Abstract
Nuclear factor-κB (NF-κB) is a pleiotropic transcription factor that generally enhances cellular resistance to apoptotic cell death. It has been shown to be constitutively active in some cancers and is being pursued as potential anticancer target. Sulfasalazine which is used clinically to treat Crohn's disease has emerged as a potential inhibitor of NF-κB and has shown promising results in two pre-clinical studies to target primary brain tumors, gliomas. Once digested, sulfasalazine is cleaved into sulfapyridine and 5-aminosalicylic acid (5-ASA; mesalamine) by colonic bacteria, and the latter, too, is reported to suppress NF-κB activity. We now show that glioma cells obtained from patient biopsies or glioma cell lines do not show significant constitutive NF-κB activation, unless exposed to inflammatory cytokines. This does not change when gliomas are implanted into the cerebrum of severe combined immundeficient mice. Nevertheless, sulfasalazine but not its cleaved form 5-ASA caused a dose-dependent inhibition of glioma growth. This effect was entirely attributable to the inhibition of cystine uptake via the system xc− cystine–glutamate transporter. It could be mimicked by S-4-carboxy-phenylglycine (S-4-CPG) a more specific system xc− inhibitor, and lentiviral expression of a constitutively active form of IκB kinase b was unable to overcome the growth retarding effects of sulfasalazine or S-4-CPG. Both drugs inhibited cystine uptake causing a chronic depletion of intracellular GSH and consequently compromised cellular redox defense which stymied tumor growth. This data suggests that system xc− is a promising therapeutic target in gliomas and possibly other cancers and that it can be pharmacologically inhibited by Sulfasalazine, an FDA-approved drug.
Keywords: glioma, glutamate, glutathione, reactive oxygen species, S-4-carboxy-phenylglycine, system xc−
The vast majority of primary brain tumors derive from glial cells or their progenitors and are collectively called gliomas. These cancers are highly resistant to radiation and chemotherapy and typically carry a dismal prognosis (Ohgaki and Kleihues 2005). Furthermore, complete surgical resection is often impossible as tumor cells diffusely infiltrate normal brain. Gliomas typically do not metastasize outside the nervous systems and their growth is physically limited by the cranial space. As the fluid filled ventricular and extracellular spaces are insufficient to accommodate the growing tumor mass, the cancer has to vacate room for its expansion. Research conducted in several laboratories over the past decade suggests that gliomas do so by killing surrounding neurons through the deliberate release of the excitatory neurotransmitter glutamate (Ye and Sontheimer 1999; Takano et al. 2001; Sontheimer 2003). Prolonged exposure of neurons to glutamate is known to cause excitotoxicity (Olney et al. 1973) and has been implicated in the death of neurons in a number of acute and chronic neurological conditions (Rothman and Olney 1987; Choi 1988). Increases in extracellular glutamate have now been demonstrated in the peritumoral space surrounding experimental gliomas in rodents (Takano et al. 2001; Behrens et al. 2000) where glioma cells deficient in glutamate release fail to grow tumors (Takano et al. 2001). Importantly, in fully ambulatory patients with malignant gliomas, significantly elevated peritumoral glutamate has been quantified by stereo-tactic microdialysis (Roslin et al. 2003) and by magnetic resonance spectroscopy in patients with oligodendrogliomas (Rijpkema et al. 2003). Glutamate released from gliomas has also been suggested to underlie peritumoral seizures (Sontheimer 2008) which are an early symptom and a common comorbidity associated with malignant glioma (Oberndorfer et al. 2002). In stark contrast to gliomas, their non-malignant glial counterparts assiduously scavenge glutamate (Danbolt 2001), ensuring that glutamate remains below excitotoxic levels (Choi 1988).
The search for a release pathway for glutamate from gliomas has converged on an amino acid transporter named system xc−, an abundant but poorly investigated transporter that transports cystine into the cell in exchange for glutamate that is being released (Ye and Sontheimer 1999; Chung et al. 2005). System xc− is a heterodimeric protein complex consisting of a catalytic light chain (xCT) and a regulatory heavy chain (4H2hc also known as CD98), which is essential for membrane localization of the transporter (Sato et al. 1999; Bassi et al. 2001). The catalytic light chain xCT which transports cystine belongs to the 12-transmembrane domain amino acid transporter protein family. It is highly expressed in gliomas where it exists in two splice variants, hxCTα and hxCTβ, both of which are up-regulated following oxidative stress (Kim et al. 2001). The principal biological role of this transporter is the import of cystine which is an essential precursor for the biosynthesis of cellular antioxidant GSH, a key regulator of cellular redox status (Balendiran et al. 2004; Wu et al. 2004). Cystine uptake via system xc− causes the obligatory release of glutamate. System xc− is the only viable pathway for cystine uptake into glioma cells (Chung et al. 2005) and hence for the production of GSH, which makes system xc− the `Achilles heel' for GSH synthesis. Indeed, pharmacological inhibition of system xc− retards glioma cell growth in vitro and in vivo (Chung et al. 2005). Specifically, chronic inhibition of the transporter causes growth arrest and induces caspase 3-mediated apoptosis which appears to result from an inability of glioma cells to defend against reactive oxygen and nitrogen species. The two most potent pharmacological inhibitors for the system xc− transporter are S-4-carboxy-phenylglycine (S-4-CPG) and sulfasalazine (SAS). SAS (trade name Azulfidine, Pharmacia Upjohn, Bridge-water, NJ, USA) is a widely used FDA-approved drug to treat ulcerative colitis, Crohn's disease, and rheumatoid arthritis. Two independent studies have successfully used SAS to reduce tumor growth in pre-clinical glioma models, one ascribing the effect of drug in inhibition of system xc− (Chung et al. 2005), the other in the inhibition of the transcription factor, NF-κB (Robe et al. 2004). NF-κB is a pleiotropic transcription factor that can activate over 150 genes involved in both innate and adaptive immunity, cell proliferation, anti-apoptosis, angiogenesis, and cell migration (Chen et al. 1999; Li and Verma 2002; Karin 2006). NF-κB can be activated by cytokines such as tumor necrosis factor-α or interleukin 18. It recruits a complex of IκB kinases leading to rapid phosphorylation and degradation of the inhibitory IκBs protein, releasing NF-κB to translocate into the nucleus to initiate gene transcription (Werner et al. 2005). A second `atypical' pathway acts independent of IκB kinases and results in the direct phosphorylation of IκBα or IκBβ by means of p38-activated casein kinase II (Kato et al. 2003). This pathway is usually triggered by DNA damage caused by radiation or chemotherapeutics (Wang et al. 2002).
