Abstract
The purpose of this essay is to overview our findings that membrane-associated calcium-independent phospholipase A2 is markedly inhibited by low, clinically relevant concentrations of anthracyclines. Our studies suggest that due to the essential role of this enzyme in membrane homeostasis, its inhibition can be one of the early culprits leading to anthracycline-induced cardiac dysfunction. The clinical importance and potential pharmaceutical use of this new phenomenon await further studies.
Keywords: Anthracyclines, Phospholipase A2, Phospholipids, Cardiomyocytes, Cardiomyopathy
Introduction
Anthracyclines are powerful anticancer antibiotics, the therapeutic efficiency of which is abridged by their prominent cardiotoxicity. Numerous studies have ascribed anthracycline cardiotoxicity to specific cellular pathways, including an increase in free radicals formation, interference of calcium dynamics, adverse effects on RNA and protein synthesis and other putative mechanisms. Unfortunately, many of these studies have been conducted using exceedingly high concentrations of the drugs, making their relevance to clinical toxicity questionable. Moreover, therapeutic interventions, based on the above mechanisms, have had limited success. Therefore there is an urgent need to explore alternative hypotheses, which can explain cardiovascular toxicity of these drugs at clinically relevant concentrations. The purpose of this essay is to overview our findings that membrane-associated calcium-independent phospholipase A2 (iPLA2), is markedly inhibited by low, clinically relevant concentrations of anthracyclines [1–4]. For the purpose of brevity we will refer to this newly observed phenomenon as AIPI (Anthracycline-Induced Phospholipase A2 Inhibition). As a result of our studies we suggest that due to the essential role of iPLA2 in membrane homeostasis, AIPI can be one of the early culprits leading to anthracycline-induced cardiac dysfunction.
Doxorubicin and oxidative stress
We start by briefly mentioning our earlier work, which was done to confirm the oxidative theory of anthracycline cardiotoxicity. Specifically we have shown that doxorubicin is toxic to cardiomyocytes with low levels of CuZn super-oxide dismutase and this effect can be correlated with the rate of superoxide anion production in the myocyte cytosolic fractions [5]. To visualize the involvement of free radicals in doxorubicin-mediated myocyte injury we used confocal laser scanning microscopy and the oxidant-sensitive fluorescent dye, 2′,7′-dichlorofluorescin. Exposure to doxorubicin produced a rapid and concentration-dependent increase in the fluorescence that appeared to occur in close proximity to the mitochondria [6, 7]. We have also shown that pretreatment of the cells with dexrazoxane inhibited doxorubicin-induced increase in oxidant-sensitive fluorescence. Although these experiments have confirmed the ability of doxorubicin to generate reactive oxygen species in intact cells, they have also suggested that at clinically relevant concentrations of the drug (<5 μM), their levels are negligibly low and thus will not directly harm the cells. We then hypothesized that reactive oxygen species generated by the drug do not directly damage tissue via oxidative stress, but act as second messengers triggering downstream events. In search of possible culprits we turned our attention to the enzymes that may be affected by low-level oxidative stress. One of the potential targets was myocardial phospholipase A2.
