Abstract
Exposure of rats to an odor of a predator can elicit an innate fear response. In addition, such exposure has been shown to activate limbic brain regions such as the amygdala. However, there is a paucity of data on the phenotypic characteristics of the activated amygdalar neurons following predator odor exposure. In the current experiments, rats were exposed to cloth which contained either ferret odor, butyric acid, or no odor for 30 minutes. Ferret odor-exposed rats displayed an increase in defensive burying versus control rats. Sections of the brains were prepared for dual-labeled immunohistochemistry and counts of c-Fos co-localized with Ca2+/calmodulin-dependent protein kinase II (CAMKII), parvalbumin, or calbindin were made in the basolateral (BLA), central (CEA), and medial (MEA) nucleus of the amygdala. Dual-labeled immunohistochemistry showed a significant increase in the percentage of CAMKII–positive neurons also immunoreactive for c-Fos in the BLA, CEA and MEA of ferret odor-exposed rats compared to control and butyric acid-exposed groups. Further results showed a significant decrease in calbindin-immunoreactive neurons that were also c-Fos-positive in the anterior portion of the BLA of ferret odor-exposed rats compared to control and butyric acid-exposed rats, whereas the MEA expressed a significant decrease in calbindin/c-Fos dual-labeled neurons in butyric acid-exposed rats compared to controls and ferret odor-exposed groups. These results enhance our understanding of the functioning of the amygdala following exposure to predator threats by showing phenotypic characteristics of activated amygdalar neurons. With this knowledge, specific neuronal populations could be targeted to further elucidate the fundamental underpinnings of anxiety and could possibly indicate new targets for the therapeutic treatment of anxiety.
Keywords: amygdala, anxiety, calbindin, CAMKII, c-Fos, ferret, parvalbumin, stress
1. Introduction
The amygdala is an important brain region in facilitating fear and anxiety, and amygdala dysregulation has been linked to anxiety-associated disorders. As a pivotal region for processing sensory stimuli and output to effector regions involved in behavioral and emotional responses (for reviews see Millan, 2003, Rosen and Donley, 2006, LeDoux, 2007, Rosen et al., 2008, Butler and Finn, 2009), the amygdala is also a prime target for anxiety-related therapeutics (for review see Mathew et al., 2008). However, the amygdala contains phenotypically-distinct neuronal populations which potentially play unique roles in the facilitation of stress and fear (McDonald, 2003, for review see Sah et al., 2003, Reznikov et al., 2008, Truitt et al., 2009). Studies to date have been limited in their ability to properly characterize the activation of these neuronal subpopulations of the amygdala following exposure to anxiety-inducing threats.
A common test of unconditioned aversion exposes a rodent to a predator odor which elicits an innate fear response even if the rodent has never encountered the predator before. Such exposure elicits defensive behaviors such as burying, excessive rearing, freezing, escape attempts, and flat-backed approaches to the source of the odor, as well as other anxiety-related behaviors (for reviews see Fendt et al., 2005, Takahashi et al., 2005). Anxiety-like responses in rodents have been reported with several odors, including cat and ferret odor (Blanchard et al., 1990, Masini et al., 2005), and 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), a component of fox urine (Vernet-Maury, 1980). The use of cat or ferret odor has repeatedly shown to induce robust fear-related behaviors in rodents (Masini et al., 2005, Staples and McGregor, 2006).
c-Fos is the protein product of the immediate-early gene, c-fos, and is widely used as a marker for neuronal activation (for review see Herrera and Robertson, 1996). In addition to eliciting fear-related behaviors, exposure to the odor of a predator has been shown to induce c-Fos expression in several limbic brain regions of rodents. For example, Staples et al. (2008b) showed increased c-Fos expression in the medial prefrontal cortex and hypothalamus (among others) following exposure to cat odor compared to control odor, while Masini et al. (2005) showed increased c-Fos mRNA expression in regions such as the basolateral and medial amygdala and the periaqueductal grey in ferret odor-exposed rats compared to controls. While such studies are valuable in terms of elucidating neural pathways involved in response to stress/anxiety, further studies are needed to determine the phenotypic and functional characteristics of the activated neurons in these different brain regions.
The amygdala consists of numerous subnuclei, each containing a heterogeneous population of neurons. McDonald et al. (2001, 2002) showed that in the basolateral amygdala (BLA), pyramidal and non-pyramidal neuronal subpopulations can be differentiated with protein markers. In the BLA, pyramidal, glutamatergic neurons contain calcium-binding protein alpha type II calcium/calmodulin-dependent protein kinase (CAMKII) (McDonald et al., 2002) and non-pyramidal GABA-containing neurons contain different types of calcium-binding proteins such as calbindin (CB) and/or parvalbumin (PV) (McDonald and Mascagni, 2001). Further studies showed direct innervation from PV-containing neurons in the BLA to the major glutamatergic pyramidal subpopulation of neurons which contain CAMKII (McDonald et al., 2002). Thus, it is possible that stressful stimuli might activate phenotypically-distinct neurons of the BLA, and this differential activation might engage distinct circuits in the amygdala. Such altered activation of neuronal subpopulations of the BLA has been observed following acute and repeated restraint stress (Reznikov et al., 2008) as well as with the expression of conditioned fear (Burghardt et al., 2006). Characterization of phenotypically distinct neuronal populations in the central and medial nuclei of the amygdala (CEA and MEA, respectively) have not been investigated as fully as with the BLA; however, studies have shown CB-positive immunoreactivity in the CEA and MEA (Pitkanen and Amaral, 1993a, Kemppainen and Pitkanen, 2000) with virtually no PV-positive immunoreactivity (Pitkanen and Amaral, 1993b, Sorvari et al., 1995, Zahm et al., 2003).
We have previously shown that exposure to ferret odor elicits anxiety-related behaviors such as freezing and defensive burying and that microinjection of various compounds into the amygdala can alter these behavioral responses (Wilson and Junor, 2008). To further elucidate the role of the amygdala in predator-induced defensive behaviors, in the present study we examined the activation of neuronal populations induced by exposure to predator odor in amygdalar subnuclei.We hypothesized that exposure of rats to predator ferret odor would activate the glutamatergic pyramidal subpopulation of neurons (CAMKII+) in the BLA and also activate distinct sets of GABAergic neuronal subpopulations (PV+ or CB+) which project an inhibitory control over these excitatory pyramidal neurons. Using dual-labeled immunohistochemistry, we sought to determine the extent of c-Fos expression in CAMKII-, PV-, and CB-positive neurons in subnuclei of the amygdala following exposure to no-odor, a noxious butyric acid odor (Hebb et al., 2004), and ferret odor in rats.
2. Experimental Procedures
2.1 Animals
Male Long-Evans rats (Harlan Laboratories, Indianapolis, IN), weighing approximately 250–300g were single-housed in an environmentally controlled animal facility on a 12:12 h light: dark cycle with lights on at 0700 hours. Purina rat chow and water were available ad libitum. All experiments were conducted during the light phase, beginning at least 2 hours after light phase onset. Animals were housed in an animal facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Animal care and use procedures were carried out in accordance with protocols written under the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of South Carolina.