The recent demonstration that some human glioma cell lines have elevated levels of nuclear NF-κB (Nagai et al. 2002) suggested that the above-discussed growth retarding effects of SAS may be mediated entirely or in parts by an inhibition of NF-κB (Wang et al. 2004) rather than by inhibiting cystine import/glutamate release via system xc−. Resolving the cellular and molecular target of SAS and the relative importance of system xc− versus NF-κB is an important question that needs to be resolved before a clinical use of SAS can be considered in patients with malignant gliomas. To address this question we used a combination of cellular and molecular tools to show that sulfasalzine inhibits system xc− activity causing growth arrest by compromising the redox status of glioma without a significant contribution of NF-κ signaling, suggesting that the principal therapeutic target for SAS in gliomas is system xc−.
Materials and methods
Chemicals
S-4-carboxy-phenylglycine was purchased from Tocris (Ellisville, MO, USA). All other reagents were purchased from Sigma Aldrich (St Louis, MO, USA) unless otherwise stated.
Cell culture
Glioma cells examined included STTG-1 (CCF-STTG1; American Type Culture Collection, Manassas, VA, USA), D54-MG, U-87MG, U-251MG, and D-65MG (all received as a gift from Dr. D. D. Bigner, Duke University, Durham, NC, USA), and two patient-derived glioblastoma multiforme (GBM) cultures established at UAB, passages 4 to 20, labeled GBM 50 and GBM 62. Glioma cells were maintained in Dulbecco's modified Eagle's medium/F12 (Media Prep, University of Alabama at Birmingham Media Preparation Facility) with 7% fetal bovine serum (Aleken Biologicals, Nash, TX, USA) and were supplemented with 2 mM glutamine. Cortical astrocytes and cortical neurons were prepared from Sprague–Dawley rats as described previously (Ye and Sontheimer 1999).
NF-κB luciferase assay
To assess NF-κB activity in glioma cells bioluminescence assay was used. Glioma cells were transfected with the NF-κB-luc reporter plasmid and plated in 96-well luminescence plates. After treatment with SAS, 5-aminosalicylic acid (5-ASA), or S-4-CPG, glioma cells were incubated with the BrightGlo luciferase subtrate (Promega, Madison, WI, USA). Luminesence was measured with a LumiStar plate reader (BMG) and normalized to total protein determined by bicinchoninic acid assay (Pierce, Rockford, IL, USA).
Immunofluorescence microscopy
For immunostaining of cultured glioma cells, cells were grown on cover slips and fixed in 4% paraformaldehyde followed by incubation for 1 h in phosphate-buffered saline (PBS) and 10% normal goat serum plus 0.1% Triton X-100. For immunostaining of mouse brain tumors, mouse brain tissue slices (100 μm) were made using a vibratome (Vibratome, St Louis, MO, USA). Cells and tissue slices were co-incubated overnight with mouse anti-p65 and rabbit anti-p50 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies; p65 and p50 constituted the two subunits of the canonical NF-κB pathway. After removing the primary antibodies, cells and tissue slices were incubated with rabbit and mouse Alexa 546 and Alexa 488 (Molecular Probes, Eugene, OR, USA or Invitrogen, Carlsbad, CA, USA). Cells and tissue slices were incubated with 4′,6-diamidino-2-phenylindole (DAPI) and mounted onto slides with Permamount solution. Cell staining was examined on a Zeiss Axiovert 200 M microscope with an 10× or 20× oil objective, and images were collected with Axiovision software (Zeiss, Thornwood, NY, USA).
Glutathione assay in situ
Intracellular levels of total GSH were determined using a GSH assay kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer's instructions. Monochlorobimane (ApoAlert GSH Detection Kit; BD Biosciences, San Jose, CA, USA) staining was performed by incubating 20-μm tissue sections from tumor-implanted mice brains with 100 μM monochlorobimane for 10 min on ice (Shih et al. 2003). Slices were washed twice with cold PBS to remove excess monochlorobimane, and fluorescence images were collected immediately at 10× magnification on a Nikon Diaphot 300 inverted microscope (Melville, NY, USA) using OpenLab software. Stained cultures were examined by the same procedure. Cells were viewed directly for fluorescence (Aqua blue; excitation, 436 nm; emission, 480 nm). Quantitative measurements of monochlorobimane/GSH adduct fluorescence were made with NIH ImageJ (National Institute of Health, Bethesda, MD, USA) by taking mean intensities from tumor regions. All values were background subtracted.
Animal studies
U-87MG glioma cells, 2.5 × 105 in 10 AL methylcellulose, were stereotactically implanted through a small burr hole using a 30-gauge Hamilton syringe into the cranium of a SCID mouse as previously described (Soroceanu et al. 1998). After 7 days, animals were randomized into three groups of five animals each. One group received 1 mL i.p. saline injections twice daily for 3 weeks. The two test groups received 8 mg of SAS in 1 mL saline twice daily for 3 weeks (SAS solution was prepared by initially solubilizing in 0.1 M NaOH, and then neutralized by titrating with 0.1 M HCl). Tumor growth and animal health were monitored. After perfusion with 4% paraformaldehyde, mouse brains were collected, rinsed, and placed in 30% sucrose.
Western blot analysis
The expression of 4F2hc (Santa Cruz Biotechnology) and xCT (CosmoBio, Koto-ku, Japan) in glioma cells were assessed by standard western blot analysis (Chung et al. 2005). Blots were visualized with enhanced chemiluminescence and exposed on hypersensitive enhanced chemiluminescence film.
Constructs
pCMV-IκB and pCMV-IκB (M) (dominant negative) were purchased from Clontech (Mountain View, CA, USA). The NF-κB-luc reporter construct contains five NF-κB binding sites upstream of the luciferase gene (Fitzgerald et al. 2001).
Lentiviral-Ikκb-EE
A full-length human IκB kinase b (Ikκb) gene was constructed by ligating the 5′ DNA fragment originated from an IMAGE cDNA clone (NIH_MGC_71) containing a partial DNA sequence of the Ikκb gene, and the 3′ DNA fragment generated by RT-PCR from the human testis cDNA library (Invitrogen). The constitutively active mutant Ikκb (Ikκb-EE) was created by substitution of Ser178 and Ser181 with glutamate. The mutant Ikκb constructs (Ikκb-EE) were cloned into a lentiviral vector pWPI (addgene), and subsequently used to generate the recombinant lentivirus (lenti-Ikκb-EE) expressing Ikκb-EE as well as green fluorescent protein (GFP).
Cystine uptake studies
L-cystine uptake was performed using 35S-L-Cystine as previously described (Ye et al. 1999). Briefly, tumor cells grown overnight were incubated for 10 min at 37°C with 0.5 μCi/mL 35S-cystine in assay buffer (122 mM NaCl, 3 mM KCl, 1.3 mM CaCl2, 1.2 mM NaH2PO4, 25 mM NaHCO3, 10 mM Glucose, and 0.4 mM MgSO4), warmed to 37°C, and saturated with 5 : 95 CO2 : O2. Uptake was terminated by three washes with ice-cold PBS. Cells were then dissolved in 0.3 M NaOH and aliquoted. 3H activity was detected in a liquid scintillation counter (Beckman Instruments, Fullerton, CA, USA) and normalized to protein contents as determined by the Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
All data were expressed as mean ± SE. The number of repeat experiments was denoted as n. Control values were derived from untreated sister cultures. Statistical evaluations of the data were performed using Student's t-test or one-way analysis of variance for data in which multiple conditions were compared.
