Abstract
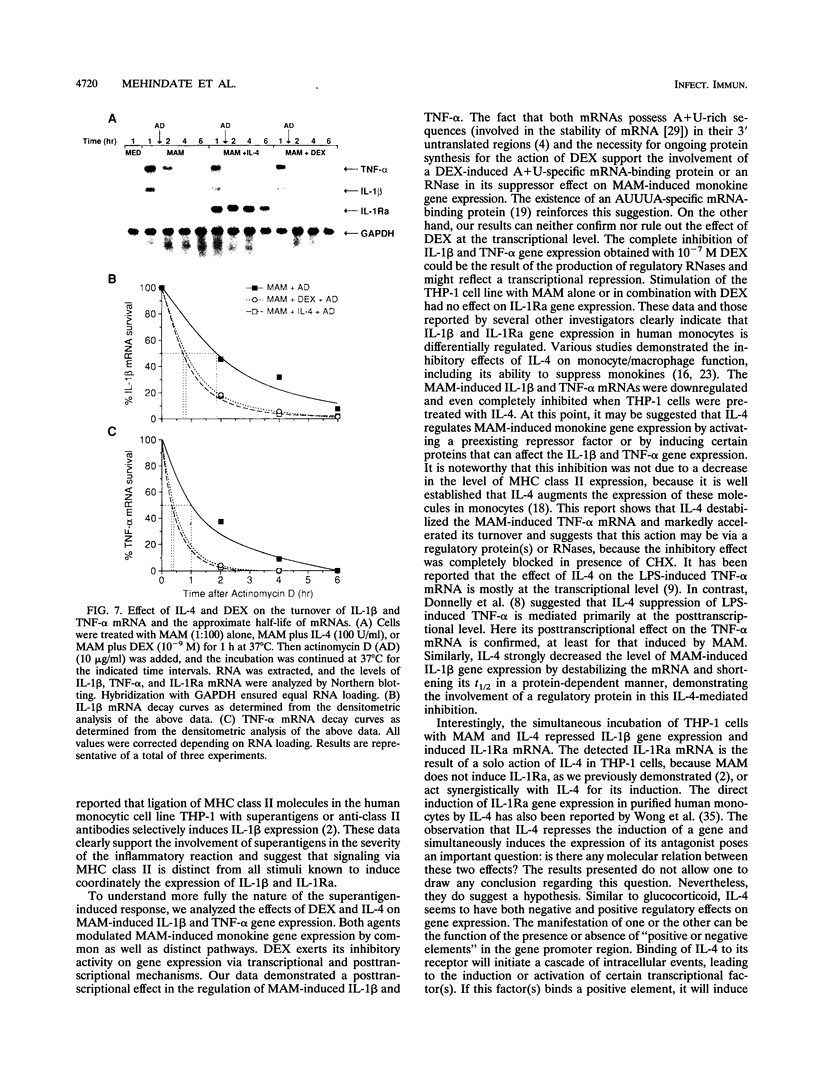
Activation of human monocytes or monocytic cell lines with all known stimuli coordinately induces the gene expression of various cytokines, including tumor necrosis factor alpha (TNF-alpha), interleukin-1 beta (IL-1 beta), and the IL-1 receptor antagonist (IL-1Ra). In contrast, superantigens induce TNF-alpha and IL-1 beta but fail to affect IL-1Ra gene expression, suggesting that activation of monocytes via major histocompatibility complex class II is distinct from other signal transduction pathways. In the present study, we analyzed the regulation of the Mycoplasma arthritidis-derived superantigen (MAM)-induced IL-1 beta and TNF-alpha gene expression by studying the effects of two different anti-inflammatory agents: dexamethasone (DEX) and the T-cell-derived cytokine IL-4. Both agents contributed to the downregulation of MAM-induced IL-1 beta and TNF-alpha gene expression. They accelerated the normal decline of the gene expression of both MAM-induced cytokines by decreasing the stability of mRNAs via the induction or enhanced synthesis of one or more regulatory proteins. In addition, IL-4, but not DEX, induced a strong and rapid expression of IL-1Ra mRNA in MAM-stimulated and unstimulated THP-1 cells in a de novo protein synthesis-independent manner. The capacity of IL-4 to induce IL-1Ra gene expression reinforces its anti-inflammatory activity. This study illustrates some of the mechanisms by which MAM-induced proinflammatory monokine gene expression can be downregulated by IL-4 and DEX.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Nagy S., Björk L., Abrams J., Holm S., Andersson U. Bacterial toxin-induced cytokine production studied at the single-cell level. Immunol Rev. 1992 Jun;127:69–96. doi: 10.1111/j.1600-065x.1992.tb01409.x. [DOI] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatila T., Geha R. S. Signal transduction by microbial superantigens via MHC class II molecules. Immunol Rev. 1993 Feb;131:43–59. doi: 10.1111/j.1600-065x.1993.tb01529.x. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R., Jones R. S., Cahill J. F. Chronic proliferative arthritis of mice induced by Mycoplasma arthritidis. I. Induction of disease and histopathological characteristics. Infect Immun. 1971 Oct;4(4):344–355. doi: 10.1128/iai.4.4.344-355.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M. K., Zagon G., Chu Z., Ravina B., Tumang J. R., Cole B. C., Friedman S. M. Human B cell differentiation induced by microbial superantigens: unselected peripheral blood lymphocytes secrete polyclonal immunoglobulin in response to Mycoplasma arthritidis mitogen. Autoimmunity. 1992;14(1):23–32. doi: 10.3109/08916939309077353. [DOI] [PubMed] [Google Scholar]
- Donnelly R. P., Fenton M. J., Kaufman J. D., Gerrard T. L. IL-1 expression in human monocytes is transcriptionally and posttranscriptionally regulated by IL-4. J Immunol. 1991 May 15;146(10):3431–3436. [PubMed] [Google Scholar]