2.2 Behavioral testing
The defensive burying test was performed as described in Wilson and Junor (2008) with the addition of 2 groups of rats which were exposed to either low or high amounts of butyric acid. A Plexiglas chamber (45 × 30 × 44 cm) filled up to a depth of 5 cm with fresh pine bedding with either a piece of untreated gauze (control), gauze with low (25 μL) or high (50-100 μL) amounts of butyric acid (based on Hebb et al., 2004), or a piece of ferret-scented towel (5 cm2) placed 2 cm above bedding, was used for this analysis (towels a generous donation from Dr. John Hines from Yale University). In a second set of experiments, rats were habituated to the novel arena for 2 consecutive days prior to testing. The habituation phase lasted for 30 minutes and was recorded onto videotape. On the test day, rats in the second set of experiments were exposed to either a piece of untreated gauze, gauze treated with a high volume (100 μL) of butyric acid, or the ferret-scented towel. In the second set of experiments, there were 3 behavioral groups; however, because the behavior data for both the first and second experiments showed similar patterns, the data were combined giving a total of 4 behavioral groups: control, low butyric, high butyric, and ferret. On the day of testing, rats were placed in the Plexiglas chamber with the odor, and behavior was recorded onto videotape for a duration of 30 minutes. Immediately after the behavioral trial, rats were placed back into the home cage. One hour 30 minutes later (2 hours from the beginning of the behavioral trial), rats were placed under deep isoflurane anesthesia and transcardially perfused with 0.1M phosphate buffer followed by 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4). The brains were removed, post-fixed overnight and transferred to 30% sucrose solution in 0.1 M phosphate buffer for cryoprotection. Serial coronal sections (45-50 micron) were cut on a microtome and stored at −20°C in anti-freezing solution (30% sucrose and 30% ethylene glycol in 0.1M phosphate buffer) until they were processed for immunohistochemistry.
2.3 Behavioral assessment
A trained rater blind to the experimental conditions assessed behavior using the Observer® XT 9.0 software package (Noldus Information Technology, Wageningen, The Netherlands). Latency to bury, duration of burying (defined as spraying bedding toward the gauze/towel), duration of tunneling (defined as the act of burrowing under the bedding), duration of freezing behavior (defined as the cessation of all movement except that needed for respiration), the number of flat-backed approaches (defined as elongation of the body while moving slowly to the source odor) to the ferret-scented cloth, the duration of walking, grooming, sniffing, the number and duration of rears (defined as number of times the animal lifted both forelimbs) and the number of escape attempts (defined as jumping towards the top of the chamber) were measured from videotapes for the duration of the 30 min test. In the absence of burying, animals were assigned a latency to bury of 30 min and duration of burying of zero.
2.4 Immunohistochemistry
Single- and dual-label immunohistochemistry (c-Fos, c-Fos and CAMKII, c-Fos and PV, or c-Fos and CB) was performed on representative sections through the rostro-caudal extent of the amygdala. Briefly, all sections were initially incubated with either rabbit (1:10000) or goat (1:1000) anti-c-Fos antibody in Tris-buffered saline (TBS) with 4% normal horse serum and 0.2% Triton X-100 for 48 h at 4°C; [Calbiochem, La Jolla, CA for rabbit, Santa Cruz Biotechnology, Inc., Santa Cruz, CA]. This was followed by incubation with a biotinylated donkey anti-rabbit or donkey anti-goat secondary antibody (1:1000) for 1.5 h at room temperature (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) and then horseradish peroxidase-conjugated streptavidin for 1 h at room temperature; (1:1600; Jackson ImmunoResearch Laboratories Inc.). c-Fos immunoreactivity was visualized by developing the sections in a nickel-cobalt intensified diaminobenzidine solution with 0.3 % hydrogen peroxide, yielding a blue-black reaction product confined to the nucleus of c-Fos-positive cells (standard mounting, dehydration, and coverslipping then proceeded for single-labeled c-Fos immunohistochemistry). After c-Fos staining, sections were incubated in mouse primary antisera directed against CAMKII (1:500; Upstate, Lake Placid, NY), PV (1:8000; Sigma-Aldrich, St. Louis, MO), or CB (1:5000; Sigma-Aldrich) at 4°C for 48 h, followed by incubation with unlabeled donkey anti-mouse secondary antibodies (1:100; Jackson ImmunoResearch Laboratories Inc.) for 2 hours at room temperature and finally with mouse peroxidase-anti-peroxidase (1:250; Covance, Florida). Immunoreactivity for CAMKII, PV, or CB was visualized by developing the sections in plain diaminobenzidine solution with 3.0 % hydrogen peroxide, yielding a brown reaction product confined to the cytoplasm of immunoreactive cells with these markers. For each marker, immunohistochemistry from amygdalar sections from the control, butyric acid-exposed and ferret odor-exposed rats were processed at the same time. After standard mounting, dehydration and coverslipping procedures, total numbers of neurons labeled with cFos, CAMK, PV or CB were counted, as well as numbers of dual-labeled neurons showing c-Fos positive nuclei and cytoplasm stained with CAMKII, PV or CB under a light microscope at 10X magnification. Subregions of the amygdala (BLA, CEA, and MEA) in the left and right hemispheres were counted from one representative section in the anterior (from Bregma: −1.80 mm to −2.30 mm) and posterior (from Bregma: −2.56 mm to −3.30 mm) portions of the amygdala (Figure 1). Neuronal counts were done by investigators blinded to the treatment condition of the sections. For single-labeled c-Fos in each region as well as single and dual-labeled CAMKII-, CB-, and PV-immunoreactivity in the MEA, counts were made with the use of a reticle encompassing an area of 0.1225 mm2 at 20x magnification to generate a density of labeled neurons per mm2. In tissue with dual-immunohistochemical staining, all labeled neurons within the boundaries of the anterior and posterior BLA and CEA were counted. The average area of the anterior/posterior BLA and CEA was then calculated from three representative samples. The total number of neurons from single and dual-labeled neurons from dual-stained tissue was then divided by the average area of the subregion to determine the density of the immunoreactive cells per mm2. Photomicrographic images were captured with a Photometrics CoolSnap digital camera (Roper Scientific, Trenton, NJ, USA) linked to a computer equipped with IPLab software. Images were collected and imported into Adobe Photoshop where minor adjustments for contrast and brightness were made.
Figure 1.
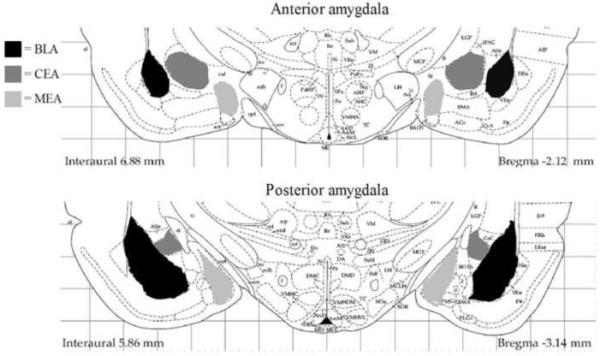
Depiction of the anatomical levels of the amygdala analyzed in this study. Separate counts were made in the anterior (−1.80 to −2.30 mm from bregma) and posterior (−2.56 to −3.30 mm from bregma) portions of the basolateral/lateral (BLA), central (CEA), and medial (MEA) nuclei of the amygdala. Figure modified from Paxinos and Watson (1998).
2.5 Experimental optimization for the medial amygdala
During our initial experiment we observed that the development time with diaminobenzidine required for assessing CAMKII immunoreactivity in the MEA was greater than the developmental time needed for BLA and CEA. Therefore, we conducted a second experiment to provide enough tissue for adequately analyzing changes in the MEA. Due to a change in husbandry procedures (bedding) at our institution, we decided to add the habituation protocol prior to testing with the odors. The patterns of single-labeled c-Fos labeling in the BLA and CEA did not differ (statistically) between habituated versus non-habituated rats nor did the patterns for double-labeled immunohistochemistry. Thus, the immunohistochemistry data presented for the BLA and CEA represent the combined data from habituated and non-habituated rats. The CAMKII immunohistochemistry data for the MEA, however, is only presented from the sections which received a longer duration of development during immunohistochemistry in animals that had been habituated to the testing arena. Note that the longer developmental time did not make it difficult to count dual labeled c-Fos/CAMKII-labeling in the BLA and CEA.