Results
Glioma cells exhibit little constitutive NF-κB activity
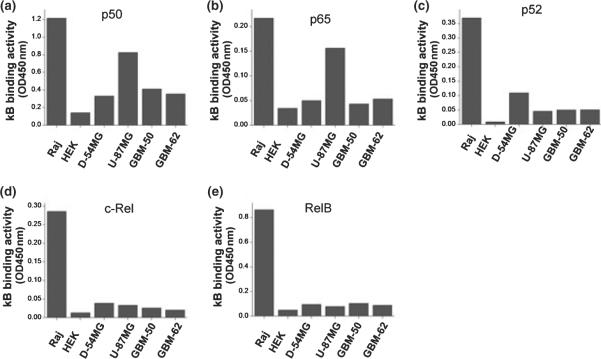
To study whether the previously observed inhibition of glioma growth by pharmacological inhibitors of system xc− may involve signaling via the NF-κB pathway, we first ascertained constitutive NF-κB activity in glioma cells using two commonly used glioma cell lines (D-54MG and U-87MG) and two cultures we established from glioblastoma patient biopsies (GBM-50 and GBM-62). Using an ELISA assay to examine which NF-κB subunit may contribute to basal NF-κB activity, we compared these four glioma cell populations and representative data are shown in Fig. 1a–e. Binding of p50 and p65, the two subunits that constitute the canonical NF-κB pathway, were detected in U-87MG cells at levels comparable with those in human Burkitt's lymphoma cells (Raj) used as positive controls. Human embryonic kidney cells served as negative controls. However, we did not find any significant κB binding activity for p50 or p65 in D-54MG, GBM-50, and GBM-62 and only U-87MG cells showed some binding. Moreover, none of the glioma cells examined showed any significant level of κB binding activity to the non-canonical NF-κB subunits p52, RelB, and c-Rel. This data suggest relatively low constitutive NF-κB activity in all but one (U-87MG) glioma line. Note, however, that we were able to demonstrate NF-κB activity with the same assay in all these glioma cells when cells were treated with tumor necrosis factor-α (data not shown), assuring that the NF-κB signaling pathway was intact in these cells. The above-observed low constitutive NF-κB activity in these glioma cells agrees with one recent report (Ferla-Bruhl et al. 2007) but disagrees with another study (Nagai et al. 2002).
Fig. 1.
Endogenous NF-κB activities among glioma cells. (a–g) ELISA. Nuclear extracts isolated from glioma cells were incubated in 96-well plates pre-coated with NF-κB oligonucleotides (active motif). NF-κB subunits bound to NF-κB oligonucleotides were probed by antibodies of NF-κB p50 (a), NF-κB p65 (b), NF-κB p52 (c), NF-κB c-Rel (d), or NF-κB RelB (e), followed by horseradish-peroxidase-conjugated secondary antibody. U-87MG cells show a significant level of p65 and p50 in the nuclear extracts compared to those of Human Burkitt's lymphoma cell line (Raj) which have constitutively active NF-κB activity. Human embryonic kidney cells served as negative controls. The data shown are representative examples that were repeated several times with qualitatively comparable endogenous NF-κB activities among glioma cells.
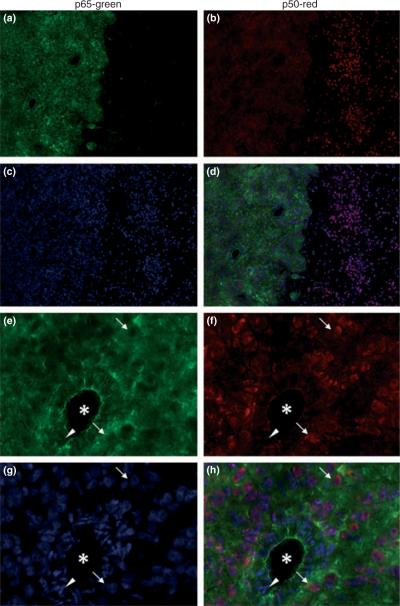
As it is plausible that the lack of NF-κB activity was related to these cells being maintained in cell culture, we next investigated whether the expression of the canonical NF-κB components can be observed in vivo, in a mouse brain tumor model used in previous studies including those examining the growth retarding effects of SAS (Robe et al. 2004). Therefore, 5 × 105 U-87MG cells were stereo-activally implanted into the cranium of SCID mice and 3 weeks after implantation, tissue slices were examined immunohistochemically. As shown for representative examples in Fig. 2, p65 immunostaining was restricted to the tumor cells, clearly demarcating the tumor mass from the neighboring brain tissue (Fig. 2a), while in the same tissue section p50 was present in both tumor cells and adjacent normal brain (Fig. 2b). The DAPI-stained cell nuclei are shown in Fig. 2c and both p50 and p65 immunoreactivity was overlayed with DAPI-stained nuclei in Fig. 2d. Interestingly, when examined at higher magnification, the majority of p65 label appeared to reside in the cytoplasm and did not colocalize with the DAPI-labeled nuclei (Fig. 2e–h with the superimposed staining in Fig. 2h), indicating relatively poor nuclear localization in tumor cells. By contrast, p50 (Fig. 2f) was mostly found in the nuclei of tumor cells (Fig. 2f–h) indicating a high level of nuclear localization. This data suggest that tumor cells in vivo maintain a low to moderate level of constitutive NF-κB activity in vivo, which agrees well with our in vitro data using the same cell line (U-87MG).
Fig. 2.
Expression of NF-κB subunits in mouse brain tumors. Brain tumor tissue slices were obtained from severe combined immundeficient mice in which U-87MG cells were injected into the brain and were co-stained with p65 antibody (a,e), p50 antibody (b,f), and DAPI (c,g). Merged images shown in d and h. p65 antibody showed exclusive staining in tumor cells, clearly demarcating tumor mass from the normal brain tissue (a). Diffuse p50 expression was observed in tumor cells as well as in neighboring normal brain cells (b). Higher magnification revealed clear difference in intracellular distribution of p65 and p50. While primarily p65 staining was strong in the cytoplasm and largely absent in nuclei of the tumor cells (arrows, e), p50 staining was weak in the cytoplasm but concentrated in areas overlapped with DAPI staining indicating localization in nuclei of tumor cells (arrows, g). In contrast, p50 is absent in nuclei of the endothelial cells (arrow head) surrounding capillary blood vessels (*). a–d: 10× magnification; e–h: 20× magnification.
