Abstract
The pathogenesis of Cryptococcus neoformans pulmonary infection in the rat was studied after intratracheal inoculation. Lungs were examined at various times following infection for histopathology in conjunction with macrophage markers, proliferating cell nuclear antigen (PCNA), and capsular glucuronoxylomannan (GXM) antigen. Serum GXM, immunoglobulin M (IgM) and IgG titers and organ fungal burden were compared with pathological findings. C. neoformans organisms were in the lung parenchyma 2 h postinoculation, and GXM antigen was present in surrounding tissues shortly thereafter. Extrapulmonary dissemination occurred early in infection. Two phases of host cellular inflammatory response were discernible: early local macrophage recruitment at 2 to 4 days followed by granulomatous inflammation, which reached maximum intensity 14 days after infection. The granulomatous phase was preceded by lymphocyte influx with macrophage proliferation and maturation into epithelioid histiocytes; this was paralleled by a shift of yeasts from extracellular to intracellular spaces. Tissue IgG deposits, serum IgG to GXM, and localization of tissue GXM immunoreactivity to epithelioid cells were noted at 2 to 4 weeks. A 10-fold decrease in lung fungal burden occurred 25 days postinfection and was associated with resolving granulomas, fewer proliferating cells, and decreased tissue GXM. The present study demonstrates that (i) C. neoformans penetrates the lung parenchyma shortly after infection; (ii) immunocompetent rats control pulmonary cryptococcosis efficiently, with minimal extrapulmonary dissemination and low levels of serum GXM; and (iii) macrophage activation is likely to play a crucial role in limiting C. neoformans infection in the rat lung.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolaños B., Mitchell T. G. Killing of Cryptococcus neoformans by rat alveolar macrophages. J Med Vet Mycol. 1989;27(4):219–228. [PubMed] [Google Scholar]
- Bolaños B., Mitchell T. G. Phagocytosis and killing of Cryptococcus neoformans by rat alveolar macrophages in the absence of serum. J Leukoc Biol. 1989 Dec;46(6):521–528. doi: 10.1002/jlb.46.6.521. [DOI] [PubMed] [Google Scholar]
- Bolaños B., Mitchell T. G. Phagocytosis of Cryptococcus neoformans by rat alveolar macrophages. J Med Vet Mycol. 1989;27(4):203–217. [PubMed] [Google Scholar]
- Casadevall A., Mukherjee J., Devi S. J., Schneerson R., Robbins J. B., Scharff M. D. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J Infect Dis. 1992 Jun;165(6):1086–1093. doi: 10.1093/infdis/165.6.1086. [DOI] [PubMed] [Google Scholar]
- Casadevall A., Mukherjee J., Scharff M. D. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J Immunol Methods. 1992 Sep 18;154(1):27–35. doi: 10.1016/0022-1759(92)90209-c. [DOI] [PubMed] [Google Scholar]
- Casadevall A., Scharff M. D. The mouse antibody response to infection with Cryptococcus neoformans: VH and VL usage in polysaccharide binding antibodies. J Exp Med. 1991 Jul 1;174(1):151–160. doi: 10.1084/jem.174.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. H., Curtis J. L., Mody C. H., Christensen P. J., Armstrong L. R., Toews G. B. Effect of granulocyte-macrophage colony-stimulating factor on rat alveolar macrophage anticryptococcal activity in vitro. J Immunol. 1994 Jan 15;152(2):724–734. [PubMed] [Google Scholar]
- Dykstra M. A., Friedman L., Murphy J. W. Capsule size of Cryptococcus neoformans: control and relationship to virulence. Infect Immun. 1977 Apr;16(1):129–135. doi: 10.1128/iai.16.1.129-135.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert T. F., Kozel T. R. Production and characterization of monoclonal antibodies specific for Cryptococcus neoformans capsular polysaccharide. Infect Immun. 1987 Aug;55(8):1895–1899. doi: 10.1128/iai.55.8.1895-1899.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland J. S. Cytokines, phagocytosis, and Mycobacterium tuberculosis. Lymphokine Cytokine Res. 1993 Apr;12(2):127–133. [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J Clin Invest. 1985 Aug;76(2):508–516. doi: 10.1172/JCI112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
