Abstract
Objective
The Framingham 10-risk of coronary heart disease (CHD) has been a widely studied estimate of cardiovascular risk in the general population. However, few studies have compared the relative risk of developing CHD in antipsychotic-treated patients with different psychiatric disorders, especially in older patients with psychotic symptoms. In this study, we compared the 10-year risk of developing CHD among middle-aged and older patients with psychotic symptoms to that in the general population.
Method
We analyzed baseline data from a study examining metabolic and cardiovascular effects of atypical antipsychotics in patients over age 40 with psychotic symptoms. After excluding patients with prior history of CHD and stroke, 179 subjects were included in this study. Among them, 68 had a diagnosis of schizophrenia, 42 mood disorder, 38 dementia, and 31 PTSD. Clinical evaluations included medical and pharmacologic treatment history, physical examination, and clinical labs for metabolic profiles. Using the Framingham 10–year risk of developing CHD based on the Framingham Heart Study (FHS), we calculated the risk CHD risk for each patient, and then compared relative risk in each psychiatric diagnosis to the risks reported in the FHS.
Results
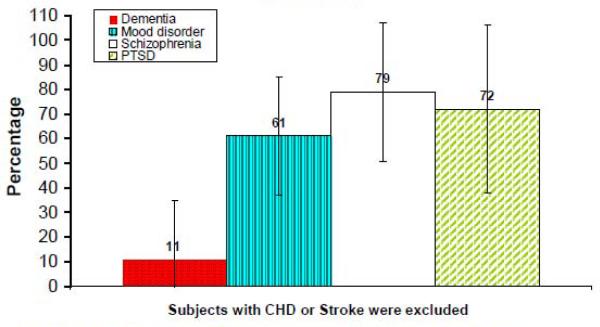
The mean age of entire sample was 63 (range 40–94) years, 68% were men. The Framingham 10-year risk of CHD was increased by 79% in schizophrenia, 72% in PTSD, 61% in mood disorder with psychosis, and 11% in dementia relative to the risk in general population from the FHS.
Conclusions
In this sample of middle-aged and older patients with psychotic symptoms, we found a significantly increased 10-year risk of CHD relative to the estimated risk from FHS, with the greatest increased risk for patients with schizophrenia and PTSD. Development of optimally tailored prevention and intervention efforts to decrease different risk components in these patients could be an important step to help decrease the risks of CHD and overall mortality in this vulnerable population.
Keywords: Framingham 10-year CHD risk, Psychotic disorders, middle aged and older patients
Introduction
Many studied have suggested that schizophrenia and other psychotic disorders are associated with increased mortality rates. One of the major causes of increased death rates in these populations is cardiovascular disease (Brown 1997, Osby et al 2000, Colton et al 2006). The CATIE study reported that schizophrenia patients had a significant increase in the Framingham 10-year risk of coronary heart disease (CHD) than matched controls, and patients on certain atypical antipsychotics (i.e.,olanzapine and quetiapine) had further increased 10-year risk of CHD (Goff DC. 2005, Daumit GL 2008). Patients with schizophrenia have been found to have higher rates of smoking, obesity, diabetes, and metabolic syndrome, which all contribute to the elevated risk of CHD (Isomaa et al 2001, Alexander et al, 2003). In recent years, there has been considerable focus on the role of atypical antipsychotics in elevating the risk of obesity, diabetes, and metabolic syndrome. Although much evidence suggests that patients with different psychiatric disorders have increased CHD risk factors including obesity, diabetes, and metabolic syndrome, few studies have directly compared the Framingham 10-year risk for CHD among different psychotic diagnoses, especially in middle aged and older psychotic patients who often have greater cardiovascular risk due to the effects of aging.
The equation of the Framingham 10-year risk for CHD, derived from the Framingham Heart Study(FHS), has been validated and widely used in different populations to predict the 10-years risk of CHD including angina, myocardial infarction, and cardiac death (Wilson et al 1998; D'Agostino, 2001). In the present study, we sought to compare the Framingham 10-year risk of CHD across diagnostic groups among middle-aged and older patients receiving atypical antipsychotics for schizophrenia, PTSD, mood disorder, or dementia with psychosis. We hypothesized that the estimated 10-year risk of CHD in patients with different psychotic disorders would be greater relative to the estimated 10-year CHD risk reported in the FHS, and there would be differential 10-year CHD risk associated with various psychiatric disorders with psychotic symptoms, with schizophrenia patients having the highest increase of the relative risk of CHD among all psychotic groups.
