Abstract
Objective and design
The objective of this study was to determine genetic differences in inflammation in these distinct inbred mouse strains.
Methods
Peritoneal leukocyte recruitment, matrix metalloproteinases and cytokines were quantified in A/J, 129/svJ, C57BL/6J, using thioglycollate or biomaterial implants as inflammatory stimuli.
Results
In response to thioglycollate, A/J had significant decreases compared to C57BL/6J in both neutrophil (86 %) and macrophage (62 %) recruitment, and 129/svJ had a significant (43 %) decrease compared to C57BL/6J in macrophage recruitment. The reduced leukocyte recruitment corresponded to reduced matrix metalloproteinase-9. In the bioimplant model, 129/svJ had a 2-fold increase in neutrophil and macrophage recruitment compared to C57BL/6J, and the increased leukocyte recruitment corresponded to elevated cytokines, monocyte inhibitory protein-2 and monocyte chemoattractant protein-1, in the lavage compared to the values for C57BL/6J.
Conclusion
Not only was leukocyte recruitment strain dependent, but the three strains had marked differences in metalloproteinases and cytokine response. In addition, there were model specific differences in the metalloproteinase and cytokine response to the two inflammatory stimuli. Thus, inflammatory cell recruitment is genetically determined and stimulus specific and may determine the susceptibility to complex diseases.
Keywords: Leukocyte recruitment, Peritoneal inflammation, Inbred mouse strains, Metalloproteinase-9, Cytokines
Introduction
Inflammation [1] is critical for host defense at sites of injury, but it is also associated with diseases including peritonitis, acute coronary disease, atherosclerosis, and autoimmune disease [2–4] in humans and mice [5–6]. The recruitment of leukocytes is central to the generation of the inflammatory response at sites of injury and infection. In addition, genetic influence on leukocyte migration is suggested by recent studies [7–10] in human populations that have identified mutations and polymorphisms in proteins that facilitate leukocyte recruitment, such as proteolytic enzymes and chemoattractants. These mutations and polymorphisms were associated with disease and suggest genetic variation in leukocyte recruitment/function may determine susceptibility to inflammatory diseases.
Three inbred mouse strains, C57BL/6J (B6), A/J, 129/svJ (129) markedly differ in their susceptibility to infection and other inflammatory diseases, and differences in susceptibility may be due to genetic variation in leukocyte recruitment to the sites of inflammation. In this study, we examined the role of genetic background on leukocyte recruitment, using two distinct models of peritoneal inflammation, the thioglycollate and biomaterial implant (bioimplant) models. While neutrophils and macrophages are recruited in both models [11, 12], the time course for neutrophil and macrophage accumulation in the peritoneal cavity is quite different in the two models. In the thioglycollate model, total neutrophils reach a peak at 6 h and decline rapidly, while macrophage number gradually increases and reaches a peak at 72 h [11]. In the bioimplant model, both total neutrophil and total macrophage number reach a maximum and plateau at 20 h [12]. In these two inflammatory models, not only was the time course of leukocyte recruitment different, but the metalloproteinase (MMP) and cytokine responses were also different. In addition, leukocyte recruitment, MMP, and cytokine response were strain-dependent in the two models. Genetic variation in leukocyte recruitment in the three inbred strains, their response in the two models, and cytokine and proteolytic enzyme responses are reported in this study.
Materials and methods
Mice
The inbred strains, B6 (#000664), 129 (#000691), A/J (#000646), FVN/B wild type (#004104), FVN/B matrix metalloproteinase-9 deficient mice (MMP-9−/−) (#01800), B6129PF2/J (#10093) mice were obtained from Jackson Laboratory (Bar Harbor, ME) at 6 wks-of-age. The wild-type (WT) strains in mixed backgrounds, 50 % B6 : 50 % 129 (M1) and 75 % B6 : 25 % 129 (M2) were derived from the development of gene-targeted mouse strains, previously described [13], and were bred in the Biological Resource Unit at Cleveland Clinic Foundation (CCF). Both male and female mice were tested between 7 and 9 wk-of-age. All animal experiments were approved by the Institutional Animal Care and Research Advisory Committee at CCF.
Thioglycollate model
Mice were injected intraperitoneally with 0.5 mL of 4 % thioglycollate (Becton Dickinson, Cockeysville, MD) and after 6 or 72 h corresponding to peak neutrophil (6 h) and macrophage (72 h) recruitment [11], peritoneal lavage fluid and cells were harvested as previously described [11]. The number of neutrophils and macrophages [14] accumulating in the lavage were determined from enzyme activity of myeloperoxidase for neutrophils [15] and non-specific esterase for macrophage/monocytes [16].
Bioimplant model
Three disks of polyethylene terephthalate film, type A, 0.127 mm thick and 1.2 cm diameter circular disks (Cadillac Plastic and Chemical Co., Birmingham, MI), were inserted into the peritoneal cavity as previously described [12]. After 20 h (peak leukocyte recruitment), lavage, cells, and disks were recovered from the mice. The numbers of adherent leukocytes from the disks and in the lavage were determined by enzyme activities, as described above. The total number of cell types responding was the sum of the cells in the lavage plus the cells on the disks.
MMPs
MMP activity was determined by subjecting the lavage collected, at 6 h and 72 h after injection of thioglycollate and 20 h after implanting the disks, to electrophoresis on a 10 % gelatin zymograph (InVitrogen, Carlsbad, CA). Identification of the MMPs was determined by the molecular weight and verified by with an MMP standard. For the Western blot characterization, the lavage was purified with gelatin-agarose (Sigma Aldrich, #G5384) and concentrated with centrifugal filter devices (Millipore, #42406). Samples were subjected to 4–20 % SDS-PAGE electrophoresis, transferred onto PVDF membranes, and after stripping the same membrane, incubated with anti-MMP-9 antibody (rabbit polyclonal, Chemicon, #AB19047). MMP-2 was detected by anti-MMP-2 antibody (BioMol, #SA384). The signals were amplified with an ABC peroxidase detection system (Vector Laboratories, # PK6101) according to manufacturer’s instruction, and visualized with enhanced chemiluminescent reagents (Amersham Biosciences, #RPN2209).