Anthracycline-induced phospholipase A2 inhibition
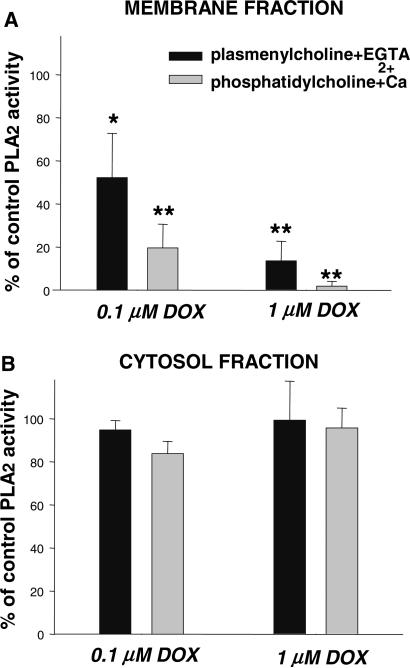
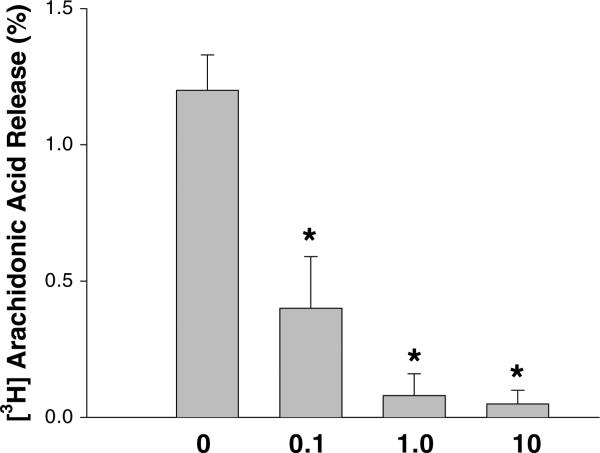
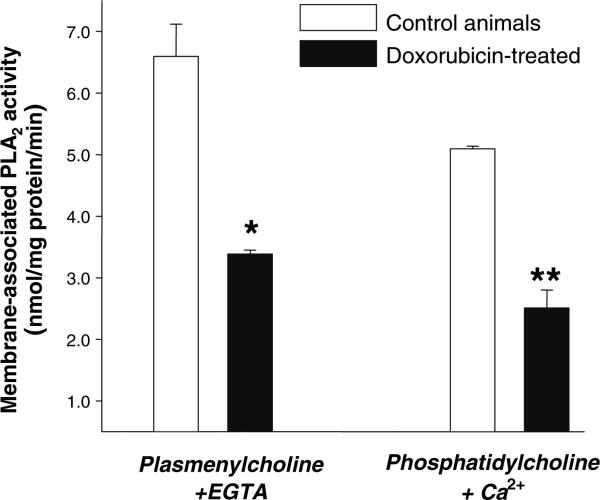
To our surprise, the measurements of total PLA2 activity in homogenates of control and doxorubicin-treated myocytes have revealed that the enzyme was markedly inhibited [1]. Importantly, the inhibition occurred at low, clinically relevant drug concentrations (as low as 0.1 μM). Our subsequent studies have shown that the effect is both concentration- and time-dependent and that the drug selectively inhibits membrane-associated PLA2 activity, without altering activity of the cytosolic enzyme (Fig. 1). The functional impact of iPLA2 inhibition was confirmed by treating cells pre-labeled with [H3]-arachidonic acid. Decrease in the baseline values of arachidonic acid (the latter being the product of iPLA2 activity) occurred minutes after doxorubicin's addition and persisted for the duration of the treatment (Fig. 2). Finally in vivo experiments, in which adult rats were intravenously injected with 4 mg/kg doxorubicin, confirmed our in vitro findings, revealing a two-fold decrease in membrane-associated Ca2+-independent iPLA2 activity in the heart tissue of drug-treated animals (Fig. 3.).
Fig. 1.
PLA2 activity in (A) membrane and (B) cytosolic fractions of control and doxorubicin-treated rat cardiomyocytes. [1]
Fig. 2.
Effect of DOX on [3H]arachidonic acid release. Myocytes were incubated with designated doxorubicin concentrations for 30 min. Basal [3H] arachidonic acid release levels remained constant in the absence of the drug. [1]
Fig. 3.
