar]
- 17.van Es JH, Clevers H. Notch and Wnt inhibitors as potential new drugs for intestinal neoplastic disease. Trends Mol Med. 2005 Nov;11(11):496–502. doi: 10.1016/j.molmed.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008 Mar;134(3):849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Tsuchiya K, Watanabe M. Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. J Gastroenterol. 2007 Sep;42(9):705–710. doi: 10.1007/s00535-007-2087-z. [DOI] [PubMed] [Google Scholar]
- 20.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, et al. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005 Sep;85(9):1139–1162. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 21.Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest. 2008 Oct;88(10):1110–1120. doi: 10.1038/labinvest.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007 Jan;56(1):61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allez M, Brimnes J, Dotan I, Mayer L. Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology. 2002 Nov;123(5):1516–1526. doi: 10.1053/gast.2002.36588. [DOI] [PubMed] [Google Scholar]
- 24.Boirivant M, Amendola A, Butera A, Sanchez M, Xu L, Marinaro M, et al. A transient breach in the epithelial barrier leads to regulatory T-cell generation and resistance to experimental colitis. Gastroenterology. 2008 Nov;135(5):1612–1623. doi: 10.1053/j.gastro.2008.07.028. e5. [DOI] [PubMed] [Google Scholar]
- 25.Deli MA. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim Biophys Acta. 2009 Apr;1788(4):892–910. doi: 10.1016/j.bbamem.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Sander GR, Powell BC. Expression of notch receptors and ligands in the adult gut. J Histochem Cytochem. 2004 Apr;52(4):509–516. doi: 10.1177/002215540405200409. [DOI] [PubMed] [Google Scholar]
- 27.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000 Jan;24(1):36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 28.Katoh M. Notch signaling in gastrointestinal tract (review) Int J Oncol. 2007 Jan;30(1):247–251. [PubMed] [Google Scholar]
- 29.Leow CC, Romero MS, Ross S, Polakis P, Gao WQ. Hath1, down-regulated in colon adenocarcinomas, inhibits proliferation and tumorigenesis of colon cancer cells. Cancer Res. 2004 Sep 1;64(17):6050–6057. doi: 10.1158/0008-5472.CAN-04-0290. [DOI] [PubMed] [Google Scholar]
- 30.Gersemann M, Becker S, Kubler I, Koslowski M, Wang G, Herrlinger KR, et al. Differences in goblet cell differentiation between Crohn's disease and ulcerative colitis. Differentiation. 2009 Jan;77(1):84–94. doi: 10.1016/j.diff.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Camerini V, Sydora BC, Aranda R, Nguyen C, MacLean C, McBride WH, et al. Generation of intestinal mucosal lymphocytes in SCID mice reconstituted with mature, thymus-derived T cells. Journal of immunology (Baltimore, Md : 1950) 1998;160(6):2608–2618. [PubMed] [Google Scholar]
- 32.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001 Apr;2(4):361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 33.Inagaki-Ohara K, Sawaguchi A, Suganuma T, Matsuzaki G, Nawa Y. Intraepithelial lymphocytes express junctional molecules in murine small intestine. Biochem Biophys Res Commun. 2005 Jun 17;331(4):977–983. doi: 10.1016/j.bbrc.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 34.Kijima M, Yamaguchi T, Ishifune C, Maekawa Y, Koyanagi A, Yagita H, et al. Dendritic cell-mediated NK cell activation is controlled by Jagged2-Notch interaction. Proc Natl Acad Sci U S A. 2008 May 13;105(19):7010–7015. doi: 10.1073/pnas.0709919105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Vos J, Couderc G, Tarte K, Jourdan M, Requirand G, Delteil MC, et al. Identifying intercellular signaling genes expressed in malignant plasma cells by using complementary DNA arrays. Blood. 2001 Aug 1;98(3):771–780. doi: 10.1182/blood.v98.3.771. [DOI] [PubMed] [Google Scholar]
- 36.Kerneis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997 Aug 15;277(5328):949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]