Cytokines and fibrinogen
Keratinocyte-derived chemokine (KC), macrophage inflammatory protein-2 (MIP-2), and monocyte chemoattractant protein 1 (MCP-1) were determined in the lavage, 6 h and 72 h after injection of thioglycollate and 20 h after implanting the disks, with immunoassay kits from R&D Systems, Minneapolis, MN, Mouse KC Immunoassay Kit (#MKC00), Mouse MIP-2 Immunoassay Kit (#MM200), and Mouse MCP-1 Immunoassay Kit (#MJE00). Fibrinogen was determined by an ELISA assay with antibodies that recognize mouse fibrinogen (HyphenBioMed, France)
Statistical analysis
Differences in values between the mouse strains, B6, A/J, 129, and the mixed strains, M1 and M2, were determined by a one-way ANOVA and a Newman-Keuls post-test. Difference in cell recruitment in the FVB/N mice was determined with t-test. A P value <0.05 was considered significant. Values are the mean ± SEM.
Results
Thioglycollate model
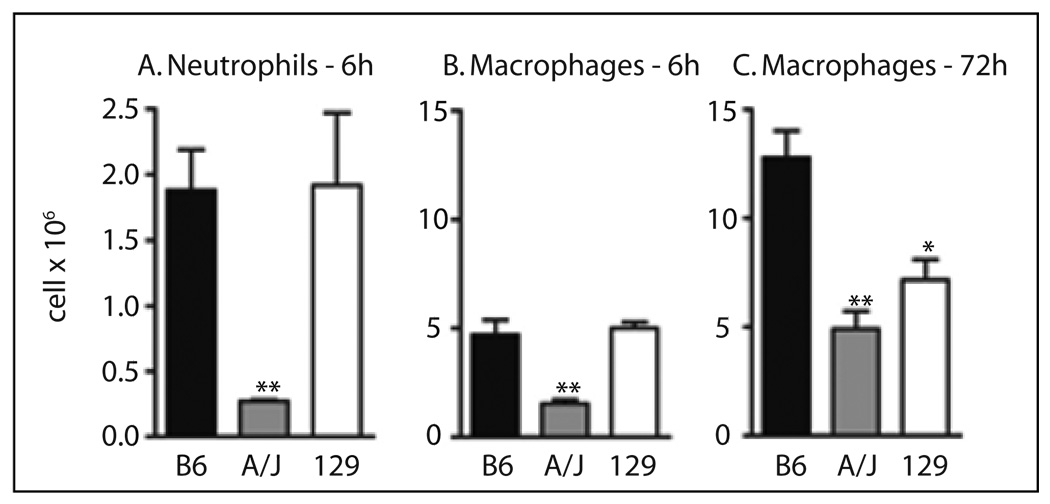
In the thioglycollate model, A/J had a marked significant (P <0.01) decrease in recruitment (Figure 1) of both neutrophils (86 %) at 6 h and macrophages (62 %) at 72 h after thioglycollate injection compared to B6. The 129 had a 44 % reduction (P <0.05) in recruitment of macrophages (Figure 1C) compared to B6, but the number of neutrophils recruited in 129 and B6 was similar (Figure 1A). The time course of macrophage recruitment was different in 129 compared to B6 and A/J. In B6 and A/J the number of macrophages was nearly three times higher at 72 h compared to 6 h (Figure 1B, 1C), but in 129 the number of macrophages recruited was similar at 6 h and 72 h. There was no significant difference in leukocytes, either total number (6–8 × 106 cells/mL) or percent neutrophils (52–60 %), lymphocytes (40–46 %), and monocytes (0–1 %) in peripheral blood between B6 and A/J or 129 in mice injected with PBS, suggesting that the number of circulating neutrophils or monocytes did not determine the leukocyte response. There were no significant (P >0.05) gender differences in leukocyte recruitment in this model.
Fig. 1.
Neutrophil and macrophage recruitment in the thioglycollate model of peritoneal inflammation. A. Neutrophil recruitment to the peritoneal cavity 6 h after injection of thioglycollate. B. Macrophage recruitment 6 h after injection of thioglycollate. C. Macrophage recruitment 72 h after injection of thioglycollate. Values are the mean ± SEM of 5–23 mice per genotype. Symbols indicate a difference between values from B6 and A/J or 129, determined by one-way ANOVA and Newman-Keuls post-test. A P-value >0.05 was considered not significant. *P <0.05, **P <0.01.
Bioimplant model
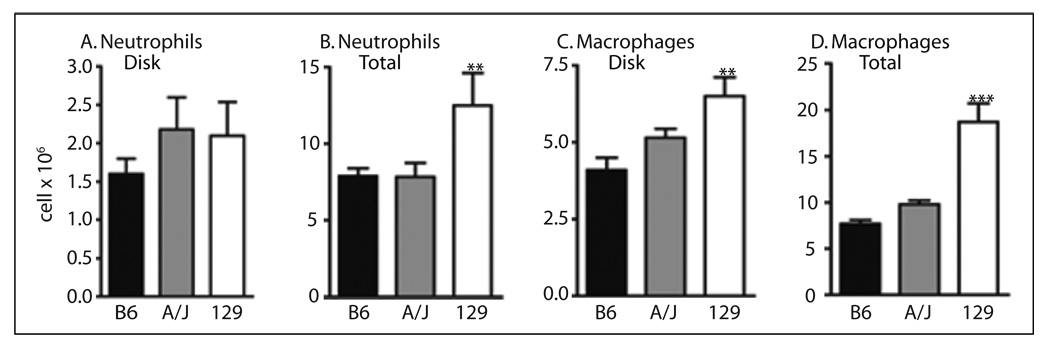
The number of cells attached to the implanted biopolymer disks was measured to ascertain if genetic background altered leukocyte adhesion (Figure 2A, B). In 129 and A/J, neutrophil adhesion (Figure 2A) to the disks was not significantly (P >0.05) different than for B6; however, the number of macrophages (Figure 2B) attached to the disks in 129 was 1.6-fold higher (P <0.001) than B6. No difference in adhesion of macrophages between the B6 and A/J was observed. Since fibrinogen is important for adhesion of leukocytes to the disks [14] in this model, plasma fibrinogen was determined in the B6, 129, and A/J and was significantly (P <0.001) higher (37 %) in 129 (2.2 ± 0.2, n = 4) than B6 (1.6 ± 0.01), n = 7), but only slightly (P <0.05) higher (6 %) in A/J thant B6 as previously reported [17]. Total neutrophil and macrophage recruitment (Figure 2C, D) was significantly (P <0.01) higher in 129 compared to B6. Neither total neutrophil or macrophage recruitment was significantly different in A/J compared to B6. Thus, in the bioimplant model, leukocyte recruitment was increased in 129, but not A/J, in contrast to the thioglycollate model where recruitment was reduced in both strains. There were no significant (P >0.05) gender differences in leukocyte recruitment in this model.
