Abstract
Background:
We explored the predictive significance of BRCA1, TXR1 and TSP1 expression in non-small-cell lung cancer (NSCLC) patients treated with docetaxel in association with cisplatin or gemcitabine.
Methods:
To analyse BRCA1, TXR1 and TSP1 mRNA expression from microdissected primary tumours of 131 patients with stage IIIB (wet) and IV NSCLC, RT–qPCR was used.
Results:
The mRNA levels of TXR1/TSP1 were inversely correlated (Spearman's test: −0.37; P=0.001). Low TXR1 mRNA levels were associated with higher response rate (RR; P=0.018), longer median progression-free survival (PFS; P=0.029) and median overall survival (mOS P=0.003), whereas high TSP1 expression was correlated with higher RR (P=0.035), longer PFS (P<0.001) and mOS (P<0.001). Higher BRCA1 mRNA expression was associated with higher RR (P=0.028) and increased PFS (P=0.021), but not mOS (P=0.4). Multivariate analysis demonstrated that low TXR1/high TSP1 expression was an independent factor for increased PFS (HR 0.49; 95% CI 0.32–0.76; P<0.001) and mOS (HR 0.37; 95% CI 0.2–0.58; P<0.001), whereas high BRCA1 expression was correlated with increased PFS (HR 0.53; 95% CI 0.37–0.78; P=0.001).
Conclusions:
These data indicate that TXR1/TSP1 and BRCA1 expression could be used for the prediction of taxanes' resistance in the treatment of NSCLC.
Keywords: BRCA1 , NSCLC, taxanes, TSP1 , TXR1
Non-small-cell lung cancer (NSCLC) is the most common visceral malignancy worldwide for both sexes, accounting for ∼1.0 million cancer deaths per year (Jemal et al, 2009). Several drugs are used for disease control but the combinations of cisplatin with taxanes, vinorelbine and gemcitabine, have been established as the new standards of care. Treatment of patients with advanced/metastatic NSCLC with regimens combining platinum compounds and taxanes have extended the median survival time to 8–11 months and the 1-year survival rate to 30–40% (Georgoulias et al, 2001; Schiller et al, 2002).
There is a growing body of evidence regarding the genetic factors that could predict response to chemotherapy in NSCLC. The BRCA1 has emerged as one of the most appealing genetic markers for the customisation of chemotherapy in NSCLC. It has multiple roles not only in DNA damage repair but also in cell cycle regulation, transcriptional control, ubiquitination and apoptosis (Georgoulias et al, 2001; Mullan et al, 2001; Kennedy et al, 2002), and it may be a regulator of mitotic spindle assembly as it colocalises to the microtubules of the mitotic spindle and to the centromeres (Lotti et al, 2002). Decreased BRCA1 mRNA expression in a breast cancer cell line led to a greater sensitivity to cisplatin and etoposide and to a greater resistance to the microtubule-interfering agents paclitaxel and vincristine (Lafarge et al, 2001). Reconstitution of wild-type BRCA1 into BRCA1-negative HCC1937 breast cancer cells resulted in a 20-fold increase in cisplatin resistance and, in contrast, a 1000- to 10 000-fold increase in sensitivity to paclitaxel and vinorelbine (Quinn et al, 2003). This differential modulating effect of BRCA1 mRNA expression was also observed in tumour cells isolated from malignant effusions of NSCLC or gastric cancer patients (Wang et al, 2008), as well as in patients with ovarian cancer (Quinn et al, 2007), where high BRCA1 mRNA levels correlated negatively with cisplatin sensitivity and positively with docetaxel sensitivity. The value of the BRCA1 as a predictive marker for the treatment customisation in NSCLC has, also, been investigated in several retrospective studies (Taron et al, 2004; Boukovinas et al, 2008) and in one prospective trial (Boukovinas et al, 2008; Rosell et al, 2009).
Another mechanism of taxane resistance, which has been recently described, suggests that overexpression of a previously unknown gene, the taxol resistance gene 1 (TXR1) or proline rich 13 (PRR13), prevents apoptosis in a human prostate cancer cell line (Lih et al, 2006). This effect was mediated by the downregulation of the antiangiogenic and proapoptotic glycoprotein thrombospondin 1 (TSP1). Moreover, the sensitivity of cells in taxanes was increased by either inactivation of TXR1 using siRNA or by activating signalling through the integrin-associated protein (CD47 receptor) (Lih et al, 2006). A retrospective study conducted, from our group in 96 adenocarcinoma NSCLC patients treated with docetaxel–gemcitabine, provided evidence for in vivo relevance of this model. Our results confirmed the in vitro evidence that overexpression of TXR1 was significantly correlated with downregulation of TSP1 expression (P<0.0001), and the expression of both genes was significantly correlated with treatment outcome (Papadaki et al, 2009).
Based on these data we decided to conduct a retrospective study in order to investigate the predictive significance of BRCA1 and TXR1–TSP1 mRNA expression in NSCLC patients treated with docetaxel-based doublets. The main goal of the study was to validate, in an independent patients' cohort, the predictive significance of TXR1–TSP1 expression in different histologies of NSCLC and across with chemotherapy regimens combining docetaxel with platinum compounds (DC) or gemcitabine (DG).
Patients and methods
Patients
A total of 131 consecutive patients with histologically confirmed stage IIIB (with pleural effusion) and IV NSCLC and available tumour material for molecular analysis, who were treated with docetaxel–gemcitabine or docetaxel–cisplatin regimens as first-line treatment, at the University Hospital of Heraklion (Crete, Greece) between January 2003 and December 2007 were enroled. The above group of patients represents an independent patients' cohort (consecutive patients, not overlapping cases with those of the previous report) and was used as a confirmatory group (patients with different histologies and different taxanes regimens) in order to validate the results reported previously (Boukovinas et al, 2008; Papadaki et al, 2009). The study has been approved by the institutional ethics committee and all patients gave their informed consent for the use of the tissue material for translational research.
