Abstract
The endocannabinoid system is a potential target for therapeutic intervention of substance abuse. Cannabinoid CB1 receptor antagonist decrease intravenous methamphetamine self-administration in animal models. This study examined whether the nucleus accumbens (NAcc) is a site of interaction between methamphetamine and the CB1 receptor antagonist AM251. Male Sprague-Dawley rats were trained to lever press then were surgically implanted with a guide cannula into the right NAcc. Rats were allowed one week to recover and then AM251 (0.1 or 1.0 μg/μL) was reverse dialyzed directly into the NAcc prior to methamphetamine (10 μg/μL) intra-accumbens self-administration. AM251 (1.0 μg/μL) reduced methamphetamine self-administration while AM251 (0.1 μg/μL) had an intermediary effect. The mechanism of self-administration attenuation is not known but could be mediated by AM251 affecting the negative feedback from the NAcc to the ventral tegmental area (VTA). This study provides evidence the endocannabinoid system is involved with rewarding effects of methamphetamine and suggests a possible therapeutic intervention for methamphetamine abuse.
Keywords: drug-taking, psychostimulant, endocannabinoid, intracranial self-administration, reverse-microdialysis
Introduction
Abuse of methamphetamine has developed into an epidemic in the United States. Surveys show nearly 12 million people have used methamphetamine at least once (SAMHSA 2005). At the neurochemical level, methamphetamine's mechanism entails binding the dopamine transporter, blocking reuptake and facilitating reverse transport of dopamine leading to increased levels in the extracellular space (Seiden et al. 1993; Kokoshka et al. 1998; Kahlig et al. 2006; Fowler et al. 2007). The mesolimbic dopamine projection from the VTA to the NAcc has a high density of dopamine transporters at the nerve terminal where psychostimulants exert a majority of their neurochemical effect (Volkow et al. 2000, Svingos et al. 1999).
Studies indicate a role of the endocannibinoid system in regulating and modulating rewarding effects of abused substances (Onaivi 2008; Parolaro and Rubino, 2008). Additionally, this system is involved in modulating synaptic plasticity in the dorsal and ventral striatum, i.e. the NAcc (a well known substrate of reward) (Freund et al. 2003; Gerdeman and Lovinger 2003; Piomelli et al. 2003). Furthermore dopamine agonists and psychostimulants increase the striatal release of endocannabinoids, suggesting an involvement in the effects of psychostimulants (Giuffrida et al. 1999; Patel et al. 2003; Centonze et al. 2004). However, the precise role of the endocannabinoid system in the behavioral effects to psychostimulants is not fully known (De Vries and Schoffelmeer 2005; Maldonado et al. 2006).
Still, the behavioral effects of endocannabinoid antagonist are emerging as modulators of drug-taking behavior. The selective CB1 receptor antagonist/inverse agonist AM251 decreases intravenous methamphetamine self-administration in Wistar rats (Vinklerova et al. 2002) and rhesus monkeys (Schindler et al. 2010). AM251 also attenuates amphetamine-induced behavioral sensitization (Corbille et al. 2007; Thiemann et al. 2008) possibly by blocking CB1 receptor activity (Landa et al. 2006) and down-stream signaling in the NAcc (Chiang et al. 2007) resulting in a modulation of monoamine levels. However AM251's involvement in relapse behavior is equivocal with inhibition of cocaine-primed relapse (Xi et al. 2006) but not methamphetamine-induced reinstatement (Boctor et al. 2007).
Reports suggest the endocannabinoid system interacts with psychostimulants in the NAcc and this interaction warrants further examination. Since the NAcc is an important locus of action of psychostimulants, the present study examined whether local AM251 pretreatment would affect acquisition of intracranial self-administration of methamphetamine into the NAcc. Similarly, self-administration of amphetamine bilaterally into the NAcc via open-ended cannulae has been shown (Hoebel et al. 1983; Phillips et al. 1994; Chevrette et al. 2002) while amphetamine and methamphetamine self-administration unilaterally into the NAcc via a microdialysis probe has been demonstrated in our laboratory (Rodriguez et al. 2003; 2005; 2008). This intracranial approach emphasizes the NAcc as the neural substrate of reward and decreases pharmacokinetic complications as would occur via systemic administration, i.e. gut absorption. The advantages of utilizing microdialysis probes versus open-ended cannulae have been addressed (Rodriguez et al. 2008) and pertain to 1) reduction of high-pressure infusions by creating a drug concentration gradient, 2) maintenance of drug concentration in localized area, and 3) allowance for concomitant delivery and collection of dialysate. Lastly, this model is important for investigating the site of action of these drugs. As well, possible clinical implications are relevant from the perspective that bilateral ablation of the NAcc is a safe procedure for surgical treatment of addiction in human (Stelten et al. 2008).