Fig. 2.
Neutrophil and macrophage recruitment in the bioimplant model of peritoneal inflammation 20 h after implanting disks. A. Number of neutrophils attached to biopolymer disks. B. Total number (attached to disk plus lavage) of neutrophils recruited. C. Number of macrophages attached to biopolymer disks. D. Total number (attached to disk plus lavage) of macrophages. Values are the mean ± SEM of 5–38 mice per genotype. Symbols indicate a difference between values from B6 and A/J or 129, determined by one-way ANOVA and Newman-Keuls post-test. **P <0.01, ***P <0.001.
Leukocyte recruitment in the B6:129 mixed background
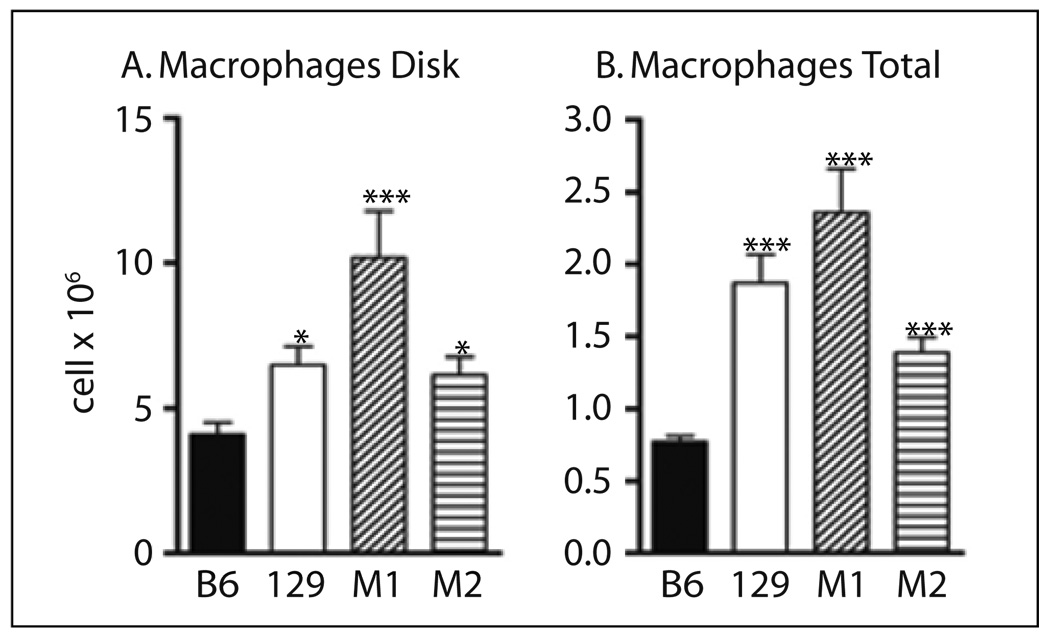
In view of the marked differences in leukocyte recruitment in the B6 and 129 in both models, the response in mice with mixed B6:129 backgrounds (M1–50 % B6: 50 % 129, M2–75 % B6: 25 % 129) was compared to B6 and 129. In the thioglycollate model no significant difference was found for neutrophils among the B6, 129, M1, or M2 strains (data not shown). For the macrophage recruitment only B6 and 129 were significantly different as in Figure 1C, and no difference was found between B6 and the mixed strains (data not shown). In the bioimplant model, total neutrophils were significantly higher (P <0.05) only in 129 compared to B6, but not between the mixed strains (data not shown) compared to B6. In the bioimplant model, disk attachment and total macrophage recruitment (Figure 3) were significantly (P <0.001) higher (~1.5–3-fold) in M1 (50 % B6: 50 % 129), M2 (75 % B6: 25 % 129) and 129 compared to B6. These results suggest that differences in gene expression in the B6 and 129 influence the macrophage recruitment in the bioimplant model. In this model, the phenotype of 129 was dominant in the mixed strains.
Fig. 3.
Macrophage recruitment in the bioimplant model in B6, 129, and mixed background strains (M1–50 % B6: 50 %129, M2–75 % B6: 25 %129. A. Number of macrophages attached to biopolymer disks. B. Total number (attached to disk plus lavage) of macrophages. Values are the mean ± SEM of 5–38 mice per genotype. Symbols indicate a difference between values from B6 and A/J or 129, determined by one-way ANOVA and Newman-Keuls post-test. *P <0.05, **P <0.01, ***P <0.001.
