Abstract
Elevated brain iron content, which has been observed in late stage human Alzheimer’s disease, is a potential target for early diagnosis. However, the time course for iron accumulation is currently unclear. Using the PSAPP mouse model of amyloid plaque formation, we conducted a time course study of metal ion content and distribution [iron (Fe), copper (Cu), and zinc (Zn)] in the cortex and hippocampus using X-ray fluorescence microscopy (XFM). We found that iron in the cortex was 34% higher than age-matched controls at an early stage, corresponding to the commencement of plaque formation. The elevated iron was not associated with the amyloid plaques. Interestingly, none of the metal ions were elevated in the amyloid plaques until the latest time point (56 weeks), where only the Zn content was significantly elevated by 38%. Since neuropathological changes in human Alzheimer’s disease are presumed to occur years before the first cognitive symptoms appear, quantification of brain iron content could be a powerful marker for early diagnosis of Alzheimer’s disease.
Keywords: Alzheimer’s disease, transgenic mice, iron, copper, zinc, amyloid plaques, cortex, hippocampus, x-ray fluorescence, imaging
1. Introduction
Alzheimer’s disease (AD) is one of the most prevalent and debilitating neurodegenerative diseases and yet it is typically diagnosed only after cognitive symptoms have appeared, which may be too late for effective treatment (Masters et al. 2006). For example, the formation of amyloid plaques, one of the hallmarks of the disease, is estimated to occur years earlier (Price and Morris 1999). Thus, early identification of pathological changes in AD may allow for treatment strategies to be implemented at pre-clinical stages when they are more likely to be effective.
One particular target stems from the observation that the AD brain is abnormally enriched in metal ions of iron (Fe), copper (Cu), and zinc (Zn) (Bush 2003; Cuajungco and Lees 1997), which could be indicative of disease severity. For example, metal ion concentrations can be measured in serum and cerebrospinal fluid, and interest in optimizing magnetic resonance imaging (MRI) sequences to non-invasively detect and quantify Fe in the brain has increased (Brass et al. 2006; Haacke et al. 2005; Stankiewicz et al. 2007). It is possible that MRI could be used to assess disease-relevant changes in Fe content before neuropathological symptoms occur in AD (Helpern et al. 2004). For example, a relationship between the transverse relaxation rate, R2, measured in vivo and Fe content in autopsied brains has been demonstrated (Thomas et al. 1993), and even better correlations with brain Fe were achieved using different MRI parameters, R2′ (Gelman et al. 1999), and T2ρ (Michaeli et al. 2007). However, little is known about metal ion distribution in plaques and non-plaque tissue during the progression of the disease.
The cortex is a complex structure associated with higher brain functions such as thought, reasoning, sensation, and language and is affected by plaque pathology and volume loss in AD (Giannakopoulos et al. 1997). The hippocampus is also of neuropathological interest because it is important for long-term memory storage and neurogenesis and one of the first and most severely affected brain regions in AD (Braak and Braak 1991). It has also been shown to be more sensitive to metal perturbation than other brain regions and has unique regulatory demands for Fe, Cu, and Zn since they are involved in synaptic plasticity (Jones et al. 2008). However, how these metal ions are affected prior to the onset of plaques cannot yet be studied in humans and remains unknown. Therefore, to examine the possibility of using abnormal brain metal ion content as a diagnostic marker of pre-clinical AD, we imaged the Fe, Cu, and Zn content and distribution in the cortex and hippocampus of the PSAPP mouse model of AD as a function of age.
The PSAPP mouse expresses a mutant human presenilin 1 (PS1) gene and a chimeric mouse/human amyloid precursor protein gene (APP), developing amyloid plaques in a similar pattern to human AD by around 6 months of age (Garcia-Alloza et al. 2006; Jankowsky et al. 2001). In this study, we imaged the metal ion content and distribution using synchrotron X-ray fluorescence microscopy (XFM), a spectroscopic technique that measures the tissue concentration of multiple metal ions simultaneously, including the histochemically reactive, loosely bound, and free ions (Paunesku et al. 2006). Importantly, brain homogenization is not required and the spatial distribution and colocalization of multiple metal ions is preserved. Quantification of brain metal ion content and distribution from the earliest stages of plaque formation may be a powerful marker for early diagnosis, assessment of treatment strategies, and/or a therapeutic target in human AD.
2. Materials and Methods
2.1 Sample Preparation
Twenty-two female B6C3-Tg(APPswe, PSEN1dE9)85 Dbo/J (PSAPP) mice and 26 age- and gender-matched B6C3F1/J control (CNT) mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA). The mice were cared for and euthanized in accordance with the guidelines set by the Brookhaven National Laboratory (BNL) Institutional Animal Care and Use Committee and were housed at the Brookhaven Laboratory Animal Facility under standard conditions. At time points representing pre-AD (13 weeks), early-AD (24 weeks), intermediate-AD (40 weeks), and late-AD (56 weeks), the mice were deeply anesthetized with 100 mg/kg 1:10 ketamine:xylazine administered by intraperitoneal injection. Afterwards, they were perfused transcardially with phosphate buffered saline, resulting in exsanguination and death. The brains were removed, frozen on dry ice, and stored at −80°C until further processing. For each sample, approximately four 30 µm thick whole brain coronal cryosections (Bregma: −1.28 – −1.64) were mounted onto 4 µm thick Ultralene film (SPEX CertiPrep, Metuchen, NJ) and dried at room temperature. The samples were kept in a dessicator until the imaging experiments were carried out.
2.2 Metal Content in the Cortex
The concentration and distribution of Fe, Cu, and Zn in a 1024 µm2 healthy, plaque free area in the cortex in both PSAPP and CNT mice (13 weeks: PSAPP=7, CNT=7; 24 weeks: PSAPP=2, CNT=7, 40 weeks: PSAPP=3, CNT=7, 56 weeks: PSAPP=4, CNT=7) were measured with the XFM at beamline X26A at the National Synchrotron Light Source (NSLS), Brookhaven National Laboratory (BNL, Upton, NY). The synchrotron X-ray beam was tuned to 12 keV using a Si(111) channel-cut monochromotor. The monochromatic beam was then collimated to 350 µm × 350 µm and then focused to approximately 6 µm × 10 µm using Rh-coated silicon mirrors in a Kirkpatrick-Baez (KB) geometry. The sample was placed at a 45° angle to the incident X-ray beam and X-ray fluorescence was detected with an energy dispersive, 9 element germanium array detector (Canberra, Meriden, CT) oriented at 90° to the incident beam. The sample was approximately 6 cm from the detector. A light microscope objective (Mitutoyo, M Plan Apo 5X) was coupled to a digital CCD camera for sample viewing. Energy dispersive spectra were collected by raster scanning the sample through the X-ray beam using a dwell time of 30 s/pixel and a step size of 4 µm to provide oversampling. Fe Kα, Cu Kα, and Zn Kα, fluorescence counts were then extracted from background-corrected energy dispersive spectra. All data were normalized to variations in incident photon flux by normalizing to changes in I0 measured by ion chamber upstream of the KB optics.