Sulfasalazine and its metabolic byproduct, 5-amino salicylic acid, but not S-4-CPG suppress NF-κB activity
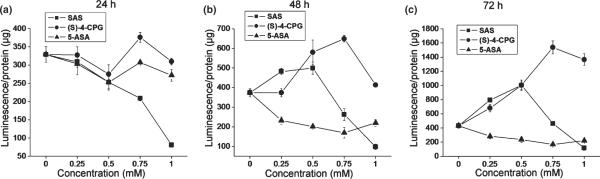
We previously demonstrated growth inhibition of glioma cells in vitro by chronic incubation with two system xc− inhibitors, namely, SAS and S-4-CPG (Chung et al. 2005), both being equipotent. If this growth suppression was mediated by NF-κB, then both system xc− inhibitors should exhibit substantial reduction of constitutive NF-κB activity of glioma cells. Furthermore, if constitutive NF-κB activity is essential for glioma growth, other NF-κB inhibitors should similarly suppress glioma growth. To examine this further we compared the effect of the two system xc− inhibitors, SAS and S-4-CPG with 5-ASA, which only inhibits NF-κB (Egan et al. 1999) without affecting system xc− on NF-κB activity and cell growth.
We chose the U-87MG cell line as model system to investigate drug effects on NF-κB activity as this cell line showed the most pronounced NF-κB activity (Fig. 1). U-87MG cells were transfected with a κB-luciferase reporter construct (κB-luc), and then incubated with SAS, S-4-CPG, and 5-ASA for 24, 48, and 72 h followed by luciferase activity measurements at the end of each treatment (Fig. 3). As previously reported in the literature (Liptay et al. 1999), SAS inhibited NF-κB activity in a dose-dependent manner following a 24 h incubation. However, SAS concentrations over 0.5 mM were required to achieve reduced NF-κB activity for prolonged incubation (48 and 72 h) under which conditions low SAS concentrations exhibited little inhibition (Fig. 3b), or even enhanced NF-κB activity (Fig. 3c). By contrast, S-4-CPG showed no effect after 24 h treatment, and only enhanced NF-κB activity for long-term incubations (48 and 72 h) at all concentrations tested. Finally, 5-ASA has also been shown to inhibit NF-κB activity by modulating p65 phosphorylation (Egan et al. 1999). Our data in Fig. 3 agrees with that finding, suggesting that prolonged treatment with 5-ASA (longer than 24 h) can suppress constitutive NF-κB activity of glioma cells even more effectively than SAS.
Fig. 3.
Effect of system xc− inhibitors on NF-κB activity of U-87MG cells. U-87MG cells were transfected with a NF-κB-dependent luciferase reporter construct (NF-κB-luc). The transfected U-87MG cells were then incubated with different concentrations of sulfasalazine, S-4-CPG, or 5-ASA for 24 h (a), 48 h (b), and 72 h (c). NF-κB activities were determined by a luciferase luminesence assay. NF-κB activity was inhibited by high dose (greater than 0.5 mM) but not with low doses (smaller than 0.5 mM) of sulfasalazine. Another system xc− inhibitor, S-4-CPG, did not inhibit but rather enhance NF-κB activity for incubations of 48 and 72 h. 5-ASA, a weak NF-κB inhibitor, repressed NF-κB activity with prolonged incubations (48 and 72 h). Error bars indicate SE. All experiments were carried out with n = 3.
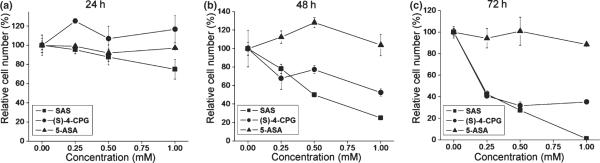
System xc− inhibitors S-4-CPG and SAS but not 5-ASA suppress glioma growth
Next, we compared the effects of the afore-mentioned pharmaceuticals on glioma cell growth. U-87MG cells were incubated with SAS, S-4-CPG, and 5-ASA for 24, 48, and 72 h over a concentration range of 0.25 to 1 mM, followed by cell counts at the end of each treatment (Fig. 4). Data were normalized to untreated sister cultures in the same experiment and expressed as relative cell number (in %). After 24 h, none of these pharmaceutical compounds showed any significant change in cell number. However, after 48 and 72 h, SAS and S-4-CPG each showed growth inhibition in a dose-dependent manner, while 5-ASA was entirely ineffective in reducing glioma growth (Fig. 4b and c). Importantly, even at low doses (0.25 mM), SAS was able to suppress glioma growth by over 60% compared to untreated controls (Fig. 4c). Yet at this concentration SAS did not show a significant reduction in NF-κB activity as shown in (Fig. 3). This suggests that the growth inhibition by low dose SAS treatment occurred independent of changes in NF-κB activity. Furthermore, chronic treatment with the system xc− inhibitor S-4-CPG suppressed cell growth equally effectively to SAS, yet S-4-CPG actually increased rather than inhibited NF-κB activity all over the entire dose range examined (Fig. 3). Finally, 5-ASA treatment which reduced NF-κB activity by over 50% (Fig. 3b and c) did not affect glioma growth. Taken together, this data strongly suggest that glioma growth inhibition by SAS occurrs independent of NF-κB.
Fig. 4.
Effects of sulfasalazine, S-4-CPG, and 5-ASA on glioma cell growth. U-87MG cells were incubated with various concentrations of sulfasalazine, S-4-CPG, or 5-ASA for 24 h (a), 48 h (b), and 72 h (c). At the end of incubations, cell numbers were determined and compared with that of untreated sister cultures. While each of the system xc− inhibitors sulfasalazine and S-4-CPG inhibited cell growth of U-87MG, the NF-κB inhibitor, 5-ASA, did not affect cell growth. Error bars indicate SE. All experiments were carried out with n = 6.