Membrane-associated iPLA2 activity in hearts from control and DOX-treated rats. Animals were given bolus injection of either saline or doxorubicin (4 mg/kg weight) and myocardial enzyme activity was assessed 4 h later [1]
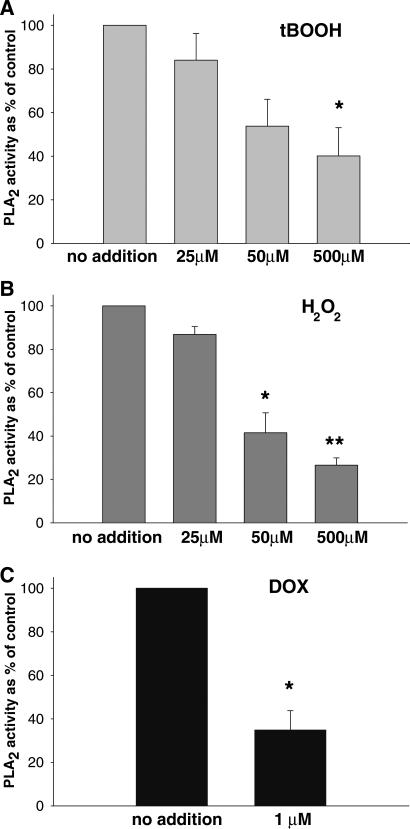
To explore the mechanism of iPLA2 inhibition we have tested whether it can be caused by the loss of membrane iPLA2 protein to the cytosol (although cytosolic activity did not increase, one can conceive translocation of the inactive form of the enzyme). However, the density of the iPLA2 bands on SDS-PAGE for the membrane and cytosol samples from doxorubicin-treated cells were identical to the bands from untreated cells and no additional bands of lower molecular weight appeared, indicating that doxorubicin treatment did not affect the amount of the actual membrane iPLA2 protein. The second possibility was a covalent modification of the iPLA2. Reducing agents blocked iPLA2 inhibition in cardiomyocyte suspensions, suggesting that the doxorubicin effect is mediated by oxidation of susceptible cysteines [1]. Notably, while tert-butyl hydroperoxide (tBOOH) and H2O2 also inhibited iPLA2 activity, it required exceedingly high concentration of the oxidants (Fig. 4) and the effect was not specific to the membrane form of the enzyme [2].
Fig. 4.
Activity of iPLA2 in membrane fraction of myocytes pre-treated with (A) tert-butyl peroxide, (B) hydrogen peroxide and (C) doxorubicin. Note the difference is effective oxidant concentrations [2]
Effect of different analogues
We then compared effects of four anthracycline analogues widely used in clinic: doxorubicin, daunorubicin, idarubicin, and epirubicin [3]. We argued that if a correlation is observed between the analogue's effect on iPLA2 activity and clinical indices of cardiotoxicity, it would serve as an important sign that this phenomenon is responsible for the deleterious effects of anthracyclines. For all examined analogues iPLA2 inhibition was concentration- and time-dependent, preceded detectable changes in cell viability and was specific to the membrane-associated enzyme. The degree of inhibition by equimolar concentrations of epirubicin and idarubicin was less than that of doxorubicin or daunorubicin. Overall the “ranking order” of analogue's ability to inhibit iPLA2 (doxorubicin ≈ daunorubicin > epirubicin > idarubicin) was in agreement with clinical reports and studies in chronic animal models. Interestingly the pecking order of the acute drug's toxicity was quite different (idarubicin > daunorubicin > epirubicin > doxorubicin). The latter sequence was derived from our data on cell necrosis observed after 24 h exposure to 20 μM of each analogue [4]. Such a discrepancy between in vitro and in vivo cardiotoxicity has been noted before, and is evident from a literature survey on comparative studies of analogues’ cardiac effects [4]. Notably, the differences in analogues’ pharmacokinetics and cardiac accumulation do not necessarily explain the discrepancies between in vivo and in vitro data [8, 9].