NIST thin-film standard reference materials (SRM) 1832 and 1833 were used for calibration and quantification of all data. The counts per second for each element were then converted into µg/cm3 by comparing the differences in X-ray absorption between the sample and the thin film standards. Finally, the concentrations in µg/cm3 were converted into molar concentrations and means and standard deviations were calculated.
A Kruskal-Wallis test was performed using SPSS v.14.0 to test for significant differences between PSAPP and CNT at each time point, and differences between time points. Post-hoc analyses on significant Kruskal-Wallis tests were performed using Mann-Whitney U tests. Wilcoxon signed-rank tests were performed for pair-wise comparisons. A significance level of 0.05 was used for all analyses.
2.3 Metal Content in the Hippocampus
The concentration and distribution of Fe, Cu, and Zn in the hippocampus of PSAPP and CNT mice (13 weeks: PSAPP=6, CNT=6; 24 weeks: PSAPP=5, CNT=6, 40 weeks: PSAPP=6, CNT=7, 56 weeks: PSAPP=5, CNT=7) were measured with XFM at the Biophysics Collaborative Access Team (BioCAT) beamline 18-ID-D (Barrea et al. In press) at the Advanced Photon Source, Argonne National Laboratory (Argonne, IL). The synchrotron X-ray beam was tuned to 12 keV using a Si(111) double crystal monochromator. The incident beam was then collimated to 600 µm × 600 µm and then focused to 30 µm using Kirkpatrick-Baez focusing mirrors. This provided an X-ray flux to the sample of approximately 1.0 × 1012 photons/s. The sample was placed at a 45° angle to the incident X-ray beam, and X-ray fluorescence was detected with a Ketek single-element silicon drift detector (80 mm2 active area) oriented at 90° to the incident beam. A light microscope objective (infinity K2/S long distance video microscope with CVF-12/3/4 objectives) was coupled to a Hitachi digital CCD camera for sample viewing in transmission geometry. Energy dispersive spectra were collected by raster scanning the sample using a dwell time of 1 s/pixel and a step size of 30 µm. Fe Kα, Cu Kα, and Zn Kα fluorescence counts were then extracted from background-corrected energy dispersive spectra. All data were normalized to variations in incident photon flux by normalizing to changes in I0 measured by an upstream ion chamber.
Elemental maps were created by fitting the full fluorescence spectrum at each pixel to modified Gaussians using MAPS software v.1.6.4.3 (Vogt 2003). Area concentrations for each element were calculated by normalizing integrated fluorescence peak intensities to fitted spectra from the thin film standards NBS 1832 and 1833 and converting the fluorescence signal to a two-dimensional concentration in µg/cm2. In order to partition the maps into different histological structures, cluster analysis was used to create unsupervised regions of interest (ROIs) in the hippocampus based on Fe, Cu, and Zn content. Two additional ROIs were created based on light microscopy to encompass the entire CA and DG areas to analyze the total metal ion content of the hippocampus. The concentrations were converted from µg/cm2 to molar units by first dividing by the sample thickness (0.003 cm) and density, which was assumed to be 1 g/cm3. That value was then divided by the molecular weight of the element.
For each ROI, the concentrations of Fe, Cu, and Zn were determined and are presented in molar units (mean ± standard deviation). A Kruskal-Wallis test was performed using SPSS v.14.0 to test for significant differences between time points. Mann-Whitney U tests were used for post-hoc analyses on significant Kruskal-Wallis tests and to test for significant differences between PSAPP and CNT at each time point. Wilcoxon signed-rank tests were performed for pair-wise comparisons. Since regional regulation is complex and may involve interactions between the metal ions (Jones et al. 2008), we also performed a correlational analysis comparing each metal ion to another in specific regions of the hippocampus using Spearman ρ analysis. A significance level of 0.01 was used for all analyses.
2.4 Metal Content in Amyloid Plaques
Amyloid plaques were also imaged using XFM at X26A and were visualized with the fluorescent dye, Thioflavin S. In order to minimize tissue disruption and optimize visualization on Ultralene film, a modified procedure based on the method used by (Guntern et al. 1992) was developed. In short, the tissue sections were first rehydrated with 50% ethanol and allowed to dry completely. A 0.006% solution of Thioflavin S in 50% ethanol was placed on the tissue sample and allowed to stand for two minutes. The sample was then rinsed with 50% ethanol and then carefully rinsed with nanopure water to remove the excess Thioflavin S solution. The sections were dried in air, and the locations of the plaques were visualized by their green fluorescence using a commercially available epifluorescence module (Navitar, Rochester, NY). All plaques imaged were 30 – 50 µm in size to ensure that the plaque extended through the whole volume probed by the X-ray beam. Approximately 2–3 plaques per animal were analyzed from 2–3 tissue sections. To confirm that Thioflavin S staining did not cause redistribution or leaching of metal ions in the sample, we scanned several areas of a tissue prior to staining with Thioflavin S. We subsequently stained the tissue and then scanned the sample again in the same area. We did not observe any substantial alterations in the Fe, Cu, or Zn abundance or distribution.