- Essner R., Rhoades K., McBride W. H., Morton D. L., Economou J. S. IL-4 down-regulates IL-1 and TNF gene expression in human monocytes. J Immunol. 1989 Jun 1;142(11):3857–3861. [PubMed] [Google Scholar]
- Friedman S. M., Crow M. K., Tumang J. R., Tumang M., Xu Y. Q., Hodtsev A. S., Cole B. C., Posnett D. N. Characterization of human T cells reactive with the Mycoplasma arthritidis-derived superantigen (MAM): generation of a monoclonal antibody against V beta 17, the T cell receptor gene product expressed by a large fraction of MAM-reactive human T cells. J Exp Med. 1991 Oct 1;174(4):891–900. doi: 10.1084/jem.174.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. H., Burgess D. R., Vitti G. F., Hamilton J. A. Interleukin-4 stimulates human monocytes to produce tissue-type plasminogen activator. Blood. 1989 Sep;74(4):1222–1225. [PubMed] [Google Scholar]
- Hart P. H., Vitti G. F., Burgess D. R., Whitty G. A., Piccoli D. S., Hamilton J. A. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci U S A. 1989 May;86(10):3803–3807. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell M. D., Diveley J. P., Lundeen K. A., Esty A., Winters S. T., Carlo D. J., Brostoff S. W. Limited T-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10921–10925. doi: 10.1073/pnas.88.23.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Brehm G., Nicklas W., Beck R., Herbst F. Biochemical characterization of the T-cell mitogen derived from Mycoplasma arthritidis. Scand J Immunol. 1986 Sep;24(3):245–249. doi: 10.1111/j.1365-3083.1986.tb02091.x. [DOI] [PubMed] [Google Scholar]
- Lew W., Oppenheim J. J., Matsushima K. Analysis of the suppression of IL-1 alpha and IL-1 beta production in human peripheral blood mononuclear adherent cells by a glucocorticoid hormone. J Immunol. 1988 Mar 15;140(6):1895–1902. [PubMed] [Google Scholar]
- Littman B. H., Dastvan F. F., Carlson P. L., Sanders K. M. Regulation of monocyte/macrophage C2 production and HLA-DR expression by IL-4 (BSF-1) and IFN-gamma. J Immunol. 1989 Jan 15;142(2):520–525. [PubMed] [Google Scholar]
- Malter J. S. Identification of an AUUUA-specific messenger RNA binding protein. Science. 1989 Nov 3;246(4930):664–666. doi: 10.1126/science.2814487. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Mourad W., Mehindate K., Schall T. J., McColl S. R. Engagement of major histocompatibility complex class II molecules by superantigen induces inflammatory cytokine gene expression in human rheumatoid fibroblast-like synoviocytes. J Exp Med. 1992 Feb 1;175(2):613–616. doi: 10.1084/jem.175.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad W., al-Daccak R., Chatila T., Geha R. S. Staphylococcal superantigens as inducers of signal transduction in MHC class II-positive cells. Semin Immunol. 1993 Feb;5(1):47–55. doi: 10.1006/smim.1993.1007. [DOI] [PubMed] [Google Scholar]
- Mukaida N., Zachariae C. C., Gusella G. L., Matsushima K. Dexamethasone inhibits the induction of monocyte chemotactic-activating factor production by IL-1 or tumor necrosis factor. J Immunol. 1991 Feb 15;146(4):1212–1215. [PubMed] [Google Scholar]