2.6 Data analysis
The four odor exposure groups were compared using a one-way analysis of variance (ANOVA) for behavioral and immunohistochemistry parameters in each region. Immunohistochemistry data were expressed as a percentage of CAMKII-, PV-, or CB-labeled neurons with c-Fos-positive nuclei in each subregion of the amygdala (see Figure 1). Total cell counts for single-labeled neurons with each marker were also compared (Table 1). In addition, the percentage of c-Fos-positive neurons with CAMKII-, PV-, or CB-labeled cell bodies was also generated (Table 2). Cell counts were performed in left and right hemispheres as well as anterior and posterior levels to assess the possibility of hemispheric and rostro-caudal differences in neuronal activation. To determine total anterior or posterior labeling, counts from the left and right were added together; similarly, to determine total left and right labeling, counts from the anterior and posterior portions of each amygdala subregion were added together; finally, to determine total labeling for a specific subregion, total counts for the left, right, anterior, and posterior portions were added together. Two-way analysis of variance (ANOVA) was initially performed to determine the effects of odor exposure, anterior and posterior differences, and the interaction on total cFos activation. Since differences in total cFos activation were found between anterior and posterior sections, one way ANOVA was then performed in dual-label experiments to determine the effects of odor exposure in each separate subregion. Due to low tissue quantities, analysis of c-Fos and CB double-staining for the low butyric acid group were not included in the final data set. Data were analyzed using GraphPad Prism software (v. 5.03; La Jolla, CA) with significance level set at alpha = 0.05. The source of significant (P<0.05) main effects was determined by post-hoc Student-Newman-Keuls (SNK) comparisons.
Table 1.
Density of neurons in subregions of the amygdala which are immunoreactive for c-FOS, CAMKII, parvalbumin, and calbindin
| Region | Treatment | c-FOS densitya |
CAMKII densityb |
PV densityb | CB densityb |
|---|---|---|---|---|---|
| Anterior BLA | control | 178±21 (11) | 17±2 (11) | 3±0 (5) | 10±2 (10) |
| Low butyric | 137±50 (4) | 23±5 (4) | 4±1 (5) | - | |
| High butyric | 149±25 (11) | 16±2 (10) | 2±0 (4) | 14±4 (11) | |
| Ferret | 237±34 (13) | 18±1 (14) | 3±0 (7) | 19±4 (11) | |
| Posterior BLA |
control | 246±31 (11) | 11±1 (11) | 6±1 (5) | 8±1 (11) |
| Low butyric | 233±70 (4) | 11±2 (4) | 4±1 (5) | - | |
| High butyric | 268±27 (11) | 11±1 (10) | 4±0 (4) | 9±1 (11) | |
| Ferret | 355±41 (13) | 12±1 (14) | 4±1 (7) | 8±1 (12) | |
| Anterior CEA | control | 129±30 (11) | 7±1 (11) | N/A | 12±3 (10) |
| Low butyric | 141±74 (4) | 4±1 (3) | - | ||
| High butyric | 149±28 (11) | 10±1 (10) | 18±4 (11) | ||
| Ferret | 187±37 (13) | 8±1 (13) | 11±2 (11) | ||
| Posterior CEA |
control | 311±59 (11) | 11±1 (11) | 10±2 (11) | |
| Low butyric | 235±91 (4) | 7±2 (4) | - | ||
| High butyric | 293±45 (11) | 13±1 (10) | 11±1 (11) | ||
| Ferret | 301±46 (13) | 12±1 (14) | 14±1 (12) | ||
| Anterior MEA |
control | 68±11 (6) | 40±7 (6) | 141±13 (4) | |
| Low butyric | - | - | - | ||
| High butyric | 51±5 (7) | 54±11 (7) | 151±5 (7) | ||
| Ferret | 75±12 (6) | 60±8 (6) | 138±15 (6) | ||
| Posterior MEA |
control | 69±7 (6) | 57±12 (6) | 145±10 (6) | |
| Low butyric | - | - | - | ||
| High butyric | 51±5 (7) | 63±14 (7) | 166±10 (7) | ||
| Ferret | 109±19* (6) | 68±10 (6) | 164±14 (6) |
P<0.05 vs. control. PV=parvalbumin, CB=calbindin. The data is presented as the density of labeled neurons in the subregion (per mm2). The numbers in parentheses are the N for each group.
From tissue slices single-labeled for c-FOS
From tissue slices double-labeled for c-FOS and CAMKII/PV/or CB
Table 2.
Percentage of c-FOS immunoreactive neurons which also contain CAMKII, parvalbumin, or calbindin
| Region | Treatment | % c-FOS with CAMKII |
% c-FOS with PV |
% c-FOS with CB |
|---|---|---|---|---|
| Anterior BLA | control | 16.6±7.2 | 9.5±1.7 | 20.4±2.3 |
| Low butyric | 22.8±8.7 | 6.5±3.8 | - | |
| High butyric | 20.9±4.4 | 22.3±9.7 | 9.2±1.7** | |
| Ferret | 41.7±4.4* | 22.2±7.8 | 9.7±2.1** | |
| Posterior BLA |
control | 14.9±5.0 | 9.8±1.6 | 19.2±2.8 |
| Low butyric | 14.3±4.7 | 7.6±3.1 | - | |
| High butyric | 16.3±4.0 | 19.5±4.8 | 17.6±2.5 | |
| Ferret | 38.8±4.3** | 10.4±2.5 | 14.8±1.6 | |
| Anterior CEA | control | 9.3±3.2 | N/A | 12.7±5.7 |
| Low butyric | 29.2±18.7 | - | ||
| High butyric | 24.2±7.0 | 7.7±2.5 | ||
| Ferret | 39.3±4.3* | 10.6±2.7 | ||
| Posterior CEA |
control | 6.0±1.9 | 15.2±3.1 | |
| Low butyric | 29.5±13.7 | - | ||
| High butyric | 16.1±4.0 | 8.5±2.0 | ||
| Ferret | 40.7±4.4*** | 11.0±2.4 | ||
| Anterior MEA |
control | 2.2±1.4 | 25.8±4.1 | |
| Low butyric | - | - | ||
| High butyric | 5.4±1.3 | 13.9±1.5* | ||
| Ferret | 10.5±2.0** | 14.7±2.7* | ||
| Posterior MEA |
control | 1.7±0.6 | 26.2±4.2 | |
| Low butyric | - | - | ||
| High butyric | 2.9±1.1 | 15.3±3.7* | ||
| Ferret | 10.8±1.8*** | 13.2±2.0* |
P<0.001
P<0.01
P<0.05 vs. control. N=4-13. PV=parvalbumin, CB=calbindin
3. RESULTS
3.1 Exposure to ferret or noxious odor elicits fear behaviors in naïve rats
Rats which had never encountered a predator ferret expressed innate fear-related behaviors upon exposure to the odor of a ferret. One-way analysis of variance (ANOVA) revealed a significant effect of odor exposure on the duration of burying (F3,38=6.540; P=0.0004) (Figure 2A) and the latency to begin burying (F3,38=4.609; P=0.0086) (Figure 2B). Student-Newman-Keuls (SNK) post-hoc test revealed that exposure to ferret odor resulted in the expression of robust innate aversive behaviors as indicated by an increase in the total duration of defensive burying (P<0.001) and a decrease in the latency to bury (P<0.05) compared to non-odor-exposed control rats. Exposure to high amounts of the noxious butyric acid odor also resulted in an increase in the duration of defensive burying (P<0.01) and a decreased latency to bury compared to non-odor-exposed rats (P<0.01), while exposure to low amounts of butyric acid resulted in a decreased latency to bury (P<0.05) but very low levels of burying behavior that did not differ from control levels (P>0.05). Further behavioral analysis showed a significant effect of odor exposure on the duration of sniffing (F3,38=14.39; P<0.0001) (Figure 2C) and the duration of grooming (F3,38=4.042; P=0.0139) (data not shown). Post-hoc analysis revealed that exposure to ferret odor or high amounts of butyric acid resulted in a significant (P<0.001) decrease in total duration of sniffing compared to controls and low amounts of butyric acid. Rats exposed to ferret odor also exhibited a significant (P<0.05) increase in the total duration of grooming compared to rats which were exposed to low amounts of butyric acid. No overall effect of odor exposure was measured on the duration of tunneling, freezing, walking, number of flat-back approaches, escape attempts, and the total number or duration of rears (data not shown).