Materials and Methods
The Institutional Animal Care and Use Committee of The University of Texas at San Antonio approved experimental protocols, which adhered to the National Institutes of Health guidelines.
Subjects
Thirty-seven male Sprague-Dawley rats (300-325g, Harlan, Indianapolis, IN) individually housed in standard laboratory cages were maintained on a 12/12h light/dark cycle (lights on at 7:00 a.m.). Animals were allowed two weeks to acclimate to the vivarium after arrival and provided food and water ad libitum. Following acclimation, 4 animals were randomly assigned as weight controls, and the remaining 33 (Ringer's, n=5; Methamphetamine, n=6; Ringer's plus 0.1 μg/μL AM251, n=5; Ringer's plus 1 μg/μL AM251, n=5; Methamphetamine plus 0.1 μg/μL AM251, n=6; Methamphetamine plus 1 μg/μL AM251, n=6) animals were food restricted 20% from control subject food intake.
Apparatus
Behavioral training occurred in operant chambers (Coulbourn Instruments, Allentown, PA) enclosed in sound-attenuating boxes (Coulbourn Instruments, Allentown, PA). One wall of each chamber was equipped with a retractable active lever (food pellet or methamphetamine contingent lever), an extended inactive lever, and a central food receptacle (45 mg, # F0021, Bioserv Dustless Precision Pellets; Frenchtown NJ). The food receptacle was located 3.5 centimeters above the barred stainless steel floor. A cue light directly above the active lever was illuminated during lever extension and dimmed while retracted. A houselight inside the sound-attenuating box was illuminated throughout the entire session. During self-administration testing the food receptacle was removed.
Autoshaping
Each daily autoshaping session consisted of 20 trials. For each trial, the retractable active lever was located outside of the chamber for 45 seconds, and then extended into the chamber for 10 seconds whereas the inactive lever remained extended throughout the session with no consequence upon press. A lever press during the 10 seconds was considered a successful trial and resulted in food pellet reinforcement; fixed ratio 1 schedule. Pellets were also dispensed when the animal failed to lever press to facilitate association between cue light and lever extension with positive reinforcement. Autoshaping continued until 18 successful trials (out of 20) for two consecutive days (95% criteria) were reached which usually occurred between 5-7 sessions.
Surgery
Eighteen to twenty-four hours following autoshaping, animals were anaesthetized with 0.1mL/100g of Nembutal (pentobarbital sodium, 50 mg/mL, Abbott Laboratories, North Chicago, IL) delivered via intraperitoneal (i.p.) injection. Animals were then placed in a stereotaxic apparatus, and a guide cannula was implanted into the right NAcc using the following coordinates from Bregma: Medial-Lateral + 1.5 mm, Anterior-Posterior +1.5 mm, Dorsal-Ventral −5.5 mm (Paxinos and Watson 1998). (Note: The microdialysis probe extended 2mm beyond the Dorsal-Ventral coordinate, from −5.5 to −7.5mm, which includes the core and shell of the NAcc.) Three surgical grade screws and dental cement held the guide cannula in place. Topical antibiotic was applied to the incision area before and after surgery and 0.1 mL of penicillin (30K units) injected intramuscularly to prevent infection. In addition, Liquid Children's Tylenol diluted in either applesauce or water was provided to minimize post-operative discomfort. One week following surgery autoshaping resumed to ensure cannula implantation did not affect the animals' ability to lever press.
