Abstract
The posterior parietal cortex (PPC) plays an integral role in visuospatial attention. Evidence suggests that neuronal activity in the PPC predicts the allocation of attention to stimuli. The present experiment tested the hypothesis that in rats performing a sustained attention task, the detection of signals, as opposed to missed signals, is associated with increased PPC unit activity. Single unit activity was recorded from the PPC of rats and analyzed individually and as a population vector for each recording session. A population of single units (28/111) showed significant activation evoked by signals on trials resulting in correct performance (hits). A smaller population of neurons (three/111) was activated on trials in which signals were not detected by the animals (misses). Analysis of populations of simultaneously recorded neurons indicated increased activation predicting signal detection; no population of neurons was activated on trials in which the animal incorrectly pressed the hit lever following nonsignals. The increased, hit-predicting activity was not modulated by signal duration or the presence of a visual distractor, although the distractor reduced the number of trials in which hit-predicting activity and subsequent correct detection occurred. These findings indicate that attentional signal processing in the PPC integrates successful detection of signals.
Keywords: attention, posterior parietal cortex, signal detection, multi-electrode physiology, neuronal ensemble
The posterior parietal cortex (PPC) is hypothesized to be part of the fronto-parietal network involved in the detection of signals. Detection can be defined as a cognitive process consisting of “… the entry of information concerning the presence of a signal into a system that allows the subject to report the existence of the signal by an arbitrary response indicated by the experimenter” (Posner et al., 1980). Evidence from patient populations with PPC brain injury indicated a role of the PPC for the reorienting of attention (Posner et al., 1984) and filtering out visual distractors (Friedman-Hill et al., 2003). Electrophysiological evidence from non-human primates suggested that the PPC executes a “matching function” between sensory input and the goal state of the subject, and has the function of shifting attention from one stimulus to another (Lynch et al., 1977; Robinson et al., 1995).
The PPC has been hypothesized to work with frontal and subcortical areas to direct spatial attention (Mesulam, 1981). Evidence from neuroimaging studies has consistently indicated activation of the PPC and prefrontal cortex (PFC) in tasks involving spatially directed attention (Corbetta et al., 1993; Coull et al., 1996; Fink et al., 1997; Gitelman et al., 1999; Lawrence et al., 2003; Lee et al., 2006). PFC and PPC contributions to attention are dissociable, however, when two response sets are competing for attentional resources. Bunge et al. (2002) have demonstrated that PPC is activated when target stimuli are flanked by distractors that either direct attention to the same (congruent) or opposite (incongruent) response as the target. The incongruent trials, in which the target and distractor cue opposing response sets, recruit PFC activation, suggesting that PFC may be recruited in resolving the conflict between these two stimulus–response associations. By contrast, both congruent and incongruent distractors recruited PPC activation, which suggests that PPC activation represents possible stimulus–response associations for further selection from the PFC. A recent study using repetitive transcranial magnetic stimulation indicated that transient disruption of human right hemisphere PPC impairs the top-down selection of targets in the left hemifield but enhances target selection in the right hemifield (Hung et al., 2005). It has been proposed that PPC provides top-down modulation of sensory areas that bias processing of attended items or locations, and both PPC and sensory areas are modulated by top-down control emanating from PFC (Miller and Cohen, 2001; Sarter et al., 2001).
Results from imaging and electrophysiological studies demonstrated that sensory parameters, such as target spatial location (Snyder, 2000), color (Rushworth et al., 2001), or preferred movement direction (Zhang and Barash, 2000) can fail to recruit PPC activity once subjects are instructed to redeploy attention toward new cues or locations (Pessoa et al., 2003). Recent evidence demonstrates that PPC involvement in attention is not entirely restricted to the spatial domain. Shomstein and Yantis (2004) demonstrated that attentional shifts from visual to auditory targets, and reciprocal shifts from auditory to visual targets, recruit PPC activation relative to a control task in which human subjects maintain attention in the current sensory stream. These investigators recently employed an auditory task to demonstrate that both spatial (i.e. left to right) and nonspatial (i.e. male to female voices) shifts in attention recruit the PPC, indicating that purely endogenous cues can activate the PPC (Shomstein and Yantis, 2006). Taken together, these results indicate that PPC subserves the shifting of attentional resources from the current stimulus–response association to a novel stimulus–response association, regardless of parameters such as response direction or the sensory properties of a target, although there are examples of exceptionally abrupt presentation of stimuli activating regions of PPC (Gottlieb et al., 1998; Powell and Goldberg, 2000).
Rats with PPC lesions exhibit impaired performance on a water maze task in which visual cues are required for navigation (Kolb and Walkey, 1987; Kolb et al., 1994; McDaniel et al., 1998). Furthermore, removal of cholinergic input to rat PPC impaired incremental conditioning, an effect that was interpreted as attentional (Bucci et al., 1998). Interestingly, unilateral PPC lesions of the rat failed to impair performance in an attentional task modeled after human attention tasks (Rosner and Mittleman, 1996), even though similar lesions produce both visual and auditory neglect (King and Corwin, 1993). The activity of parietal neurons in animals performing an attentional task has yet to be assessed, and a neurophysiological approach could provide further insight into the role of PPC in signal detection in rats. Using the sustained attention paradigm, this study tested the hypothesis that the firing rate of PPC neurons is selectively activated during accurate signal detection. Thus, it was expected that “hits” (correctly detected signals) would be associated with greater PPC unit activity than would other responses.
The maintenance of attentional performance under challenging conditions requires the optimization of input processing and the filtering of irrelevant stimuli (Kozak et al., 2006; Sarter et al., 2006). The presentation of visual distractors has been hypothesized to increase “background noise,” as reflected by impairments in attentional task performance. Bilateral removal of cholinergic input to the PPC impaired the performance on signal trials in the presence of a distractor; performance on nonsignal trials remained unaffected (Givens, unpublished observations). Furthermore, distractor-induced performance is associated with augmented increases in cortical acetylcholine (ACh) efflux (Himmelheber et al., 2001; Kozak et al., 2006), and cholinergically-mediated increases in prefrontal neuronal activity (Gill et al., 2000), which may reflect an involvement of cholinergic input to the PFC in suppressing the processing of these distractors. The present study investigated whether neural activity associated with the visual signal is influenced by a visual distractor. By testing the effects of two conditions within each session, one in which signal presentation is conducted in a relatively noise-free environment and one in which a visual distractor is present, this study was designed to investigate how different levels of attentional demand can affect stimulus processing in the PPC.
In addition to assessing the activity of single PPC neurons, the role of multiple neuronal encoding of signal detection was evaluated by means of population analysis techniques. The spiking activity of single units exhibits considerable variability, requiring multiple trials of an event for an experimenter to derive meaningful correlates. Principal component analysis (PC) is one method of extracting or decoding possible stimulus (Nicolelis et al., 1998; Matell et al., 2003), motor (Wessberg et al., 2000; Carmena et al., 2003, 2005; Cohen and Nicolelis, 2004; Santucci et al., 2005), or mnemonic (Deadwyler and Hampson, 2004) information from a neuronal ensemble with relatively few trials. In this experiment, we investigated the first principal component (PC1) from multiple, simultaneously recorded neurons in order to complement the standard analysis of the single units.