Specimens' characteristics and assay methods
In order to ensure the validity of the specimen and select the most appropriate area for microdissection, all paraffin-embedded tumours were reviewed by two independent pathologists (EL and ES). Serial sections of 5 μm thickness were prepared and then stained with nuclear Fast Red (Sigma-Aldrich, St Louis, MO, USA). Cancer cells were procured using an Eppendorf piezoelectric microdissector (Eppendorf, Hamburg, Germany) (Harsch et al, 2001).
The pellet of microdissected cells was subsequently submitted for RNA extraction with Trizol LS (Invitrogen, Carlsbad, CA, USA), and the SuperScript III Reverse Transcriptase (Invitrogen) was used to prepare cDNA from 50 ng of total RNA for each gene as previously described (Papadaki et al, 2009). The quality of the extracted RNA was evaluated with amplification of β-actin before RT–qPCR. Only samples with cycle quantification (Cq) <30 were considered suitable for further analysis based on the validation experiments for the performance of the set of primers and probes (Supplementary Figure 1a and b). The primers and probe sets were designed using Primer Express 2.0 Software (AB, Foster City, CA, USA). All primers and probes sequence were previously reported (Boukovinas et al, 2008; Papadaki et al, 2009). Relative cDNA quantification for BRCA1, TXR1, TSP1 and β-actin as an internal reference gene was done using the ABI Prism 7900HT Sequence Detection System (AB) (Supplementary Figures 2a–d).
Relative gene expression quantification was performed according to the comparative Ct method using β-actin as an endogenous control and commercial RNA controls (mRNA from lung and liver; Stratagene, La Jolla, CA, USA) as calibrators. In addition, RNA extracted from FFPE lung tissue of a normal individual (who was operated after an accident) was used as internal standard during the whole experiment. Final results were determined as follows: 2−(ΔCt sample−ΔCt calibrator), where ΔCT values of the calibrator and sample were determined by subtracting the CT value of the target gene from the value of the reference gene. In all experiments, only triplicates with s.d. of the Ct value <0.25 were accepted, according to the manufacturer suggestions (AB 7900 and SDS 2.3 User guide; AB). In addition, genomic DNA contamination of each sample has been excluded by non-reverse transcription of RNA.
Study design and statistics
This study was a retrospective analysis aiming to explore the predictive significance of BRCA1, TXR1 and TSP1 mRNA expression in patients with NSCLC treated with front-line DG or DC regimen, in an independent, confirmatory group of patients. All appropriate specimens of the primary tumour with >100 cells per section were included in the analysis. Objective responses were recorded according to the RECIST criteria (Therasse et al, 2000). All efficacy results were assessed on an intention-to-treat basis. Median progression-free survival (PFS) was measured from the date of first-line therapy initiation to the first radiographic documentation of disease progression or death and median overall survival (mOS) was calculated from the date of diagnosis of metastatic disease to death from any cause. Quantitative PCR analyses yielded values that were expressed as ratios between two absolute measurements (gene of interest: internal reference gene). Cutoff points were calculated according to the median value for the mRNA expression of each gene (Rosell et al, 2003; Taron et al, 2004). Samples with mRNA expression above or equal to the median were considered as samples with high expression, whereas those with value below the median as samples with low expression. All laboratory analyses were performed blinded to the clinical data.
Associations between treatment response and mRNA expression or baseline characteristics were assessed using Fisher's exact test for dichotomous variables or logistic regression for continuous variables. Kaplan–Meier curves were used to describe the proportion of subjects who remained free of events over the follow-up period. Associations between prognostic factors and PFS or OS were examined using Cox proportional hazards regression models; we report hazard ratio (HR) estimates and their 95% confidence intervals (CIs).
Cox regression models with interaction terms were used to assess whether mRNA expression effects varied across treatment subgroups. For each gene mRNA expression, two hypotheses were tested: (1) whether effect on first-line PFS and mOS varied according to regimen (DG or DC); and (2) whether the effect on first-line PFS and mOS varied according to tumour histology (squamous or non-squamous). All reported P-values were two sided and not adjusted for multiple testing.
Results
Patients' characteristics
Clinical data and representative samples from the primary tumours were collected from 131 consecutive patients treated with docetaxel-containing doublets in our centre. Successful amplification of both genes was achieved in all 131 specimens. Patient characteristics were all typical for NSCLC and are summarised in Table 1a.
Table 1a. Patients' characteristics.
|
Patients treated with DG or DC
|
||
|---|---|---|
| Number | % | |
| Gender | ||
| Male | 106 | 88 |
| Female | 25 | 12 |
| Age (years) | ||
| Median | 60 | |
| Range | 37–78 | |
| Performance status (ECOG) | ||
| 0 | 67 | 51 |
| 1 | 52 | 40 |
| 2 | 12 | 9 |
| Stage | ||
| IIIB (wet) | 35 | 27 |
| IV | 96 | 73 |
| Histology | ||
| Adenocarcinoma | 68 | 52 |
| Squamous | 56 | 43 |
| Other | 7 | 5 |
| Regimen | ||
| Docetaxel cisplatin | 64 | 49 |
| Docetaxel Gemcitabine | 67 | 51 |
| Response rate (CR+PR) | 40 | 30 |
| PFS (months, 95% CI) | 4.2 (2.7–5.7) | |
| Median OS (months, 95% CI) | 11.1 (9.7–14.6) | |
Abbreviations: CR=complete response; PR=partial response; PFS=progression-free survival; OS=overall survival; CI=confidence interval; ECOG=Eastern Cooperative Oncology Group; DG=docetaxel and gemcitabine; DC=docetaxel and cisplatin.
In an intention-to-treat analysis, complete response (CR) was observed in 2 (2%) and partial response (PR) in 38 (28%) patients (overall response rate (RR) 30% 95% CI 24.3–39.2%). After a median follow-up period of 9.7 months (range 1.3–84.5), the median PFS was 4.2 months (95% CI 2.7–5.7) and the median OS was 11.1 months (95% CI 9.7–14.6).