XFM data from the plaques were normalized to tissue protein density using Fourier transform infrared microspectroscopy (FTIRM) at beamline U10B at the NSLS using previously described methods (Leskovjan et al. 2009). Briefly, a Thermo Nicolet Magna 860 FTIR spectrometer, coupled to a Continuum IR microscope (Thermo Nicolet, Madison, WI), was used with synchrotron light as the infrared source. The regions to be imaged were the same as those imaged using XFM and the stage was raster scanned through this area with a step size of 4 µm. At each point, an absorbance spectrum was collected in transmission mode in the mid-infrared spectral range (4000–800 cm−1) with a spectral resolution of 8 cm−1 and 128 scans co-added. To examine the relative protein content in the plaques compared to the non-plaque tissue, the Amide II protein band of each spectrum was integrated from 1490 – 1580 cm−1. A Matlab routine was then used to mask out the plaque area based on the Zn Kα fluorescence, which corresponded well with the Thioflavin S staining. These elevated Zn regions were used to create a mask that defined the plaque area. There were approximately 50–100 pixels per plaque. In order to obtain a relationship between metal ion content and protein concentration and to ensure that any changes in protein density within the plaque were taken into account, we normalized the metal ion content to the relative amount of protein in each plaque. Specifically, we calculated the relative increase in protein content in the plaque compared to the non-plaque area using the integrated area of the Amide II IR band. To normalize the metal ion content in each plaque to protein density, a ratio of metal/protein content for each plaque was then calculated.
It should be noted that tissue density normalization was only performed for the plaques. We did not find significant differences in tissue density between the non-plaque cortical and hippocampal regions, possibly due to a lack of neurodegeneration in this mouse model, so this normalization was not performed for these regions.
3. Results
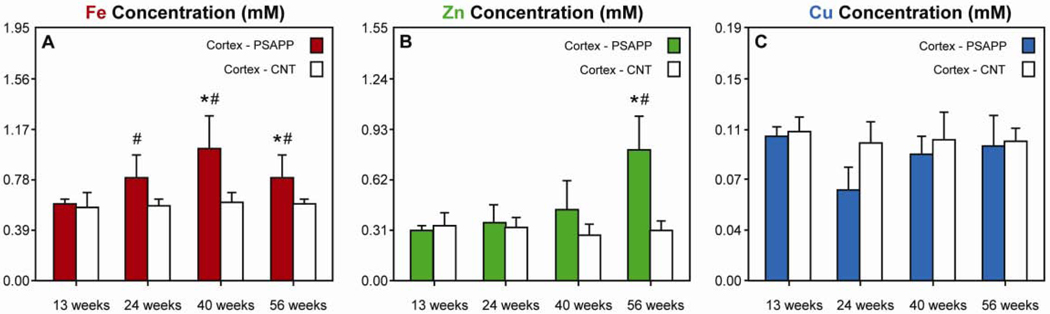
The means and standard deviations of Fe, Zn, and Cu content in healthy (non-plaque) cortex and hippocampus in both control (CNT) and PSAPP mice over time are reported in Tables 1, 2, and 3. Results showed that Fe content in the cortex of PSAPP mice was altered from the beginning stages of plaque formation; specifically, cortical Fe content was higher in PSAPP mice than age-matched control (CNT) mice at 24, 40, and 56 weeks-old by 39%, 68%, and 32%, respectively (p < 0.05, Fig. 1A). Moreover, Fe content in the cortex increased over time in the PSAPP mice; specifically, Fe content increased from 13 weeks-old to 40 and 56 weeks-old by 71% and 34%, respectively (p < 0.05). Zn content in PSAPP mice increased from 13 weeks-old to 56 weeks-old by 167% (p < 0.05) and was 158% higher than CNT mice at 56 weeks-old (p < 0.05, Fig 1B). In contrast, Cu content remained constant (Fig. 1C). No changes in metal ion content were observed in CNT mice.
Table 1.
Fe Content in PSAPP and CNT Mice (µM)
| ROI | 13 weeks | 24 weeks | 40 weeks | 56 weeks |
|---|---|---|---|---|
| Total | ||||
| Hippocampus | ||||
| PSAPP | 260 ± 29 (N = 6) |
341 ± 31* (N = 5) |
369 ± 89 (N = 6) |
291 ± 15‡,# (N = 5) |
| CNT | 299 ± 54 (N = 6) |
380 ± 122 (N = 6) |
300 ± 74 (N = 7) |
354 ± 21 (N = 7) |
| Dendritic Layer | ||||
| PSAPP | 249 ± 27 | 324 ± 32* | 352 ± 85 | 280 ± 13‡,# |
| CNT | 288 ± 53 | 360 ± 122 | 285 ± 70 | 342 ± 21 |
| PCL | ||||
| PSAPP | 468 ± 37 | 710 ± 71* | 688 ± 181 | 468 ± 68‡,# |
| CNT | 546 ± 81 | 880 ± 246 | 649 ± 200 | 726 ± 96 |
| CA3 | ||||
| PSAPP | 225 ± 29 | 294 ± 22* | 346 ± 88 | 291 ± 43 |
| CNT | 265 ± 69 | 345 ± 117 | 244 ± 57 | 395 ± 19 |
| Hilus | ||||
| PSAPP | 237 ± 28 | 281 ± 24 | 343 ± 71* | 326 ± 31*, # |
| CNT | 265 ± 55 | 335 ± 98 | 242 ± 50 | 270 ± 19 |
| Cortex | ||||
| PSAPP | 590 ± 40 | 790 ± 180 | 1010 ± 260* | 790 ± 180*, # |
| CNT | 570 ± 10 | 570 ± 50 | 600 ± 70 | 600 ± 40 |
significantly different from 13 weeks
significantly different from 24 weeks
significantly different from CNT
Table 2.
Zn Content in PSAPP and CNT Mice (µM)
| ROI | 13 weeks | 24 weeks | 40 weeks | 56 weeks |
|---|---|---|---|---|
| Total | ||||
| Hippocampus | ||||
| PSAPP | 378 ± 40 (N = 6) |
427 ± 85 (N = 5) |
462 ± 104 (N = 6) |
410 ± 80 (N = 5) |
| CNT | 472 ± 105 (N = 6) |
467 ± 79 (N = 6) |
349 ± 65 (N = 7) |
376 ± 23 (N = 7) |
| Dendritic Layer | ||||
| PSAPP | 294 ± 33 | 334 ± 68 | 360 ± 83 | 312 ± 59 |
| CNT | 366 ± 79 | 370 ± 65 | 270 ± 54 | 297 ± 20 |
| PCL | ||||
| PSAPP | 374 ± 36 | 401 ± 48 | 438 ± 084 | 411 ± 114 |
| CNT | 452 ± 64 | 463 ± 103 | 320 ± 57 | 345 ± 29 |
| CA3 | ||||
| PSAPP | 752 ± 91 | 814 ± 142 | 950 ± 178 | 868 ± 207 |
| CNT | 893 ± 193 | 979 ± 198 | 762 ± 160 | 774 ± 53 |
| Hilus | ||||
| PSAPP | 1173 ± 161 | 1415 ± 303 | 1700 ± 266* | 1757 ± 540*,# |
| CNT | 1497 ± 238 | 1547 ± 393 | 1244 ± 345 | 1150 ± 98 |
| Cortex | ||||
| PSAPP | 300 ± 30 | 360 ± 110 | 430 ± 180* | 800 ± 200*,# |
| CNT | 330 ± 80 | 330 ± 50 | 280 ± 70 | 310 ± 60 |
significantly different from 13 weeks
significantly different from 24 weeks
significantly different from CNT
Table 3.