Mutated, constitutively active NF-κB activity does not rescue cell from growth inhibitory effect of SAS
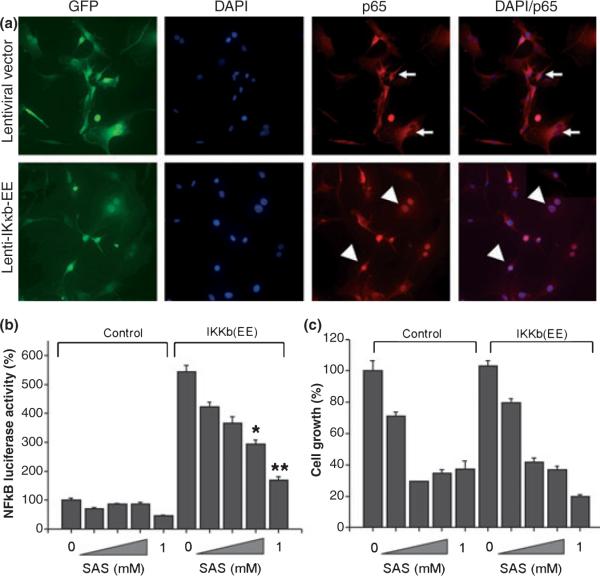
Although low concentrations of SAS (0.25 mM) had only minimal effects on NF-κB activity, it is possible that such small decreases may still have profound effects on cell growth. This would then suggest, however, that artificially driving NF-κB activity during SAS exposure should prevent growth inhibition by SAS. To test this possibility, we constructed a recombinant lentivirus co-expressing a constitutively active form of IκB kinase b (Iκκb) and GFP reporter. IκB kinase b is an essential component of the NF-κB signaling pathway, and activated Ikκb catalyzes the phosphorylation of target sites on IκB, causing a dissociation of IκB from the NF-κB-IκB complex (Hayden and Ghosh 2008). After dissociation from the complex, NF-κB molecules translocate into the nucleus and bind to κB elements, resulting in transcriptional activation of various downstream genes. The constitutively active form of Iκκb was constructed by substituting serine with glutamate at amino acids 178 and 181 (Iκκb-EE), and subsequently used to generate recombinant lentivirus expressing Iκκb-EE and GFP. Expression of Ikκb-EE dramatically increased NF-κB-driven luciferase activity (Fig. 5b) as reported previously (Mercurio et al. 1997), and promoted nuclear translocation of p65 subunit in the majority of U-87MG cells infected with the recombinant lentivirus carrying Ikκb-EE (Fig. 5a). This showed that expression of Ikκb-EE constitutively activated NF-κB activity in glioma cells. In order to test whether maintaining NF-κB activity higher than control can reverse SAS-induced glioma growth inhibition, we first examined whether expression of Ikκb-EE can maintain elevated NF-κB activity in the presence of SAS. U-87MG cells were infected for 24 h with the recombinant viruses, and subsequently transfected with an NF-κB-luc reporter construct. The cells were then incubated with culture media in the presence or absence of various concentrations of SAS. After 24 h incubation, NF-κB-driven luciferase activity was measured and normalized to luciferase activity of U-87MG cells infected with the recombinant lentivrus without Ikκb-EE transgene. As shown in Fig. 5b, SAS treatment exhibited a dose-dependent inhibition of NF-κB activity in Ikκb-EE expressing cells, consistent with previous observation demonstrating that SAS is a competitive inhibitor of phosphorylation activity of Ikκb as well as Ikκb-EE (Weber et al. 2000). However, Ikκb-EE expression still maintained much higher NF-κB activity than that of untreated cells or those infected with a control lentivirus, even in the presence of SAS. Using these cells we next asked whether expression of Ikκb-EE can rescue the growth inhibition by SAS. As shown in Fig. 5c, cells expressing the Ikκb-EE showed a similar growth inhibition by a 48-hour incubation with SAS as control cells. This data strongly suggests that the growth suppression by SAS was not mediated by an inhibition of NF-κB activity.
Fig. 5.
Artifically elevated levels of NF-κB through lentiviral over-expression does not reverse cell growth inhibition by sulfasalazine. (a) U-87MG cells were infected with either control GFP reporter lentivirus or hIkκb-EE lentivirus and cultured for 24 h. The cells were then fixed and the intracellular distribution of p65 was visualized with anti-p65 antibody and DAPI staining. In cells infected with control lentivirus, the majority of p65 resides in the cytoplasm and is absent in the nucleus. In cells infected with hIkκb-EE lentivirus, the majority of cells exhibit intense nuclear staining of nucleus with anti-p65 antibody indicating nuclear translocation of p65 and NF-κB activation. (b) Comparison of sulfasalazine effects on NF-κB activities of U-87MG cells infected with control lentivirus and hIkκb-EE lentivirus: U-87MG cells infected with the control lentivirus or the hIkκb-EE lentivirus for 24 h and transfected with the NF-κB-luc plasmid and incubated with various concentrations of sulfasalazine for 24 h. Expression of hIkκb-EE increases NF-κB activity more than five-fold over endogenous NF-κB activity. Although sulfasalazine inhibited NF-κB activity in a dose-dependent manner in cells expressing hIkκb-EE, the expression of hIkκb-EE maintained a higher level of NF-κB activity well above endogenous NF-κB activity of U-87MG cells. Error bars indicate SE. All experiments were carried out with n = 3. *p < 0.001, **p < 0.05. (c) Comparison of sulfasalazine effects on cell growth of U-87MG cells infected with the control lenti-virus and the hKκb-EE lentivirus: U-87MG cells were infected with the control lentivirus or the hIkκb-EE lentivirus for 24 h, and then incubated with various concentrations of sulfasalazine. After 48 h incubation, cell numbers were counted and compared with that of control. Even though the expression of hIkκb-EE maintained elevated level of NF-κB activity in the presence of sulfasalazine, it did not rescue cells from growth inhibition by sulfasalazine. Error bars indicate SE. All experiments were carried out with n = 3.
Sulfasalazine and S-4-CPG but not 5-ASA reduce cystine uptake and deplete intracellular GSH
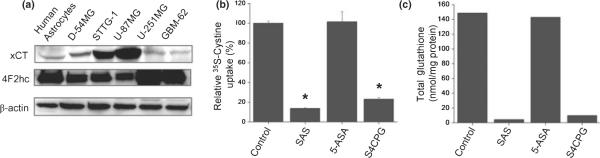
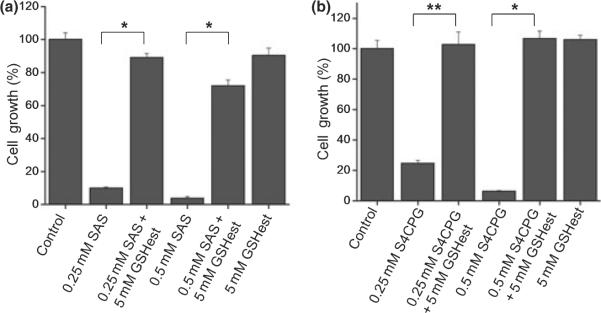
We previously showed a time- and dose-dependent depletion of intracellular GSH by treatment of gliomas with SAS that was directly attributable to the inhibition of cystine uptake via system xc−. This transporter requires an obligatory association of catalytic subunit xCT with the regulatory subunit 4F2hc (Sato et al. 1999). Both subunits were expressed, albeit to variable degrees, in the glioma cell lines studied here as well as in patient-derived primary gliomas and non-malignant human astrocytes (Fig. 6a). To directly compare the relative effect of SAS, S-4-CPG, and 5-ASA on this transporter, we determined the cellular uptake of 35S-cystine in the presence or absence of these drugs. As shown in Fig. 6b, both SAS and S-4-CPG reduced 35S-cystine uptake by over 80% while 5-ASA was without effect. If the cystine uptake was destined to be used for the synthesis of GSH we should see a similar reduction in cellular GSH levels. Indeed, as illustrated in Fig. 6c, a 24-hour treatment with either SAS or S-4-CPG resulted in >90% decrease in cellular GSH levels when compared with untreated controls, yet 5-ASA was once again without effect. These results were consistent with the notion that the above observed growth inhibition of glioma cells was because of inhibition of cystine uptake and an ensuing depletion of intracellular GSH. This interpretation is further supported by data in Fig. 7 showing that a membrane-permeable GSH ethyl ester was able to completely rescue cell growth even in the continued presence of SAS (Fig. 7a) or S-4-CPG (Fig. 7b), and this was the case for U-87MG cells as well as patient-derived GBM-62 cells.