Genes' mRNA expression levels and response to treatment
The median mRNA expression levels were 4.28 (range 0.86–42.45) for BRCA1, 1.21 (range 0.02–7.8) for TXR1 and 0.24 (range 0.02–1.87) for TSP1. There was no correlation between age, gender, performance status or stage of disease and BRCA1, TXR1 or TSP1 mRNA levels (all P-values >0.05). In comparison with non-squamous tumours (median 3.62; P=0.001), BRCA1 was significantly higher in squamous tumours (median 8.1),whereas TXR1 and TSP1 expression was almost identical between the two histology groups (Table 1b) and in the same range with those reported for the previous patients' cohort (Papadaki et al, 2009). Furthermore, we observed the same inverse correlation between TXR1 and TSP1 mRNA expression (Spearman's test −0.37; P=0.001). For the BRCA1, as the expression values were significantly higher in squamous cell carcinomas in comparison with the adenocarcinomas (P=0.001), the cutoff values were calculated according to median expression levels in each group of squamous and non-squamous histology. Using these cutoff values, low (below the median) tumoural BRCA1 expression was observed in 28 (50%) squamous and 38 (50%) non-squamous specimens, whereas high (above or equal to the median) in 28 (50%) and 37 (50%) patients with squamous and non-squamous tumours, respectively.
Table 1b. BRCA1, TXR1 and TSP1 tumoural mRNA expression.
| All patients | Squamous | Non-squamous | P-value a | |
|---|---|---|---|---|
| No. of patients (%) | 131 (100) | 56 (43) | 75 (52) | |
| BRCA1 | ||||
| Expression value, median (range) | 4.28 (0.86–42.45) | 8.1 (1.73–42.45) | 3.62 (0.72–39.31) | 0.001 |
| High expression | 65 (50) | 28 (50) | 37 (50) | |
| Low expression | 66 (50) | 28 (50) | 38 (50) | |
| TXR1 | ||||
| Expression value, median (range) | 1.21 (0.02–7.8) | 1.19 (0.02–6.7) | 1.21 (0.12–7.8) | 0.92 |
| High expression | 65 (50) | 28(50) | 36 (50) | |
| Low expression | 66 (50) | 28(50) | 38 (50) | |
| TSP1 | ||||
| Expression value, median (range) | 0.24 (0.02–1.87) | 0.23 (0.02–1.54) | 0.24 (0.02–1.87) | 1.0 |
| High expression | 65 (50) | 28 (50) | 36 (50) | |
| Low expression | 66 (50) | 28 (50) | 38 (50) | |
Abbreviations: BRCA1=breast cancer 1 gene; TSP1=thrombospondin 1; TXR1=taxol resistance gene 1.
Mann–Whitney U-test, P-value.
BRCA1, TXR1, and TSP1 expression levels and treatment outcome
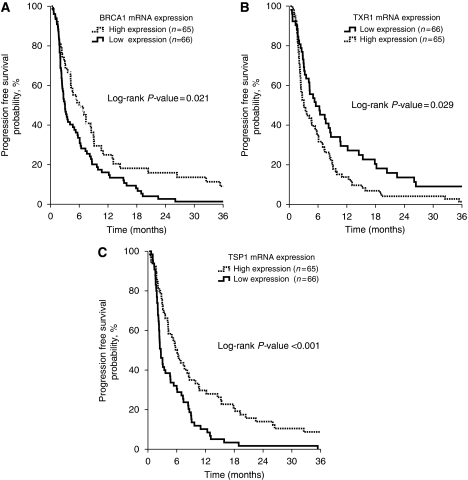
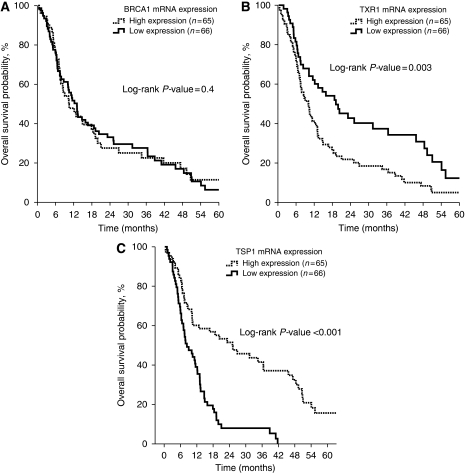
Table 2 summarises the treatment outcomes according to BRCA1, TXR1 and TSP1 mRNA expression. Patients with high BRCA1 mRNA expression had increased PFS (6.0 vs 3.0 months; P=0.021; Figure 1A) and RR (42 vs 20% P=0.028) in comparison with those with low BRCA1 mRNA levels. Conversely, there was no difference in terms of OS according to the BRCA1 mRNA expression (10.5 vs 11.2 months; P=0.4; Figure 2A). Patients with low TXR1 expression experienced a longer PFS (5.5 vs 3.0 months; P=0.029; Figure 1B), OS (19.1 vs 10.0 months; P=0.003; Figure 2B) and RR (45 vs 18%, P=0.018) when compared with patients whose tumours had high TXR1 mRNA expression. In addition, patients with high TSP1 expression presented longer PFS (6.1 vs 2.6 months; P<0.001; Figure 1C), OS (25.1 vs 8.4 months; P<0.001; Figure 2C) and RR (41 vs 22% P=0.035) when compared with patients with low TSP1 mRNA expression.