Drug preparation
One hundred milligrams of methamphetamine sulfate (Sigma-Aldrich, St. Louis, MO) dissolved in 10 mL of lactated Ringer's solution (B. Braun Medical Inc., Irvine, CA) obtained a concentration of 10 μg/μL. The methamphetamine concentration is based on studies in our lab that demonstrated positive reinforcement at the 10 μg/μL, above the threshold dose of 3 and not causing concern of systemic dosing by 30 μg/μL concentrations for intracranial dialysis (Rodriguez et al. 2003 & 2005; Ricoy et al. 2009). Ten milligrams of AM251 [N-(piperidin-1-yl)-5-(4-indophonyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide] (Sigma-Aldrich, St. Louis, MO) was dissolved into 10 mL of dimethyl sulfoxide (DMSO, Fischer Chemicals, Fair Lawn, NJ), divided into 0.5mL aliquots and stored at −20°C. Each test day, an aliquot was thawed and diluted in 4.5 mL of nanopure water to obtain the 0.1 μg/μL concentration of AM251. In order to prepare 1 μg/μL AM251, 10 mg AM251 was dissolved in 1 mL DMSO, divided into 0.1 mL aliquots and stored at −20°C. By adding one of these aliquots to 0.9 mL of NANOpure water (molecular grade, Thomas Scientific, Swedesboro, NJ), 1 μg/μL AM251 was prepared fresh daily. A 10% DMSO solution was also prepared daily by combining 0.1 mL of DMSO with 0.9 mL of NANOpure water. DMSO was used as the vehicle control for pretreatment.
Self-Administration
Each animal was fitted with a vest which attached to a tether (11.5 inches) connected to a swivel at the top of the chamber. The tether protected the drug infusion line connecting the one ml syringe and microdialysis probe (0.24-mm outer diameter and 2.00-mm membrane length, CMA, North Chelmsford, MA). A syringe pre-loaded with either lactated Ringer's solution (vehicle) or methamphetamine was placed in an infusion pump (Harvard Apparatus, Holliston, MA), and infused at a rate of 2 μL/minute into the microdialysis probe upon a lever press. Immediately before each session, the dummy cannula was replaced with a microdiaylsis probe and AM251 (0.1 μg/μL or 1.0 μg/μL) or 10% DMSO (administered to the Ringer's and methamphetamine only groups to control for AM251's vehicle solution) was dialyzed into the NAcc for five minutes. Following pretreatment, subjects were moved to an operant chamber containing the preloaded infusion lines of methamphetamine or Ringer's solution. Methamphetamine self-administration sessions consisted of 30 trials. Each trial consisted of a 1-minute time out (active lever retracted), a 45-second active lever extension period with cue light illuminated, a 1-minute dialysis period of methamphetamine (20μg/2μL/dialysis) or Ringer's solution following a press, and a 1-minute wait. If the rat failed to press the active lever, then the 1-minute wait period immediately followed the 45-second self-administration period. Sessions continued for 10 days.
Histology Verification
Animals were anesthetized with 1 mL i.p. injection of Nembutal (50 mg/mL) then transcardially perfusion with physiological saline followed by paraformaldehyde solution. The brains were extracted, placed in formaldehyde and sectioned (200 microns) using a vibratome through the level of the NAcc to assess proper placement of the microdialysis probe.
Data Analysis
Averages and standard error of the mean were calculated for Ringer's solution pretreatment with 10% DMSO or AM251 (0.1μg/μL or 1.0 μg/μL) and methamphetamine pretreatment with 10% DMSO or AM251 (0.1μg/μL or 1.0 μg/μL). Sigma Stat was used to calculate 2-way ANOVA with repeated measures, with pretreatment and treatment as factors. Student Newman-Keuls post hoc tests were used to determine differences between experimental groups (p<0.05).
Results
Probe Placement
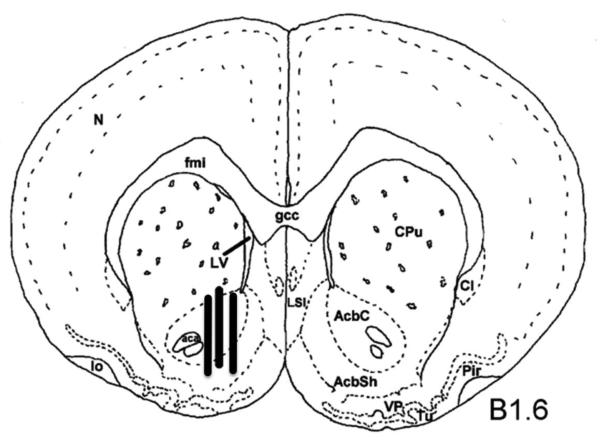
Histology results show all animals included in the self-administration study had correct probe placement, the probe tips were within the core and shell subterritories of the NAcc. Improper probe placement was verified in one subject of the methamphetamine group and thus removed from study. Figure 1 is a composite illustration of the microdialysis probe tip locations for all 32 animals showing the lateral variations with no anterior/posterior difference.