EXPERIMENTAL PROCEDURES
Subjects and apparatus
Male Long-Evans rats weighing 250–300 g at the start of the experiment (Harlan, Indianapolis, IN, USA) were housed singly in wire cages in a climate-controlled vivarium on a 12-h light/dark cycle (lights on at 6:30 a.m.). The rats received a water reinforcer during daily training sessions, and were allowed limited access to water in their home cages (5–20 min/day) following behavioral testing in order to maintain at least 85% of their unrestricted body weight with free access to food throughout the training. All animals were trained 5–7 days a week and received extra water supplement on non-training days. Animals were handled before and throughout training and testing. Animal care and experimentation were performed in accordance with protocols approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee and were in compliance with the NIH Guide for the Care and Use of Laboratory Animals. The minimum number of rats necessary to achieve the study aims was used in these experiments. Pain and distress to the animals was also minimized, but treated, if and when it did occur.
The rats were initially trained in standard operant chambers (28 cm length×21 cm width×27 cm height) that were located inside sound and light attenuating shells (64 cm×41 cm×41 cm; Med Associates, East Fairfield, VT, USA). The front panel of the operant chamber had three panel lights (2.8 W) centered 6 cm above three response levers, and a house light (2.8 W) located 5 cm above the center stimulus light. The response levers were located 7 cm above the grid floor. On the back panel were located a tone generator and a water dispenser that delivered one drop (60 μl) of water as a reinforcer into a recessed water port (5 cm width×3 cm depth×5 cm height). After reaching stable performance at a criterion level, the rats were transferred into a different operant chamber that was adapted for electrophysiological recording. The basic configuration of this chamber was the same as the training chamber except that it had taller walls that were made of plastic instead of metal. The water port was also bigger (6.5 cm×6 cm×13 cm) to allow room for the head-mounted recording equipment, and had installed an infrared emitter and detector at its entrance. The operant chamber was housed in a larger light and sound attenuating shell (59 cm×39 cm×54 cm).
Behavioral training
In an effort to better study the neural substrate of attention, the sustained attention task modeled after human vigilance paradigms (Parasuraman et al., 1987) was developed (McGaughy and Sarter, 1995; Bushnell, 1999). This task has previously demonstrated the necessity of the cholinergic input to the cortex in maintaining the detection of visual signals, whereas nonsignal performance remained unaffected (McGaughy et al., 1996; McGaughy and Sarter, 1999). Training in the sustained visual attention task was done in four stages. A house light remained illuminated during all phases of training. The rats were initially trained on a two lever-press paradigm with an FR-1 schedule of reinforcement. Animals were not rewarded for more than five consecutive presses on a single lever in order to prevent side bias. After the rats made at least 50 responses on each lever during a 1 h session for three consecutive days, the rats were trained in a sustained visual attention task.
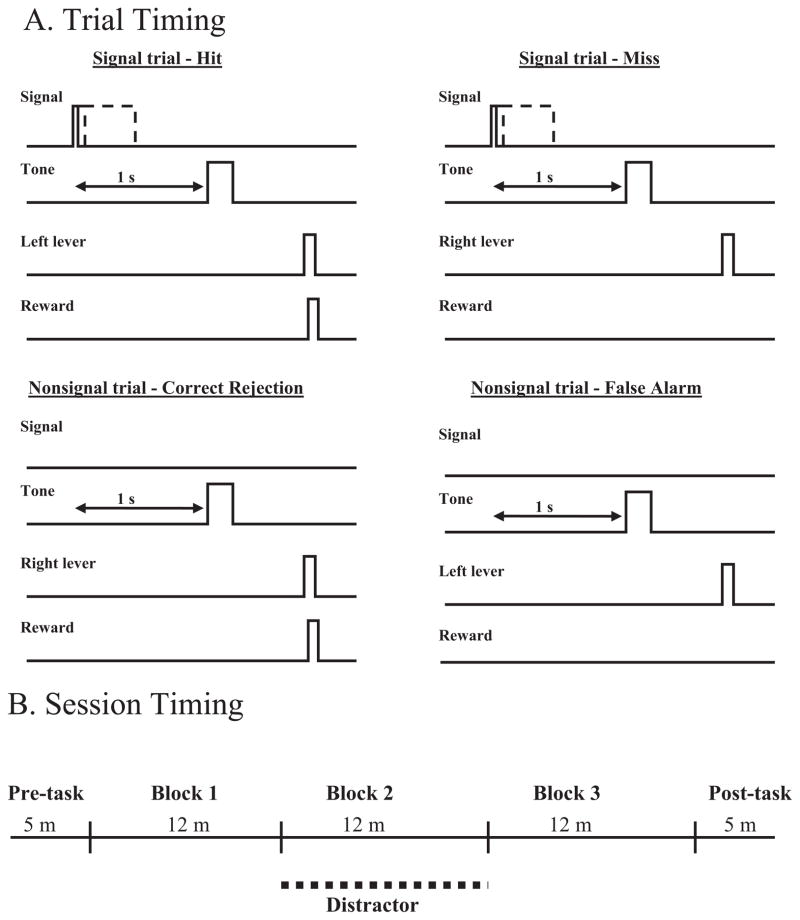
The rules of the sustained attention task were presented in the second stage (Fig. 1.) Animals were required to detect the presence of signal events (illumination of the central panel light for 500 ms) and correctly reject non-signal events (central panel light remained off). Both types of events were followed 1 s later by a 200 ms tone that initiated a 4 s response window. Following the presentation of the signal, a left lever press was considered a correct response, scored as a Hit (H). A right lever press on a signal trial was an incorrect response and recorded as a Miss (M). On non-signal trials, a right lever press was the correct response, scored as a correct rejection (CR), whereas a left lever press during non-signal trials was incorrect and scored as a false alarm (FA). Correct responses were followed by a water reward, and incorrect responses were not rewarded. If a response is not made within 4 s, the trial is scored as an omission. Either a response or an omission initiated a variable intertrial interval (12±3 s). At this stage of training, an incorrect response led to a correction trial in which the same type of trial was repeated for up to five consecutive times or until the rat made a correct response. Each behavioral training session included a 36 min task period, divided into three 12 min blocks of trials, that was both preceded and followed by 5 min, task-free periods. The criterion for performance was accuracy of 70% or higher on both signal and nonsignal trials with less than 30% omissions for three consecutive days.
Fig. 1.
Illustration of the response rules and recording epochs analyzed (A), and timeline of testing session (B). (A) Signals are illuminations of a panel light (25, 50, or 500 ms), which are followed 1 s later by tone (250 s), which opens the 4 s response window. For signal trials, left lever responses are rewarded (hits) whereas right lever responses are not (misses). Nonsignal trials have no signal light; a computer marker indicates the beginning of a trial, followed 1 s later by the tone opening the response window. Right lever responses following the tone are rewarded (correct rejections) and left lever responses are not (false alarms). A response or an omission (no response in 4 s) initiates a variable ITI (10±3 s) for the next trial. The 1 s interval prior to the tone on all trials (as represented by the double-sided arrow) is analyzed to test attention related activation of the PPC prior to a response. (B) During non-distractor blocks 1 and 3 the animal must detect visual signals and correctly reject nonsignals while a house light above the operant chamber remains continuously lit. During the second 12 min block of trials the house light flashes at 0.5 Hz, increasing the “background noise” in the task.