Methods
We examined baseline data from an ongoing NIMH-funded study examining metabolic, cardiovascular, and cerebrovascular effects (MCCE) of atypical antipsychotics in patients over age 40, who had psychotic symptoms that, according to the patients' own treating psychiatrists, needed treatment with atypical antipsychotics. This investigation is being conducted at the NIMH-funded Advanced Center for Innovation in Services and Intervention Research for the study of older patients with psychosis at the University of California, San Diego (UCSD) and Veterans Affairs San Diego Healthcare System (VASDHS). The study has been approved by the UCSD IRB, and all the participants have provided a written informed consent. Participants enrolled in this study complete a baseline evaluation and have follow-up assessments at six weeks, and then every three months for up to two years. The present paper is restricted to baseline data from the available sample. In addition to sociodemographic information, the data used in the analysis included: Medical history and use of psychotropic and other medications, as well as physical examination; Anthropomorphic measurements for obesity; and Clinical labs for metabolic profiles.
Subjects
The patients were recruited from psychiatric clinics at the UCSD and VASDHS, as well as from nursing homes and board-and-care homes in San Diego County. All the patients were diagnosed by their primary psychiatrists, many of whom are on the clinical faculty of UCSD. The patients met DSM-IV criteria for schizophrenia/ schizoaffective disorder, or psychosis associated with mood disorder, dementia, or PTSD, or psychotic disorder not otherwise specified (American Psychiatric Association, 1994). Patients with active substance abuse in the past 30 days or unstable medical conditions were excluded. In all 323 subjects enrolled upon this analysis, we excluded 120 subjects with history of CHD and stroke from baseline since this study was intended to examine the 10-year risk of CHD and these subjects already had the specified conditions. In addition, we excluded 24 patients with diagnoses of psychotic disorder not otherwise specified or others in the final analysis due to the small number of patients in these groups. We had 179 subjects included in the final analysis.
Assessment
The detailed description of clinical assessments and lab testing for the study has been published elsewhere (Jin, et al. 2008). In brief, the baseline assessment included detailed medical history, use of psychotropic and other medications, and neurological and general physical examination. The clinical assessment and physical exam were carried out by two trained physician assistants (PAs) who worked specifically for the study. In the medical history assessment, for example, the PAs obtained detailed past and present medical history about medical illnesses, known risk factors for cardiac or metabolic abnormalities (e.g., smoking status), and treatment for these conditions. Physical comorbidity was evaluated with the Cumulative Illness Rating Scale for Geriatrics (CIRS-G); (Miller MD 1992). All blood samples were collected by a nurse working in the General Clinical Research Center (GCRC) at UCSD, and the laboratory testing was done at the UCSD Medical Center-certified clinical laboratory. The blood for the chemistry panel that included fasting plasma glucose and lipid panel (total, HDL, and LDL cholesterol as well as triglycerides) was drawn in the early morning, after at least 12 hours of fasting.
Relative 10-year CHD risk calculation
Based on the FHS, (Wilson, et al. Circulation 1998), following variables including LDL level, HDL level, blood pressure, diabetes and smoking status were used to calculate the 10-year CHD risk. For each of these components, we first calculated the total risk points based on age group and gender specific stratified levels of LDL, HDL, blood pressure as well as status of diabetes and smoker. Next, the risk points were converted to corresponding percentage of 10-year CHD risk. For example, if the total risk points were 5 or 9, the 10-year CHD risk will be 9% or 22%, respectively. The detailed calculation formula of risk points and corresponding percentage of 10-year CHD risk can be found at website (http://www.framinghamheartstudy.org/risk/coronary.html). For each study participant, we then calculated a relative risk by comparing the participant's 10-year risk to that of the average person from the same age group in the FHS. (Actual risk rate from this study divided by norm risk rate in general population from the FHS).
Statistical Analysis
The demographic characteristics and each component of the Framingham 10-year CHD risk factors were analyzed with descriptive analysis. Between group comparisons of categorical variables were made using chi-square tests, while comparisons on continuous variables were performed with analysis of variance (ANOVA) with Welch option using SPSS statistical software (SPSS-PC, Windows V12.0.1). All comparisons were two-tailed, with p-values <.05 considered statistically significant.
For Framingham relative risk score calculation, we first calculated 10-year CHD risk and relative risk as described above. For each diagnostic group, we then calculated a mean relative risk and compared the relative risks for each diagnostic group with 95% confidence interval (95% CI) to general population risk adjusted to zero.