Table 2. Tumoural expression of BRCA1, TXR 1 and TSP1 mRNA and treatment efficacy.
|
PFS (months)
|
OS (months)
|
RR, N (%)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Genes | No. of patients | Median (95% CI) | P-value a | Median (95% CI) | P-value a | CR+PR (%) | SD+PD(%) | P-value |
| BRCA1 low | 66 (50) | 3.0 (2.3–3.7) | 0.021 | 10.5 (6.2–14.8) | 0.4 | 20 | 80 | 0.028 |
| BRCA1 high | 65 (50) | 6.0 (3.5–8.5) | 11.2 (7.2–15.3) | 42 | 58 | |||
| TXR1 low | 66 (50) | 5.5 (2.1–8.9) | 0.029 | 19.1 (10.4–27.9) | 0.003 | 45 | 55 | 0.018 |
| TXR1 high | 65 (50) | 3.0 (1.8–4.0) | 10.0 (7.4–12.7) | 18 | 82 | |||
| TSP1 low | 66 (50) | 2.6 (2.0–3.3) | <0.001 | 8.4 (5.2–11.6) | <0.001 | 22 | 78 | 0.035 |
| TSP1 high | 65 (50) | 6.1 (4.4–7.7) | 25.1 (11.1–39.2) | 41 | 59 | |||
Abbreviations: BRCA1=breast cancer 1 gene; CR=complete response; RR=response rate; PR=partial response; PFS=progression-free survival; OS=overall survival; CI=confidence interval; SD=stable disease; PD=progressive disease; TSP1=thrombospondin 1; TXR1=taxol resistance gene 1.
Log-rank P-value.
Figure 1.
Progression-free survival (PFS) according to BRCA1 (A), TXR1 (B) and TSP1 (C) mRNA expression.
Figure 2.
Median OS according to BRCA1 (A), TXR1 (B) and TSP1 (C) mRNA expression.
Genes' mRNA expression and treatment outcome according to histological subtype and first-line regimen used
The correlation between high BRCA1 mRNA expression and increased PFS was significant for both patients with squamous and non-squamous histology (interaction test P=0.61), whereas no significant correlation with mOS was found for either histological subtype (interaction test P=0.97; Table 3A). Similarly, the correlation between high TXR1 mRNA expression and decreased PFS (interaction test P=0.21) and mOS (interaction test P=0.19) was comparable (Table 3A). Finally, high TSP1 mRNA expression retained its predictive significance for increased PFS (interaction test P=0.34) and mOS (interaction test P=1.0) among patients with squamous and non-squamous histology (Table 3A).
Table 3. Correlation of tumoural expression of BRCA1, TXR1 and TSP1 mRNA and treatment efficacy (a) in different histological subtypes and (b) across taxane-based regimens.
|
PFS (months)
|
OS (months)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Genes | No. of patients | Median (95% CI) | Log-rank P-value | Interaction P-value | Median (95% CI) | Log-rank P -value | Interaction P-value | |
| (A) | ||||||||
| Histology | ||||||||
| Squamous | BRCA1 low | 28 (50) | 4.0 (1.5–7.6) | 0.047 | 0.61 | 11.3 (5.5–17.2) | 0.99 | 0.97 |
| BRCA1 high | 28 (50) | 7.4 (4.7–10.1) | 9.8 (4.8–17.0) | |||||
| Non-squamous | BRCA1 low | 38 (51) | 3.8 (2.1–5.5) | 0.041 | 11.2 (8.3–16.1) | 0.91 | ||
| BRCA1 high | 37 (49) | 7.2 (4.3–9.1) | 11.1 (8.8–15.6) | |||||
| Squamous | TXR1 low | 28 (50) | 7.1 (3.4–9.7) | 0.038 | 0.21 | 16.4 (10.9–19.5) | 0.022 | 0.19 |
| TXR1 high | 28 (50) | 4.2 (1.6–7.8) | 8.3 (2.6–12.1) | |||||
| Non-squamous | TXR1 low | 38 (51) | 6.4 (3.8–9.6) | 0.027 | 23.2 (10.2–38.1) | 0.007 | ||
| TXR1 high | 37 (49) | 3.3 (1.9–3.6) | 8.7 (5.2–12.3) | |||||
| Squamous | TSP1 low | 28 (50) | 3.6 (1.4–7.3) | 0.019 | 0.34 | 9.0 (4.6–13.5) | 0.001 | 1.0 |
| TSP1 high | 28 (50) | 6.1 (3.8–10.6) | 25.2 (10.2–47.8) | |||||
| Non-squamous | TSP1 low | 38 (51) | 2.3 (2.1–2.5) | 0.001 | 7.9 (3.0–12.7) | 0.001 | ||
| TSP1 high | 37 (49) | 6.3 (3.2–10.3) | 23.2 (10.4–41.8) | |||||
| (B) | ||||||||
| Regimen | ||||||||
| Docetaxel–gemcitabine | BRCA1 low | 34 (51) | 3.5 (2.1–4.8) | 0.013 | 0.006 | 11.1 (6.8–15.5) | 0.458 | 0.613 |
| BRCA1 high | 33 (49) | 6.2 (4.1–8.5) | 10.2 (3.9–17.6) | |||||
| Docitaxel–cisplatin | BRCA1 low | 32 (50) | 6.8 (3.7–10.0) | 0.874 | 11.2 (8.3–16.1) | 0.912 | ||
| BRCA1 high | 32 (50) | 6.1 (4.5–7.7) | 11.1 (8.8–15.6) | |||||
| Docetaxel–gemcitabine | TXR1 low | 34 (51) | 7.2 (4.9–9.7) | 0.024 | 0.56 | 15.8 (8.2–25.9) | 0.013 | 0.387 |
| TXR1 high | 33 (49) | 3.3 (2.1–4.4) | 8.5 (4.1–12.8) | |||||
| Docitaxel–cisplatin | TXR1 low | 32 (50) | 7.4 (4.2–10.3) | 0.032 | 23.2 (10.2–38.1) | 0.007 | ||
| TXR1 high | 32 (50) | 3.7 (1.4–5.2) | 8.7 (3.9–12.3) | |||||
| Docetaxel–gemcitabine | TSP1 low | 34 (51) | 3.4 (2.1–4.8) | 0.007 | 0.48 | 7.5 (5.4–11.4) | 0.006 | 0.931 |
| TSP1 high | 33 (49) | 6.8(4.6–9.7) | 26.7 (10.9–43.6) | |||||
| Docitaxel–cisplatin | TSP1 low | 32 (50) | 4.4 (1.6–8.1) | 0.002 | 9.1 (5.4–12.7) | 0.002 | ||
| TSP1 high | 32 (50) | 7.6 (4.3–12.8) | 36.4 (16.7–50.1) | |||||
Abbreviations: BRCA1=breast cancer 1 gene; PFS=progression-free survival; OS=overall survival; CI=confidence interval; TSP1=thrombospondin 1; TXR1=taxol resistance gene 1.