Figure 2.
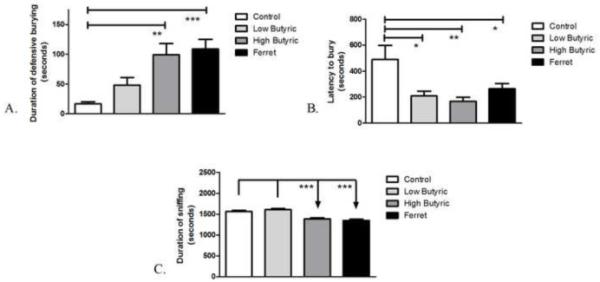
Anxiety measures in the defensive burying task after exposure to butyric acid and ferret odor. The total duration of odor exposure was 30 minutes. High amounts (50-100 uL) of butyric acid and ferret odor exposure increased the (A) duration of defensive burying while low (25 μL), high amounts of butyric acid and ferret odor exposure decreased the (B) latency to begin burying. High amounts of butyric acid and ferret odor exposure reduced the (C) total duration of sniffing. **P<0.001, **P<0.01, *P<0.05 (N=5-13).
3.2 Exposure to ferret odor increases the activation of CAMKII-positive neurons of the basolateral, central, and medial nuclei of the amygdala
The major glutamatergic population of the basolateral amygdala, indicated phenotypically as expressing high levels of CAMKII immunoreactivity (McDonald et al., 2002), showed increased activation following exposure to ferret odor. One-way ANOVA showed a significant effect of odor-exposure on the percentage of CAMKII-positive neurons containing c-Fos immunoreactivity in the BLA (F3,38=11.96; P<0.0001) (Figure 3A). Post-hoc analysis revealed a significant increase in the percentage of CAMKII-positive neurons with c-Fos in ferret-odor-exposed compared with control- (P<0.001), low butyric acid- (P<0.01), and high butyric acid-odor-exposed rats (P<0.001). Furthermore, a significant effect of odor-exposure on the percentage of CAMKII-positive neurons containing c-Fos immunoreactivity was observed in the CEA (F3,38=6.073; P=0.0019) (Figure 3B) and MEA (F2,18=6.073; P<0.0001). A significant increase in the percentage of CEA CAMKII-positive neurons with c-Fos was seen in ferret-odor-exposed versus control- (P<0.01), low butyric acid- (P<0.05), and high butyric acid-odor-exposed rats (P<0.01). There was also a significant (P<0.001) increase in the percentage of MEA CAMKII-positive neurons with c-Fos in ferret-odor-exposed versus control- and high butyric acid-exposed rats. As seen in Table 1, the number of CAMKII- positive neurons did not differ between treatment conditions, except in the anterior CEA (F3,38=4.415; P<0.01), and none of the odor-treatment groups differed significantly from control groups in the number of CAMKII-labeled cells in any region. The difference in anterior CEA is likely due to low number of CAMKII positive neurons and the small sample size in the low butyric acid group, and post-hoc analysis showed that only the high butyric acid-exposed group differed significantly compared to the low butyric acid-exposed group in the CEA (P<0.01).
Figure 3.
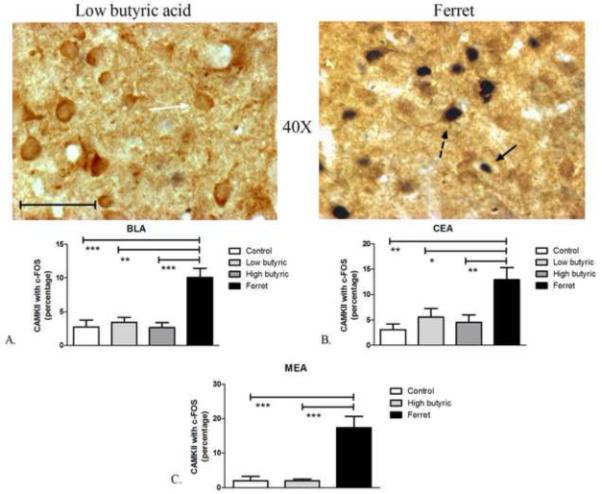
Odor exposure-induced changes in c-Fos expression of CAMKII neurons. (Top) Representative photomicrograph with CAMKII (brown) and c-Fos (blue/black) labeling in low butyric acid (left) and ferret odor-exposed (right) rats. Exposure to ferret odor increased the percentage of CAMKII-positive neurons with c-Fos in the BLA (Bottom, A), CEA (Bottom, B), and MEA (Bottom, C) compared to control, low and/or high butyric acid-exposure. ***P<0.01, **P<0.01, *P<0.05 (N=5-13). Scale bar represents approximately 50 microns. The white arrow points to a single-labeled CAMKII neuron, the black arrow points to a single-labeled c-Fos neuron, and the dashed black arrow points to a double-labeled CAMKII/c-Fos neuron.
To determine the relative phenotypic proportions of c-Fos labeled neurons, we also investigated the percentage of c-Fos labeled neurons which contain CAMKII-, PV, or CB-labeled cell bodies (Table 2). As seen in Table 1, amygdalar subregions showed increased c-Fos density after odor exposure. There was a significant effect of odor-exposure on the density of single-labeled c-Fos neurons in the posterior MEA (F2,18=6.463; P=0.0088) and two-way ANOVA indicated a significant effect of odor exposure in BLA (F3,67=3.114; P=0.0316), as well as differences between anterior and posterior BLA in the density of c-Fos labeled neurons (F1,67=13.26; P=0.0005). More critically, there was a significant effect of odor exposure on the percentage of c-Fos neurons with CAMKII in the BLA (F3,38=6.496; P=0.0013), CEA (F3,38=11.14; P<0.0001), and MEA (F2,18=12.09; P=0.0006). In each subregion, exposure to ferret odor significantly increased the percentage of c-Fos-labeled neurons with CAMKII compared to control (BLA: P<0.01; CEA: P<0.001; MEA: P<0.001), low (BLA: P<0.05; CEA: P<0.01) and high (BLA: P<0.01; CEA: P<0.001; MEA: P<0.01) butyric acid-exposed groups.
3.3 Exposure to ferret odor does not activate PV-positive neurons in the basolateral amygdala
The PV-immunoreactive neurons in the BLA are GABAergic, non-pyramidal interneurons (McDonald and Betette, 2001, McDonald and Mascagni, 2001). We observed dense PV-positive neurons in the BLA, but no PV-positive neurons in the CEA or MEA, so results are confined to the BLA regions. The activation of PV-positive neurons, which are expressed exclusively in the lateral/basolateral nucleus in the amygdala, showed no significant change due to differential odor exposure (F3,17=0.556; P=0.65). The density of PV-positive neurons in amygdala subregions (Table 1), and the percentage of PV-immunoreactive neurons colocalized with c-Fos were similar in all groups as shown in Figure 4. In addition, the percentage of c-Fos-immunoreactive neurons colocalized with PV was similar between groups as shown in Table 2.