Results
The sociodemographic and clinical characteristics of the four diagnostic groups (schizophrenia, mood disorder, dementia, and PTSD) are presented in Table 1. The mean age of the total sample was 63.1 (range 40–94) years, 68% were men, and 89% were currently taking antipsychotics. The dementia group was older (p<.01), and both dementia and mood disorder groups had higher percentages of women (p<.01) than schizophrenia and PTSD groups. The schizophrenia and PTSD groups had a lower percentage of Caucasians compared to dementia and mood disorder patients (p<.01). There were also significant differences in diastolic blood pressure (p<.01) and HDL cholesterol (p<.05) among the four diagnostic groups, with schizophrenia and PTSD patients tending to have higher diastolic blood pressure and dementia patients having the highest HDLs. As expected, the mean durations of illness was significantly shorter in the dementia group compared to the other groups (p<.001). Similarly, the duration of being on antipsychotic treatment was significantly different (p<.001) with shortest in dementia and longest in patients with schizophrenia. The percentage of concurrent use of antidepressants and mood stabilizers were no significant different but concurrent use of anti-anxiety medication was significant different (p<.01) and patients with PTSD and mood disorder showed higher percentage of using anti-anxiety medications. The proportions of patients currently taking anti-diabetic drugs (p<.05) or statins (p<.05) differed significantly though patients taking anti-hypertensive drugs did not differ among the four patient groups. The schizophrenia group had highest rate of current smoking relative to other psychiatric disorders with psychotic symptoms (p<.001).
Table 1.
Demographic and clinical characteristics of middle-aged and older patients with 4 different psychotic disorders.
| Diagnostic groups |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| CHARACTERISTIC | Dementia w/psychosis (D) N=38 | Mood d/o w/psychosis (M) N=42 | Schizophrenia (S) N=68 | PTSD (P) N=31 | p-value* | ||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 77.4 | 9.1 | 62.9 | 12.6 | 56.9 | 9.7 | 59.6 | 11.2 | <.001 |
| Education level (years) | 11.6 | 6.65 | 12.9 | 4.6 | 12.8 | 2.64 | 14.0 | 2.24 | .244 |
| Duration of illness (years) | 8.2 | 16.0 | 31.7 | 25.9 | 30.7 | 20.6 | 32.4 | 16.2 | <.001 |
| Duration on antipsychotics (months) | 52.8 | 60.1 | 154.0 | 157.7 | 263.7 | 163.0 | 151.3 | 137.7 | <.001 |
| Body Mass Index (BMI) | 26.4 | 5.5 | 30.0 | 8.0 | 30.4 | 6.8 | 31.1 | 4.3 | <.001 |
| Diastolic blood pressure (mmHg) | 67.3 | 8.4 | 71.6 | 7.9 | 73.1 | 9.6 | 74.3 | 9.7 | .005 |
| Systolic blood pressure (mmHg) | 123.3 | 17.2 | 125.6 | 16.8 | 125.2 | 18.4 | 131.6 | 15.7 | .151 |
| Total cholesterol (mg/dlL) | 184.3 | 39.4 | 194.1 | 56.7 | 186.2 | 41.1 | 192.6 | 48.6 | .846 |
| Fasting glucose (mg/dL) | 96.9 | 17.0 | 102.2 | 28.1 | 123.8 | 79.6 | 114.6 | 47.0 | .088 |
| HDL Cholesterol (mg/dL) | 52.2 | 17.6 | 43.7 | 12.3 | 44.5 | 17.1 | 45.9 | 14.1 | .042 |
| Triglyceride (mg/dL) | 108.5 | 67.9 | 169.8 | 176.4 | 152.1 | 118.1 | 131.6 | 77.8 | .082 |
| Categorical variable s** | N | % | N | % | N | % | N | % | |
|---|---|---|---|---|---|---|---|---|---|
| Gender | |||||||||
| Male | 23 | 60 | 20 | 52 | 51 | 75 | 27 | 87 | <.01 |
| Female | 15 | 40 | 22 | 48 | 17 | 25 | 4 | 13 | |
| Ethnicity | |||||||||
| White | 29 | 76 | 35 | 83 | 41 | 60 | 15 | 48 | <.01 |
| Non-white | 9 | 24 | 7 | 17 | 27 | 40 | 16 | 52 | |
| Antipsychotic status at enrollment | |||||||||
| None | 9 | 24 | 3 | 7 | 3 | 4 | 3 | 10 | <.01 |
| First generation AP | 0 | 0 | 1 | 2 | 6 | 9 | 0 | 0 | |
| Second generation AP | 29 | 76 | 38 | 90 | 59 | 87 | 28 | 90 | |
| On antidepressants | 22 | 58 | 26 | 62 | 33 | 49 | 22 | 71 | .181 |
| On anti-anxiety drugs | 3 | 8 | 15 | 36 | 15 | 22 | 13 | 42 | <.01 |
| On mood stablizers | 7 | 18 | 16 | 38 | 19 | 28 | 7 | 23 | .228 |
| On anti-diabetic drugs | 4 | 12 | 6 | 14 | 19 | 29 | 12 | 41 | .330 |
| On statins | 16 | 49 | 16 | 38 | 19 | 30 | 17 | 59 | .031 |
| On anti-hypertension drugs | 16 | 47 | 18 | 44 | 27 | 44 | 20 | 67 | .145 |
| Currently smoker | 3 | 8.1 | 13 | 31 | 33 | 49 | 9 | 29 | <.001 |
ANOVA with Welch corrections for continues variables and chi-square for categorical variables.