Likewise, the correlation between high TXR1 mRNA expression and decreased PFS (interaction test P=0.56) and mOS (interaction test P=0.38) was observed for patients receiving either DG or DC regimens (Table 3B). Moreover, PFS (interaction test P=0.48) and mOS (interaction test P=0.93) were significantly correlated with TSP1 mRNA expression in either DG or DC treatment regimens (Table 3B). In contrast, high BRCA1 mRNA expression was significantly correlated with increased PFS only in patients treated with DG regimen but not in those treated with DC (interaction test P=0.006); on the contrary, no such significant association with mOS was observed in either treatment group (interaction test P=0.61; Table 3B). In addition, 42 (63%) patients treated with first-line DG received second-line treatment with a cisplatin-based combination; BRCA1 mRNA expression was significantly correlated with PFS (5.7 vs 2.2 months for patients with low and high mRNA expression; P=0.01) in this subgroup of patients.
Univariate and multivariate analyses
Univariate analysis demonstrated that high TXR1 (P=0.002) mRNA expression and stage IV (P=0.02) as diagnosis factors were significantly associated with decreased PFS, whereas high TSP1 (P<0.001) and BRCA1 (P=0.02) mRNA expression were associated with increased PFS. In addition, performance status of 2 (P=0.04), high TXR1 (P=0.003) and low TSP1 (P<0.001) mRNA expression were significantly associated with decreased OS (Table 4A). Cox proportional hazard analysis revealed that the combined expression of TXR1 and TSP1 (low TXR1/high TSP1 expression) (HR 0.49; 95% CI 0.32–0.76; P<0.001) and BRCA1 (HR 0.53; 95% CI 0.37–0.78; P=0.001) expression emerged as independent factors associated with increased PFS (Table 4B). Moreover, performance status (2 vs 0–1; HR 1.92; 95% CI 1.02–3.60; P=0.04) and TXR1 and TSP1 (low TXR1/high TSP1 expression; HR 0.37; 95% CI 0.23–0.58; P<0.001) were independent prognostic factors for OS (Table 4b).
Table 4a. Univariate analysis for PFS and OS.
| HR | 95% CI | P-value | |
|---|---|---|---|
| PFS | |||
| BRCA1 expression (high vs low) | 0.65 | 0.45–0.94 | 0.02 |
| TXR1 expression (high vs low) | 1.61 | 1.17–2.43 | 0.002 |
| TSP1 expression (high vs low) | 0.49 | 0.34–0.72 | <0.001 |
| TXR1–TSP1 expression (TXR1 low/TSP1 high vs others) | 0.60 | 0.39–0.90 | 0.01 |
| PS (2 vs 0–1) | 1.66 | 0.89–3.10 | 0.13 |
| Age (>70 years vs ⩽70 years) | 1.17 | 0.77–1.84 | 0.48 |
| Gender (male vs female) | 1.30 | 0.72–2.33 | 0.38 |
| Stage (IV vs IIIβ) | 1.90 | 1.15–2.58 | 0.02 |
| OS | |||
| BRCA1 expression (high vs low) | 1.02 | 0.69–1.50 | 0.93 |
| TXR1 expression (high vs low) | 1.83 | 1.23–2.73 | 0.003 |
| TSP1 expression (high vs low) | 0.34 | 0.22–0.53 | <0.001 |
| TXR1–TSP1 expression (TXR1 low/TSP1 high vs others) | 0.38 | 0.24–0.62 | <0.001 |
| PS (2 vs 0–1) | 1.92 | 1.02–3.58 | 0.04 |
| Age (>70 years vs ⩽70 years) | 1.27 | 0.79–2.04 | 0.33 |
| Gender (male vs female) | 1.57 | 0.77–3.59 | 0.18 |
| Stage (IV vs IIIβ) | 1.73 | 1.03–2.24 | 0.04 |
Abbreviations: BRCA1=breast cancer 1 gene; PFS=progression-free survival; PS=performance status; OS=overall survival; CI=confidence interval; HR=hazard ratio; TSP1=thrombospondin 1; TXR1=taxol resistance gene 1. Values shown in bold are statistically significant.
Table 4b. Multivariate analysis for time to tumour progression and OS.
| HR | 95% CI | P-value | |
|---|---|---|---|
| PFS | |||
| Stage (IV vs IIIβ) | 1.41 | 0.94–1.66 | 0.09 |
| BRCA1 expression (high vs low) | 0.53 | 0.37–0.78 | 0.001 |
| TXR1–TSP1 expression (TXR1 low/TSP1 high vs others) | 0.49 | 0.32–0.76 | <0.001 |
| OS | |||
| Stage (IV vs IIIβ) | 1.42 | 0.92–2.74 | 0.2 |
| TXR1–TSP1 expression (TXR1 low/TSP1 high vs others) | 0.37 | 0.23–0.58 | <0.001 |
| PS (0–1 vs 2) | 1.92 | 1.02–3.60 | 0.04 |
Abbreviations: BRCA1=breast cancer 1 gene; PFS=progression-free survival; PS=performance status; OS=overall survival; CI=confidence interval; HR=hazard ratio; TSP1=thrombospondin 1; TXR1=taxol resistance gene 1. Values shown in bold are statistically significant.