MMP response
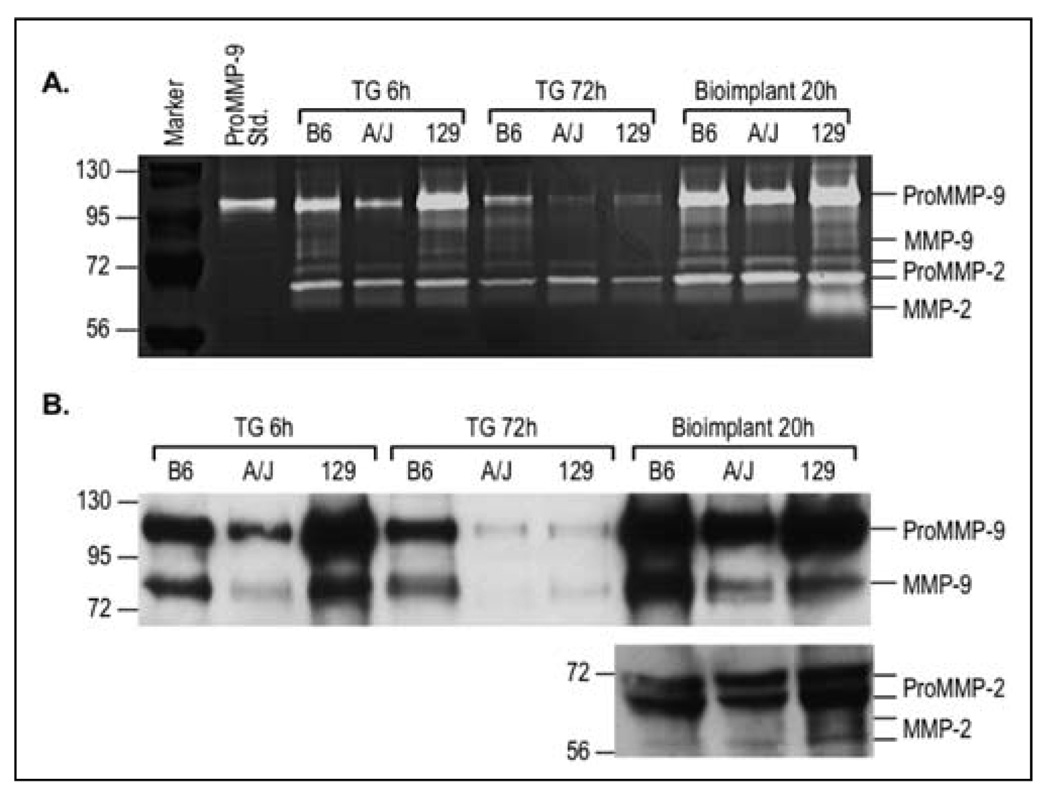
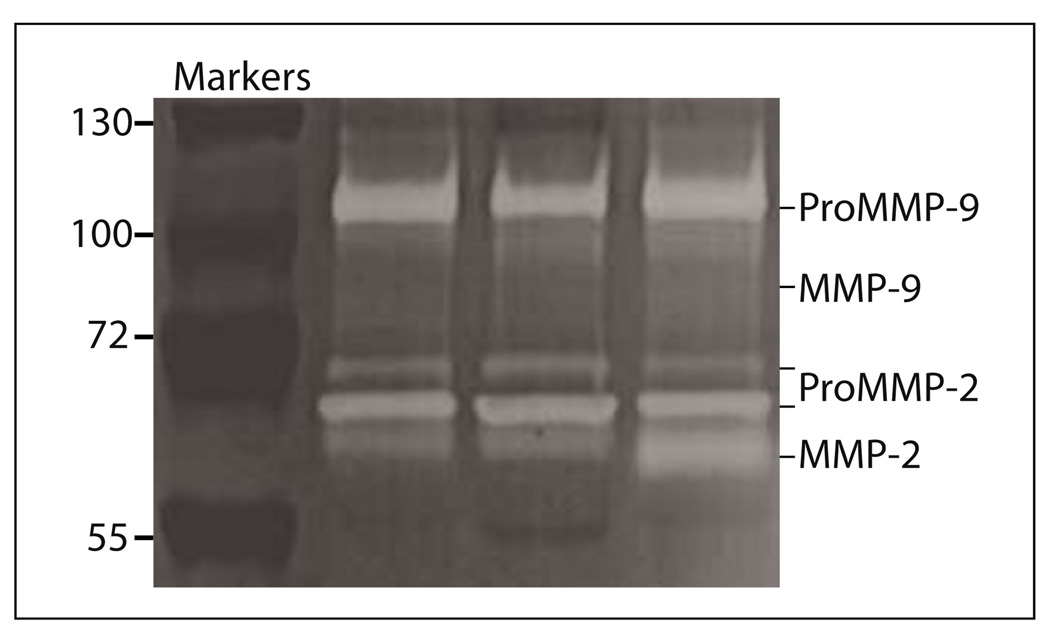
To determine if differences in proteolytic enzymes could be a mechanism explaining the differences in leukocyte recruitment among the inbred strains and between the models, MMPs were evaluated in the lavage after administration of the inflammatory stimuli. MMPs are released from cells in an inactive form, designated ProMMP, and a proteolytically cleaved form, the active form, designated MMP. ProMMP-9, ProMMP-2, MMP-9, and MMP-2 were detected by gelatin zymography (Figure 4A) and quantified relative to B6 bands (Table 1). At 6 h, both ProMMP-9 (63 %) and MMP-9 (37 %) were lower in the lavage from the A/J compared to B6. At 72 h the reduced ProMMP-9 (30 %) and MMP-9 (7 %) were still lower in A/J than B6. In 129, MMP-9 was 75 % at 6 h and 24 % at 72 h of the values for B6. The lavage was further subjected to Western blotting with antibodies that recognized both ProMMP-9 and MMP-9 or ProMMP-2 and MMP-2 (Figure 4B), and similar results were observed as for the zymograph. The reduced MMP-9 in the A/J lavage corresponded to the reduced macrophage recruitment at 6 and 72 h after thioglycollate injection. The reduced MMP-9 in 129 at 72 h after thioglycollate injection also corresponded to reduced macrophage recruitment. MMP-9 may be more closely related to the macrophage recruitment than neutrophil recruitment, since the neutrophils were either not different or not detected in the A/J or 129 compared to B6 (Table 2). In the bioimplant model, MMP-9 was only slightly reduced in both A/J and 129 compared to B6, and did not correspond to either neutrophil or macrophage recruitment. In the 129 there was a marked increase in MMP-2 (Figure 4A, B), and MMP-2 was also found in mixed background (B6: 129) mice (Figure 5). B6 or A/J had no detectable MMP-2, only the ProMMP-2. This increase in MMP-2 in 129 and a mixed background strain may contribute to the elevated recruitment of both neutrophils and macrophages in the bioimplant model.