Figure 4.
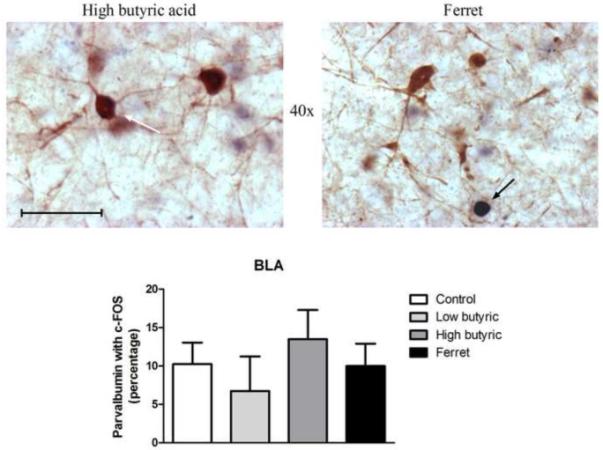
(Top) Representative photomicrograph with parvalbumin (brown) and c-Fos (blue/black) labeling in high butyric acid (left) and ferret odor-exposed (right) rats. Parvalbumin-staining was isolated to the BLA. (Bottom) No changes in c-Fos expression in parvalbumin neurons following differential odor exposure (N=4-7). Scale bar represents approximately 50 microns. The white arrow points to a single-labeled parvalbumin neuron, and the black arrow points to a single-labeled c-Fos neuron.
3.4 Exposure to ferret-odor reduces CB-positive-neuronal activation in the anterior portion of the basolateral amygdala
CB-positive neurons in the anterior portions of the BLA, which are exclusively non-pyramidal GABAergic interneurons (McDonald, 1997, McDonald and Mascagni, 2001), showed significantly less activation following exposure to ferret odor compared to non-odor-exposed controls. One-way ANOVA showed a significant effect of odor-exposure on the percentage of CB-positive neurons with c-Fos in the anterior portion of the BLA (F2,28=12.42; P=0.0002; Figure 5A), but not in the posterior regions of the BLA (F2,33=0.7455; P=0.4828). Post-hoc analysis revealed a significant decrease in CB-positive neuronal activation in the anterior BLA of both ferret- (P<0.001) and high butyric acid-exposed rats (P<0.001) compared to non-odor-exposed control rats. One-way ANOVA showed a significant effect of odor exposure on the percentage of CB-positive neurons with c-Fos in the MEA as well (F2,16=12.45; P=0.0008). Surprisingly, exposure to high levels of butyric acid resulted in a significant decrease (P<0.01) in CB-positive neurons with c-Fos in the MEA compared to controls and ferret-odor exposed rats. There were no significant effects of odor exposure on the percentage of CB-positive neurons with c-Fos in the CEA (F2,16=3.069; P=0.0785), the number of CB-positive neurons (Table 1), or the percentage of c-Fos-immunoreactive neurons colocalized with CB in any subregion (Table 2).
Figure 5.
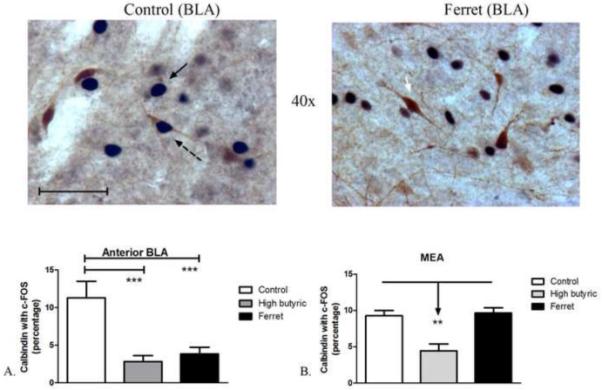
Odor exposure-induced changes in c-Fos expression in calbindin neurons. (Top) Representative photomicrograph with calbindin (brown) and c-Fos (blue/black) labeling in control (left) and ferret odor-exposed (right) rats. (Bottom, A) Exposure to high amounts of butyric acid (50-100 μL) and ferret odor decreased the percentage of calbindin-positive neurons with c-Fos in the anterior portion of the BLA compared to control odor-exposure. (Bottom, B) Exposure to high amounts of butyric acid decreased the percentage of calbindin-positive neurons with c-Fos in the MEA compared to control odor- and ferret odor-exposed rats. ***P<0.001, **P<0.01 (N=4-11). Scale bar represents approximately 50 microns. The white arrow points to a single-labeled calbindin neuron, the black arrow points to a single-labeled c-Fos neuron, and the dashed black arrow points to a double-labeled calbindin/c-Fos neuron.
4. DISCUSSION
Our results indicate that exposure of rats to a either a predator odor or high amounts of a noxious control odor elicits aversive behaviors such as increased defensive burying and a decrease in the latency to bury. Rats which had been exposed to the ferret-scented towel, but not high amounts of butyric acid, expressed increased co-localization of c-Fos, a marker of neuronal activation, with CAMKII in glutamatergic pyramidal neurons in the BLA. Furthermore, the anterior BLA of rats exposed to a ferret-scented towel or high amounts of butyric acid displayed significantly less co-localization of c-Fos with CB in GABAergic non-pyramidal neurons, but no changes in the activation of the GABAergic neuronal populations which contained PV in the BLA (anterior or posterior). Further results also showed an increased co-localization of c-Fos with CAMKII in the CEA and MEA of rats which had been exposed to ferret odor compared to controls.
The increase in aversive behaviors in rats upon exposure to a ferret odor corroborates a number of previous studies which showed similar behavioral effects (Masini et al., 2005, Masini et al., 2006, for review see Campeau et al., 2008, Wilson and Junor, 2008, Masini et al., 2009, Weinberg et al., 2009, Masini et al., 2010). Olfactory receptors which are specific to predator threats are processed by unique zones in the olfactory bulb (Kobayakawa et al., 2007), which project to and activate limbic brain regions such as the amygdala following exposure to predator odors (Hebb et al., 2002). The specific type of odor has previously been shown to be an important factor in eliciting an aversive behavioral response. For example, studies of TMT by Vernet-Maury (1980, 1984) and others (for review see Fendt et al., 2005) have shown that TMT elicits anxiety-induced responses such as increased corticosterone release, freezing, and urination. However, recent studies by Staples et al. (2006, 2008b) and others have suggested little to no avoidance behaviors in rats exposed to TMT. The olfactory bulb projects to the amygdala and recent evidence suggests that the MEA plays a prominent role in the expression of aversive behaviors following exposure to ferret odor as lesions to the MEA disrupted hypothalamo-pituitary-adrenocortical axis activation following ferret odor exposure – an effect not observed with CEA lesions (Masini et al., 2009). However, we have previously shown that the CEA can regulate rat aversive behaviors to ferret odor through opioid-dependent mechanisms (Wilson and Junor, 2008). The CEA is the major amygdalar output nucleus to downstream autonomic effector regions and receives intra-amygdalar neuronal projections from the BLA. However, previous studies investigating the activation of subnuclei of the amygdala following predator exposure have yielded mixed results. For example, Masini et al. (2005) showed an increase in mRNA expression for c-Fos following ferret odor exposure in the rat BLA and posteroventral MEA, but not the CEA – an effect corroborated with a similar study by Staples et al. (2008b) using cat odor. In contrast, Dielenberg et al. (2001b) showed that rat exposure to cat odor increased c-Fos expression in the MEA and CEA, but not the BLA. While these studies are important in elucidating overall activation of distinct brain regions, they do not indicate the functional and phenotypic characteristics of the activated neurons. On this point, one must also consider the difference between mRNA and protein as the dependent measure. Whether mRNA changes result in increased protein expression may depend on the duration, specific type, and context of the odor exposure and the timing of the analysis. Furthermore, when comparing results consideration should be made regarding the type of species (rats vs. mice), different strains (ie. Sprague-Dawley vs. Long-Evans), and differences in methodology (ie. habituation to new environment, type of arena, etc.) – all of which could partially account for differences in behavior and c-Fos expression between studies.