In the third stage, correction trials were removed and an approximately equal number of signal and nonsignal trials were presented for each 36 min session. The criterion for advancing to the final stage of training was accurate performance of 70% or higher on both signal and nonsignal trials with less than 30% omissions for three consecutive days.
During the final stage of training, the task was modified to further tax attention. Variations in signal duration and increases in the event rate have been shown to reduce attentional performance in humans (Parasuraman et al., 1987). Signals of three durations (25, 50, and 500 ms) were presented with nonsignal trials and the intertrial interval was reduced to 10±3s. Criterion was raised to 75% correct responses on both types of trials and less than 25% omissions. Each behavioral testing session included a 36 min task period, divided into three 12 min blocks of trials, that was both preceded and followed by 5 min, task-free periods.
After reaching criterion performance for three consecutive days, animals performed in a distractor session, which was identical to the final stage of training except that a distractor (house light flashing at 0.5 Hz) was presented during the second 12 min block of the task period. Previous studies using this task have demonstrated that this distractor impairs performance on signal and nonsignal trials (Gill et al., 2000). Each rat received at least three distractor-testing sessions that were separated by at least two baseline testing sessions. After the third distractor testing session, the rats were transferred to an operant chamber equipped for electrophysiological recording, and were required again to reach criterion levels of performance on the final training stage which was used for all recording sessions. All rats in this study required 3–6 months of training. The rats underwent surgery following one additional distractor session in this new environment.
Recording electrodes
Recording stereotrodes were prepared by immersing a pair of 20 μm tungsten wires (California Fine Wire, Grofer Beach, CA, USA) in epoxy and baking them at 200 °C for 1 h. Two stereotrodes were then inserted into a 30-ga cannula (18 mm) and extended 0.7–1.0 mm beyond the distal end of the cannula. The electrode guide cannula were attached to a moveable micro-drive that consisted of an insulated carrier that was tapped and fitted with threaded rods, which when turned lowered the electrodes through the brain during post-surgical testing. The two fine wires from each stereotrode were separated from each other on the proximal end of the guide cannula, stripped of insulation and wrapped around separate lead wires. The connection was covered with nickel print to ensure electrical conduction, and covered with epoxy in order to insulate and protect it. The impedance of the fine wires ranged from 50 to 300 kΩ. Polyethylene glycol was then heated to 60 °C and applied to the electrodes to prevent the bending of the stereotrodes as they entered the brain.
Electrode cannula implantation
Rats were anesthetized with a ketamine/xylazine cocktail (90 mg/kg and 10 mg/kg respectively). Isoflurane gas was used to supplement the anesthesia later in the surgery. Body heat was maintained at approximately 37 °C with a thermal pad (Deltaphase IV, Braintree, MA, USA). The electrodes were implanted unilaterally into the right or left PPC of two rats using the stereotaxic coordinates A/P −4.5 mm, M/L ±4.5 mm from bregma, and D/V −1.0 mm from the dura surface (Paxinos and Watson, 1998). Electrodes were also implanted bilaterally in four rats using the coordinates A/P −4.3 mm, M/L ±2.5 mm, and D/V −1.0 mm from the dura surface. One Teflon-coated, 250 μm, stainless steel electrode (A-M Systems, Everitt, WA, USA) was implanted into the left parietal cortex and served as a reference ground. A separate, similar electrode (250 μm) was wrapped around a machine screw on the skull surface and served as an animal ground. The ground, reference, and recording electrodes from the microdrive were epoxied to a plastic headstage connector. The microdrive, ground electrodes, and headstage connector were affixed to the skull with screws and dental cement. Lidocaine and antibiotics were applied to the wound immediately after surgery and twice daily for 3 days postoperatively. All rats received either cefadroxil (30 mg/kg) twice daily for 3 days postoperatively, or a single (0.5 ml) i.m. injection of amoxicillin during surgery. Rats were allowed one week to recover from surgery in their home cages with free access to food and water, after which access to water was gradually reduced before resumption of behavioral testing and neurophysiological recording.
Neurophysiological recording sessions
Electrical potentials were collected with a head-mounted operational amplifier and sent via a cable to a commutator that relayed the signal to four-channel differential amplifiers (A-M Systems). The analog signals were amplified (10,000×), bandpass-filtered (low pass, 300 Hz, high pass; 5 kHz), and digitized by an analog-to-digital board (DT2821, 250 kHz). Signals that exhibited peak amplitude that exceeded a user-defined threshold on either electrode of the stereotrodes were sampled at 25 kHz using Discovery software (Datawave Technologies, Longmont, CO, USA). Prior to each testing session, multiple unit activity on each stereotrode was separated into single units based on the clustering of signals on pair-wise plots between parameters extracted from the wave-form on both electrodes of the stereotrodes, including: peak amplitude, peak phase angle, valley amplitude, valley phase angle, peak to valley amplitude, and spike width.
The isolated single units were recorded during one baseline session and one distractor session. The microdrive was advanced after the presentation of a distractor testing session, in order to maximize the number of neurons recorded from a single rat. The software controlling the behavioral task sent a pulse to a clock board to signal each behaviorally relevant event (signal light, tone, house light, right and left lever presses during the response window and the ITI, water delivery, water port entry) with 0.1 ms precision. The behavioral software also sent pulses to the clock board to code for the start and end of each trial, for signal duration and non-signal events.
Histology
Within a week following the final electrophysiological recording session, animals were anesthetized with pentobarbital (50 mg/kg) and the final recording site was marked with a small electrolytic lesion (−15 μA for 30 s on each channel of the four stereotrodes) using a stimulator and isolation unit (Grass Instruments, Quincy, MA, USA). Animals were then given a supplemental injection of pentobarbital (100 mg/kg) and transcardially perfused with 0.9% cold saline followed by 10% buffered formalin. The brains were post-fixed for 24 h in 10% buffered formalin and transferred to a 30% sucrose solution in 0.1 M phosphate buffer, pH 7.4. Each brain was sliced into 50 μm sections around the recording site, mounted, stained for Nissl substance (Cresyl Violet), and examined for electrode placements.
Behavioral measures
The behavioral measures generated for statistical analysis included response accuracy on signal trials [hits/(hits+misses)] and on nonsignal trials [correct rejection/(correct rejections+false alarms)], response latency (latency from tone onset to lever press for each type of response), total errors of omission (omits/total trials), errors of omission on signal (signal trial omits/total signal trials) and nonsignal (nonsignal trial omits/total nonsignal trials) trials, and response lever side bias [(hits+false alarms)/(total # of responses)]. The behavioral measures were generated from both distractor testing sessions and baseline sessions. Baseline testing sessions with >40% overall omissions or distractor sessions with more than 50% omissions within the distractor block were excluded from the statistical analyses.