Figure 1.
Front view of a coronal plane of the rat brain redrawn showing placement of the microdialysis probe in the NAcc (B1.6 denotes +1.6 mm anterior to bregma, Paxinos and Watson 1998). The locations of the 2-mm dialysis tips were viewed within a series of thick coronal sections through the frontal lobe and NAcc for all animals under a dissecting microscope. The final placements were sketched onto line drawings from the atlas and summarized in this illustration.
AM251 Effects on Methamphetamine Self-Administration
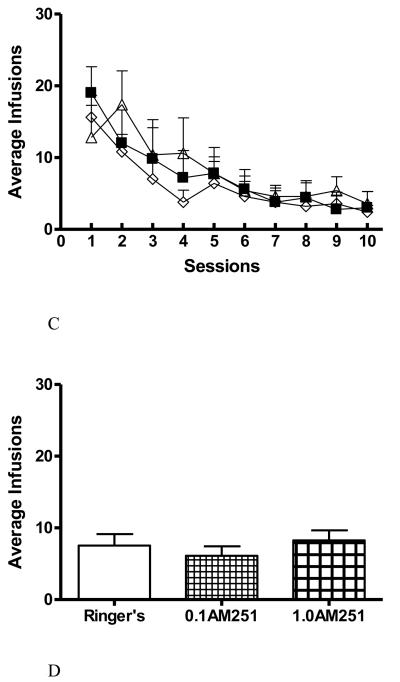
Mean number of lever presses (successful trials resulting in drug infusions) for the animals that self-administered Ringer's solution or methamphetamine after pretreatment with 10% DMSO is shown in Figure 2A. An effect of treatment (F(1,72) =12.523; p=0.008) and sessions (F(9,99)=10.063; p<0.001) was determined. The Newman-Keuls posthoc revealed the methamphetamine group self-administered more than the Ringer's control group during Sessions 2, 3, 4, 5, 6, 7, 8, 9 and 10 (*, p <0.001), and the overall average of presses for all sessions were 22.2 ± 3.2 (methamphetamine group) versus 7.5 ± 3.1 (Ringer's group) was significant (*, p<0.05, Figure 2B). Comparison between the methamphetamine groups pretreated with 10% DMSO or 1 μg/μL AM251 (Figure 2A) revealed an effect of AM251 pretreatment (F(1,81)=7.860; p=0.021) and sessions (F(9,109)=8.590; p<0.001). The Newman-Keuls posthoc showed pretreatment with 1 μg/μL AM251 decreased active lever pressing in sessions 3, 5, 6, 8, 9 and 10 (p <0.001, #), and the overall average of presses for all sessions were 22.2 ± 3.2 (methamphetamine group) versus 12.4 ± 2.1 (1 μg/μL AM251 pretreated methamphetamine group) was significant (#, p<0.05, Figure 2B). The 0.1 μg/μL AM251 pretreatment was associated with lowered lever pressing but did not reach significance for any session nor overall (14.5 ± 3.6 average of presses across all sessions) and therefore was considered as a subthreshold dose. The Ringer's control groups pretreated with 10 % DMSO or AM251 (0.1 or 1 μg/μL) were not significantly different from each other (Figure 2C and D).
Figure 2.
Line and bar graphs show results for lever pressing by session and treatments (A) and averaged across all sessions (B). A) Examining cumulative lever pressing by session demonstrates the temporal delay before AM251 at 1.0 μg/μL (triangles, n=6) had an effect on methamphetamine self-administration into the NAcc. The pound sign above sessions indicate significant difference from corresponding methamphetamine-vehicle sessions (circles, n=5); p-values are given in results. Note how the points for AM251 at 0.1 μg/μL (diamonds, n=6) are positioned between the methamphetamine (asterisks indicate significant differences from control) and Ringer's control group (squares, n=5); this low AM251 effect was not significantly different from any other group including the higher AM251 concentration. B) Bar graph of cumulative data across all sessions reveals robust suppression of lever presses for methamphetamine caused by the higher AM251 pretreatment. C & D) Line graph of each session and bar graph of cumulative lever presses across all sessions, respectively, for Ringer's control groups pretreated with 10 % DMSO (squares, n=5), AM251 0.1 μg/μL (open diamond, n=6) or AM251 1.0 μg/μL (open triangles, n=6) in (C) and Ringer's groups pretreated with DMSO, AM251 0.1 or 1.0 μg/μL in (D).