Cu Content in PSAPP and CNT Mice (µM)
| ROI | 13 weeks | 24 weeks | 40 weeks | 56 weeks |
|---|---|---|---|---|
| Total | ||||
| Hippocampus | ||||
| PSAPP | 72 ± 10 (N = 6) |
74 ± 10 (N = 5) |
91 ± 15 (N = 6) |
80 ± 13 (N = 5) |
| CNT | 76 ± 14 (N = 6) |
87 ± 23 (N = 6) |
70 ± 16 (N = 7) |
78 ± 12 (N = 7) |
| Dendritic Layer | ||||
| PSAPP | 76 ± 10 | 76 ± 11 | 95 ± 16 | 82 ± 14 |
| CNT | 77 ± 14 | 88 ± 23 | 71 ± 16 | 79 ± 13 |
| PCL | ||||
| PSAPP | 56 ± 7 | 62 ± 12 | 68 ± 9 | 66 ± 11 |
| CNT | 66 ± 14 | 77 ± 24 | 52 ± 13 | 58 ± 9 |
| CA3 | ||||
| PSAPP | 58 ± 9 | 66 ± 6 | 77 ± 11 | 71 ± 6 |
| CNT | 70 ± 18 | 81 ± 22 | 66 ± 17 | 75 ± 13 |
| Hilus | ||||
| PSAPP | 66 ± 10 | 72 ± 9 | 81 ± 12 | 78 ± 12 |
| CNT | 75 ± 12 | 89 ± 21 | 81 ± 25 | 101 ± 32 |
| Cortex | ||||
| PSAPP | 100 ± 10 | 70 ± 20 | 90 ± 10 | 100 ± 20 |
| CNT | 110 ± 10 | 110 ± 20 | 100 ± 20 | 100 ± 10 |
significantly different from 13 weeks
significantly different from 24 weeks
significantly different from CNT
Figure 1.
Metal ion content in the cortex in PSAPP (colored bars) and CNT mice (white bars). (A) Fe content in the cortex of PSAPP mice was altered from the beginning stages of plaque formation and was significantly higher in PSAPP mice than CNT mice at 24 weeks (PSAPP = 0.79 ± 0.18; CNT = 0.57 ± 0.05), 40 weeks (PSAPP = 1.01 ± 0.26; CNT = 0.60 ± 0.07), and 56 weeks (PSAPP = 0.79 ± 0.18; CNT = 0.60 ± 0.04). Fe content also significantly increased by 71% from 13 weeks-old to 40 weeks-old and by 34% from 13 weeks-old to 56 weeks-old. (B) Zn content in PSAPP mice increased from 13 weeks to 56 weeks and was also 161% higher than CNT mice at 56 weeks. (C) Cu remained constant in both the CNT and PSAPP mice. [Error bars = SD. * indicates significantly different from 13 week-old PSAPP mice (p < 0.05); # indicates significantly different from CNT mice at the same time point (p < 0.05).]
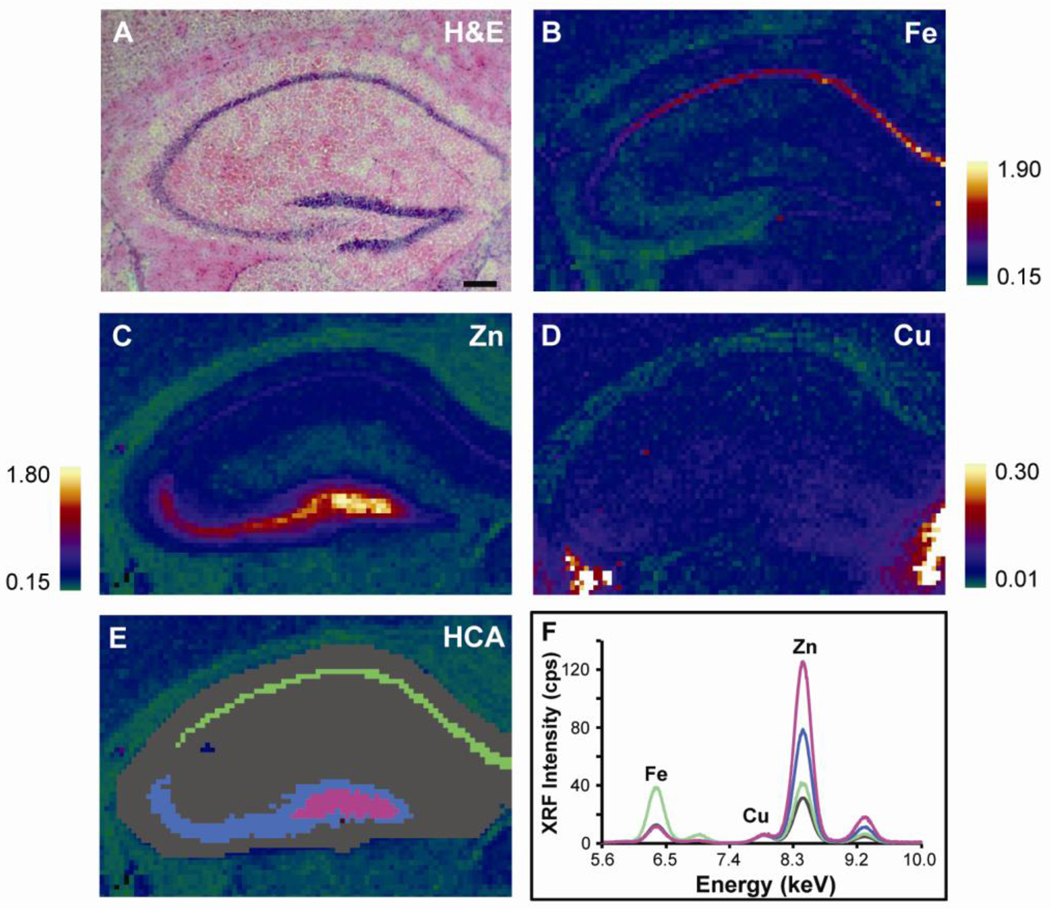
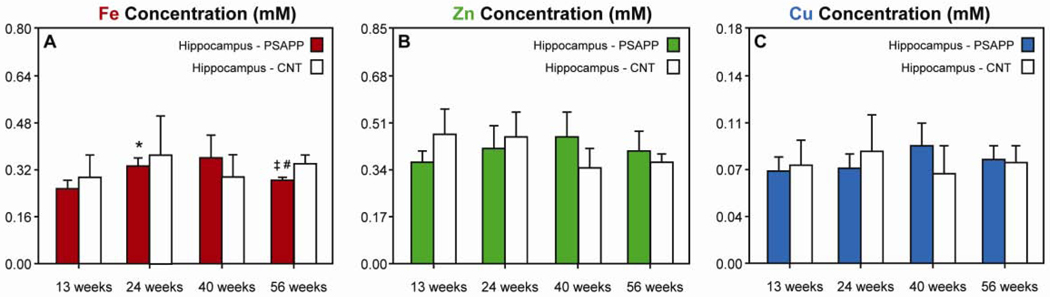
We then examined the metal ion concentration and distribution in the hippocampus of PSAPP and CNT mice as a function of age (Fig. 2). In all mice, Fe was highest in the region corresponding to the pyramidal cell layer (PCL, Fig. 2B), Zn was highest in the hilus of the dentate gyrus (Fig. 2C), while Cu was generally uniformly distributed (Fig. 2D). Hierarchical cluster analysis was used to quantify metal ion content in separate regions of the hippocampus, which included the PCL, cornu ammonis 3 (CA3), the hilus, and the combined CA and dentate gyrus (DG) regions, containing mainly granule and pyramidal cells and their dendrites, which we termed the dendritic layer (Fig. 2E). When integrated over the entire hippocampus, Fe content increased in PSAPP mice from 13 to 24 weeks of age by 31% (p < 0.01, Fig 3A). However, after this initial increase in Fe, we found that from 24 to 56 weeks-old Fe content in these regions then decreased by 21% (p < 0.01) and was 22% lower than in the CNT mice at 56 weeks-old (p < 0.01). This trend was also observed in the dendritic layer and PCL (Table 1). In the hilus, Fe content increased by 38% from 13 to 56 weeks-old and was 17% higher than CNT mice at 56 weeks-old (Table 1). Thus, in contrast to Fe content in the cortex, Fe content in the hippocampus showed a fluctuating pattern, while no changes were observed in CNT mice.