Fig. 4.
Representative zymography and western blots of the lavage after stimulation. A. 6 and 72 h after thioglycollate (TG) injection and 20 h after disk insertion. Note only the 129 mice had a band for MMP-2 (66 kDa) 20 h after biopolymer disks were inserted. Molecular weight markers and ProMMP-9 standard (105 kDa) are included in the first two lanes on the left. B. Western blotting of the lavage from the thioglycollate (TG) and bioimplant models. After electrophoresis, MMPs were determined by specific antibodies for MMP-9 and MMP-2 that recognize both pro and active forms. Lavage from three mice per strain were tested and run in duplicate for both zymographs and Western blotting.
Table 1.
MMPs in the lavage after thioglycollate and bioimplant stimulation.
| B6 (%) | A/J (%) | 129 (%) | |
|---|---|---|---|
| Thioglycollate – 6 h | |||
| ProMMP-9 | 100 | 63 (59–67)*** | 77 (47–107) |
| MMP-9 | 100 | 37 (23–51)** | 75 (71–79)*** |
| ProMMP-2 | 100 | 101 (98–101) | 100 (98–101) |
| Thioglycollate – 72 h | |||
| ProMMP-9 | 100 | 30 (21–39)*** | 58 (32–84)* |
| MMP-9 | 100 | 7 (3–10)*** | 24 (15–34)** |
| ProMMP-2 | 100 | 99 (97–100) | 96 (92–100)* |
| Bioimplant – 20 h | |||
| ProMMP-9 | 100 | 86 (72–99)* | 94 (80–108) |
| MMP-9 | 100 | 78 (70–87)** | 85 (69–107) |
| ProMMP-2 | 100 | 89 (71–107) | 91 (47–134) |
Density of bands on zymographs were determined and expressed as percent of the B6 values. Values are the mean and 95 % confidence interval of 3–4 mice per strain analyzed in triplicate.
Symbols indicate P <0.05,
P <0.01,
P <0.001 compared to B6 determined by a t-test.
Table 2.
Summary of differences in three inbred strains.
| Neutrophils | Macrophages | MMP-9 | MIP-2 | MCP-1 | |
|---|---|---|---|---|---|
| Thioglycollate – 6 h | |||||
| A/J | Decrease | Decrease | Decrease | NC | NC |
| 129 | NC | NC | Decrease | NC | NC |
| Thioglycollate – 72 h | |||||
| A/J | ND | Decrease | Decrease | NC | NC |
| 129 | ND | Decrease | Decrease | NC | NC |
| Bioimplant – 20 h | |||||
| A/J | NC | NC | Decrease | NC | Increase |
| 129 | Increase | Increase | NC | NC | Increase |
Values significantly (P <0.05) increased or decreased compared to values for B6 mice. NC = no significant change. ND = not detected.
Fig. 5.
Zymography of the lavage 72 h After thioglycollate stimulation in B6:129 mixed background mice, B6129PF2. The left lane is the molecular weight markers and the other 3 lanes are lavage from 3 different mice representative of 5 mice assayed. MMP-2 was present in all five samples, but not in lavage from B6 mice as in Figure 4A.
Leukocyte recruitment in MMP-9 deficient mice
MMP-9 is a proteinase that degrades extracellular matrix, and is required in many inflammatory models [18–20]. ProMMP-9 and MMP-9 were reduced in both A/J and 129 mice and to determine if MMP-9 played a significant role in thioglycollate stimulation of leukocyte recruitment, MMP-9−/− mice were tested. Wild-type and MMP-9 deficient mice in a FVN/B background were stimulated with thioglycollate and macrophage recruitment determined after 72 h. Macrophage (cells × 106, mean ± SEM, n = 13–15) recruitment was ~3-fold higher (P <0.05) in MMP-9+/+ (8.2 ± 0.2) than MMP-9−/− (2.7 ± 0.4). These results suggest that the reduced MMP-9 in the two strains, A/J and 129, may have contributed to their reduced macrophage recruitment in the thioglycollate model.