Discussion
These results demonstrate pretreatment with the cannabinoid CB1 receptor antagonist AM251 reduces acquisition of methamphetamine intra-accumbens self-administration. The methamphetamine self-administering group pretreated with AM251 still displayed prototypical stereotypic behaviors produced by methamphetamine alone, such as head sways and hyperlocomotion, which indicate that AM251 did not completely reverse the effects of methamphetamine but only reduced lever pressing for drug infusions. The results demonstrate a threshold dose response to AM251, with only the higher concentration being effective and the lower being subthreshold in reducing methamphetamine self-administration during the acquisition phase. Attenuated self-administration was not evident from day one suggesting the mechanism of AM251 action requires time to be effective. Results could be due to antagonistic effects locally within the NAcc on the CB1 receptors and second messengers altering the rewarding properties of methamphetamine also in the NAcc.
Another possible explanation of results is based on previous studies that show reinforcing stimuli increase extracellular dopamine in the NAcc, activating GABAergic interneurons, which inhibit dopaminergic somas in the VTA through negative feedback (Koob and Swerdlow, 1988). Diminished GABAergic neurotransmission has been shown to occur when the CB1 receptors are stimulated (Ameri 1999). Thus methamphetamine self-administration could potentially facilitate endocannabinoid release in the NAcc resulting in disinhibited dopaminergic somas in the VTA and increased neuronal firing (Giuffrida et al. 1999; Patel et al. 2003; Centonze et al. 2004). AM251 may inhibit this process by allowing GABAergic feedback to occur resulting in decreased exocytotic release of dopamine (Heimer et al. 1991; Raham et al. 2002). Hence, this would diminish the rewarding properties of methamphetamine (the concomitant release of dopamine by reverse transport and exocytosis) and cause the subsequent reduction of self-administration observed in this study. However, considering that extracellular dopamine was not measured in our study renders this interpretation speculative. On the contrary, a recent finding report AM251 blocks the dopamine transporter independent of CB1 receptor activity (Price et al. 2008). This could explain reduced rewarding properties of methamphetamine since AM251 would compete for dopamine transporter occupancy and ultimately reduce reverse transport of dopamine.
Alternatively, AM251 has been shown to cause nausea and vomiting as a side effect in various species (Darmani 2001; McLaughlin et al. 2005; Van Sickle et al. 2001; Sharkey et al. 2007) following systemic administration. Therefore, the attenuation of methamphetamine-induced self-administration may have resulted from the summation of the aversive effects of AM251 and the rewarding properties of methamphetamine, rather than a direct effect of blockade of the CB1 receptor on dopamine release. However, considering that our drug administration technique involved local application into the NAcc renders this interpretation unlikely, i.e., no systemic effects of AM251. Another argument takes into consideration AM251's inverse agonistic potential (for review see, Pertwee 2007; Bergman et al. 2008). Although speculative, the 2 doses of AM251 administered here may have acted as a neutral CB1 receptor antagonists at the low concentration and exhibit additional CB1 inverse agonist activity only at the higher concentration. Furthermore, there exists a suggestion that ligands may have two sites of action on the CB1 receptor, one at which they displace agonists to produce antagonism and another at which they somehow induce inverse agonism, perhaps through an allosteric mechanism (for review see, Sim-Selley 2003). Indeed, there is now evidence not only that the CB1 receptor contains an allosteric site but also that SR141716A, AM251 and AM281 can produce inverse cannabimimetic effects by acting on such sites to slow the rate at which an agonist dissociates from the CB1 receptor (Price et al. 2004).