Fig. 6.
Sulfasalazine and S-4-CPG but not 5-ASA inhibit cystine uptake and deplete intracellular glutathione. (a) Expression of xCT, 4F2hc, with β-actin as loading control was compared among various glioma cells by western blot analysis. (b,c) Effects of sulfasalazine, S-4-CPG, and 5-ASA on cytine uptake (b) and intracellular glutathione (c) in U-87MG cells. Treatment of sulfasalazine (0.25 mM) and S-4-CPG (0.25 mM) drastically reduced L-35S-cystine uptake followed by depletion of intracellular glutathione, while 5-ASA (0.25 mM) was without effect on cystine uptake and intracellular glutathione levels. Error bars indicate SE. All experiments were carried out with n = 3. *p < 0.00005.
Fig. 7.
Inhibition of glioma growth by system xc− blockers requires depletion of intracellular GSH. Co-incubation of GSH ethylester, the membrane permeable form of GSH rescued U-87MG cells from growth inhibition by either sulfasalazine (a) or S-4-CPG (b). Error bars indicate SE. All experiments were carried out with n = 3. *p < 0.00001, **p < 0.01.
Sulfasalazine reduces GSH levels but does not cause a significant change in the expression or nuclear localization of NF-κB subunits in mouse brain tumors in vivo
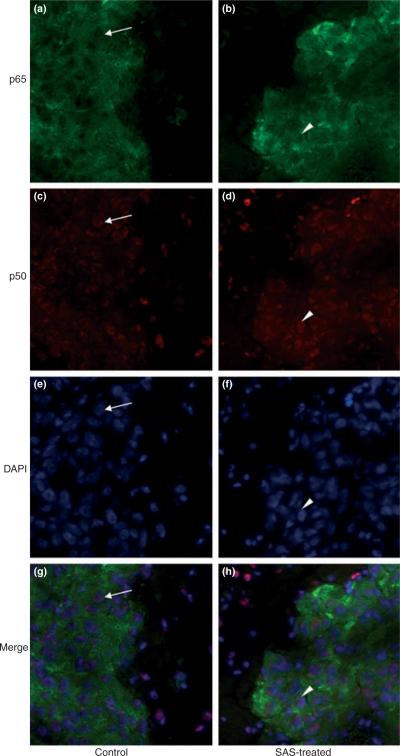
A final aspect of this study pertains to assess the role that NF-κB plays in glioma growth in vivo. To this extent we are drawing on a previous study where we demonstrated that SAS treatment reduced tumor size in SCID mice bearing xenografted human gliomas (Chung et al. 2005). While our data above suggests that growth-retarding effects of SAS do not invoke NF-κB signaling, it is possible that the situation is different in vivo. To assess this possibility, we examined expression and intracellular distribution of p65 and p50 in tissue sections obtained from brain tumors of mice that were treated with 8 mg SAS given in 1 mL saline by i.p. for 3 weeks, and compared them with control mice which received only saline. As shown in Fig. 8, SAS treatment did not reduce overall expression of p65 or p50 in the tumor tissue. Also, lack of the presence of p65 in the nuclei of tumor cells was not changed nor was the nuclear localization of p50. These results clearly demonstrated that SAS treatment did not result in significant changes in overall expression and distribution of p65 and p50 suggesting that SAS does not cause a significant inhibition of the canonical NF-κB activity of brain tumor in vivo. To demonstrate more directly that in vivo activity of SAS is a reduction of system xc− activity, which in turn regulates cellular GSH synthesis, we examined intracellular GSH levels in tumor tissue from the above animals. As shown in Fig. S1, GSH levels in tumor tissues from SAS-treated mice was significantly reduced (p < 0.01) compared with untreated control mice, indicating once again that that SAS inhibited cystine uptake destined for incorporation into GSH in the tumor in vivo.
Fig. 8.
Treatment of tumor bearing mice in vivo with SAS does not exhibit a significant change in the overall expression or nuclear localization of NF-κB p65 and p50. Microscopic images of brain tumor slices obtained from control mice (a,c,e,g) and SAS-treated mice (b,d,f,h) co-stained with p65 antibody (a,b), p50 antibody (c,d), and DAPI (e,f). Merged images are shown in panel g and h. Overall intensity of p65 and p50 staining in SAS-treated brain tumors (a,c) did not differ significantly from the control group (b,d). Also, SAS treatment showed little change in intracellular localization of p65 and p50 (arrowhead) compared with saline-treated control (arrow).
Discussion
This study was motivated by the recent finding that SAS, a pleiotropic drug clinically used to treat a number of chronic inflammatory conditions, was also effective in slowing brain tumor growth in vivo (Robe et al. 2004; Chung et al. 2005). While exciting, the mechanism of action of SAS in this disease remains controversial. On the one hand, a growing body of literature suggests that SAS targets the NF-κB signaling pathway and suppresses apoptotic cell death (Wahl et al. 1998; Liptay et al. 1999; Weber et al. 2000; Lo et al. 2008b). On the other hand, some studies propose that SAS has a different molecular target, namely, the system xc− amino acid transporter that imports cystine in exchange for glutamate for the cellular synthesis of GSH (Narang et al. 2003; Chung et al. 2005; Lo et al. 2008b; Chen et al. 2009). Furthermore, reports in the literature on the constitutive activity and significance of NF-κB in gliomas are conflicting, with at least one report supporting (Nagai et al. 2002) and another refuting this notion (Ferla-Bruhl et al. 2007).
Using several widely studied glioma cell lines as well as patient-derived glioma cells we show that NF-κB shows at best low to moderate activity in gliomas, a finding in agreement with another report in the literature (Ferla-Bruhl et al. 2007). Importantly, this does not change upon implantation of these cancer cells into host animals, suggesting that it is not environmentally determined. Furthermore, we showed that even if induced to supra normal levels through lentiviral over-expression of a constitutively active IκB kinase b, NF-κB activity did not protect glioma cells from the growth-retarding effects of SAS. Instead, we showed that SAS inhibited cellular cystine uptake via system xc−. Cystine is destined for the synthesis of the cellular antioxidant GSH and not surprisingly therefore system xc− inhibition depletes cellular GSH, compromising the cells' redox status. Exogenous GSH can fully rescue glioma cells from SAS. Our data hence fully support a potential clinical use of SAS in glioma patients while proposing that the cellular target relevant in this disease is system xc−-mediated cystine uptake, without a significant involvement of NF-κB signaling in this drug therapy approach. Recent studies on lymphoma (Gout et al. 2001) and mammary carcinomas (Narang et al. 2003), prostate (Doxsee et al. 2007), and pancreatic cancer (Lo et al. 2008a) came to the same conclusion yet in vastly different cancer types. Hence it seems possible that this mechanism of action may be more widespread among cancers.