Chronic administration of anthracyclines and its effect on phospholipid content
Our next step was to examine whether iPLA2 activity is altered during chronic administration of anthracyclines. These experiments employed a rat model of anthracycline cardiomyopathy, which involves weekly injections of doxorubicin [10]. This protocol results in a weight loss, increased heart/body ratio, structural lesions, decreased ejection fraction and eventually leads to the development of congestive heart failure [11]. We employed low, subtoxic cumulative doses of the drug [8] mg/kg) at which no prominent cardiac lesions were observed (they starts to appear at >15 mg/kg in rat [10]). The goal was to observe changes in iPLA2 activity and membrane composition before the development of cardiomyopathic lesions. Indeed, we saw a marked decrease in myocardial iPLA2 activity in animals chronically treated with doxorubicin [4]. Interestingly, a hypothesis that chronically decreased myocardial membrane-associated iPLA2 activity can be detrimental to the heart function was supported by recent data that myocardial infarction decreases membrane iPLA2 protein and activity [12]. Although reasons for such a decrease remain to be established, one may speculate that myocardial iPLA2 loss is detrimental for both anthracycline-treated and post-infarcted subjects.
Our second goal while conducting chronic animal studies was to observe possible changes in phospholipid content. But why should one expect changes in phospholipid composition in the hearts of doxorubicin-treated animals? The reasons are as follows: The membrane phospholipids are in a constant dynamic state of deacetylation/acetylation with different incorporation rates for individual fatty acids [13]. The iPLA2 has shown substrate preference for plasmalogens and arachidonylated phoshopholipids [14, 15]. A certain degree of substrate spec-ificity was also observed for AIPI itself [1, 2]. Together this implies that chronic inhibition of the enzyme is highly likely to alter phospholipid composition of both sarco-lemma and sarcoplasmic reticulum. To test this assumption we have quantified the phospholipid content of control rats and animals chronically treated with doxorubicin. After HPLC separation of phospholipids into individual classes, the choline (CGP) and ethanolamine glycerophospholipids (EGP) were separated by reverse-phase HPLC, and individual molecular species were collected and quantified using the microphosphate assay. Doxorubicin treatment resulted in significant alterations in the distribution of fatty acyl moieties esterified to the sn-2 position of CGP while EGP-esterified fatty acids remained unaffected. The effect included a selective loss of 20:4 and 18:1 fatty acids in diacyl CGPs and increase in esterified 20:4 and 18:2 fatty acids in choline plasmalogens. The elevation in the amount of arachidonate and linoleate (20:4 and 18:2) esterified to the sn-2 position of plasmalogens is consistent with the notion that iPLA2 displays selectivity for plasmalogens, therefore enzyme inhibition may affect de-esterification of these species. More studies are required to understand the specificity of these changes as well as why mainly choline glycerophospholipids are affected. Notably the above described changes have been seen at low cumulative dose of 8 mg/kg at which very few structural lesions or functional changes occur. Therefore we conclude that doxorubicin-induced phospholipid changes appear to be both specific and early signs, which precede morphological alterations.
Effect on cell survival
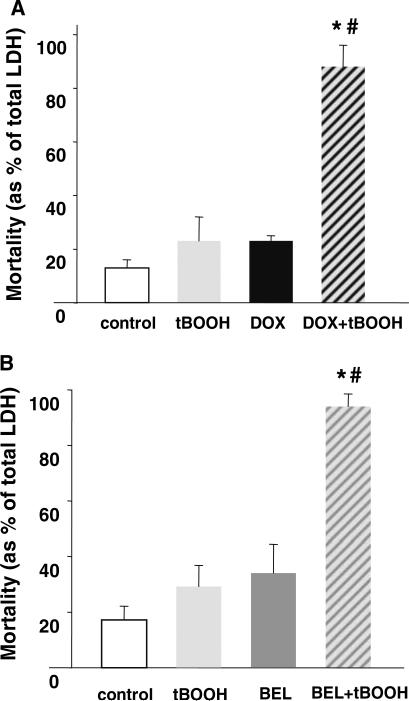
Finally we sought direct evidence that a reduction in iPLA2 influenced myocyte survival/function [16]. Cardiomyocytes were pretreated with the iPLA2 inhibitor bromoenol lactone (BEL) followed by additions of low micromolar concentration of tert-butyl hydroperoxide (tBOOH). While either the oxidant or the iPLA2 inhibitor itself had little effects on cells, acting together they significantly decreased cell viability (Fig. 5). The effects of doxorubicin were essentially identical to the effect of the iPLA2 inhibitor, i.e., drug-treated myocytes become more susceptible to the low-level oxidative stress. These data suggest the following explanation. Under normal, non-stressed conditions cells survived even if most of the iPLA2 activity was inhibited. In the samples treated with oxidant only, the remaining iPLA2 and/or the restoration of membrane enzyme activity between the applications alleviated induced lipid peroxidation, preserving the cells. However, during the combined oxidant/inhibitor treatment, inhibition of the detoxification pathway under conditions of stress proved to be lethal.