Some column numbers and % may not match with total subject numbers due to missing data on certain variables.
Framingham 10-year risk of CHD relative to estimate risk from FHS
Using the Framingham 10–year estimate risk of CHD reported in the FHS, (Wilson, et al. Circulation 1998), we compared estimated 10-year risk ratio from the risk found in this study to the risk estimated from FHS in each psychotic diagnosis. The results suggested that the Framingham 10-year risk of CHD was increased by 79% (95% CI: 50%–107%) in schizophrenia, 72% (95% CI: 34%–109%) in PTSD, 61% (95% CI: 36%–86%) in mood disorder, and 11% (95% CI: −.14%–35%) in dementia.
Discussion
Our study suggests that the estimated Framingham 10-year risk of CHD was increased by at least 50% in middle aged and older patients with schizophrenia, PTSD, and mood disorder, with the dementia group having only a slightly increased of risk relative to the general population from the FHS. The schizophrenia and PTSD groups had the highest estimated increase in the 10-year risk of CHD relative to other psychiatric diagnoses. Our observed 70% estimated increase in the risk of CHD in our schizophrenia sample was consistent from CATIE study which found that the 10-year CHD risk in younger adult schizophrenia patients was increased relative to general population (Goff DC et al, 2005). Our study also found that middle aged and older PTSD patients had a substantial increase in 10-year risk of CHD relative to the population from the FHS. Recent findings have suggested that patients with PTSD have increased risk of metabolic syndrome, glucose dysregulation, and hypertension. (Pia, 2009, Jin et al 2009). All these factors are considered as cardiovascular risk factors that could contribute to the increase of 10-year risk of CHD. Since a majority of the PTSD patients enrolled in our study were taking antipsychotics at the time of study enrollment, as per our study inclusion criteria, our sample should not be considered as a typical sample of PTSD patients. Though there was no direct evidence suggesting an association between use of antipsychotic and 10-year risk of CHD, it is possible that PTSD itself, along with associated behaviors, are primarily related to increased risk of CHD, and that antipsychotics further increase that risk. Our middle aged and older patients with mood disorders showed a moderate increase in 10-year risk of CHD compared to the risk found in FHS, whereas the patients with dementia only showed a slight increase in this risk. This suggests that patients with different psychiatric disorders with psychotic symptoms carry somewhat different 10-year risks of CHD.
Our study has several limitations. First, this was a convenience sample referred from different clinics, and it may not be representative of total populations of patients with the respective conditions. Second, our sample size for each psychotic diagnosis was relatively small, and all diagnoses were based on clinical assessment rather than on a structured interview such as the Structured Clinical Interview for DSM-IV or SCID (APA 1994). Third, there may be other demographic and clinical factors that are different in our study sample compared to those in the FHS. Finally, use of different atypical antipsychotics may have different impacts on metabolic outcomes contributing to different 10-year risk of CHD. However, due to the sample size, we were unable to compare patients on different drugs within each diagnostic group.
Despite these limitations, our results suggest that middle-aged and elderly patients with psychosis have significantly increased 10-year risk of CHD relative to the estimated risk of CHD in general population found from FHS. The schizophrenia and PTSD groups appeared to have the highest increased risk of CHD. Development of optimally tailored prevention and intervention efforts to decrease different risk components such as smoking, hypertension and other factors in these patients could be an important step to help decrease the risks of CHD and overall mortality in this vulnerable population.