Figure 2.
Spatial distribution of Fe, Cu, and Zn in the hippocampus of PSAPP and CNT mice measured using XFM. (A) H & E stained hippocampal brain section from a PSAPP mouse. XFM images of (B) Fe, (C) Zn and (D) Cu in a serial tissue section. Units are mM. Scale bar = 300 µm. (E) Hierarchical cluster analysis (HCA) was used to create unsupervised regions of interest (ROIs) based on Fe, Cu, and Zn content in order to compare metal content in separate regions of the hippocampus. On average, four clusters were required to separate the images into histological ROIs where the dendritic layer is gray, the PCL is green, CA3 is blue, and the hilus is magenta. These areas corresponded to distinct anatomical regions of the hippocampus defining four distinct regions of the hippocampus. (F) Average XFM spectra from each ROI.
Figure 3.
Metal ion content in the hippocampus in PSAPP (colored bars) and CNT mice (white bars). No changes in metal ion content were observed in age-matched control (CNT) mice at any time point. (A) Fe content in PSAPP mice increased by 31% from 13 to 24 weeks and decreased by 15% from 24 to 56 weeks. At 56 weeks, Fe content was 18% lower than in CNT mice at the same time point (p < 0.01). (B) Zn and (C) Cu content remained constant in PSAPP mice. [Error bars = SD. * indicates significantly different from 13 week-old PSAPP mice (p < 0.01); ‡ indicates significantly different from 24 week-old PSAPP mice (p < 0.01); # indicates significantly different from CNT mice at the same time point (p < 0.01).]
Unlike Fe content, the Zn and Cu contents did not change as a function of age in the hippocampus of either PSAPP or CNT mice (Figs. 3B, C; Tables 2, 3). However, late-stage changes in Zn content were observed in the hilus where the concentration increased by 50% from 13 to 56 weeks of age (p < 0.01) and was 53% higher than in CNT mice at 56 weeks of age (p < 0.01, Table 2).
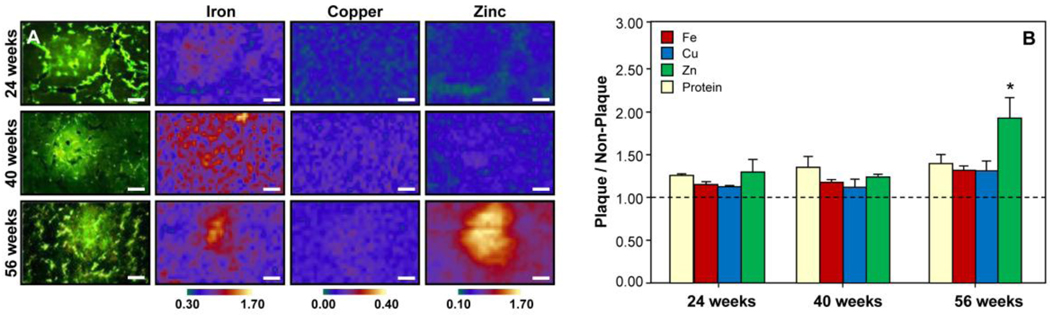
The XFM images of the Fe, Cu, and Zn distributions in representative plaques at 24, 40, and 56 weeks-old in the PSAPP mice are shown in Fig. 4A. There were no plaques observed at the 13 week-old time point. Before 56 weeks-old, none of the plaques showed significantly elevated metal ion content (Fig. 4A). At 56 weeks-old, the plaques showed elevated Fe, Cu, and Zn content (Fig. 4B). However, the protein density in the plaques also increased by 11% from 24 to 56 weeks-old (Fig. 4B). Thus, when normalized to protein density, Cu and Fe were actually lower in the plaques than the surrounding tissue and only the Zn content was significantly elevated in the plaques (38% increase, p < 0.05). A similar increase in Zn was observed in the cortex and the hilus of the hippocampus (Table 2) and indicates that increased Zn (and not Fe or Cu) is a late-stage effect of advanced disease pathology (Religa et al. 2006).