Fig. 5.
Potentiation of tert-butyl peroxide toxicity by doxorubicin and iPLA2 inhibitor BEL [3]
Linkage to other mechanisms of anthracycline cardiotoxicity
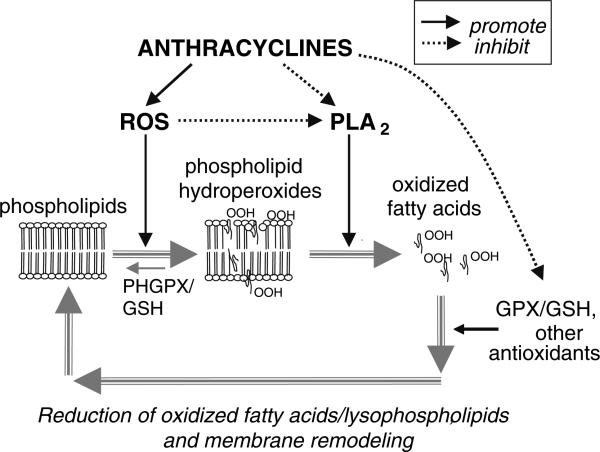
All together our data lead us to suggest that AIPI (Anthracycline-Induced iPLA2 Inhibition) can be linked to chronic anthracycline cardiotoxicity as illustrated in Fig. 6. Phospholipid hydroperoxides are not susceptible to direct reduction by cytosolic glutathione peroxidase. Instead, the oxidized sn-2 fatty acyl groups must first be hydrolyzed by PLA2, and then glutathione peroxidase can act on liberated fatty acid hydroperoxides. Therefore, the ability of cells to deal with anthracycline-enhanced lipid peroxidation is severely compromised by AIPI. It also explains how anthracyclines can lead to measurable lipid peroxidation [17], while no significant increases in free radical formation have been detected at clinically relevant doxorubicin concentrations [6, 18]. Another selenium-dependent enzyme - membrane-bound phospholipid hydroperoxide glutathione peroxidase (PHGPX) has been shown to reduce phospholipid hydroperoxides in situ without the necessity of prior hydrolysis by PLA2 [19]. However, the activity of PHGPX in cardiac muscle is a hundred times lower than the activity of soluble glutathione peroxidase [20]. Interestingly, a significant increase in membrane-associated PHGPX activity (with no changes in the cytosolic enzyme) was found in the heart tissue of rats treated with doxorubicin [21], suggesting some adaptive mechanisms to deal with increased phospholipid peroxides when PLA2 activity is diminished.
Fig. 6.
Proposed role of AIPI (Anthracycline-induced iPLA2 inhibition) in phospholipid turnover and repair cycle. ROS- reactive oxygen species, GPX – glutathione peroxidase, PHGPX – phospholipid hydroperoxide glutathione peroxidase, GSH – glutathione
Another point where oxidative stress is likely to be involved is iPLA2 inhibition itself via oxidation of cysteines [1]. Thus, the fact that an increase in tissue antioxidant capacity is capable of alleviating anthracycline toxicity may be due: (i) relief of iPLA2 inactivation by restoring active cysteines; and (ii) detoxification of accumulated phospholipid peroxides. Therefore, the AIPI-based mechanism allows one to narrow down the previous oxyradical hypothesis to more specific pathways.