Figure 1. Increased Framingham 10-year risk of developing CHD among middle-aged and older patients with different psychiatric disorders relative to the risk in US population*.
* The Us population 10-year risk of CHD was based on estimation from Framingham Heart Study and the population estimated risk was adjusteded as zero
Acknowledgements
We like to thank Rebecca Daly for her assistant in data analysis and Tran Liu for clinical data collection. We also thank AstraZeneca, Bristol-Myers Squibb, Eli Lilly, and Janssen that donated quetiapine, aripiprazole, olanzapine, and risperidone, respectively, for this NIMH-funded study.
This study is supported, in part, by the National Institute of Mental Health grants (MH071536, T32 MH019934-12 and P30 MH080002-01), and by the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander CM, Landsman PB, Teutsch SM, Haffner SM. Third National Health and Nutrition Examination Survey (NHANES III); National Cholesterol Education Program (NCEP). NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003 May;52(5):1210–4. doi: 10.2337/diabetes.52.5.1210. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders IV. American Psychiatric Publishing Inc.; Arlington: 1994. [Google Scholar]
- Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–70. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- Brown S. Excess mortality of schizophrenia: a meta-analysis. Br. J. Psychiatry. 1997;171:502–508. doi: 10.1192/bjp.171.6.502. [DOI] [PubMed] [Google Scholar]
- Cardenas J, Frye MA, et al. Modal subcomponents of metabolic syndrome in patients with bipolar disorder. J Affect Disord. 2008;106(1–2):91–7. doi: 10.1016/j.jad.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Casey DE. Metabolic issues and cardiovascular disease in patients with psychiatric disorders. Am J Med. 2005;118(Suppl 2):15S–22S. doi: 10.1016/j.amjmed.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National High Blood Pressure Education Program Coordinating Committee The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Colton CW, Manderscheid RW, et al. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42. [PMC free article] [PubMed] [Google Scholar]
- D'Agostino RB, Sr., Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- Daumit GL, Goff DC, Meyer JM, Davis VG, Nasrallah HA, McEvoy JP, Rosenheck R, Davis SM, Hsiao JK, Stroup TS, Lieberman JA. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res. 2008;105(1–3):175–87. doi: 10.1016/j.schres.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini A, Frank E, et al. Metabolic syndrome in bipolar disorder: findings from the Bipolar Disorder Center for Pennsylvanians. Bipolar Disord. 2005;7(5):424–30. doi: 10.1111/j.1399-5618.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- Goff DC, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schiophr Res. 2005;80(1):45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Heiskanen TH, Niskanen LK, et al. Metabolic syndrome and depression: a cross-sectional analysis. J Clin Psychiatry. 2006;67(9):1422–7. doi: 10.4088/jcp.v67n0913. [DOI] [PubMed] [Google Scholar]
- Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001 Apr;24(4):683–9. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- Jakovljevic M, Crncevic Z, et al. Mental disorders and metabolic syndrome: a fatamorgana or warning reality? Psychiatr Danub. 2007;19(1–2):76–86. [PubMed] [Google Scholar]
- Jin H, et al. Association of Post-Traumatic Stress Disorder with Increased Prevalence of Metabolic Syndrome. J Clin Psychopharmacol. 2008;29(3):210–5. doi: 10.1097/JCP.0b013e3181a45ed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Meyer JM, et al. Atypical antipsychotics and glucose dysregulation: a systematic review. Schizophr Res. 2004;71(2–3):195–212. doi: 10.1016/j.schres.2004.03.024. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Meyer JM, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CF., 3rd Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res. 1992;41(3):237–48. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- Osby U, Correia N, Brandt L, Ekbom A, Sparen P. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. BMJ. 2000;321:483–484. doi: 10.1136/bmj.321.7259.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner Pia S, Crawford Eric F, Haji Uzair A, Afari Niloofar, Hauger Richard L, Dashevsky Boris A, Horn Paul S, Nunnink Sarah E, Baker Dewleen G. The association of posttraumatic stress disorder and metabolic syndrome: a study of increased health risk in veterans. BMC Medicine. 2009;7:1. doi: 10.1186/1741-7015-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. Centers for Disease Control and Prevention. National Center for Health Statistics U.S. Government Printing Office; Washington, DC: Health, United States, 2009. 2009
- Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Wirshing DA, Boyd JA, et al. The effects of novel antipsychotics on glucose and lipid levels. J Clin Psychiatry. 2002;63(10):856–65. doi: 10.4088/jcp.v63n1002. [DOI] [PubMed] [Google Scholar]