Discussion
Downregulation of TSP1 through TXR1 overexpression has been proposed as a novel mechanism that modulates the cellular cytotoxicity of taxanes in vitro (Lih et al, 2006); indeed, in a previous report we have confirmed the clinical relevance of this mechanism in a group of patients with lung adenocarcinomas treated with a chemotherapy regimen combining docetaxel and gemcitabine (Papadaki et al, 2009). In this we evaluated the predictive significance of TXR1 overexpression/TSP1 downregulation, together with BRCA1 mRNA expression, in samples from patients with all histologies of NSCLC treated with either docetaxel/gemcitabine or docetaxel/cisplatin combinations.
We observed that the median expression values of TXR1 and TSP1 were almost identical between squamous and non-squamous carcinomas (P-value=0.92 and 1.0, respectively) and we confirmed that overexpression of TXR1 was significantly correlated with downregulation of TSP1 expression (P=0.001). In addition, multivariate analysis revealed that the favourable genotype (low TXR1/high TSP1 expression) was an independent prognostic factor for increased PFS and survival, and was associated with a 51 and 63% reduction of the risk for progression or death, respectively. It is interesting to note that the predictive value of TXR1 and TSP1 expression for PFS and median OS was retained irrespectively of the tumour histology. All these results are in agreement with the published in vitro and in vivo data regarding the role of TXR1–TSP1 expression in taxanes' resistance (Lih et al, 2006; Papadaki et al, 2009).
In contrast, median BRCA1 expression was significantly different in squamous and non-squamous tumours (P=0.001), as has been previously reported (Rosell et al, 2007). Overexpression of BRCA1 was significantly correlated with higher RR and PFS but not with mOS. The predictive value of BRCA1 in PFS remained significant in both squamous and non-squamous groups (interaction test P=0.61). On the contrary, overexpression of BRCA1 was significantly correlated with increased PFS in patients treated with DG but not in those treated with DC regimen (interaction test P=0.006). These results are in agreement with the current evidence for the differential predictive value of BRCA1, as low BRCA1 expression confers increased sensitivity to cisplatin (Husain et al, 1998; Lafarge et al, 2001; Quinn et al, 2003, 2007) and resistance to antimicrotubule drugs such as docetaxel (Quinn et al, 2007), whereas high BRCA1 expression leads to resistance to cisplatin (Husain et al, 1998; Lafarge et al, 2001; Taron et al, 2004; Rosell et al, 2009) and sensitivity to docetaxel (Quinn et al, 2007; Boukovinas et al, 2008; Rosell et al, 2009). In addition, BRCA1 overexpression is significantly correlated with those of ERCC1 and RRM1 in several studies (Rosell et al, 2007; Boukovinas et al, 2008; Wang et al, 2008; Bartolucci et al, 2009), providing another explanation for the lack of association of BRCA1 with OS.
High BRCA1 mRNA expression has been associated with increased risk of relapse in patients with early (stage IB–IIB) NSCLC (Bartolucci et al, 2009), whereas BRCA1 haplotype could predict the outcome of NSCLC cancer patients treated with platinum-based chemotherapy, especially of those with squamous cell histology (Kim et al, 2008); these findings could explain the lack of significant association between BRCA1 expression and efficacy of the DC regimen observed in this study. Similarly, BRCA1 protein expression was not a predictive factor for response to treatment in patient with operable NSCLC treated with DC in the neoadjuvant setting (Kang et al, 2010). Finally, the poor correlation of BRCA1 with OS, especially in patients treated with DG, could be partially explained by the fact that second-line cisplatin chemotherapy is more effective in patients with low BRCA1 expression as has been previously reported (Boukovinas et al, 2008), as was the case in the present study.
The effect of TXR1–TSP1 mRNA expression on taxanes' cytotoxicity is independent of MDR phenotype (the ability of tumour cells to efflux taxanes through the upregulation of the ATP-dependent cell membrane glycoproteins) (Gottesman and Ling, 2006), as the overexpression of TXR1 did not affect the cellular accumulation of [3H]-labelled-paclitaxel in the resistant cells and did not reduce the sensitivity to other agents that are also expelled from tumour cells by the MDR (Lih et al, 2006). Also, it seems to be independent from tubulin formation (the mutations of the β-tubulin gene that may interfere with the taxane-binding sites to microtubules) (Giannakakou et al, 1997, 2000a, 2000b; Kavallaris et al, 1997), as quantitative biochemical analysis of cell lines resistant to taxanes has shown the same tubulin dynamics as that of the parental (sensitive) cells and no increase in microtubules' isoforms that are commonly upregulated in tubulin-related resistance to taxanes (Gottesman and Ling, 2006; Lih et al, 2006).
Owing to the lack of a non-taxane-treated control group in our study, we cannot confirm that the effect of TSP1 expression was taxane specific and not a simple predictive marker for response to chemotherapy. In many human cancers, TSP1 expression is inversely correlated with progression, and it was also found to be an independent prognostic indicator (Neal et al, 2006; Guerrero et al, 2008). However, using cDNA microarrays, it has been previously shown that the induction of TSP1 in docetaxel-treated head and neck squamous cell carcinoma cell lines increased cytotoxicity (Yoo et al, 2002). The TSP1 is considered a proapoptotic protein through the activation of CD47 cell surface receptor (Lih et al, 2006), which has previously been shown to result in not only caspase-independent (Mateo et al, 1999) but also caspase-dependent apoptosis (Manna et al, 2005). In addition, TSP1 is a strong inhibitor of angiogenesis and decreased levels of circulating TSP1 in certain inbred mouse strains are correlated with increased circulating endothelial precursors and susceptibility to cancers (Shaked et al, 2005). In NSCLC, reduced protein expression of TSP1 has been associated with increased microvessels count and unfavourable prognosis (Yamaguchi et al, 2002). On the other hand, overexpression of TSP1 has also been associated with aggressiveness and increased angiogenesis in lung cancer (Ioachim et al, 2006).