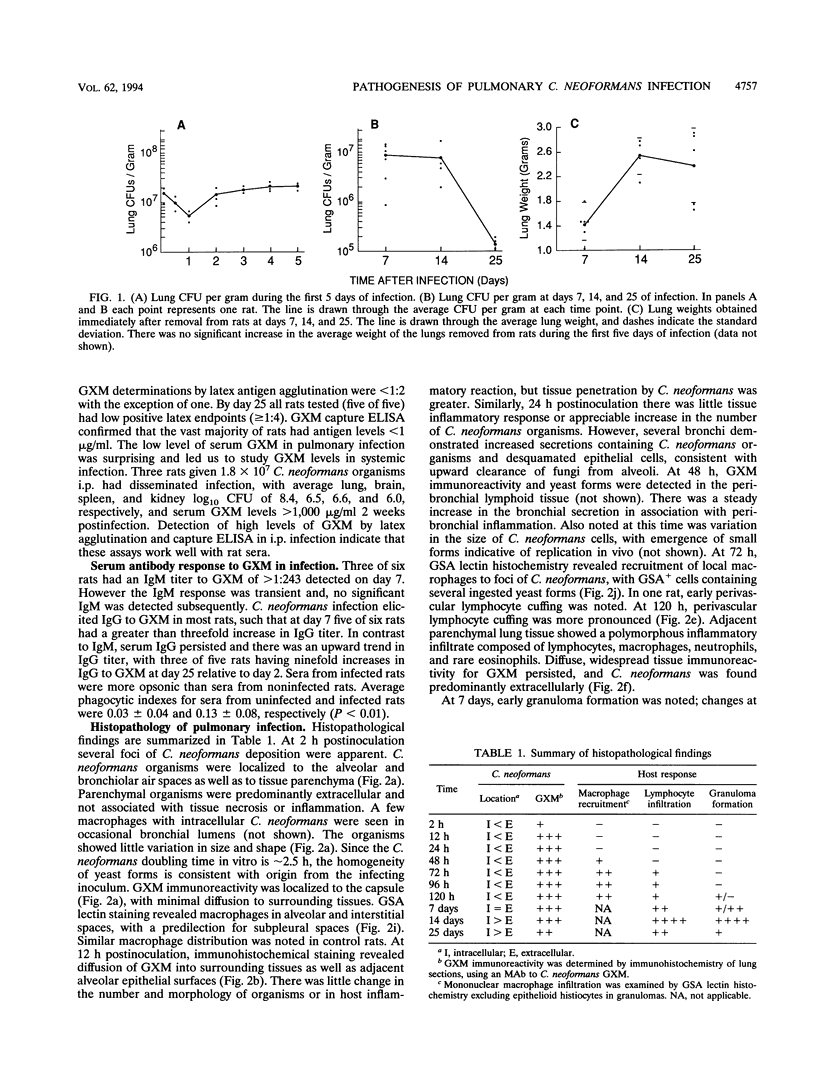
- Graybill J. R., Ahrens J., Nealon T., Paque R. Pulmonary cryptococcosis in the rat. Am Rev Respir Dis. 1983 May;127(5):636–640. doi: 10.1164/arrd.1983.127.5.636. [DOI] [PubMed] [Google Scholar]
- Hill J. O., Dunn P. L. A T cell-independent protective host response against Cryptococcus neoformans expressed at the primary site of infection in the lung. Infect Immun. 1993 Dec;61(12):5302–5308. doi: 10.1128/iai.61.12.5302-5308.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffnagle G. B., Yates J. L., Lipscomb M. F. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J Exp Med. 1991 Apr 1;173(4):793–800. doi: 10.1084/jem.173.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffnagle G. B., Yates J. L., Lipscomb M. F. T cell-mediated immunity in the lung: a Cryptococcus neoformans pulmonary infection model using SCID and athymic nude mice. Infect Immun. 1991 Apr;59(4):1423–1433. doi: 10.1128/iai.59.4.1423-1433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Gotschlich E. C. The capsule of cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982 Oct;129(4):1675–1680. [PubMed] [Google Scholar]
- Kwon-Chung K. J., Bennett J. E. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am J Epidemiol. 1984 Jul;120(1):123–130. doi: 10.1093/oxfordjournals.aje.a113861. [DOI] [PubMed] [Google Scholar]
- Lipscomb M. F. Lung defenses against opportunistic infections. Chest. 1989 Dec;96(6):1393–1399. doi: 10.1378/chest.96.6.1393. [DOI] [PubMed] [Google Scholar]
- McDonnell J. M., Hutchins G. M. Pulmonary cryptococcosis. Hum Pathol. 1985 Feb;16(2):121–128. doi: 10.1016/s0046-8177(85)80060-5. [DOI] [PubMed] [Google Scholar]
- Merkel G. J., Cunningham R. K. The interaction of Cryptococcus neoformans with primary rat lung cell cultures. J Med Vet Mycol. 1992;30(2):115–121. [PubMed] [Google Scholar]
- Mukherjee J., Zuckier L. S., Scharff M. D., Casadevall A. Therapeutic efficacy of monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan alone and in combination with amphotericin B. Antimicrob Agents Chemother. 1994 Mar;38(3):580–587. doi: 10.1128/aac.38.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Lee S., Mukherjee J., Scharff M. D., Casadevall A. Monoclonal antibodies to Cryptococcus neoformans capsular polysaccharide modify the course of intravenous infection in mice. Infect Immun. 1994 Mar;62(3):1079–1088. doi: 10.1128/iai.62.3.1079-1088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negroni R., Costa M. R., Finquelievich J. L., Iovannitti C., Agorio I., Tiraboschi I. N., Loebenberg D. Treatment of experimental cryptococcosis with SCH 39304 and fluconazole. Antimicrob Agents Chemother. 1991 Jul;35(7):1460–1463. doi: 10.1128/aac.35.7.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R., Lang S. D., Durack D. T. Influence of agglutinating antibody in experimental cryptococcal meningitis. Br J Exp Pathol. 1981 Dec;62(6):595–599. [PMC free article] [PubMed] [Google Scholar]
- Scrimgeour E. M., Purohit R. G. Chronic pulmonary cryptococcosis in a Rattus rattus from Rabaul, Papua New Guinea. Trans R Soc Trop Med Hyg. 1984;78(6):827–828. doi: 10.1016/0035-9203(84)90034-8. [DOI] [PubMed] [Google Scholar]
- Taelman H., Bogaerts J., Batungwanayo J., Van de Perre P., Lucas S., Allen S. Failure of the cryptococcal serum antigen test to detect primary pulmonary cryptococcosis in patients infected with human immunodeficiency virus. Clin Infect Dis. 1994 Jan;18(1):119–120. doi: 10.1093/clinids/18.1.119. [DOI] [PubMed] [Google Scholar]