In the current study, we show two key findings regarding the phenotypic characteristics of the activated neurons in the BLA following exposure to predator odor: (1) an increase in CAMKII-positive neuronal activation and (2) a decrease in CB-positive neuronal activation in the anterior portion of the BLA. The increase in glutamatergic CAMKII-positive neuronal population suggests an increase in excitation to downstream effector regions. The increase in activation of CAMKII-positive neurons in the CEA, which receives projections from the BLA, appears to support this conjecture. We find it somewhat surprising that PV-positive neurons in the BLA were not activated following exposure to ferret odor as they do receive perisomatic innervations from CAMKII-positive pyramidal neurons in the BLA (McDonald et al., 2005). It is possible that PV-positive neurons also receive inhibitory connections from other BLA or non-BLA neuronal subtypes. For example, inhibitory vasoactive intestinal peptide (VIP)-positive neurons in the BLA have been shown to innervate CB-positive neurons in the anterior BLA (McDonald and Pearson, 1989, Muller et al., 2003). PV-positive neurons are a subpopulation of CB-positive neurons (McDonald and Betette, 2001) and thus it is possible that they receive excitatory (via CAMKII-positive neurons) and inhibitory (via VIP-positive or other) connections within the network, though this has not been shown directly. Previous studies have also shown that rats develop contextual fear with an environment previously associated with odor from the fur of a cat (Dielenberg et al., 2001a), but not odor which originated from cat feces or with TMT (Blanchard et al., 2003). Further experiments to determine if in fact the rats are forming a negative association with the contextual arena due to the exposure to ferret odor would be of interest.
Both CB and PV-positive neurons in the BLA have previously been shown to be mostly non-pyramidal and GABAergic (McDonald and Betette, 2001, Muller et al., 2006). Furthermore, CB and PV-positive neurons in the BLA innervate and control CAMKII pyramidal neurons and seem to control circuit outputs (McDonald and Betette, 2001, McDonald et al., 2002). The decrease in activation in CB-positive neurons in the anterior portions of the BLA upon exposure to ferret odor suggests a decrease in inhibitory control of these CB neurons on the pyramidal, CAMKII-positive neurons of the BLA with the expression of predator odor-induced anxiety. Approximately 80% of PV-positive neurons contain CB and 60% of CB-positive neurons contain PV in the entire BLA (McDonald and Betette, 2001). The high levels of CB co-localization with c-Fos in the control group compared to the ferret group without a similar effect on PV neuronal populations may be due to the fact that small populations of CB-positive neurons in the anterior BLA are not colocalized with PV, but are colocalized with the neuropeptide somatostatin (SOM) (McDonald and Mascagni, 2002). Studies with SOM have shown no co-localization with PV but approximately 90% co-localization with CB in the BLA (McDonald and Mascagni, 2002). Thus, interesting future studies would be to determine if SOM-positive interneurons in the anterior BLA also exhibit decreased activation upon exposure to predator odor. The lack of change in the posterior regions of the BLA may be related to the fact that many pyramidal neurons show CB immunoreactivity in this region suggesting that the reduced neuronal activation of CB positive interneuron populations in the posterior BLA is offset by the activation CB-positive pyramidal neurons (McDonald et al., 2002). Another major interneuronal subpopulation of the BLA contains the calcium binding protein calretinin (CR). CR-positive neurons in the BLA are highly co-localized with GABA but are mostly distinct from CB, PV and SOM (McDonald and Mascagni, 2001, 2002). However, previous studies have shown no change in activation of CR-positive neurons following exposure to acute or repeated restraint stress (Reznikov et al., 2008). In any rate, in addition to investigating SOM-positive neuronal activation following exposure to ferret odor, interesting future studies would also investigate activation of CR-positive neuronal activation in the BLA. Activation of this neuronal population might represent another interesting difference between predator odor exposure and restraint stress.
Previous studies have shown that rats exposed to acute restraint stress display a significant increase in the percentage of CAMKII, PV and CB neurons which co-localized with c-Fos in the BLA (Reznikov et al., 2008). This would suggest differential activation of PV and CB containing interneurons based on the type of stressor (i.e., predator odor vs. restraint stress). Further supporting this hypothesis are the results of the current study which showed an increase in CAMKII-positive neuronal activation in the BLA in the ferret odor-exposed group and not the high butyric acid-exposed group. Given that neuronal activation of CB positive neurons in the BLA was similar between the high butyric and ferret odor-exposed groups, the possibility exists that anxiogenic (restraint, predator) stimuli are processed by CAMKII-positive projection neurons whereas noxious stimuli such as butyric acid are processed by both projection neurons and interneuronal populations. Most interestingly, CAMKII- containing neurons in the BLA and CEA were activated by exposure to a ferret odor, but not high levels of butyric acid, despite the similar behavioral defensive burying response in these two groups. This might suggest that activation of these neurons is associated with the detection of anxiogenic stimuli, rather than the specific behavior output, while other neuronal populations in the amygdala help determine the behavioral output. This would be supported by the activation of BLA CAMKII- containing projection neurons with both restraint stress (Reznikov et al., 2008) and predator odor (present study).
In addition to altered activation of BLA neurons, our data also show differential effects of odor exposure on neuronal populations in the MEA. In contrast to the BLA, populations of the neurons in the MEA have not been characterized as thoroughly. Our data show that exposure to ferret odor increases activation of CAMKII-positive neurons in the MEA. This result is not surprising considering that multiple studies have shown increased neuronal activation in the MEA following exposure to predator odor (Staples et al., 2008a, Staples et al., 2008b, Samuelsen and Meredith, 2009b, a). It has been suggested that when predator odor is detected by olfactory receptors the accessory olfactory bulb processes the information and activates the medial amygdala via glutamatergic projections (Hunt et al., 2009). We also show that exposure to high levels of butyric acid reduces the percentage of CB-positive neurons with c-Fos compared to controls and ferret odor-exposed rats. This result is a little bit more difficult to explain mostly due to the lack of studies which have characterized projections to and from CB-positive MEA neurons. We can speculate that the reduction is, at least in part, due to the reduction in sniffing behaviors observed in high butyric acid-exposed rats that somehow does not correlate with the reduction of sniffing in ferret odor-exposed rats. More studies are needed to confirm these hypotheses as well as on the elucidation of the anatomy of neuronal subpopulations of the MEA.
There are suggestions that the amygdala does not play a major role in mediating responses to predators and most studies show little to no increase in c-Fos expression in the BLA upon exposure to predator odor (for review see Wallace and Rosen, 2001, Rosen et al., 2008). Studies have shown, however, that lesions of the BLA significantly reduced aversive behaviors upon exposure to predator odor (Takahashi et al., 2007). Further, while we show an increase in c-Fos expression in CAMKII-positive neurons in the BLA after ferret odor exposure, we also show a corresponding decrease in c-Fos expression in CB-positive c-Fos neurons in the BLA, which could explain the lack of differences in immediate early gene activation in some studies. The data presented here suggest a possible mechanistic role for the CB-positive interneurons and CAMKII-positive projection neurons in the BLA in predator odor-induced aversion, and suggest that distinct neuronal populations in the amygdala may be activated by different anxiogenic stimuli.