Cytokine response
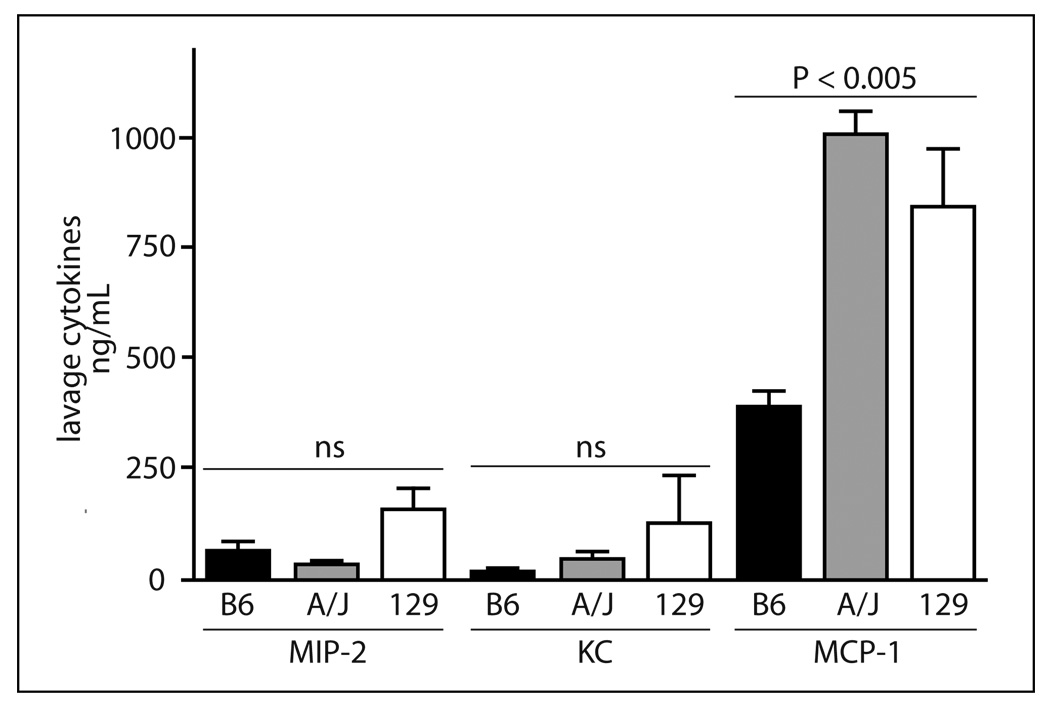
While differences in the MMPs corresponded to leukocyte recruitment in the thioglycollate model, they did not account for the differences in cell recruitment in the bioimplant model. Another important regulator of leukocyte migration signals [1] is the chemoattractant gradient in the tissues. To determine if genetic differences in cytokine release could explain differences in leukocyte recruitment in the bioimplant model, MIP-2 and KC, important neutrophil cytokines [21–23], and MCP-1, the critical macrophage cytokine [24], were determined in the lavage after administration of the inflammatory stimuli. No significant differences were found for the three cytokines in the lavage at 6 or 72 h after thioglycollate injection (data not shown). However, in the bioimplant model (Figure 6) a significant increase (P <0.01) was found in the lavage for MCP-1 in A/J and 129 compared to B6. MCP-1 was 2–3-fold higher in A/J and 129 than B6. There was a trend for increased MIP-2 and KC in 129 compared to B6.
Fig. 6.
Cytokines determined in lavage 20 h after insertion of the disks in the bioimplant model. Values expressed as pg/mL are the mean ± SEM of 3–4 mice per strain. Results of a one-way ANOVA are indicated for each cytokine. A Newman-Keuls post-test for the MCP-1 values indicated both A/J and 129 values were significantly (P <0.01) different than B6 values.
Discussion
Results of this study (summarized in Table 2) demonstrate that the three inbred strains had notable differences in the responses of leukocyte recruitment, MMP-9 expression and activation, and chemoattractants in the two inflammatory models. Mouse strain, leukocyte (neutrophils or macrophages), and model dependent genetic differences were identified. In the thioglycollate model, reduced macrophage recruitment in A/J and 129 corresponded to reduced expression and activation of MMP-9. In the bioimplant model, macrophage and neutrophil recruitment were increased in 129 with increased cytokines and increased activation of MMP-2. These results suggest that genetic factors control leukocyte recruitment in a model and strain specific fashion. This is the first study to identify genetic background variation in MMP-9 in the thioglycollate model, and MMP-2 and MCP-1 in the bioimplant model.
In the thioglycollate model, A/J had reduced MMP-9 at the time of peak neutrophil recruitment (6 h) and peak macrophage recruitment (72 h) that corresponded to reduced neutrophil and macrophage recruitment. Furthermore, in the both A/J and 129 at 72 h, MMP-9 and ProMMP-9 were reduced and MMP was lower than ProMMP, suggesting that proteolytic activation as well as release of the ProMMP-9 are reduced. The reduced proteolytic activity may be the important factor since at 6 h in 129, macrophage recruitment was not different than for B6, and the reduced MMP-9 and ProMMP-9 were similar (~76 %). A deficiency of plasminogen and its active form plasmin, a major proteolytic enzyme of MMP-9 activation [25, 26], reduce leukocyte migration in both the thioglycollate and bioimplant inflammatory models [11, 12] Although A/J [17] have higher plasma plasminogen, this strain also has higher levels of plasminogen activator inhibitor-1 and α2-antiplasmin. The high levels of plasmin inhibitors may contribute to the reduced plasmin activation of MMP-9. If the source of MMP-9 is leukocytes and the number in the lavage is reduced, this may explain a reduced ProMMP-9, but would not explain its reduced activation since plasminogen is found in high concentrations in plasma and interstitial fluid and its activators are expressed in many cell types. In this study, we have demonstrated support for a role of MMP-9 in macrophage recruitment in the thioglycollate model. Conflicting results [25–31] of the inflammatory response have been observed with MMP-9 deficient mice and it is not clear if the differences are due to genetic background (129, B6: 129, B6:Swiss; Balb/c, FVB/N) or differences in the inflammatory stimulus. Our results indicate that genetic background is an important determinate of the response to inflammatory stimuli.
Clearly, the mechanism of leukocyte recruitment in the thioglycollate and bioimplant models is different. In the bioimplant model, 129 had an increase in MMP-2. None of the other mice stimulated with bioimplants or thioglycollate had this increase in MMP-2. Although MMP-2 and MMP-9 are structurally similar, striking differences have been observed in their function. In contrast to MMP-9, MMP-2 plays a role in tissue remodeling that is required for wound healing and has a protective role in the development of colitis [32]. In the bioimplant model, the increased MMP-2 may play a role in the tissue healing after the implant insertion. The activation of MMP-2 takes place on the cell surface and is dependent on MT-MMPs [33]. Genetic variation in the activation of MMP-2 in 129 may contribute to the increased cell migration in the bioimplant model. The increases in cytokines in 129 corresponded to increased neutrophil and macrophage recruitment. However, the increase of MCP-1 in A/J did not influence macrophage recruitment in the bioimplant model where both neutrophil and macrophage recruitment were similar to B6. In addition, while the active MMP-9 was also reduced in A/J in the bioimplant model, the magnitude was not sufficient to alter leukocyte number, suggesting MMP-9 may not be important in the bioimplant model. There may be other factors in A/J that negate the increased MCP-1 response.