Percentage data were angularly transformed (Zar, 1996) before analysis to correct for the skewed distribution of percentage scores. Repeated-measures ANOVAs were conducted on the behavioral data using testing session (baseline and distractor) and trial blocks (1–3) as within subjects factors for the dependent measures of signal response accuracy, nonsignal response accuracy, errors of omission, and reaction time on signal and nonsignal trials, as well as response lever side bias. The repeated-measures ANOVAs on the behavioral data from signal trials also included signal duration (25, 50, and 500 ms) as an additional within-subjects factor when analyzing the dependent measures of response accuracy, errors of omission, and response latency. A Huyn-Feldt correction was applied to all repeated measures to control for possible violations of the sphericity assumption of homogeneity of variances, (Vasey and Thayer, 1987). A significance level of α = 0.01 was used in all analyses.
Neurophysiological measures
Single units exhibiting more than 300 total spikes during the recording sessions in which the behavioral performance met the criteria were used for the electrophysiological analysis. The mean firing rate for each single unit was calculated for the entire session and for each of the 5 min pre- and post-task periods, and for each 12 min block of trials. Analysis was based on the changes in the firing rate of single neurons, as assessed by peri-event time histograms (PETH), were calculated for each single unit.
Ensemble analysis
Since the firing rate of single neurons in vivo typically ranges between 1 and 5 Hz (Markus et al., 1995) several trials of a task are required for proper analysis of behavioral correlates of single unit data. When few trials are recorded in a session (e.g. misses following the 500 ms signal in the current data set), the activity of the population of all simultaneously recorded neurons from a session may better encode the detection of stimuli or other task parameters better than any single neuron. PC is an approach that can provide insight into how information may be encoded in simultaneously recorded neuronal populations (Nicolelis et al., 1999).
The number of spike trains recorded from multiple neurons in a 46 min task constitutes a large data set with high dimensionality. Briefly, PC is a linear transformation that reduces high dimensional data into principal component features representing a majority of the variance in the data set (Chapin and Nicolelis, 1999). A population of neurons is analyzed as an i×j matrix, in which i is the number of neurons and j is the number of 20 ms recording bins for each neuron. A correlation matrix was then produced from the Pearson correlation coefficient between vectors j and i. The eigenvalues and eigenvectors are calculated from this correlation vector, the PC1 representing the first eigenvector, which accounts for the greatest amount of variance in the data set. The remaining data matrix is rotated around the PC1 axis to find the second principal component, which has the greatest possible remaining variance in the matrix. This process is repeated until a set of orthogonal vectors is produced equaling the number of original neurons entered. The first few principal components contain most of the significant total variance, and the PC1 is thought to contain the magnitude of the variance, which has been sufficient for extracting motor commands from motor cortex (Chapin et al., 1999). Since none of the original information is lost in this transformation, the PC1 can be analyzed using standard statistical techniques.
Statistical analysis of single unit and ensemble activity
The firing rate of single units and ensembles was summed in 20 ms bins and PETHs were constructed for hits, misses, correct rejections, and false alarms. PETHs were constructed at each signal duration on hit and miss trials, and the modulatory effect of the visual distractor was tested by comparing pre-signal to post-signal activity in baseline conditions (the first and third block of trials for each testing session) and in the presence of the visual distractor (the second block of trials).
The spiking activity (spikes/s) of each single unit and PC1 neuronal ensemble was analyzed at each trial type (hits, misses, false alarms, correct rejections) using paired samples t-tests to compare the firing rate in the 1 s span following the signal to the firing rate of the 1 s span in the ITI prior to the trial onset. Additional paired samples t-tests investigated the effects of signal duration and visual distractor on neuronal correlates.
RESULTS
Behavioral effects of signal duration and distractor
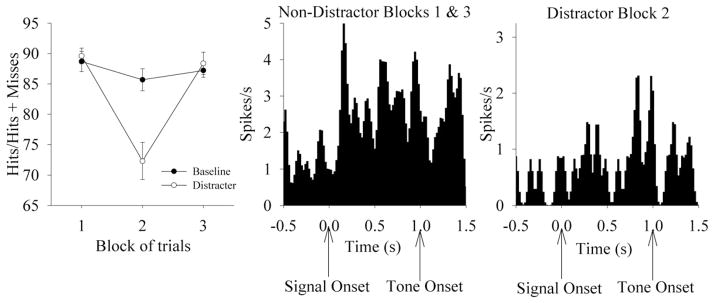
The detection of brief visual signals during attentional testing was signal duration-dependent (F2,10 = 74.05, P<0.01), with an increased relative number of hits occurring across increased signal duration. The presentation of a visual distractor (flashing house light at 0.5 Hz during trial block 2) decreased the relative number of hits and correct rejections (Fig. 5), as indicated by a Distractor×Block interaction on performance for signal and nonsignal trials [Signal: Distractor; (F1,5 = 8.33, P<0.01); Block; (F2,10 = 10.77, P<0.01)]. One-way ANOVAs revealed that signal and nonsignal performance was diminished only during the distractor blocks (F2,10 = 15.29, P<0.01). Distractor sessions do not affect omissions, nor was there a significant interaction between Distractor and Block on omissions. Reaction time was shortest for hits (hits 0.86±0.11 s), but reaction time for hits was not significantly shorter than for correct rejections (Correct Rejections 1.12±0.12 s; F1,5 = 6.34; P>0.01). Reaction time for hits was significantly shorter than reaction time for misses (Misses 1.30±0.10 s; F1,5 = 18.32; P<0.01). Reaction time for correct rejections was not significantly different than reaction time for false alarms (False alarms 1.24 ± 0.09 s; F1,5 = 1.90; P>0.01).
Fig. 5.
Behavioral performance and neurophysiological responses in the presence and absence of a visual distractor. Left: Sustained attention performance across blocks of trials during Baseline and testing conditions, as expressed in relative number of hits (mean±S.E.M.) across trial blocks under baseline and distractor testing conditions. Middle. Detection-associated activity of a single neuron during the first and third (non-distractor) blocks of trials (40 hit trials, 89% accuracy). The firing rate of this unit increases following the signal on hit trials. Format is the same as Fig. 3. Right: Detection-associated activity of this neuron following the signal in the presence of the visual distractor (19 hit trials, 70% accuracy).
Detection-related PPC single unit and ensemble activity
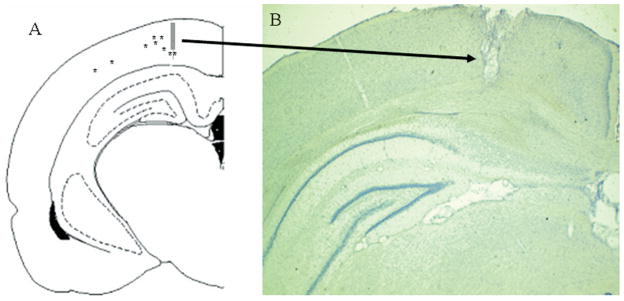
For all six of the animals included in the statistical analyses electrode placements were within the deep layers of the PPC, as illustrated in Fig. 2. All placements were 2.5–4.3 mm lateral from midline and 4.5 mm posterior to bregma. During each recording session, three to 14 units were isolated from the multiple unit activity recorded during distractor testing sessions. The mean spontaneous firing rate (±S.E.M.) of PPC units was 0.77±0.10 spikes/s, with a range from 0.077–7.08 spikes/s. Paired t-tests indicated that 28 of the 111 neurons (25%) collected displayed an increase in unit activity following the presentation of the signal relative to activity in the ITI (using a threshold of t49 = 2.80, P<0.01). Smaller, separate populations were activated on miss (three/111), correct rejection (seven/111), and false alarm (11/111) trials. Neuronal activation on hit trials was highest (as measured by spikes/s) at 660 ms after the onset of the cue, and peak activation was >600% of the mean firing rate (mean pre-signal firing rate: 1.49 spikes/s, peak firing rate following signal on hit trials: 9.86 spikes/s). Neural activity was at or below baseline firing rate during the response. The latency of this activation is similar to that of PPC neurons in primates interpreted to represent top-down evaluation of a signal (Zhang and Barash, 2004). Analysis of these neurons confirmed that their activity was not significantly different from baseline on miss trials. Unit activity in this population was not significantly modulated on correct rejections or false alarms for these neurons (all below t49 = 2.80, P>0.01).