Figure 4.
Metal ion content in amyloid plaques at 24, 40, and 56 weeks. (A) Thioflavin S-stained PSAPP mouse brain tissue and corresponding XFM images of Fe, Cu, and Zn in the plaques and surrounding non-plaque tissue. All scale bars are 25 µm. (B) Ratio of Fe, Cu, Zn, and protein content in the plaque vs. non-plaque tissue. At 56 weeks, the plaques showed elevated Fe, Cu, and Zn content. However, the protein density in the plaques also increased by 11% from 24 to 56 weeks. Thus, when normalized to protein density, Cu and Fe were actually lower in the plaques than the surrounding tissue and only the Zn content was significantly elevated by 38% in the plaques (p < 0.05). [* indicates significantly different from 40 week-old mice (p < 0.05).]
4. Discussion
There is growing evidence to suggest that imbalances of metal ions play an important role in the pathology of AD (Lee et al. 1999; Lovell et al. 1998; Miller et al. 2006; White et al. 2006). For example, increased Fe (Smith et al. 2010) and Zn (Religa et al. 2006) have been observed in the cortex of human AD patients. Moreover, recent studies suggest that elevated brain Fe could be used as a diagnostic tool in AD (Bartzokis et al. 2000; Schenck et al. 2006; Smith et al. 2010). However, most human studies examine brain tissue at the endstage and little is known about metal ion content prior to and during the progression of plaque pathology. Therefore, we conducted a time course study using the PSAPP mouse model of plaque formation in AD, from pre-plaque formation to late-stage plaque formation, and showed that increased Fe content in the cortex is evident at 24-weeks of age, i.e. at the first signs of plaque formation, as well as spatial working memory impairments (Trinchese et al. 2004), in these mice. Interestingly, the increased Fe content was not associated with amyloid plaques.
Increased Fe in the brain has been consistently observed in AD and a number of studies suggest that brain Fe content is altered in AD (Bartzokis et al. 2000; Connor et al. 1992; Deibel et al. 1996). The present results also showed that Fe content in the cortex was significantly higher in the PSAPP mice than in the CNT mice starting from 24 weeks of age and progressively increased as the mice aged. This is in agreement with a recent study by Smith and colleagues showing that Fe content in the cortex was increased at the earliest detectable signs of cognitive decline in AD in humans and in mild cognitive impairment (MCI), a condition thought to be an early precursor to AD (Smith et al. 2010). In addition, Fe content in the hippocampus increased from 13 weeks-old to 24 weeks-old, which is in agreement with previous results indicating that Fe content in human hippocampus was also increased in patients with MCI (House et al. 2006). However, after this initial increase in Fe, we found that from 24 to 56 weeks-old Fe content in these regions then decreased and was lower than in the CNT mice at 56 weeks-old. Thus, decreased hippocampal Fe content appears to be a late-stage marker in the PSAPP mice. In contrast to our results, another study using double transgenic APP/PS1 mice did not find any significant changes in Fe content with age in the cortex or hippocampus (El Tannir El Tayara et al. 2006). However, that MRI study only examined mice in two different age ranges: young (27 to 45 weeks) and old (60–86 weeks), much older than our early-AD time point. Since the mice in the present study were younger, we were able to show changes in cortical Fe content in PSAPP mice at 40 and 56 weeks-old relative to 13 weeks-old. Moreover, that these changes were not observed in the CNT mice suggests a possible relationship of brain iron content to plaque pathology.
Increased Zn content in the hilus (in addition to the cortex) also appears to be a late-stage marker. This increase might correspond to an increase in the zinc transporter protein, ZnT3, which is abundant in the hilus and has been shown to increase in this same mouse strain (Zhang et al. 2008), whereas other mouse models of AD lacking ZnT3 are devoid of hilar Zn and show reduced plaque load (Lee et al. 2002; Linkous et al. 2008).
We next examined the time course of metal accumulation specifically in the PSAPP plaques since previous studies have found elevations of these metals in plaques in endstage human AD (Lovell et al. 1998; Miller et al. 2006) and in transgenic mice (Lee et al. 2002; Leskovjan et al. 2009; Suh et al. 2000), but the time course of metal accumulation was never determined. In the present study, only the Zn content was significantly elevated in the plaques at 56 weeks-old, further indicating that increased Zn is a late-stage effect of advanced plaque pathology (Religa et al. 2006). Interestingly, Fe did not accumulate in the plaques at any time point, which suggests that the early increase in Fe that was observed in the cortex arises not from an interaction with Aβ, but from some other mechanism, such as a disruption in the expression of other brain Fe metabolism, transport, or storage proteins. The Fe storage protein, ferritin, is thought to remain constant in human AD brain even though Fe content increases, which is in contrast to normal aging (Bartzokis et al. 2004; Griffiths et al. 1999). For example, ferritin isolated from AD and Parkinson’s patients had higher Fe content than that from control brains (Bartzokis et al. 2004; Griffiths et al. 1999). Therefore, it is possible that the increased Fe observed in PSAPP mice, but not in CNT mice, represents overloaded ferritin related to plaque pathology and not age.
The observed increases in cortical Fe at the beginning stages of plaque formation in PSAPP mice may have direct clinical relevance for early detection of the pathological symptoms of AD. Though previous studies have shown elevated Fe in the cortex and hippocampus in AD (Magaki et al. 2007), it is unclear when excess Fe begins to accumulate. We showed that Fe content was increased in the cortex of PSAPP mice starting at 24-weeks of age. Significantly, the clear increase in cortical Fe, in contrast to the fluctuating content in the hippocampus, makes the cortex a promising starting point for clinical diagnosis.