Despite the fact that the results of this study should be interpreted with caution because of the retrospective nature of the study, it seems that the TXR1–TSP1 mRNA expression could be used as predictive markers for patients with NSCLC treated with docetaxel-based chemotherapy. The next step should be the evaluation of the significance of TXR1/TSP1 expression in taxanes' chemosensitivity in other tumour types such as breast and ovarian cancer, where taxanes are commonly used in the daily clinical practice. If this will be the case, the clinical relevance of the TXR1/TSP1 expression should be further validated in prospective adequately designed clinical trials.
Acknowledgments
This study was partially supported by grants from the Cretan Association for Biomedical Research (CABR), Pfizer Hellas and Janssen-Cilag Hellas.
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Bartolucci R, Wei J, Sanchez JJ, Perez-Roca L, Chaib I, Puma F, Farabi R, Mendez P, Roila F, Okamoto T, Taron M, Rosell R (2009) XPG mRNA expression levels modulate prognosis in resected non-small-cell lung cancer in conjunction with BRCA1 and ERCC1 expression. Clin Lung Cancer 10: 47–52 [DOI] [PubMed] [Google Scholar]
- Boukovinas I, Papadaki C, Mendez P, Taron M, Mavroudis D, Koutsopoulos A, Sanchez-Ronco M, Sanchez JJ, Trypaki M, Staphopoulos E, Georgoulias V, Rosell R, Souglakos J (2008) Tumor BRCA1, RRM1 and RRM2 mRNA expression levels and clinical response to first-line gemcitabine plus docetaxel in non-small-cell lung cancer patients. PLoS One 3: e3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgoulias V, Papadakis E, Alexopoulos A, Tsiafaki X, Rapti A, Veslemes M, Palamidas P, Vlachonikolis I (2001) Platinum-based and non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a randomised multicentre trial. Lancet 357: 1478–1484 [DOI] [PubMed] [Google Scholar]
- Giannakakou P, Gussio R, Nogales E, Downing KH, Zaharevitz D, Bollbuck B, Poy G, Sackett D, Nicolaou KC, Fojo T (2000a) A common pharmacophore for epothilone and taxanes: molecular basis for drug resistance conferred by tubulin mutations in human cancer cells. Proc Natl Acad Sci USA 97: 2904–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakakou P, Poy G, Zhan Z, Knutsen T, Blagosklonny MV, Fojo T (2000b) Paclitaxel selects for mutant or pseudo-null p53 in drug resistance associated with tubulin mutations in human cancer. Oncogene 19: 3078–3085 [DOI] [PubMed] [Google Scholar]
- Giannakakou P, Sackett DL, Kang YK, Zhan Z, Buters JT, Fojo T, Poruchynsky MS (1997) Paclitaxel-resistant human ovarian cancer cells have mutant beta-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem 272: 17118–17125 [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Ling V (2006) The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett 580: 998–1009 [DOI] [PubMed] [Google Scholar]
- Guerrero D, Guarch R, Ojer A, Casas JM, Ropero S, Mancha A, Pesce C, Lloveras B, Garcia-Bragado F, Puras A (2008) Hypermethylation of the thrombospondin-1 gene is associated with poor prognosis in penile squamous cell carcinoma. BJU Int 102: 747–755 [DOI] [PubMed] [Google Scholar]
- Harsch M, Bendrat K, Hofmeier G, Branscheid D, Niendorf A (2001) A new method for histological microdissection utilizing an ultrasonically oscillating needle: demonstrated by differential mRNA expression in human lung carcinoma tissue. Am J Pathol 158: 1985–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain A, He G, Venkatraman ES, Spriggs DR (1998) BRCA1 up-regulation is associated with repair-mediated resistance to cis-diamminedichloroplatinum(II). Cancer Res 58: 1120–1123 [PubMed] [Google Scholar]
- Ioachim E, Michael MC, Salmas M, Damala K, Tsanou E, Michael MM, Malamou-Mitsi V, Stavropoulos NE (2006) Thrombospondin-1 expression in urothelial carcinoma: prognostic significance and association with p53 alterations, tumour angiogenesis and extracellular matrix components. BMC Cancer 6: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59: 225–249 [DOI] [PubMed] [Google Scholar]
- Kang CH, Jang BG, Kim DW, Chung DH, Kim YT, Jheon S, Sung SW, Kim JH (2010) The prognostic significance of ERCC1, BRCA1, XRCC1, and [beta]III-tubulin expression in patients with non-small cell lung cancer treated by platinum- and taxane-based neoadjuvant chemotherapy and surgical resection. Lung Cancer 68: 478–483 [DOI] [PubMed] [Google Scholar]
- Kavallaris M, Kuo DY, Burkhart CA, Regl DL, Norris MD, Haber M, Horwitz SB (1997) Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest 100: 1282–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RD, Quinn JE, Johnston PG, Harkin DP (2002) BRCA1: mechanisms of inactivation and implications for management of patients. Lancet 360: 1007–1014 [DOI] [PubMed] [Google Scholar]
- Kim HT, Lee JE, Shin ES, Yoo YK, Cho JH, Yun MH, Kim YH, Kim SK, Kim HJ, Jang TW, Kwak SM, Kim CS, Ryu JS (2008) Effect of BRCA1 haplotype on survival of non-small-cell lung cancer patients treated with platinum-based chemotherapy. J Clin Oncol 26: 5972–5979 [DOI] [PubMed] [Google Scholar]