Fig. 2.
Illustration and example of the final recording sites for moveable stereotrodes into the PPC. (A) A schematic illustration of the final placement of the moveable electrodes, which extended 1.0 mm beyond the guide cannula and were located at AP −4.5, within ML ±2.5–4.0, and DV 1.0. (B) An example showing the final placement of electrode and cannula. The electrolytic lesion and tract produced by the electrode cannula are readily visible (arrow).
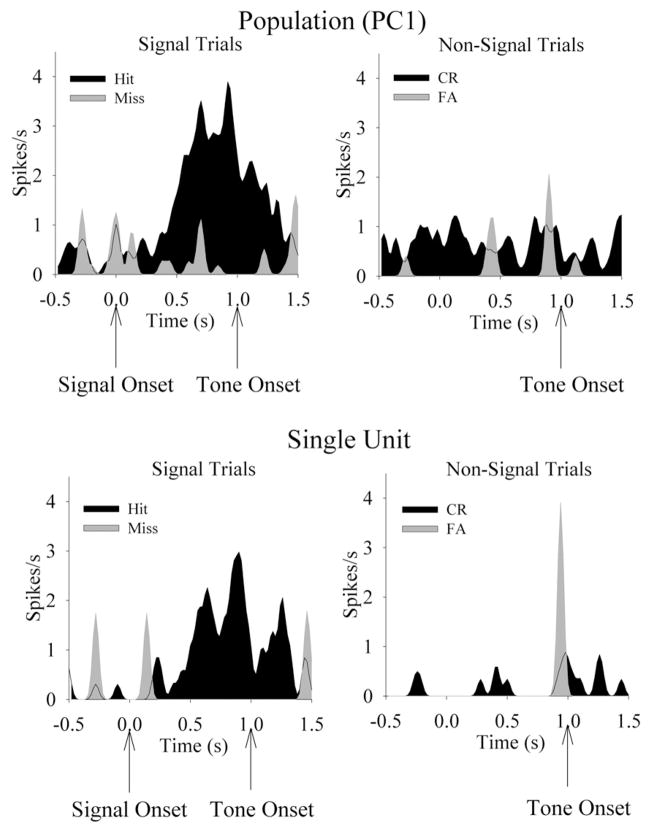
Neuronal population vectors were constructed and their activity compared from before and after the presentation of the signal trials or nonsignal references. Eleven of 20 vectors (55%) significantly increased activation following the signal on hit trials (t49 = 2.80, P<0.01, see Fig. 3). Interestingly, some populations of neurons, in which no single neuron was significantly activated, were still able to produce a robust signal-induced activation following the principal component transformation. The latency of peak activation for the population vectors was similar to that of single neurons (620 ms), as well as inhibition during the response phase of the task. Only one of 20 (5%) vectors showed significant increases in the firing rate following the signal on miss trials (t49 > 2.80, P<0.01). On nonsignal trials, two/20 vectors (10%) increased in activity following the presentation of the nonsignal on correct rejection trials (t49 > 2.80, P<0.01). No vectors showed modulation on false alarms trials, and no population vectors showed significant decreases in firing rate activity during task performance.
Fig. 3.
Detection of visual signals activates single units and the PC1 of PPC neuronal populations. The PETHs (20 ms/bin) are from a linear population vector constructed from 11 simultaneously recorded PPC neurons from the same rat. Top left: The presentation of the visual signal elicits a significant increase in firing rate from this population on trials in which the rat performs a hit response, yet signal fails to modulate the firing rate on trials in which the rat performs a miss response. Top right: Activity during the same epoch during nonsignal trials indicates no change in firing rate for this population. Bottom left: A single unit from the same population of simultaneously recorded neurons shows significant detection-associated activation prior to hit, but not miss, responses. Bottom right: This unit is not modulated during nonsignal trials.
Effects of signal duration on unit and population activity
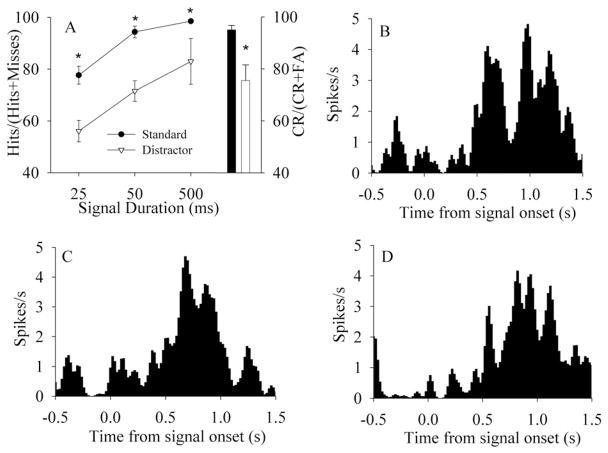
Analysis of hit trials by each signal duration indicates that 53% (15/28) of single units were activated following 25 ms signals, 67% (19/28) of neurons were activated by the 50 ms signal, and 61% (17/28) of neurons were activated by the 500 ms signal (t49 > 2.80, P<0.01). Population vectors demonstrated detection-associated activity for 25 ms signals (found in six of nine sessions), 50 ms signals (eight of nine sessions), and 500 ms signals (seven of nine sessions) (all t49 > 2.80, P<0.01). Varying signal durations did not change the response profile of PPC activity, suggesting that rat PPC activity does not encode stimulus parameters per se, but rather reflects the detection of signals and perhaps the initiation of a response plan. Analysis of the effects of signal duration on the detection-related activation of neuronal populations indicated that detection-associated activation was independent of signal duration. Short (25 ms) signals activated seven of nine neuronal populations prior to hit performance, medium (50 ms) signals activated eight of nine neuronal populations prior to hit performance, and long signals (500 ms) activated seven of nine neuronal populations (Fig. 4). Analysis of the activity of the parietal population indicates no significant modulation of neural activity to the onset or offset of the visual distractor (all P>0.10, paired samples t-test).
Fig. 4.
Behavioral performance and neurophysiological responses at different signal durations. (A) Signal duration dependent performance during testing sessions in the presence (white triangles) and absence (black circles) of a distractor. The relative number of hits (mean±S.E.M.) and correct rejections (mean±S.E.M.) also indicate a main effect of distractor on performance on signal and nonsignal trials. The black bar indicates nonsignal performance on baseline trials; the white bar indicates nonsignal performance in the presence of a distractor. (B–D) The PC1 of a population vector of 12 simultaneously recorded PPC neurons from the same task-performing rat during hit trials. The onset of the signal light occurs at 0.0, followed 1 s later by the onset of the tone allowing the rat to respond. (B) Detection-associated excitatory activity of this population following 25 ms signals. (C) Detection-associated excitatory activity of this population following the presentation of 50 ms signals. (D) Detection-associated excitatory activity following the presentation of 500 ms signals.