Conclusions
In conclusion, this study demonstrated that AM251 administered into the NAcc reduced acquisition of intracranial methamphetamine self-administration in rats. These findings extend the results of Vinklerova et al. (2002), which demonstrated that AM251 decreased intravenous methamphetamine self-administration during the maintenance phase. Additionally, the findings reported here are in agreement with a recent study in rhesus monkeys showing that AM251 attenuates intravenous methamphetamine self-administration (Schindler et al. 2010). Our findings demonstrate a localized delivery of methamphetamine and AM251 emphasizing the importance of the NAcc in regulating psychostimulant rewarding behaviors, which makes this study distinct from those two studies. However, we have not specifically assessed the contribution of the individual NAcc subregions as the microdialysis probe was present in both the core and shell. Nonetheless, the interactions between the dopamine and endocannabinoid systems more than likely involve dopamine neuron modulation by either intrinsic or extrinsic GABAergic neurons, which contain cannabinoid receptors. These results suggest that antagonizing the cannabinoid system may provide a novel approach to treat methamphetamine abuse.
Research Highlights.
Pretreatment with AM251, cannabiniod antagonist, into the nucleus accumbens decreases methamphetamine self-administration into the nucleus accumbens.
Findings illustrate modulating the endocannabinoid system influences drug-taking behavior.
Acknowledgments
We sincerely thank Mr. Pedro Galvan for his work in establishing the operant system and methods for the self-administration procedure. Supported by DA 04195, RR 013646-06A1, the Ewing Halsell Foundation, and the Kleberg Foundation to JLM and a Minority Supplement to JSR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergman J, Delatte MS, Paronis CA, Vemuri K, Thakur GA, Makriyannis A. Some effects of CB1 antagonists with inverse agonist and neutral biochemical properties. Physiol Behav. 2008;93(4-5):666–70. doi: 10.1016/j.physbeh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Boctor SY, Martinez JL, Jr., Koek W, France CP. The cannabinoid CB1 receptor antagonist AM251 does not modify methamphetamine reinstatement of responding. Eur J Pharmacol. 2007;571:39–43. doi: 10.1016/j.ejphar.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA. Delta-9-tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB1 receptor antagonist/inverse agonist SR141716A. Neuropsychopharmacology. 2001;24:198–203. doi: 10.1016/S0893-133X(00)00197-4. [DOI] [PubMed] [Google Scholar]
- Centonze D, Battista N, Rossi S, Mercuri NB, Finazzi-Agro A, Bernardi G, Calabresi P, Maccarrone M. A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal GABAergic transmission. Neuropsychopharmacology. 2004;29:1488–1497. doi: 10.1038/sj.npp.1300458. [DOI] [PubMed] [Google Scholar]
- Chevrette J, Stellar JR, Hesse GW, Markou A. Both the shell of the nucleus accumbens and the central nucleus of the amygdala support amphetamine self-administration in rats. Pharmacol Biochem Behav. 2002;71:501–507. doi: 10.1016/s0091-3057(01)00686-4. [DOI] [PubMed] [Google Scholar]
- Chiang YC, Chen JC. The role of the cannabinoid type 1 receptor and down-stream cAMP/DARPP-32 signal in the nucleus accumbens of methamphetamine-sensitized rats. J Neurochem. 2007;103:2505–17. doi: 10.1111/j.1471-4159.2007.04981.x. [DOI] [PubMed] [Google Scholar]
- Corbillé AG, Valjent E, Marsicano G, Ledent C, Lutz B, Hervé D, Girault JA. Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. J Neurosci. 2007;27:6937–47. doi: 10.1523/JNEUROSCI.3936-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN. Cannabinoid CB1 receptors control conditioned drug seeking. Trends Pharmacol Sci. 2005;26:420–426. doi: 10.1016/j.tips.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Kroll C, Ferrieri R, Alexoff D, Logan J, Dewey SL, Schiffer W, Schlyer D, Carter P, King P, Shea C, Xu Y, Muench L, Benveniste H, Vaska P, Volkow ND. PET studies of d-methamphetamine pharmacokinetics in primates: comparison with l-methamphetamine and (--)-cocaine. J Nucl Med. 2007;48:1724–32. doi: 10.2967/jnumed.107.040279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Lovinger DM. Emerging roles for endocannabinoids in long-term synaptic plasticity. Br J Pharmacol. 