Currently, there is considerable interest in optimizing MRI sequences to non-invasively detect and quantify excessive Fe in the brain for the purposes of early diagnosis and treatment monitoring (Brass et al. 2006; Haacke et al. 2005; Stankiewicz et al. 2007). Since Fe concentration must be at least on the order of 0.1 mM in order to affect MRI contrast (Schenck 2003), our results indicate that the Fe concentration in the PSAPP cortex is well within reported values detectable by MRI. Moreover, we observe a 34% increase in cortical Fe content in the early stages of the disease, making detection by MRI a promising avenue for future research. Further advancement of MRI techniques in conjunction with other imaging techniques to identify amyloid plaques (e.g. positron emission tomography) may allow for early diagnosis through sensitive quantification of brain Fe deposition at pre-clinical or early stage AD and related diseases so that more effective treatments can be developed and monitored. However, understanding the significance of altered Fe levels the AD brain is critical in determining if Fe is a primary contributor to AD or a secondary consequence of disease progression. Future work should involve disentangling the cause of elevated Fe in AD brain from other possible causes of excess Fe in the brain and longitudinal examinations of brain Fe in human patients should be conducted.
Research Highlights
Brain metal content is a potential target for early diagnosis and treatment of AD.
In the PSAPP mouse, Fe was elevated in the cortex at the onset of plaque formation.
The elevated iron was not associated with the amyloid plaques.
Fe, Cu, and Zn were not elevated in PSAPP plaques at time points up to 56 weeks.
Only the Zn content was elevated at 56 wks, representing a marker of late stage AD.
Acknowledgements
We thank J. Collins for her skillful technical assistance with the animal dissection. This work was funded by NIH Grant R01-GM66873. The NSLS is funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract DE-AC02-98CH10886. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. BioCAT (APS Beamline 18-ID) is a National Institutes of Health-supported Research Center RR-08630.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrea RA, Gore D, Kujala N, Karanfil C, Kozyrenko S, Heurich R, Vukonich M, Huang R, Paunesku T, Woloschak G, Irving TC. Fast-scanning high flux microprobe for biological X-ray fluorescence microscopy and microXAS. J. Synchrotron Radiation. 2010;17:522–529. doi: 10.1107/S0909049510016869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Cummings J, Holt LE, Hance DB, Henderson VW, Mintz J. In vivo evaluation of brain iron in Alzheimer disease using magnetic resonance imaging. Arch. Gen. Psychiatry. 2000;57:47–53. doi: 10.1001/archpsyc.57.1.47. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Tishler TA, Shin IS, Lu PH, Cummings JL. Brain ferritin iron as a risk factor for age at onset in neurodegenerative diseases. Ann. N Y Acad. Sci. 2004;1012:224–236. doi: 10.1196/annals.1306.019. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brass SD, Chen NK, Mulkern RV, Bakshi R. Magnetic resonance imaging of iron deposition in neurological disorders. Top. Magn. Reson. Imaging. 2006;17:31–40. doi: 10.1097/01.rmr.0000245459.82782.e4. [DOI] [PubMed] [Google Scholar]
- Bush AI. The metallobiology of Alzheimer's disease. Trends Neurosci. 2003;26:207–214. doi: 10.1016/S0166-2236(03)00067-5. [DOI] [PubMed] [Google Scholar]
- Connor JR, Snyder BS, Beard JL, Fine RE, Mufson EJ. Regional distribution of iron and iron-regulatory proteins in the brain in aging and Alzheimer's disease. J. Neurosci. Res. 1992;31:327–335. doi: 10.1002/jnr.490310214. [DOI] [PubMed] [Google Scholar]
- Cuajungco MP, Lees GJ. Zinc and Alzheimer's disease: is there a direct link? Brain Res. Brain. Res. Rev. 1997;23:219–236. doi: 10.1016/s0165-0173(97)00002-7. [DOI] [PubMed] [Google Scholar]
- Deibel MA, Ehmann WD, Markesbery WR. Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer's disease: possible relation to oxidative stress. J. Neurol. Sci. 1996;143:137–142. doi: 10.1016/s0022-510x(96)00203-1. [DOI] [PubMed] [Google Scholar]
- El Tannir El Tayara N, Delatour B, Le Cudennec C, Guegan M, Volk A, Dhenain M. Age-related evolution of amyloid burden, iron load, and MR relaxation times in a transgenic mouse model of Alzheimer's disease. Neurobiol. Dis. 2006;22:199–208. doi: 10.1016/j.nbd.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, Purcell SM, Betensky RA, Raju S, Prada C, Greenberg SM, Bacskai BJ, Frosch MP. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol. Dis. 2006;24:516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Gelman N, Gorell JM, Barker PB, Savage RM, Spickler EM, Windham JP, Knight RA. MR imaging of human brain at 3.0 T: preliminary report on transverse relaxation rates and relation to estimated iron content. Radiology. 1999;210:759–767. doi: 10.1148/radiology.210.3.r99fe41759. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Hof PR, Michel JP, Guimon J, Bouras C. Cerebral cortex pathology in aging and Alzheimer's disease: a quantitative survey of large hospital-based geriatric and psychiatric cohorts. Brain Res. Brain. Res. Rev. 1997;25:217–245. doi: 10.1016/s0165-0173(97)00023-4. [DOI] [PubMed] [Google Scholar]
- Griffiths PD, Dobson BR, Jones GR, Clarke DT. Iron in the basal ganglia in Parkinson's disease. An in vitro study using extended X-ray absorption fine structure and cryo-electron microscopy. Brain. 1999;122:667–673. doi: 10.1093/brain/122.4.667. [DOI] [PubMed] [Google Scholar]
- Guntern R, Bouras C, Hof PR, Vallet PG. An improved thioflavine S method for staining neurofibrillary tangles and senile plaques in Alzheimer's disease. Experientia. 1992;48:8–10. doi: 10.1007/BF01923594. [DOI] [PubMed] [Google Scholar]