Our data show that in the BLA, CAMKII-, PV-, or CB-positive neurons represent approximately 60-80% of all activated neurons following odor exposure. Thus, these three populations of neurons represent the majority of neurons activated by exposure to predator odor in the BLA. In contrast, in the CEA and MEA, these cell types represent approximately 20-40% and 10%, respectively, of all activated neurons. The question remains then as to what phenotypic characteristics comprise the remaining percentage of activated neurons in the CEA and MEA. The lack of PV-immunoreactive neurons in the CEA and MEA confirms previous reports (Pitkanen and Amaral, 1993b, Sorvari et al., 1995, Zahm et al., 2003). Previous data suggests that most neurons in the CEA contain neuropeptides such as neuropeptide Y, enkephalin, and somatostatin, corticotropin releasing factor and others (Simantov et al., 1977, Barden et al., 1981, Fischman and Moldow, 1982, Smith et al., 1985) and that these neuropeptide-containing neurons may represent phenotypically and functionally distinct populations. Similarly, the MEA also has been shown to be abundant in neuropeptide-containing neurons. Interesting future studies would be to examine the activation of phenotypically distinct neurons in the CEA and MEA with a focus on the neuropeptides contained in the activated neurons.
One point of consideration remains in regards to whether the expression of the predator odor-induced anxiety is mediated through the activation of the distinct amygdalar neuronal populations. Tests which can selectively inhibit or activate selected neuronal populations would aid in determining whether a causal or passive role exists for the distinct neuronal populations in the expression of innate fear. Furthermore, c-Fos protein expression is not a direct measure of neuronal activation, and other immediate early genes could show distinct patterns of neuronal activation.
CONCLUSION
The study presented here enhances our knowledge of the amygdala circuitry involved in the expression of innate fear induced by the odor of a natural predator. We show that distinct neuronal populations in the amygdala are activated by different stressors/anxiogenic stimuli, suggesting that these stimuli activated unique neuronal circuits through this brain area. Such knowledge is important for the fundamental understanding of fear and anxiety and could be used to determine viable targets for anxiety-related disorders.
Research highlights.
Exposure of rats to ferret odor induces an innate fear response of defensive burying
Exposure of rats to ferret odor activates discrete CAMKII-positive subpopulations of the basolateral, central, and medial amygdala
A reduction in co-localization of c-Fos with calbindin in the basolateral amygdala was observed in rats exposed to ferret odor
ACKNOWLEDGEMENTS
This work was funded by an NIMH RO1 (MH063344) and an R21 (AA017361) grant awarded to MAW and JRF. Additionally, we acknowledge funding from Science Undergraduate Research Fellowship (SURF) for EMO and MIH-MBRS-RISE at (Universidad del Este) (UNE) 5R25GM066250 for PBV. The authors wish to thank Dr. Alexander J. McDonald for insightful comments on the content of the manuscript. Thanks to Pumpkin, Zooey, Valentine and Dr. John Hines (Yale University) for supplying ferret-scented towels.
ABBREVIATIONS
- CAMKII
Ca2+/calmodulin-dependent protein kinase II
- BLA
basolateral amygdala
- CEA
central nucleus of the amygdala
- MEA
medial nucleus of the amygdala
- PV
parvalbumin
- CB
calbindin
- TMT
2,5-dihydro-2,4,5-trimethylthiazoline
- VIP
vasoactive intestinal peptide
- CR
calretinin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTEREST The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Barden N, Merand Y, Rouleau D, Moore S, Dockray GJ, Dupont A. Regional distributions of somatostatin and cholecystokinin-like immunoreactivities in rat and bovine brain. Peptides. 1981;2:299–302. doi: 10.1016/s0196-9781(81)80123-4. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Markham C, Yang M, Hubbard D, Madarang E, Blanchard RJ. Failure to produce conditioning with low-dose trimethylthiazoline or cat feces as unconditioned stimuli. Behav Neurosci. 2003;117:360–368. doi: 10.1037/0735-7044.117.2.360. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Weiss SM, Meyer S. The effects of ethanol and diazepam on reactions to predatory odors. Pharmacol Biochem Behav. 1990;35:775–780. doi: 10.1016/0091-3057(90)90357-n. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Pasumarthi RK, Wilson MA, Fadel J. Alterations in fear conditioning and amygdalar activation following chronic wheel running in rats. Pharmacol Biochem Behav. 2006;84:306–312. doi: 10.1016/j.pbb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol. 2009;88:184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Campeau S, Nyhuis TJ, Sasse SK, Day HE, Masini CV. Acute and chronic effects of ferret odor exposure in Sprague-Dawley rats. Neurosci Biobehav Rev. 2008;32:1277–1286. doi: 10.1016/j.neubiorev.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dielenberg RA, Carrive P, McGregor IS. The cardiovascular and behavioral response to cat odor in rats: unconditioned and conditioned effects. Brain Res. 2001a;897:228–237. doi: 10.1016/s0006-8993(01)02227-2. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. “When a rat smells a cat”: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001b;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T, Lowry CA, Apfelbach R, McGregor IS. TMT-induced autonomic and behavioral changes and the neural basis of its processing. Neurosci Biobehav Rev. 2005;29:1145–1156. doi: 10.1016/j.neubiorev.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Fischman AJ, Moldow RL. Distribution of CRF-like immunoreactivity in the rabbit. Peptides. 1982;3:841–843. doi: 10.1016/0196-9781(82)90025-0. [DOI] [PubMed] [Google Scholar]
- Hebb AL, Zacharko RM, Dominguez H, Trudel F, Laforest S, Drolet G. Odor-induced variation in anxiety-like behavior in mice is associated with discrete and differential effects on mesocorticolimbic cholecystokinin mRNA expression. Neuropsychopharmacology. 2002;27:744–755. doi: 10.1016/S0893-133X(02)00354-8. [DOI] [PubMed] [Google Scholar]
- Hebb AL, Zacharko RM, Gauthier M, Trudel F, Laforest S, Drolet G. Brief exposure to predator odor and resultant anxiety enhances mesocorticolimbic activity and enkephalin expression in CD-1 mice. Eur J Neurosci. 2004;20:2415–2429. doi: 10.1111/j.1460-9568.2004.03704.x. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Hunt GE, Van Nieuwenhuijzen PS, Chan-Ling T, McGregor IS. ‘When an old rat smells a cat’: A decline in defense-related, but not accessory olfactory, Fos expression in aged rats. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Kemppainen S, Pitkanen A. Distribution of parvalbumin, calretinin, and calbindin-D(28k) immunoreactivity in the rat amygdaloid complex and colocalization with gamma-aminobutyric acid. J Comp Neurol. 2000;426:441–467. doi: 10.1002/1096-9861(20001023)426:3<441::aid-cne8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, Mori K, Sakano H. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr Biol. 2007;17:R868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Masini CV, Garcia RJ, Sasse SK, Nyhuis TJ, Day HE, Campeau S. Accessory and main olfactory systems influences on predator odor-induced behavioral and endocrine stress responses in rats. Behav Brain Res. 2010;207:70–77. doi: 10.1016/j.bbr.2009.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini CV, Sasse SK, Garcia RJ, Nyhuis TJ, Day HE, Campeau S. Disruption of neuroendocrine stress responses to acute ferret odor by medial, but not central amygdala lesions in rats. Brain Res. 2009;1288:79–87. doi: 10.1016/j.brainres.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini CV, Sauer S, Campeau S. Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence. Behav Neurosci. 2005;119:280–292. doi: 10.1037/0735-7044.119.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masini CV, Sauer S, White J, Day HE, Campeau S. Non-associative defensive responses of rats to ferret odor. Physiol Behav. 2006;87:72–81. doi: 10.1016/j.physbeh.2005.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Price RB, Charney DS. Recent advances in the neurobiology of anxiety disorders: implications for novel therapeutics. Am J Med Genet C Semin Med Genet. 2008;148C:89–98. doi: 10.1002/ajmg.c.30172. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Calbindin-D28k immunoreactivity in the rat amygdala. J Comp Neurol. 1997;383:231–244. [PubMed] [Google Scholar]