Effects of distractor on unit and population activity
Neither the onset nor the offset of the house light elicited a response in unit (t49 < 2.80, P>0.01) or population activity (t49 < 2.80, P>0.01). As stated in the behavioral results, the visual distractor impaired the ability of rats to detect the signal, resulting in a greater number of misses. The distractor reduced the relative number of trials that activate the PPC, and reduced the relative number of hit trials. For correctly detected signals, detection-related activity was found in all three blocks of the testing session, in both the presence and absence of the visual distractor (Fig. 5). This was the case for the activity recorded from single units and population vectors. Specifically, 23 of the 28 detection-related neurons (82%) were activated in the first, non-distractor block of trials, 16 of 28 neurons (57%) showed detection-related activation in the presence of the distractor in the second block of trials. The persistence of the signal detection-associated activation of PPC neurons in the presence and absence of the visual distractor suggests that task-relevant stimuli which are detected by the rat activate PPC neurons, possibly initiating a specific response plan among alternatives.
DISCUSSION
The aim of this study was to assess the role of rat PPC in sustained attention performance. The observation that both single neurons and the PC1 of neural ensembles from the PPC increased neural activity following signal presentation (but not nonsignal trials) is further evidence of the role of PPC in the signal detection process. Furthermore, and importantly, this increase was primarily observed when the rat made a correct “hit” response. PPC activation following signal presentation on “hit” trials did not reflect the duration of the signal. Although the correct detection of signals was a function of signal duration, the detection of shorter signals evoked unit activity similar to longer signals. Both single neurons and the PC1 of a simultaneously recorded population exhibited signal detection-associated activation.
The present data support the hypothesis that attention-demanding stimuli which require a correct operant response recruit PPC neuronal activity. This detection-associated activity continued in the presence of the visual distractor for a majority of single unit and population vector correlates. Activation of a small group of neurons was found on trials in which the animal produces a false alarm (incorrect detection of a signal during a nonsignal trial). This false alarm activity may reflect a false detection of a signal and thus the animal responded as if a signal had been presented. Alternatively, this result may reflect the stochastic nature of spiking neurons with few trials (<10), as analysis of the PC1 of neuronal ensembles revealed no activation during false alarm trials. The onset and offset of the visual distractor itself did not elicit activation of PPC neurons. Thus, these neurons do not simply respond to a visual stimulus, because they fail to modulate activity in response to the visual distractor light, and they fail to activate on Miss trials, in which a visual stimulus is presented that is identical to those presented on Hit trials. Rather, these neurons respond selectively to visual signals that are both behaviorally-relevant and correctly-detected.
Accurate performance in the sustained attention task is directly related to signal duration. Increased unit activity in the pre-response period is an indicator of accurate performance. The unit activity for detected signals is similar for different durations, suggesting that weaker signals are less likely to recruit the PPC, resulting in a lower rate of detection. This finding indicates that the detection of the visual stimuli is the most salient feature activating neuronal responses in the PPC, and not the properties of the stimulus, which supports the hypothesis that the parietal cortex is involved in maintaining behaviorally-relevant signals for attentional processing necessary for a response (Bunge et al., 2002). The lack of differences in unit activity between signals of different duration indicates that the stimulus properties per se are not encoded in the PPC, but that only those signals which activate the attentional network drive unit and PC1 activity.
The visual distractor failed to elicit a response from the detection-associated neurons, despite being in the same modality as the signal. One interpretation of this finding is that the distractor light itself is not processed discretely as a signal but rather truly contributes to the “background noise” in the task (Sarter et al., 2001). This is consistent with the behavioral data in which there is not only an increase in false alarms, but also an increase in misses. In humans, the PPC has been shown to be involved in the discrimination of target stimuli in the presence of flanking distracters (Friedman-Hill et al., 2003). The finding that signal-related PPC activation remains intact in the presence of a distractor for a majority of this population of neurons, and the lack of an effect of the visual distractor, correspond with the hypothesis that top-down suppression of irrelevant stimuli is necessary for task performance (Bentley et al., 2003; Friedman-Hill et al., 2003).
Cortical activation of PPC during the redirection of attention
Early electrophysiological work in primates suggested that the PPC was involved in sensorimotor integration (Mountcastle et al., 1974; Lynch et al., 1977). Recent work has defined more precisely the roles of regions within the PPC. Multiple representations of space (e.g. retinotopic and somatotopic) are hypothesized to be represented within different regions of primate PPC (Colby and Goldberg, 1999). The abrupt onset of visual stimuli not required for task performance activates certain PPC neurons, indicating bottom up recruitment of the PPC allocating attention to the stimulus (Gottlieb et al., 1998; Bisley et al., 2004). There is also considerable evidence that regions of PPC initiate response plans specific to eye or limb movements (Scherberger et al., 2005). Interestingly, other studies have shown that PPC activation can have both a short-latency activation followed by long-latency activation (Zhang and Barash, 2000), indicating that both exogenously recruited and endogenously deployed attention may be represented by activity within the same PPC neuron. In the current study, latency of peak population activity following the signal was as early as 130 ms, which may be representing stimulus-driven activity. However, many of the single neurons and neuronal populations showed peak activation ~600 ms, which is more consistent with evidence indicating that PPC activation represents top down deployment of attentional resources.
Results from this study may help to explain some of seemingly contradictory results from previous experiments investigating rat PPC in attention. It has been posited that the PPC is essential in processing multimodal information related to attention and spatial orientation (Corwin and Reep, 1998). Lesions of rat PPC result in spatial deficits in allocentric, but not egocentric, maze performance, that is, rats were impaired at using distal cues to navigate through space (Kolb and Walkey, 1987). Unilateral lesions of rat PPC also produce contralateral neglect, an effect that is transient for auditory and tactile cues but persists with visual cues (King and Corwin, 1993). However, in a task more specifically designed to assess reaction time to valid and invalid cues, PPC lesions have no effect (Rosner and Mittleman, 1996). Similarly, both excitotoxic lesion of PPC (Ward and Brown, 1997) and removal of PPC cholinergic input (Muir et al., 1996), failed to disrupt attentional performance in reaction time tasks. These discrepancies may be explained in part by the fact that tasks which exclusively present signal trials only require one stimulus–response association, whereas the PPC may be required in tasks in which a subject must switch from one association to another based on how well it can monitor incoming stimuli (Bunge et al., 2002; Sarter et al., 2005). As mentioned in the introduction, evidence from human neuroimaging experiments suggests that it is not the detection of stimuli but the shifting of the allocation of attentional resources that activates parietal regions (Rushworth et al., 2001; Yantis et al., 2002; Shomstein and Yantis, 2004, 2006). The allocation of attentional resources may result in a covert shift (Posner et al., 1984, 1987) or an overt response plan (Kalaska, 1996; Andersen et al., 1997; Snyder et al., 1998; Bunge et al., 2001; Buneo et al., 2002). The detection-associated PPC neuronal activity from the present study is evidence for a role of rat PPC in using visual cues to direct a specific response among alternatives.