The calcium hypothesis, which implicates abnormal Ca++in cycling, with ryanodine receptors as the major culprit, can also be linked to AIPI. Several independent laboratories have shown that chronic doxorubicin treatment causes an impairment of calcium-induced-calcium-release [22, 23]. The latter requires a coordinated effort from L-type calcium channels, ryanodine receptors, SERCA and other membrane proteins [24]. Thus, it is intriguing to suggest that AIPI-induced changes in the phospholipid environment adversely affect the finely-tuned system of calcium-induced-calcium-release with a resulting decrease in contractility and cardiac function.
Cardiac sarcolemmal cis-unsaturated fatty acid sensitive phospholipase D (cis-UFA phospholipase D) is modulated by iPLA2 activity via intramembrane release of cis-UFA [12, 25]. As phospholipase D-derived phosphatidic acid influences intracellular Ca2+ concentration and contractile performance [26], changes in iPLA2 activity may contribute to abnormal function of the failing heart. Thus, the AIPI might contribute to the defective Ca2+ handling and contractile performance of the failing heart via decreased activity of cis-UFA phospholipase D.
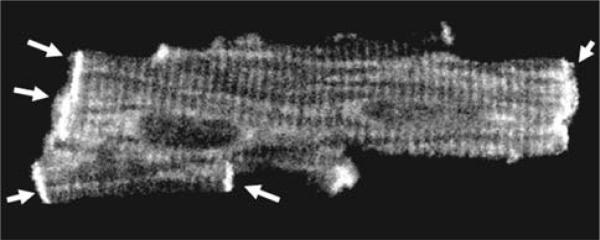
We have recently observed an abundance of iPLA2 protein within the intercalated disc areas of cardiac myocytes (Fig. 7). This finding suggests that these sites possess high metabolic activity of iPLA2, which may be important for the maintenance of complex protein-phospholipid structures at the cellular interface. Since ruptured intercalated disks have been observed in electron micrographs from subjects with anthracycline cardiomyopathy, it is tempting to suggest a link between AIPI, intercalated disk stability, titin attachment and/or cardiac remodeling.
Fig. 7.
Immunostaining of adult rat cardiac myocyte for iPLA2 (polyclonal antibody from Cayman Chemicals). The cross-striated pattern suggests presence of iPLA2 protein in the T-tubules. The arrows point to the abundance of the protein in the intercalated disks
Interestingly, the AIPI also allows one to explain the “paradoxically” decreased circulating levels of conjugated dienes and hydroperoxides shown to occur after intravenous administration of doxorubicin to cancer patients [27]. This effect is likely to be a direct manifestation of AIPI, which decreases the release of conjugated dienes and hydroperoxides from oxidized cardiac membranes. Therefore, the above mentioned study may provide the first indirect evidence that AIPI occurs in humans.
Conclusion
In summary, we have shown that low micromolar concentrations of anthracyclines inhibit iPLA2, a major housekeeping enzyme. A series of follow-up studies led us to suggest that this phenomenon may serve as one of the initial steps in a series of events leading to anthracycline cardiomyopathy. While intriguing, this hypothesis awaits further experimental confirmation. Its’ clinical importance as well as potential pharmaceutical application also remain to be explored.
Acknowledgments
The authors thank Dr. Ara Arutunyan for helpful discussions and the National Institutes of Health for the financial support.
Contributor Information
Luther Swift, Pharmacology and Physiology Department, The George Washington University, 2300 Eye Street, Washington, DC 20037, USA.
Jane McHowat, Department of Pathology, Saint Louis University School of Medicine, St. Louis, MO 63104, USA.
Narine Sarvazyan, Pharmacology and Physiology Department, The George Washington University, 2300 Eye Street, Washington, DC 20037, USA phynas@gwumc.edu.