- Paliard X., West S. G., Lafferty J. A., Clements J. R., Kappler J. W., Marrack P., Kotzin B. L. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991 Jul 19;253(5017):325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- Posnett D. N. Do superantigens play a role in autoimmunity? Semin Immunol. 1993 Feb;5(1):65–72. doi: 10.1006/smim.1993.1009. [DOI] [PubMed] [Google Scholar]
- Rink L., Kirchner H. Mycoplasma arthritidis-derived superantigen. Chem Immunol. 1992;55:137–145. [PubMed] [Google Scholar]
- Rink L., Nicklas W., Alvarez-Ossorio L., Koester M., Kirchner H. Differential induction of tumor necrosis factor alpha in murine and human leukocytes by Mycoplasma arthritidis-derived superantigen. Infect Immun. 1994 Feb;62(2):462–467. doi: 10.1128/iai.62.2.462-467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Standiford T. J., Strieter R. M., Chensue S. W., Westwick J., Kasahara K., Kunkel S. L. IL-4 inhibits the expression of IL-8 from stimulated human monocytes. J Immunol. 1990 Sep 1;145(5):1435–1439. [PubMed] [Google Scholar]
- Thornhill M. H., Kyan-Aung U., Haskard D. O. IL-4 increases human endothelial cell adhesiveness for T cells but not for neutrophils. J Immunol. 1990 Apr 15;144(8):3060–3065. [PubMed] [Google Scholar]
- Tobler A., Meier R., Seitz M., Dewald B., Baggiolini M., Fey M. F. Glucocorticoids downregulate gene expression of GM-CSF, NAP-1/IL-8, and IL-6, but not of M-CSF in human fibroblasts. Blood. 1992 Jan 1;79(1):45–51. [PubMed] [Google Scholar]
- Waage A., Bakke O. Glucocorticoids suppress the production of tumour necrosis factor by lipopolysaccharide-stimulated human monocytes. Immunology. 1988 Feb;63(2):299–302. [PMC free article] [PubMed] [Google Scholar]
- Wong H. L., Costa G. L., Lotze M. T., Wahl S. M. Interleukin (IL) 4 differentially regulates monocyte IL-1 family gene expression and synthesis in vitro and in vivo. J Exp Med. 1993 Mar 1;177(3):775–781. doi: 10.1084/jem.177.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H. L., Lotze M. T., Wahl L. M., Wahl S. M. Administration of recombinant IL-4 to humans regulates gene expression, phenotype, and function in circulating monocytes. J Immunol. 1992 Apr 1;148(7):2118–2125. [PubMed] [Google Scholar]
- Woodland D. L., Blackman M. A. How do T-cell receptors, MHC molecules and superantigens get together? Immunol Today. 1993 May;14(5):208–212. doi: 10.1016/0167-5699(93)90164-G. [DOI] [PubMed] [Google Scholar]
- al-Daccak R., Mehindate K., Hébert J., Rink L., Mecheri S., Mourad W. Mycoplasma arthritidis-derived superantigen induces proinflammatory monokine gene expression in the THP-1 human monocytic cell line. Infect Immun. 1994 Jun;62(6):2409–2416. doi: 10.1128/iai.62.6.2409-2416.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Daccak R., Mehindate K., Poubelle P. E., Mourad W. Signalling via MHC class II molecules selectively induces IL-1 beta over IL-1 receptor antagonist gene expression. Biochem Biophys Res Commun. 1994 Jun 15;201(2):855–860. doi: 10.1006/bbrc.1994.1779. [DOI] [PubMed] [Google Scholar]