Functional implications
The PPC is an integral part of a larger distributed neuronal network mediating attentional function (Mountcastle et al., 1974; Mesulam, 1981). In primates, PPC neuronal activation can result from the sudden onset of a target stimulus (Gottlieb et al., 1998), or can reflect top-down activation required for more effortful attentional processing (Zhang and Barash, 2000, 2004). The present study indicates that visual signals requiring an operant response will recruit PPC neuronal activation, and this effect is not found for a visual distractor. The distractor did reduce the number of correctly detected signals, however, with a corresponding loss of signal-related activation of PPC neurons. Medial prefrontal (PFC) cholinergic inputs mediate maintenance of task performance in the presence of this visual distractor (Gill et al., 2000; Sarter et al., 2001). Glutamatergic and cholinergic activation of the PFC influences PPC regulation of ACh release, suggesting a possible mechanism by which PFC can exert “top-down” control of attention (Nelson et al., 2005; Sarter et al., 2005, 2006). Future research will be directed toward dissociating the contributions of PFC afferent input to the PPC during attentional performance. We are currently exploring these hypotheses in experiments in which PPC neuronal activity is recorded from animals performing attentional tasks as the integrity of the cholinergic input to the PFC is challenged.
Acknowledgments
This research was supported by PHS grants NS37026 and KO2MH01072 (M.S.).
Abbreviations
- ACh
acetylcholine
- PC
principal component analysis
- PC1
first principal component
- PETH
peri-event time histograms
- PFC
prefrontal cortex
- PPC
posterior parietal cortex
References
- Andersen RA, Snyder L, Bradley DC, Xing J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci. 1997;20:303–330. doi: 10.1146/annurev.neuro.20.1.303. [DOI] [PubMed] [Google Scholar]
- Bentley P, Vuilleumier P, Theil CM, Driver J, Dolan R. Cholinergic enhancement modulates neural correlates of selective attention and emotional processing. Neuroimage. 2003;20:58–70. doi: 10.1016/s1053-8119(03)00302-1. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Krishna BS, Goldberg ME. A rapid and precise on-response in posterior parietal cortex. J Neurosci. 2004;24:1833–1838. doi: 10.1523/JNEUROSCI.5007-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci DJ, Holland PC, Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J Neurosci. 1998;18:8038–8046. doi: 10.1523/JNEUROSCI.18-19-08038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buneo CA, Jarvis MR, Batista AP, Andersen RA. Direct visuomotor transformations for reaching. Nature. 2002;416:632–636. doi: 10.1038/416632a. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JDE. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JDE. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage. 2002;17:1562–1571. doi: 10.1006/nimg.2002.1252. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ. Detection of visual signals by rats: effects of signal intensity, event rate, and task type. Behav Process. 1999;46:141–150. doi: 10.1016/s0376-6357(99)00030-3. [DOI] [PubMed] [Google Scholar]
- Carmena JM, Lebedev MA, Henriquez CS, Nicolelis MAL. Stable ensemble performance with single-neuron variability during reaching movements in primates. J Neurosci. 2005;25:10712–10716. doi: 10.1523/JNEUROSCI.2772-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena JM, Lebedev MA, Crist R, O’Doherty JE, Santucci DM, Dimitrov DF, Patil PG, Henriquez CS, Nicolelis MAL. Learning to control a brain-machine interface for reaching and grasping by primates. PLoS Biol. 2003;1:e2. doi: 10.1371/journal.pbio.0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin JK, Nicolelis MAL. Principal component analysis of neuronal ensemble activity reveals multidimensional somatosensory representations. J Neurosci Methods. 1999;94:121–140. doi: 10.1016/s0165-0270(99)00130-2. [DOI] [PubMed] [Google Scholar]
- Chapin JK, Moxon KA, Markowitz RS, Nicolelis MAL. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat Neurosci. 1999;2:664–670. doi: 10.1038/10223. [DOI] [PubMed] [Google Scholar]
- Cohen D, Nicolelis MAL. Reduction of single-neuron firing uncertainty by cortical ensembles during motor skill learning. J Neurosci. 2004;24:3574–3582. doi: 10.1523/JNEUROSCI.5361-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci. 1999;22:319–349. doi: 10.1146/annurev.neuro.22.1.319. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Peterson SE. A PET study of visuospatial attention. J Neurosci. 1993;13:1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JV, Reep RL. Rodent posterior parietal cortex as a component of a cortical network mediated directed spatial attention. Psychobiology. 1998;26:87–102. [Google Scholar]
- Coull JT, Frith CD, Frackowiak RSJ, Grasby PM. A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia. 1996;34:1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Hampson RE. Differential but complementary mnemonic functions of the hippocampus and subiculum. Neuron. 2004;42:465–476. doi: 10.1016/s0896-6273(04)00195-3. [DOI] [PubMed] [Google Scholar]
- Fink G, Halligan PW, Marshall JC, Frith CD, Frackowiak RSJ, Dolan R. Neural mechanisms involved in the processing of global and local aspects of hierarchically organized visual stimuli. Brain. 1997;120:1779–1791. doi: 10.1093/brain/120.10.1779. [DOI] [PubMed] [Google Scholar]
- Friedman-Hill SR, Robertson LC, Desimone R, Ungerleider LG. Posterior parietal cortex and the filtering of distractors. Proc Nat Acad Sci U S A. 2003;100:4263–4268. doi: 10.1073/pnas.0730772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill TM, Sarter M, Givens B. Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. J Neurosci. 2000;20:4745–4757. doi: 10.1523/JNEUROSCI.20-12-04745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim Y-H, Meyer JR, Mesulam M-M. A large-scale distributed network for covert spatial attention. Brain. 1999;122:1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Kusunoki M, Goldberg ME. The representation of visual salience in monkey parietal cortex. Nature. 1998;391:481–484. doi: 10.1038/35135. [DOI] [PubMed] [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP. The effects of manipulations of attentional demand on cortical acetylcholine release. Cogn Brain Res. 2001;12:353–370. doi: 10.1016/s0926-6410(01)00064-7. [DOI] [PubMed] [Google Scholar]
- Hung J, Driver J, Walsh V. Visual selection and posterior parietal cortex: effects of repetitive transcranial magnetic stimulation on partial report analyzed by Bundesen’s theory of visual attention. J Neurosci. 2005;25:9602–9612. doi: 10.1523/JNEUROSCI.0879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaska JF. Parietal cortex area 5 and visuomotor behavior. Can J Physiol Pharmacol. 1996;74:483–498. [PubMed] [Google Scholar]
- King V, Corwin JV. Comparisons of hemi-inattention produced by unilateral lesions of the posterior parietal cortex or medial agranular prefrontal cortex in rats: neglect, extinction, and the role of stimulus distance. Behav Brain Res. 1993;54:117–131. doi: 10.1016/0166-4328(93)90070-7. [DOI] [PubMed] [Google Scholar]
- Kolb B, Walkey J. Behavioural and anatomical studies of the posterior parietal cortex in the rat. Behav Brain Res. 1987;23:127–145. doi: 10.1016/0166-4328(87)90050-7. [DOI] [PubMed] [Google Scholar]