- Haacke EM, Cheng NY, House MJ, Liu Q, Neelavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W, Obenaus A. Imaging iron stores in the brain using magnetic resonance imaging. Magn. Reson. Imaging. 2005;23:1–25. doi: 10.1016/j.mri.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Helpern JA, Jensen J, Lee SP, Falangola MF. Quantitative MRI assessment of Alzheimer's disease. J. Mol. Neurosci. 2004;24:45–48. doi: 10.1385/JMN:24:1:045. [DOI] [PubMed] [Google Scholar]
- House MJ, St Pierre TG, Foster JK, Martins RN, Clarnette R. Quantitative MR imaging R2 relaxometry in elderly participants reporting memory loss. Am. J. Neuroradiol. 2006;27:430–439. [PMC free article] [PubMed] [Google Scholar]
- Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, Copeland NG, Borchelt DR. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol. Eng. 2001;17:157–165. doi: 10.1016/s1389-0344(01)00067-3. [DOI] [PubMed] [Google Scholar]
- Jones LC, Beard JL, Jones BC. Genetic analysis reveals polygenic influences on iron, copper, and zinc in mouse hippocampus with neurobiological implications. Hippocampus. 2008;18:398–410. doi: 10.1002/hipo.20399. [DOI] [PubMed] [Google Scholar]
- Lee JY, Cole TB, Palmiter RD, Suh SW, Koh JY. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc. Natl. Acad. Sci. U S A. 2002;99:7705–7710. doi: 10.1073/pnas.092034699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Mook-Jung I, Koh JY. Histochemically reactive zinc in plaques of the Swedish mutant β-amyloid precursor protein transgenic mice. J. Neurosci. 1999;19:RC10. doi: 10.1523/JNEUROSCI.19-11-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leskovjan AC, Lanzirotti A, Miller LM. Amyloid plaques in PSAPP mice bind less metal than plaques in human Alzheimer's disease. Neuroimage. 2009;47:1215–1220. doi: 10.1016/j.neuroimage.2009.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkous DH, Flinn JM, Koh JY, Lanzirotti A, Bertsch PM, Jones BF, Giblin LJ, Frederickson CJ. Evidence that the ZNT3 protein controls the total amount of elemental zinc in synaptic vesicles. J. Histochem. Cytochem. 2008;56:3–6. doi: 10.1369/jhc.6A7035.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer's disease senile plaques. J. Neurol. Sci. 1998;158:47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- Magaki S, Raghavan R, Mueller C, Oberg KC, Vinters HV, Kirsch WM. Iron, copper, and iron regulatory protein 2 in Alzheimer's disease and related dementias. Neurosci. Lett. 2007;418:72–76. doi: 10.1016/j.neulet.2007.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters CL, Cappai R, Barnham KJ, Villemagne VL. Molecular mechanisms for Alzheimer's disease: implications for neuroimaging and therapeutics. J. Neurochem. 2006;97:1700–1725. doi: 10.1111/j.1471-4159.2006.03989.x. [DOI] [PubMed] [Google Scholar]
- Michaeli S, Oz G, Sorce DJ, Garwood M, Ugurbil K, Majestic S, Tuite P. Assessment of brain iron and neuronal integrity in patients with Parkinson's disease using novel MRI contrasts. Mov. Disord. 2007;22:334–340. doi: 10.1002/mds.21227. [DOI] [PubMed] [Google Scholar]
- Miller LM, Wang Q, Telivala TP, Smith RJ, Lanzirotti A, Miklossy J. Synchrotron-based infrared and X-ray imaging shows focalized accumulation of Cu and Zn co-localized with β-amyloid deposits in Alzheimer's disease. J. Struct. Biol. 2006;155:30–37. doi: 10.1016/j.jsb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Paunesku T, Vogt S, Maser J, Lai B, Woloschak G. X-ray fluorescence microprobe imaging in biology and medicine. J. Cell. Biochem. 2006;99:1489–1502. doi: 10.1002/jcb.21047. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann. Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Religa D, Strozyk D, Cherny RA, Volitakis I, Haroutunian V, Winblad B, Naslund J, Bush AI. Elevated cortical zinc in Alzheimer disease. Neurology. 2006;67:69–75. doi: 10.1212/01.wnl.0000223644.08653.b5. [DOI] [PubMed] [Google Scholar]
- Schenck JF. Magnetic resonance imaging of brain iron. J. Neurol. Sci. 2003;207:99–102. doi: 10.1016/s0022-510x(02)00431-8. [DOI] [PubMed] [Google Scholar]
- Schenck JF, Zimmerman EA, Li Z, Adak S, Saha A, Tandon R, Fish KM, Belden C, Gillen RW, Barba A, Henderson DL, Neil W, O'Keefe T. High-field magnetic resonance imaging of brain iron in Alzheimer disease. Top. Magn. Reson. Imaging. 2006;17:41–50. doi: 10.1097/01.rmr.0000245455.59912.40. [DOI] [PubMed] [Google Scholar]
- Smith MA, Zhu X, Tabaton M, Liu G, McKeel DW, Jr, Cohen ML, Wang X, Siedlak SL, Dwyer BE, Hayashi T, Nakamura M, Nunomura A, Perry G. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J Alzheimers Dis. 2010;19:363–372. doi: 10.3233/JAD-2010-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankiewicz J, Panter SS, Neema M, Arora A, Batt CE, Bakshi R. Iron in chronic brain disorders: imaging and neurotherapeutic implications. Neurotherapeutics. 2007;4:371–386. doi: 10.1016/j.nurt.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SW, Jensen KB, Jensen MS, Silva DS, Kesslak PJ, Danscher G, Frederickson CJ. Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer's diseased brains. Brain Res. 2000;852:274–278. doi: 10.1016/s0006-8993(99)02096-x. [DOI] [PubMed] [Google Scholar]
- Thomas LO, Boyko OB, Anthony DC, Burger PC. MR detection of brain iron. Am. J. Neuroradiol. 1993;14:1043–1048. [PMC free article] [PubMed] [Google Scholar]
- Trinchese F, Liu S, Battaglia F, Walter S, Mathews PM, Arancio O. Progressive age-related development of Alzheimer-like pathology in APP/PS1 mice. Ann. Neurol. 2004;55:801–814. doi: 10.1002/ana.20101. [DOI] [PubMed] [Google Scholar]
- Vogt S. MAPS: A set of software tools for analysis and visualization of 3D X-ray fluorescence data sets. J. Phys. IV. 2003;104:635–638. [Google Scholar]
- White AR, Barnham KJ, Bush AI. Metal homeostasis in Alzheimer's disease. Expert. Rev. Neurother. 2006;6:711–722. doi: 10.1586/14737175.6.5.711. [DOI] [PubMed] [Google Scholar]
- Zhang LH, Wang X, Zheng ZH, Ren H, Stoltenberg M, Danscher G, Huang L, Rong M, Wang ZY. Altered expression and distribution of zinc transporters in APP/PS1 transgenic mouse brain. Neurobiol. Aging. 2008;31:74–87. doi: 10.1016/j.neurobiolaging.2008.02.018. [DOI] [PubMed] [Google Scholar]