One may argue that from a clinical point of view the mechanism of action of SAS is not really relevant. We would vehemently disagree with this notion. A number of drugs have been developed or are at various stages of development to suppress NF-κB activity in an effort to reduce its positive effects on cell survival. Many of these are specifically targeted as anticancer drugs (Nakanishi and Toi 2005; Karin 2006). Our data suggest that none of these drugs would be able to inhibit glioma growth as they do not target system xc−, albeit they may be effective in other cancers. We demonstrate this by one example, 5-ASA, a frequently used NF-κB inhibitor. 5-ASA is a cleavage product of SAS that retains most of the beneficial anti-inflammatory effects of SAS for the treatment of Crohn's disease. However, the ineffectiveness of 5-ASA to slow glioma growth also highlights the importance of SAS reaching the tumor in its uncleaved chemical form. Neither of its cleavage products 5-ASA nor sulphapyridine are effective. Indeed, a non-cleavable form of SAS would offer the greatest benefit as only 30% of the ingested dose of SAS escapes the gut and colon uncleaved (Hoult 1986). Such a drug `Susalimode' was used in a clinical trial to treat multiple sclerosis in the late 1980s (Noseworthy et al. 1998) but while patients showed a reduction in the frequency of relapses in the first year; unfortunately, Susalimode did not provide a long-term benefit for multiple sclerosis patients.
Gliomas are among the most difficult tumors to treat, in part, because of their relentless growth and diffuse invasion. Hence new treatments are desperately needed. Most often, drugs in clinical trials have poor specificity or are difficult to produce and deliver. Most glioma trials do not advance past early experimental stages. The above-observed growth retarding effects through an inhibition of GSH synthesis is quite exciting as the drug of choice is readily available, can be given orally and has an excellent and well known side-effect profile. SAS has been in use for over 40 years to treat ulcerative colitis and a number of inflammatory conditions including rheumatoid arthritis. Gliomas, particularly, low to intermediate grade tumors (WHO grades II and III) are metabolically very active cells and hence produce a significant load of endogenous reactive oxygen species (ROS) associated with mitochondrial function. Not surprisingly, therefore, gliomas generate significant amounts of GSH to scavenge these reactive oxygen species products. This endogenous defense is lost upon SAS treatment. Current treatment strategies routinely include chemo- and radiation treatments that could also benefit from being administered in combination with SAS. Of note, cellular toxicity from ionizing radiation is attributed to OH radicals which are scavenged by GSH, and the multidrug resistance transporter that effectively removes chemotherapeutic drugs from the cytoplasm of tumor cells requires a conjugation of xeno-biotics to GSH. This pathway, too, would be inhibited by the action of SAS on system xc− proposed here under. In light of this we argue in favor of adjuvant use of SAS with radiation and/or chemotherapy. Finally, recent studies suggest that system xc− may function to release glutamate which in turn promotes invasiveness of tumors by acting in an autocrine or paracrine way on α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptors on invading glioma cells (Lyons et al. 2007). This pathway, too, is disrupted by SAS blocking glutamate release via system xc−. Hence the therapeutic potential of SAS or other system xc− inhibitor is tremendous and may offer hope for a more successful treatment of this devastating cancer in the future.
Supplementary Material
Acknowledgements
The authors wish to thank Naomi Logsdon for constructive criticism on the manuscript. This work was supported by NIH grant RO1-NS052634.
Abbreviations used
- 5-ASA
5-aminosalicylic acid
- DAPI
4′,6-diamidino-2-phenylindole
- GBM
glioblastoma multiforme
- GFP
green fluorescent protein
- PBS
phosphate-buffered saline
- S-4-CPG
S-4-carboxy-phenylglycine
- SAS
sulfasalazine
- SCID
severe combined immundeficient
Footnotes
Supporting Information Additional Supporting Information may be found in the online version of this article:
Figure S1 Sulfasalazine reduces GSH levels in vivo as judged by MCB-GSH imaging of mouse tumor tissue indicating SAS inhibits cystine uptake in vivo.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Balendiran GK, Dabur R, Fraser D. The role of glutathione in cancer. Cell Biochem. Funct. 2004;22:343–352. doi: 10.1002/cbf.1149. [DOI] [PubMed] [Google Scholar]
- Bassi MT, Gasol E, Manzoni M, Pineda M, Riboni M, Martin R, Zorzano A, Borsani G, Palacin M. Identification and characterisation of human xCT that co-expresses, with 4F2 heavy chain, the amino acid transport activity system xc−. Pflugers Arch. 2001;442:286–296. doi: 10.1007/s004240100537. [DOI] [PubMed] [Google Scholar]
- Behrens PF, Langemann H, Strohschein R, Draeger J, Hennig J. Extracellular glutamate and other metabolites in and around RG2 rat glioma: an intracerebral microdialysis study. J. Neurooncol. 2000;47:11–22. doi: 10.1023/a:1006426917654. [DOI] [PubMed] [Google Scholar]
- Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin. Chem. 1999;45:7–17. [PubMed] [Google Scholar]
- Chen RS, Song YM, Zhou ZY, et al. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene. 2009;28:599–609. doi: 10.1038/onc.2008.414. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system [Review] Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, Gillespie GY, Sontheimer H. Inhibition of cystine uptake disrupts the growth of primary brain tumors. J. Neurosci. 2005;25:7101–7110. doi: 10.1523/JNEUROSCI.5258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Doxsee DW, Gout PW, Kurita T, et al. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate. 2007;67:162–171. doi: 10.1002/pros.20508. [DOI] [PubMed] [Google Scholar]
- Egan LJ, Mays DC, Huntoon CJ, Bell MP, Pike MG, Sandborn WJ, Lipsky JJ, McKean DJ. Inhibition of interleukin-1-stimulated NF-kappaB RelA/p65 phosphorylation by mesalamine is accompanied by decreased transcriptional activity. J. Biol. Chem. 1999;274:26448–26453. doi: 10.1074/jbc.274.37.26448. [DOI] [PubMed] [Google Scholar]