- Kolb B, Buhrmann K, McDonald R, Sutherland RJ. Dissociation of the medial prefrontal, posterior parietal, and posterior temporal cortex for spatial navigation and recognition memory in the rat. Cereb Cortex. 1994;4:664–680. doi: 10.1093/cercor/4.6.664. [DOI] [PubMed] [Google Scholar]
- Kozak R, Bruno JP, Sarter M. Augmented prefrontal acetylcholine release during challenged attentional performance. Cereb Cortex. 2006;16:9–17. doi: 10.1093/cercor/bhi079. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15:1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- Lee KH, Choi YY, Gray JR, Cho SH, Chae J-H, Lee S, Kim K. Neural correlates of superior intelligence: stronger recruitment of posterior parietal cortex. Neuroimage. 2006;29:578–586. doi: 10.1016/j.neuroimage.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Lynch JC, Mountcastle VB, Talbot WH, Yin TCT. Parietal lobe mechanisms for directed visual attention. J Neurophysiol. 1977;40:362–389. doi: 10.1152/jn.1977.40.2.362. [DOI] [PubMed] [Google Scholar]
- Markus E, Qin Y, Leonard B, Skaggs W, McNaughton B, Barnes C. Interactions between location and task affect the spatial and directional firing of hippocampal neurons. J Neurosci. 1995;15:7079–7094. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matell MS, Meck WH, Nicolelis MAL. Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behav Neurosci. 2003;117:760–773. doi: 10.1037/0735-7044.117.4.760. [DOI] [PubMed] [Google Scholar]
- McDaniel WF, Williams LB, Cullen MA, Compton DM. Turn-signal utilization by rats with either unilateral or bilateral posterior parietal cortex injuries. Psychobiology. 1998;26:143–152. [Google Scholar]
- McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine and benzodiazepine receptor ligands. Psychopharmacology. 1995;117:340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Effects of ovariectomy, 192 IgG-saporin-induced cortical cholinergic deafferentation, and administration of estradiol on sustained attention performance in rats. Behav Neurosci. 1999;113:1216–1232. doi: 10.1037//0735-7044.113.6.1216. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110:247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J Neurophysiol. 1974;38:871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex. 1996;6:470–480. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Nelson C, Sarter M, Bruno J. Prefrontal cortical modulation of acetylcholine release in posterior parietal cortex. Neuroscience. 2005;132:347–359. doi: 10.1016/j.neuroscience.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL, Stambaugh CR, Brisben A, Laubach M. Methods for simultaneous multisite neural ensemble recordings. In: Nicolelis MAL, editor. Methods for neural ensemble recordings. New York: CRC Press; 1999. pp. 193–212. [Google Scholar]
- Nicolelis MAL, Ghazanfar AA, Stambaugh CR, Oliveira LM, Laubach M, Chapin JK, Nelson RJ, Kaas JH. Simultaneous encoding of tactile information by three primate cortical areas. Nat Neurosci. 1998;1:621–630. doi: 10.1038/2855. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Warm JS, Dember WN. Vigilance: taxonomy and utility. In: Mark LS, Warm JS, Huston RL, editors. Ergonomics and human factors: recent research. New York: Springer Verlag; 1987. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Neuroimaging studies of attention: from modulation of sensory processing to top-down control. J Neurosci. 2003;23:3990–3998. doi: 10.1523/JNEUROSCI.23-10-03990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Snyder C, Davidson B. Attention and detection of signals. J Exp Psychol. 1980;109:160–174. [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FA, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FA, Rafal RD. How do the parietal lobes direct covert attention? Neuropsychologia. 1987;25:135–145. doi: 10.1016/0028-3932(87)90049-2. [DOI] [PubMed] [Google Scholar]
- Powell KD, Goldberg ME. Response of neurons in the lateral intraparietal area to a distractor flashed during the delay period of a memory-guided saccade. J Neurophysiol. 2000;84:301–310. doi: 10.1152/jn.2000.84.1.301. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Bowman EM, Kertzman C. Covert orienting of attention in macaques. II. Contributions of parietal cortex. J Neurophysiol. 1995;74:698–712. doi: 10.1152/jn.1995.74.2.698. [DOI] [PubMed] [Google Scholar]
- Rosner AL, Mittleman G. Visuospatial attention in the rat and posterior parietal cortex lesions. Behav Brain Res. 1996;79:69–77. doi: 10.1016/0166-4328(95)00263-4. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Paus T, Sipila PK. Attention systems and the organization of the human parietal cortex. J Neurosci. 2001;21:5262–5271. doi: 10.1523/JNEUROSCI.21-14-05262.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci DM, Kralik JD, Lebedev MA, Nicolelis MAL. Frontal and parietal cortical ensembles predict single-trial muscle activity during reaching movements in primates. Eur J Neurosci. 2005;22:1529. doi: 10.1111/j.1460-9568.2005.04320.x. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: The neurobiology of attentional effort. Brain Res Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Scherberger H, Jarvis MR, Andersen RA. Cortical local field potential encodes movement intentions in the posterior parietal cortex. Neuron. 2005;46:347–354. doi: 10.1016/j.neuron.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Shomstein S, Yantis S. Control of attention shifts between vision and audition in human cortex. J Neurosci. 2004;24:10702–10706. doi: 10.1523/JNEUROSCI.2939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomstein S, Yantis S. Parietal cortex mediates voluntary control of spatial and nonspatial auditory attention. J Neurosci. 2006;26:435–439. doi: 10.1523/JNEUROSCI.4408-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder L. Moving forward by looking away. Nature. 2000;408:921–923. doi: 10.1038/35050192. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Change in motor plan, without a change in the spatial locus of attention, modulates activity in posterior parietal cortex. J Neurophysiol. 1998;79:2814–2819. doi: 10.1152/jn.1998.79.5.2814. [DOI] [PubMed] [Google Scholar]
- Vasey M, Thayer J. The continuing problem of false positives in repeated measures ANOVA in psychology: a multivariate solution. Psychophysiology. 1987;24:479–486. doi: 10.1111/j.1469-8986.1987.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Ward NM, Brown VJ. Deficits in response initiation, but not attention, following excitotoxic lesions of posterior parietal cortex in the rat. Brain Res. 1997;775:81–90. doi: 10.1016/s0006-8993(97)00915-3. [DOI] [PubMed] [Google Scholar]
- Wessberg J, Stambaugh CR, Kralik JD, Beck PD, Laubach M, Chapin JK, Kim J, Biggs SJ, Srinivasan MA, Nicolelis MAL. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature. 2000;408:361–365. doi: 10.1038/35042582. [DOI] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci. 2002;5:995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. Englewood Cliffs, NJ: Prentiss Hall; 1996. [Google Scholar]
- Zhang M, Barash S. Neuronal switching of sensorimotor transformations for antisaccades. Nature. 2000;408:971–975. doi: 10.1038/35050097. [DOI] [PubMed] [Google Scholar]
- Zhang M, Barash S. Persistent LIP activity in memory antisaccades: working memory for a sensorimotor transformation. J Neurophysiol. 2004;91:1424–1441. doi: 10.1152/jn.00504.2003. [DOI] [PubMed] [Google Scholar]