- Lafarge S, Sylvain V, Ferrara M, Bignon YJ (2001) Inhibition of BRCA1 leads to increased chemoresistance to microtubule-interfering agents, an effect that involves the JNK pathway. Oncogene 20: 6597–6606 [DOI] [PubMed] [Google Scholar]
- Lih CJ, Wei W, Cohen SN (2006) Txr1: a transcriptional regulator of thrombospondin-1 that modulates cellular sensitivity to taxanes. Genes Dev 20: 2082–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti LV, Ottini L, D'Amico C, Gradini R, Cama A, Belleudi F, Frati L, Torrisi MR, Mariani-Costantini R (2002) Subcellular localization of the BRCA1 gene product in mitotic cells. Genes Chromosomes Cancer 35: 193–203 [DOI] [PubMed] [Google Scholar]
- Manna PP, Dimitry J, Oldenborg PA, Frazier WA (2005) CD47 augments Fas/CD95-mediated apoptosis. J Biol Chem 280: 29637–29644 [DOI] [PubMed] [Google Scholar]
- Mateo V, Lagneaux L, Bron D, Biron G, Armant M, Delespesse G, Sarfati M (1999) CD47 ligation induces caspase-independent cell death in chronic lymphocytic leukemia. Nat Med 5: 1277–1284 [DOI] [PubMed] [Google Scholar]
- Mullan PB, McWilliams S, Quinn J, Andrews H, Gilmore P, McCabe N, McKenna S, Harkin DP (2001) Uncovering BRCA1-regulated signalling pathways by microarray-based expression profiling. Biochem Soc Trans 29: 678–683 [DOI] [PubMed] [Google Scholar]
- Neal CP, Garcea G, Doucas H, Manson MM, Sutton CD, Dennison AR, Berry DP (2006) Molecular prognostic markers in resectable colorectal liver metastases: a systematic review. Eur J Cancer 42: 1728–1743 [DOI] [PubMed] [Google Scholar]
- Papadaki C, Mavroudis D, Trypaki M, Koutsopoulos A, Stathopoulos E, Hatzidaki D, Tsakalaki E, Georgoulias V, Souglakos J (2009) Tumoral expression of TXR1 and TSP1 predicts overall survival of patients with lung adenocarcinoma treated with first-line docetaxel-gemcitabine regimen. Clin Cancer Res 15: 3827–3833 [DOI] [PubMed] [Google Scholar]
- Quinn JE, James CR, Stewart GE, Mulligan JM, White P, Chang GK, Mullan PB, Johnston PG, Wilson RH, Harkin DP (2007) BRCA1 mRNA expression levels predict for overall survival in ovarian cancer after chemotherapy. Clin Cancer Res 13: 7413–7420 [DOI] [PubMed] [Google Scholar]
- Quinn JE, Kennedy RD, Mullan PB, Gilmore PM, Carty M, Johnston PG, Harkin DP (2003) BRCA1 functions as a differential modulator of chemotherapy-induced apoptosis. Cancer Res 63: 6221–6228 [PubMed] [Google Scholar]
- Rosell R, Perez-Roca L, Sanchez JJ, Cobo M, Moran T, Chaib I, Provencio M, Domine M, Sala MA, Jimenez U, Diz P, Barneto I, Macias JA, de Las PR, Catot S, Isla D, Sanchez JM, Ibeas R, Lopez-Vivanco G, Oramas J, Mendez P, Reguart N, Blanco R, Taron M (2009) Customized treatment in non-small-cell lung cancer based on EGFR mutations and BRCA1 mRNA expression. PLoS One 4: e5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell R, Scagliotti G, Danenberg KD, Lord RV, Bepler G, Novello S, Cooc J, Crino L, Sanchez JJ, Taron M, Boni C, De MF, Tonato M, Marangolo M, Gozzelino F, Di CF, Rinaldi M, Salonga D, Stephens C (2003) Transcripts in pretreatment biopsies from a three-arm randomized trial in metastatic non-small-cell lung cancer. Oncogene 22: 3548–3553 [DOI] [PubMed] [Google Scholar]
- Rosell R, Skrzypski M, Jassem E, Taron M, Bartolucci R, Sanchez JJ, Mendez P, Chaib I, Perez-Roca L, Szymanowska A, Rzyman W, Puma F, Kobierska-Gulida G, Farabi R, Jassem J (2007) BRCA1: a novel prognostic factor in resected non-small-cell lung cancer. PLoS One 2: e1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346: 92–98 [DOI] [PubMed] [Google Scholar]
- Shaked Y, Bertolini F, Man S, Rogers MS, Cervi D, Foutz T, Rawn K, Voskas D, Dumont DJ, Ben-David Y, Lawler J, Henkin J, Huber J, Hicklin DJ, D'Amato RJ, Kerbel RS (2005) Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis; Implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell 7: 101–111 [DOI] [PubMed] [Google Scholar]
- Taron M, Rosell R, Felip E, Mendez P, Souglakos J, Ronco MS, Queralt C, Majo J, Sanchez JM, Sanchez JJ, Maestre J (2004) BRCA1 mRNA expression levels as an indicator of chemoresistance in lung cancer. Hum Mol Genet 13: 2443–2449 [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van GM, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216 [DOI] [PubMed] [Google Scholar]
- Wang L, Wei J, Qian X, Yin H, Zhao Y, Yu L, Wang T, Liu B (2008) ERCC1 and BRCA1 mRNA expression levels in metastatic malignant effusions is associated with chemosensitivity to cisplatin and/or docetaxel. BMC Cancer 8: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Sugio K, Ondo K, Yano T, Sugimachi K (2002) Reduced expression of thrombospondin-1 correlates with a poor prognosis in patients with non-small cell lung cancer. Lung Cancer 36: 143–150 [DOI] [PubMed] [Google Scholar]
- Yoo GH, Piechocki MP, Ensley JF, Nguyen T, Oliver J, Meng H, Kewson D, Shibuya TY, Lonardo F, Tainsky MA (2002) Docetaxel induced gene expression patterns in head and neck squamous cell carcinoma using cDNA microarray and PowerBlot. Clin Cancer Res 8: 3910–3921 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.