References
- 1.McHowat J, Swift LM, Arutunyan A, Sarvazyan N. Clinical concentrations of doxorubicin inhibit activity of myocardial membrane-associated, calcium-independent phospholipase A(2). Cancer Research. 2001;61:4024–4029. [PubMed] [Google Scholar]
- 2.McHowat J, Swift LM, Sarvazyan N. Oxidant-induced inhibition of myocardial calcium-independent phospholipase A2. Cardiovascular Toxicology. 2001;1:309–316. doi: 10.1385/ct:1:4:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swift L, McHowat J, Sarvazyan N. Inhibition of Membrane-associated Calcium-independent Phospholipase A2 as a Potential Culprit of Anthracycline Cardiotoxicity. Cancer Research. 2003;63:5992–5998. [PubMed] [Google Scholar]
- 4.McHowat J, Swift LM, Crown KN, Sarvazyan NA. Changes in phospholipid content and myocardial calcium-independent phospholipase A2 activity during chronic anthracycline administration. The Journal of Pharmacology and Experimental Therapeutics. 2004;311:736–741. doi: 10.1124/jpet.104.069419. [DOI] [PubMed] [Google Scholar]
- 5.Sarvazyan NA, Askari A, Huang WH. Effects of doxorubicin on cardiomyocytes with reduced level of superoxide dismutase. Life Science. 1995;57:1003–1010. doi: 10.1016/0024-3205(95)02036-i. [DOI] [PubMed] [Google Scholar]
- 6.Sarvazyan N. Visualization of doxorubicin-induced oxidative stress in isolated cardiac myocytes. The American Journal of Physiology. 1996;271:H2079–H2085. doi: 10.1152/ajpheart.1996.271.5.H2079. [DOI] [PubMed] [Google Scholar]
- 7.Swift LM, Sarvazyan N. Localization of dichlorofluorescin in cardiac myocytes: implications for assessment of oxidative stress. American Journal Of Physiology. Heart and Circulatory Physiology. 2000;278:H982–H990. doi: 10.1152/ajpheart.2000.278.3.H982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss RB. The anthracyclines: will we ever find a better doxorubicin? Seminars in Oncology. 1992;19:670–686. [PubMed] [Google Scholar]
- 9.Weiss RB, Sarosy G, Clagett-Carr K, Russo M, Leyland-Jones B. Anthracycline analogs: the past, present, and future. Cancer Chemotherapy and Pharmacology. 1986;18:185–197. doi: 10.1007/BF00273384. [DOI] [PubMed] [Google Scholar]
- 10.Czarnecki CM. Animal models of drug-induced cardiomyopathy. Comparative Biochemistry And Physiology C. 1984;79:9–14. doi: 10.1016/0742-8413(84)90154-3. [DOI] [PubMed] [Google Scholar]
- 11.Della Torre P, Podesta A, Pinciroli G, Iatropoulos MJ, Mazue G. Long-lasting effect of dexrazoxane against anthracycline cardiotoxicity in rats. Toxicologic Pathology. 1996;24:398–402. doi: 10.1177/019262339602400402. [DOI] [PubMed] [Google Scholar]
- 12.McHowat J, Tappia PS, Liu S-Y, McCrory R, Panagia V. Redistribution and abnormal activity of phospholipase A2 isoenzymes in postinfarct congestive heart failure. American Journal Of Physiology. Cell Physiology. 2001;280:C573–C580. doi: 10.1152/ajpcell.2001.280.3.C573. [DOI] [PubMed] [Google Scholar]
- 13.Sevanian A. Lipid damage and repair. In: Davies KJ, editor. Oxidative damage and repair. Pergamon Press; New York: 1988. pp. 543–549. [Google Scholar]
- 14.dMcHowat J, Creer MH. Calcium-independent phospholipase A2 in isolated rabbit ventricular myocytes. Lipids. 1998;33:1203–1212. doi: 10.1007/s11745-998-0324-5. [DOI] [PubMed] [Google Scholar]