It was surprising to find that total macrophage recruitment was markedly increased in the 129 in the bioimplant model. White et al [34] also reported that 129 have a reduced macrophage response to thioglycollate compared to B6. In the bioimplant model, the number of macrophages attached to the disks was higher in the 129, suggesting that there may be differences in adhesion receptors [35] cell adhesion molecules [36] or fibrinogen, the major adhesive substrate. Tang et al [14, 36] reported fibrinogen coated disks elicited a greater attachment of cells to the biopolymer disks than albumin or hypofibrinogenemic plasma, and in fibrinogen deficient mice [12] the attachment of both neutrophils and macrophages to the biopolymer disks were markedly reduced. There was no difference in the cells recruited to the lavage in the fibrinogen deficient mice, but in the plasminogen deficient mice attachment of cells to the disk is not altered only a reduction in the cells recruited to the lavage, suggesting that the attachment of cells to the disks and recruitment to the lavage occur by different mechanisms. Plasma fibrinogen is slightly higher in 129 than B6, but the amount of fibrinogen bound to the disks is small and the plasma difference is not likely to contribute to differences in attachment of cells to the disks. In 129, increases in adhesion of cells to the bioimplants, and increases in cytokines and MMP-2 may contribute to the increased leukocyte recruitment in the bioimplant model.
In summary, this study of two models of inflammation demonstrates marked differences in leukocyte recruitment in the 129 in the two models, and marked reduction in both neutrophils and macrophages in A/J in the thioglycollate model, but not the bioimplant model. These results show genetically determined factors contribute to leukocyte recruitment in peritoneal inflammation. Moreover, genetic differences in MMP and cytokines and their expression and activation may profoundly alter leukocyte recruitment in inflammation and ultimately the risk of disease. Our study in the three inbred strains provides a basis to dissect new genetic determinants of inflammatory response and disease risk using mouse genetics.
Acknowledgements
The authors thank Dr. Edward Plow for his helpful discussion and review of the manuscript and Dr. Yelena Shchurina for assistance with the thioglycollate model, and R. Lewis and N. Klimczak for assistance with manuscript preparation. This study was supported by grants from NIH, T32 HL07914 (AS), HL17964, HL65205, HL078701 (JHP).
References
- 1.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt DL, Chew DP, Lincoff AM, Simoons ML, Harrington RA, Ommen SR, Jia G, Topol EJ. Effect of revascularization on mortality associated with an elevated white blood cell count in acute coronary syndromes. Am J Cardiol. 2003;92:136–140. doi: 10.1016/s0002-9149(03)00527-7. [DOI] [PubMed] [Google Scholar]
- 3.Imahara SD, O’Keefe GE. Genetic determinants of the inflammatory response. Curr Opin Crit Care. 2004;10:318–324. doi: 10.1097/01.ccx.0000140942.42247.7e. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Raya A, Abou-Raya S. Inflammation: a pivotal link between autoimmune diseases and atherosclerosis. Autoimmun Rev. 2006;5:331–337. doi: 10.1016/j.autrev.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Delano DL, Montesinos MC, D’Eustachio P, Wiltshire T, Cronstein BN. An interaction between genetic factors and gender determines the magnitude of the inflammatory response in the mouse air pouch model of acute inflammation. Inflammation. 2005;29:1–7. doi: 10.1007/s10753-006-8962-6. [DOI] [PubMed] [Google Scholar]
- 6.Nadeau JH, Arbuckle LD, Skamene E. Genetic dissection of inflammatory responses. J Inflamm. 1995;45:27–48. [PubMed] [Google Scholar]
- 7.Natividad A, Cooke G, Holland MJ, Burton MJ, Joof HM, Rockett K, Kwiatkowski DP, Mabey DC, Bailey RL. A coding polymorphism in matrix metalloproteinase 9 reduces risk of scarring sequelae of ocular Chlamydia trachomatis infection. BMC Med Genet. 2006;7:40. doi: 10.1186/1471-2350-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakashima K, Hirota T, Obara K, Shimizu M, Doi S, Fujita K, Shirakawa T, Enomoto T, Yoshihara S, Ebisawa M, Matsumoto K, Saito H, Suzuki Y, Nakamura Y, Tamari M. A functional polymorphism in MMP-9 is associated with childhood atopic asthma. Biochem Biophys Res Commun. 2006;344:300–307. doi: 10.1016/j.bbrc.2006.03.102. [DOI] [PubMed] [Google Scholar]
- 9.Kurzawski M, Pawlik A, Czerny B, Domanski L, Rozanski J, Drozdzik M. Frequencies of the common promoter polymorphisms in cytokine genes in a Polish population. Int J Immunogenet. 2005;32:285–291. doi: 10.1111/j.1744-313X.2005.00524.x. [DOI] [PubMed] [Google Scholar]
- 10.Bjarnadottir K, Eiriksdottir G, Aspelund T, Gudnason V. Examination of genetic effects of polymorphisms in the MCP-1 and CCR2 genes on MI in the Icelandic population. Atherosclerosis. 2006;188:341–346. doi: 10.1016/j.atherosclerosis.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Ploplis VA, French EL, Carmeliet P, Collen D, Plow EF. Plasminogen deficiency differentially affects recruitment of inflammatory cell populations in mice. Blood. 1998;91:2005–2009. [PubMed] [Google Scholar]