- McDonald AJ. Encyclopedia of the Neurological Sciences. Elsevier Science; 2003. Amygdala; pp. 124–128. [Google Scholar]
- McDonald AJ, Betette RL. Parvalbumin-containing neurons in the rat basolateral amygdala: morphology and co-localization of Calbindin-D(28k) Neuroscience. 2001;102:413–425. doi: 10.1016/s0306-4522(00)00481-4. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala. Neuroscience. 2001;105:681–693. doi: 10.1016/s0306-4522(01)00214-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Res. 2002;943:237–244. doi: 10.1016/s0006-8993(02)02650-1. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Mania I, Rainnie DG. Evidence for a perisomatic innervation of parvalbumin-containing interneurons by individual pyramidal cells in the basolateral amygdala. Brain Res. 2005;1035:32–40. doi: 10.1016/j.brainres.2004.11.052. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Muller JF, Mascagni F. GABAergic innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. J Comp Neurol. 2002;446:199–218. doi: 10.1002/cne.10204. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Pearson JC. Coexistence of GABA and peptide immunoreactivity in non-pyramidal neurons of the basolateral amygdala. Neurosci Lett. 1989;100:53–58. doi: 10.1016/0304-3940(89)90659-9. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Synaptic connections of distinct interneuronal subpopulations in the rat basolateral amygdalar nucleus. J Comp Neurol. 2003;456:217–236. doi: 10.1002/cne.10435. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, editors. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- Pitkanen A, Amaral DG. Distribution of calbindin-D28k immunoreactivity in the monkey temporal lobe: the amygdaloid complex. J Comp Neurol. 1993a;331:199–224. doi: 10.1002/cne.903310205. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Amaral DG. Distribution of parvalbumin-immunoreactive cells and fibers in the monkey temporal lobe: the amygdaloid complex. J Comp Neurol. 1993b;331:14–36. doi: 10.1002/cne.903310103. [DOI] [PubMed] [Google Scholar]
- Reznikov LR, Reagan LP, Fadel JR. Activation of phenotypically distinct neuronal subpopulations in the anterior subdivision of the rat basolateral amygdala following acute and repeated stress. J Comp Neurol. 2008;508:458–472. doi: 10.1002/cne.21687. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Donley MP. Animal studies of amygdala function in fear and uncertainty: relevance to human research. Biol Psychol. 2006;73:49–60. doi: 10.1016/j.biopsycho.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Pagani JH, Rolla KL, Davis C. Analysis of behavioral constraints and the neuroanatomy of fear to the predator odor trimethylthiazoline: a model for animal phobias. Neurosci Biobehav Rev. 2008;32:1267–1276. doi: 10.1016/j.neubiorev.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. Categorization of biologically relevant chemical signals in the medial amygdala. Brain Res. 2009a;1263:33–42. doi: 10.1016/j.brainres.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. The vomeronasal organ is required for the male mouse medial amygdala response to chemical-communication signals, as assessed by immediate early gene expression. Neuroscience. 2009b;164:1468–1476. doi: 10.1016/j.neuroscience.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simantov R, Kuhar MJ, Uhl GR, Snyder SH. Opioid peptide enkephalin: immunohistochemical mapping in rat central nervous system. Proc Natl Acad Sci U S A. 1977;74:2167–2171. doi: 10.1073/pnas.74.5.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Parent A, Kerkerian L, Pelletier G. Distribution of neuropeptide Y immunoreactivity in the basal forebrain and upper brainstem of the squirrel monkey (Saimiri sciureus) J Comp Neurol. 1985;236:71–89. doi: 10.1002/cne.902360107. [DOI] [PubMed] [Google Scholar]
- Sorvari H, Soininen H, Paljarvi L, Karkola K, Pitkanen A. Distribution of parvalbumin-immunoreactive cells and fibers in the human amygdaloid complex. J Comp Neurol. 1995;360:185–212. doi: 10.1002/cne.903600202. [DOI] [PubMed] [Google Scholar]
- Staples LG, Hunt GE, van Nieuwenhuijzen PS, McGregor IS. Rats discriminate individual cats by their odor: possible involvement of the accessory olfactory system. Neurosci Biobehav Rev. 2008a;32:1209–1217. doi: 10.1016/j.neubiorev.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Staples LG, McGregor IS. Defensive responses of Wistar and Sprague-Dawley rats to cat odour and TMT. Behav Brain Res. 2006;172:351–354. doi: 10.1016/j.bbr.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Staples LG, McGregor IS, Apfelbach R, Hunt GE. Cat odor, but not trimethylthiazoline (fox odor), activates accessory olfactory and defense-related brain regions in rats. Neuroscience. 2008b;151:937–947. doi: 10.1016/j.neuroscience.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Hubbard DT, Lee I, Dar Y, Sipes SM. Predator odor-induced conditioned fear involves the basolateral and medial amygdala. Behav Neurosci. 2007;121:100–110. doi: 10.1037/0735-7044.121.1.100. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci Biobehav Rev. 2005;29:1157–1167. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Truitt WA, Johnson PL, Dietrich AD, Fitz SD, Shekhar A. Anxiety-like behavior is modulated by a discrete subpopulation of interneurons in the basolateral amygdala. Neuroscience. 2009;160:284–294. doi: 10.1016/j.neuroscience.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet-Maury E. Olfaction Taste VII. VII 1980. Trimethyl-thiazoline in fox feces: A natural alarming substance for the rat. [Google Scholar]
- Vernet-Maury E, Polak EH, Demael A. Structure-activity relationship of stress-inducing odorants in the rat. Journal of Chemical Ecology. 1984;10:1007–1018. doi: 10.1007/BF00987509. [DOI] [PubMed] [Google Scholar]
- Wallace KJ, Rosen JB. Neurotoxic lesions of the lateral nucleus of the amygdala decrease conditioned fear but not unconditioned fear of a predator odor: comparison with electrolytic lesions. J Neurosci. 2001;21:3619–3627. doi: 10.1523/JNEUROSCI.21-10-03619.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MS, Bhatt AP, Girotti M, Masini CV, Day HE, Campeau S, Spencer RL. Repeated ferret odor exposure induces different temporal patterns of same-stressor habituation and novel-stressor sensitization in both hypothalamic-pituitary-adrenal axis activity and forebrain c-fos expression in the rat. Endocrinology. 2009;150:749–761. doi: 10.1210/en.2008-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Junor L. The role of amygdalar mu-opioid receptors in anxiety-related responses in two rat models. Neuropsychopharmacology. 2008;33:2957–2968. doi: 10.1038/sj.npp.1301675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Grosu S, Irving JC, Williams EA. Discrimination of striatopallidum and extended amygdala in the rat: a role for parvalbumin immunoreactive neurons? Brain Res. 2003;978:141–154. doi: 10.1016/s0006-8993(03)02801-4. [DOI] [PubMed] [Google Scholar]