- 15.McHowat J, Creer MH. Selective plasmalogen substrate utilization by thrombin-stimulated Ca(2+)-independent PLA(2) in cardiomyocytes. American Journal Of Physiology. Heart and Circulatory Physiology. 2000;278:H1933–H1940. doi: 10.1152/ajpheart.2000.278.6.H1933. [DOI] [PubMed] [Google Scholar]
- 16.Hazen SL, Zupan LA, Weiss RH, Getman DP, Gross RW. Suicide inhibition of canine myocardial cytosolic calcium-independent phospholipase A2. Mechanism-based discrimination between calcium-dependent and -independent phospholipases A2. The Journal of Biological Chemistry. 1991;266:7227–7232. [PubMed] [Google Scholar]
- 17.Thayer WS. Serum lipid peroxides in rats treated chronically with adriamycin. Biochemical Pharmacology. 1984;33:2259–2263. doi: 10.1016/0006-2952(84)90664-6. [DOI] [PubMed] [Google Scholar]
- 18.Malisza KL, McIntosh AR, Sveinson SE, Hasinoff BB. Semiquinone free radical formation by daunorubicin aglycone incorporated into the cellular membranes of intact Chinese hamster ovary cells. Free Radical Research. 1996;24:9–18. doi: 10.3109/10715769609087995. [DOI] [PubMed] [Google Scholar]
- 19.Thomas JP, Maiorino M, Ursini F, Girotti AW. Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation. In situ reduction of phospholipid and cholesterol hydroperoxides. The Journal of Biological Chemistry. 1990;265:454–461. [PubMed] [Google Scholar]
- 20.Bermano G, Nicol F, Dyer JA, et al. Tissue-specific regulation of selenoenzyme gene expression during selenium deficiency in rats. Biochemistry Journal. 1995;311:425–430. doi: 10.1042/bj3110425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jotti A, Maiorino M, Paracchini L, Piccinini F, Ursini F. Protective effect of dietary selenium supplementation on delayed cardiotoxicity of adriamycin in rat: is PHGPX but not GPX involved? Free Radical Biology and Medicine. 1994;16:283–288. doi: 10.1016/0891-5849(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 22.Boucek RJ, Jr., Miracle A, Anderson M, Engelman R, Atkinson J, Dodd DA. Persistent effects of doxorubicin on cardiac gene expression. Journal of Molecular and Cellular Cardiology. 1999;31:1435–1446. doi: 10.1006/jmcc.1999.0972. [DOI] [PubMed] [Google Scholar]
- 23.Pessah IN, Schiedt MJ, Shalaby MA, Mack M, Giri SN. Etiology of sarcoplasmic reticulum calcium release channel lesions in doxorubicin-induced cardiomyopathy. Toxicology. 1992;72:189–206. doi: 10.1016/0300-483x(92)90112-r. [DOI] [PubMed] [Google Scholar]
- 24.Bers DM. Calcium and cardiac rhythms: physiological and pathophysiological. Circulation Research. 2002;90:14–17. [PubMed] [Google Scholar]
- 25.Liu SY, Tappia PS, Dai J, Williams SA, Panagia V. Phospholipase A2-mediated activation of phospholipase D in rat heart sarcolemma. Journal of Molecular and Cellular Cardiology. 1998;30:1203–1214. doi: 10.1006/jmcc.1998.0685. [DOI] [PubMed] [Google Scholar]
- 26.Xu YJ, Panagia V, Shao Q, Wang X, Dhalla NS. Phosphatidic acid increases intracellular free Ca2+ and cardiac contractile force. American Journal of Physiology. 1996;271:H651–H659. doi: 10.1152/ajpheart.1996.271.2.H651. [DOI] [PubMed] [Google Scholar]
- 27.Minotti G, Mancuso C, Frustaci A, et al. Paradoxical inhibition of cardiac lipid peroxidation in cancer patients treated with doxorubicin. Pharmacologic and molecular reappraisal of anthracycline cardiotoxicity. The Journal of Clinical Investigation. 1996;98:650–661. doi: 10.1172/JCI118836. [DOI] [PMC free article] [PubMed] [Google Scholar]