- 12.Busuttil SJ, Ploplis VA, Castellino FJ, Tang L, Eaton JW, Plow EF. A central role for plasminogen in the inflammatory response to biomaterials. J Thromb Haemost. 2004;2:1798–1805. doi: 10.1111/j.1538-7836.2004.00916.x. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P, Schoonjans L, Kieckens L, Ream B, Degen J, Bronson R, De Vos R, Van den Oord JJ, Collen D, Mulligan RC. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368:419–424. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- 14.Tang L, Eaton JW. Fibrin(ogen) mediates acute inflammatory responses to biomaterials. J Exp Med. 1993;178:2147–2156. doi: 10.1084/jem.178.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Himmelhoch SR, Evans WH, Mage MG, Peterson EA. Purification of myeloperoxidases from the bone marrow of the guinea pig. Biochemistry. 1969;8:914–921. doi: 10.1021/bi00831a022. [DOI] [PubMed] [Google Scholar]
- 16.Torres JL, Rush RS, Main AR. Physical and chemical characterization of a horse serum carboxylesterase. Arch Biochem Biophys. 1988;267:271–279. doi: 10.1016/0003-9861(88)90032-x. [DOI] [PubMed] [Google Scholar]
- 17.Hoover-Plow J, Shchurin A, Hart E, Sha J, Hill AE, Singer JB, Nadeau JH. Genetic background determines response to hemostasis and thrombosis. BMC Blood Disord. 2006;6:6. doi: 10.1186/1471-2326-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van den Steen PE, Opdenakker G, Wormald MR, Dwek RA, Rudd PM. Matrix remodelling enzymes, the protease cascade and glycosylation. Biochim Biophys Acta Gen Subj. 2001;1528:61–73. doi: 10.1016/s0304-4165(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 19.Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol. 2003;28:12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- 20.Tarlton JF, Whiting CV, Tunmore D, Bregenholt S, Reimann J, Claesson MH, Bland PW. The role of up-regulated serine proteases and matrix metalloproteinases in the pathogenesis of a murine model of colitis. Am J Pathol. 2000;157:1927–1935. doi: 10.1016/S0002-9440(10)64831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol. 1995;155:722–729. [PubMed] [Google Scholar]
- 22.Ramos CD, Fernandes KS, Canetti C, Teixeira MM, Silva JS, Cunha FQ. Neutrophil recruitment in immunized mice depends on MIP-2 inducing the sequential release of MIP-1alpha, TNF-alpha and LTB(4) Eur J Immunol. 2006;36:2025–2034. doi: 10.1002/eji.200636057. [DOI] [PubMed] [Google Scholar]
- 23.Roche JK, Keepers TR, Gross LK, Seaner RM, Obrig TG. CXCL1/KC and CXCL2/MIP-2 are critical effectors and potential targets for therapy of Escherichia coli O157:H7-associated renal inflammation. Am J Pathol. 2007;170:526–537. doi: 10.2353/ajpath.2007.060366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Li N, Diaz LA, Shipley M, Senior RM, Werb Z. Synergy between a plasminogen cascade and MMP-9 in autoimmune disease. J Clin Invest. 2005;115:879–887. doi: 10.1172/JCI23977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 27.McMillan SJ, Kearley J, Campbell JD, Zhu XW, Larbi KY, Shipley JM, Senior RM, Nourshargh S, Lloyd CM. Matrix metalloproteinase-9 deficiency results in enhanced allergen-induced airway inflammation. J Immunol. 2004;172:2586–2594. doi: 10.4049/jimmunol.172.4.2586. [DOI] [PubMed] [Google Scholar]
- 28.Betsuyaku T, Shipley JM, Liu Z, Senior RM. Neutrophil emigration in the lungs, peritoneum, and skin does not require gelatinase B. Am J Respir Cell Mol Biol. 1999;20:1303–1309. doi: 10.1165/ajrcmb.20.6.3558. [DOI] [PubMed] [Google Scholar]
- 29.Kolaczkowska E, Chadzinska M, Scislowska-Czarnecka A, Plytycz B, Opdenakker G, Arnold B. Gelatinase B/matrix metalloproteinase-9 contributes to cellular infiltration in a murine model of zymosan peritonitis. Immunobiology. 2006;211:137–148. doi: 10.1016/j.imbio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Renckens R, Roelofs JJ, Florquin S, de Vos AF, Lijnen HR, van’t VC, van der PT. Matrix metalloproteinase-9 deficiency impairs host defense against abdominal sepsis. J Immunol. 2006;176:3735–3741. doi: 10.4049/jimmunol.176.6.3735. [DOI] [PubMed] [Google Scholar]
- 31.Cataldo DD, Tournoy KG, Vermaelen K, Munaut C, Foidart JM, Louis R, Noel A, Pauwels RA. Matrix metalloproteinase-9 deficiency impairs cellular infiltration and bronchial hyperresponsiveness during allergen-induced airway inflammation. Am J Pathol. 2002;161:491–468. doi: 10.1016/S0002-9440(10)64205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garg P, Rojas M, Ravi A, Bockbrader K, Epstein S, Vijay-Kumar M, Gewirtz AT, Merlin D, Sitaraman SV. Selective ablation of matrix metalloproteinase-2 exacerbates experimental colitis: contrasting role of gelatinases in the pathogenesis of colitis. J Immunol. 2006;177:4103–4112. doi: 10.4049/jimmunol.177.6.4103. [DOI] [PubMed] [Google Scholar]
- 33.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 34.White P, Liebhaber SA, Cooke NE. 129X1/SvJ mouse strain has a novel defect in inflammatory cell recruitment. J Immunol. 2002;168:869–874. doi: 10.4049/jimmunol.168.2.869. [DOI] [PubMed] [Google Scholar]
- 35.Tang L, Ugarova TP, Plow EF, Eaton JW. Molecular determinants of acute inflammatory responses to biomaterials. J Clin Invest. 1996;97:1329–1334. doi: 10.1172/JCI118549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang L, Jiang W, Welty SE. The participation of P- and E-selectins on biomaterial-mediated tissue responses. J Biomed Mater Res. 2002;62:471–477. doi: 10.1002/jbm.10271. [DOI] [PubMed] [Google Scholar]