- Ferla-Bruhl K, Westhoff MA, Karl S, Kasperczyk H, Zwacka RM, Debatin KM, Fulda S. NF-kappaB-independent sensitization of glioblastoma cells for TRAIL-induced apoptosis by proteasome inhibition. Oncogene. 2007;26:571–582. doi: 10.1038/sj.onc.1209841. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Palsson-McDermott EM, Bowie AG, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- Gout PW, Buckley AR, Simms CR, Bruchovsky N. Sulfasalazine, a potent suppressor of lymphoma growth by inhibition of the x(c)− cystine transporter: a new action for an old drug. Leukemia. 2001;15:1633–1640. doi: 10.1038/sj.leu.2402238. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hoult JR. Pharmacological and biochemical actions of sulphasalazine. Drugs. 1986;32(Suppl 1):18–26. doi: 10.2165/00003495-198600321-00005. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Kato T, Jr, Delhase M, Hoffmann A, Karin M. CK2 is a C-terminal IkappaB kinase responsible for NF-kappaB activation during the UV response. Mol. Cell. 2003;12:829–839. doi: 10.1016/s1097-2765(03)00358-7. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kanai Y, Chairoungdua A, Cha SH, Matsuo H, Kim DK, Inatomi J, Sawa H, Ida Y, Endou H. Human cystine/glutamate transporter: cDNA cloning and upregulation by oxidative stress in glioma cells. Biochim. Biophys. Acta. 2001;1512:335–344. doi: 10.1016/s0005-2736(01)00338-8. [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Liptay S, Bachem M, Hacker G, Adler G, Debatin KM, Schmid RM. Inhibition of nuclear factor kappa B and induction of apoptosis in T-lymphocytes by sulfasalazine. Br. J. Pharmacol. 1999;128:1361–1369. doi: 10.1038/sj.bjp.0702937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo M, Ling V, Wang YZ, Gout PW. The xc− cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance. Br. J. Cancer. 2008a;99:464–472. doi: 10.1038/sj.bjc.6604485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo M, Wang YZ, Gout PW. The x(c)− cystine/glutamate antiporter: a potential target for therapy of cancer and other diseases. J. Cell. Physiol. 2008b;215:593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H. Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res. 2007;67:9463–9471. doi: 10.1158/0008-5472.CAN-07-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio F, Zhu H, Murray BW, et al. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- Nagai S, Washiyama K, Kurimoto M, Takaku A, Endo S, Kumanishi T. Aberrant nuclear factor-kappaB activity and its participation in the growth of human malignant astrocytoma. J. Neurosurg. 2002;96:909–917. doi: 10.3171/jns.2002.96.5.0909. [DOI] [PubMed] [Google Scholar]
- Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat. Rev. Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells. Anti-cancer Res. 2003;23:4571–4579. [PubMed] [Google Scholar]
- Noseworthy JH, O'Brien P, Erickson BJ, et al. The Mayo Clinic–Canadian Cooperative trial of sulfasalazine in active multiple sclerosis. Neurology. 1998;51:1342–1352. doi: 10.1212/wnl.51.5.1342. [DOI] [PubMed] [Google Scholar]
- Oberndorfer S, Schmal T, Lahrmann H, Urbanits S, Lindner K, Grisold W. The frequency of seizures in patients with primary brain tumors or cerebral metastases. An evaluation from the Ludwig Boltzmann Institute of Neuro-Oncology and the Department of Neurology, Kaiser Franz Josef Hospital, Vienna. Wien. Klin. Wochenschr. 2002;114:911–916. [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oli-godendroglial gliomas. J. Neuropathol. Exp. Neurol. 2005;64:479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- Olney JW, Ho OL, Rhee V, DeGubareff T. Letter: neurotoxic effects of glutamate. N. Engl. J. Med. 1973;289:1374–1375. 23. doi: 10.1056/NEJM197312202892519. [DOI] [PubMed] [Google Scholar]
- Rijpkema M, Schuuring J, van der MY, van der GM, Bernsen H, Boerman R, van der KA, Heerschap A. Characterization of oligodendrogliomas using short echo time 1H MR spectroscopic imaging. NMR Biomed. 2003;16:12–18. doi: 10.1002/nbm.807. [DOI] [PubMed] [Google Scholar]
- Robe PA, Bentires-Alj M, Bonif M, et al. In vitro and in vivo activity of the nuclear factor-kappaB inhibitor sulfa-salazine in human glioblastomas. Clin. Cancer Res. 2004;10:5595–5603. doi: 10.1158/1078-0432.CCR-03-0392. [DOI] [PubMed] [Google Scholar]
- Roslin M, Henriksson R, Bergstrom P, Ungerstedt U, Bergenheim AT. Baseline levels of glucose metabolites, glutamate and glycerol in malignant glioma assessed by stereotactic microdialysis. J. Neurooncol. 2003;61:151–160. doi: 10.1023/a:1022106910017. [DOI] [PubMed] [Google Scholar]
- Rothman SM, Olney JW. Excitotoxicity and the NMDA receptor. TINS. 1987;10:299–302. doi: 10.1016/0166-2236(95)93869-y. [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J. Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H. Malignant gliomas: perverting glutamate and ion homeostasis for selective advantage. Trends Neurosci. 2003;26:543–549. doi: 10.1016/j.tins.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Sontheimer H. A role for glutamate in growth and invasion of primary brain tumors. J. Neurochem. 2008;105:287–295. doi: 10.1111/j.1471-4159.2008.05301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soroceanu L, Gillespie Y, Khazaeli MB, Sontheimer H. Use of chlorotoxin for targeting of primary brain tumors. Cancer Res. 1998;58:4871–4879. [PubMed] [Google Scholar]
- Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat. Med. 2001;7:1010–1015. doi: 10.1038/nm0901-1010. [DOI] [PubMed] [Google Scholar]
- Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J. Clin. Invest. 1998;101:1163–1174. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Zhang X, Li JJ. The role of NF-kappaB in the regulation of cell stress responses. Int. Immunopharmacol. 2002;2:1509–1520. doi: 10.1016/s1567-5769(02)00058-9. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang H, Zhang W, Huang HJ, Liao WS, Fuller GN. Analysis of the activation status of Akt, NFkappaB, and Stat3 in human diffuse gliomas. Lab. Invest. 2004;84:941–951. doi: 10.1038/labinvest.3700123. [DOI] [PubMed] [Google Scholar]
- Weber CK, Liptay S, Wirth T, Adler G, Schmid RM. Suppression of NF-kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology. 2000;119:1209–1218. doi: 10.1053/gast.2000.19458. [DOI] [PubMed] [Google Scholar]
- Werner SL, Barken D, Hoffmann A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science. 2005;309:1857–1861. doi: 10.1126/science.1113319. [DOI] [PubMed] [Google Scholar]
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J. Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59:4383–4391. [PubMed] [Google Scholar]
- Ye ZC, Rothstein JD, Sontheimer H. Compromised glutamate transport in human glioma cells: reduction mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J. Neurosci. 1999;19:10767–10777. doi: 10.1523/JNEUROSCI.19-24-10767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.