2003;140:781–789. doi: 10.1038/sj.bjp.0705466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neurosci. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Hoebel BG, Monaco AP, Hernandez L, Aulisi E, Stanley BG, Lenard L. Self-injection of amphetamine directly into the brain. Psychopharmacology (Berl) 1983;81:158–163. doi: 10.1007/BF00429012. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, Galli A. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc Natl Acad Sci. 2005;102:3495–500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoshka JM, Vaughan RA, Hanson GR, Fleckenstein AE. Nature of methamphetamine-induced rapid and reversible changes in dopamine transporters. Eur J Pharmacol. 1998;361:269–75. doi: 10.1016/s0014-2999(98)00741-9. [DOI] [PubMed] [Google Scholar]
- Koob GF, Swerdlow NR. The functional output of the mesolimbic dopamine system. Ann N Y Acad Sci. 1988;537:216–27. doi: 10.1111/j.1749-6632.1988.tb42108.x. [DOI] [PubMed] [Google Scholar]
- Landa L, Sulcova A, Slais K. Involvement of cannabinoid CB1 and CB2 receptor activity in the development of behavioural sensitization to methamphetamine effects in mice. Neuro Endocrinol Lett. 2006;27:63–9. [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Winston KM, Limebeer CL, Parker LA, Makriyannis A, Salamone JD. The cannabinoid antagonist AM 2511 produces food avoidance and behavioras associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology. 2005;180(2):286–293. doi: 10.1007/s00213-005-2171-0. [DOI] [PubMed] [Google Scholar]
- Onaivi ES. An endocannabinoid hypothesis of drug reward and drug addiction. Ann N Y Acad Sci. 2008;1139:412–21. doi: 10.1196/annals.1432.056. [DOI] [PubMed] [Google Scholar]
- Parolaro D, Rubino T. The role of the endogenous cannabinoid system in drug addiction. Drug News Perspect. 2008;21:149–57. [PubMed] [Google Scholar]
- Patel S, Rademacher DJ, Hillard CJ. Differential regulation of the endocannabinoids anandamide and 2-arachidonylglycerol within the limbic forebrain by dopamine receptor activity. J Pharmacol Exp Ther. 2003;306:880–888. doi: 10.1124/jpet.103.054270. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th Ed. Academic Press; New York: 1998. [Google Scholar]
- Phillips GD, Robbins TW, Everitt BJ. Bilateral intra-accumbens self-administration of d-amphetamine: Antagonism with intra-accumbens SCH-23390 and sulpiride. Psychopharmacology (Berl) 1994;114:477–485. doi: 10.1007/BF02249339. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Price DA, Owens WA, Gould GG, Frazer A, Roberts JL, Daws LC, Giuffrida A. CB1-independent inhibition of dopamine transporter activity by cannabinoids in mouse dorsal striatum. J Neurochem. 2007;101(2):389–96. doi: 10.1111/j.1471-4159.2006.04383.x. [DOI] [PubMed] [Google Scholar]
- Price TJ, Patwardhan A, Akopian AN, Hargreaves KM, Flores CM. Modulation of trigeminal sensory neuron activity by the dual cannabinoidvanilloid agonists anandamide, N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide. Br J Pharmacol. 2004;141(7):1118–30. doi: 10.1038/sj.bjp.0705711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005;76(12):1307–24. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Rahman S, McBride WJ. Involvement of GABA and cholinergic receptors in the nucleus accumbens on feedback control of somatodendritic dopamine release in the ventral tegmental area. J Neurochem. 2002;80:646–54. doi: 10.1046/j.0022-3042.2001.00739.x. [DOI] [PubMed] [Google Scholar]
- Results from the 2004 National Survey on Drug Abuse: National Findings. Rockville, MD: (Office of Applied Studies, NSDUH Series H-28, DHHS Publication No. SMA 05-4062). [Google Scholar]
- Ricoy U, Martinez JL., Jr Local hippocampal methamphetamine-induced reinforcement. Front Behav Neurosci. 2009;3:47. doi: 10.3389/neuro.08.047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JS, Boctor SY, Phelix CP, Martinez JL., Jr F344 Rats Self-Administer Methamphetamine but not Amphetamine into the Nucleus Accumbens via Revese Microdialysis. Abstr: Soc Neurosci Prog. 2003:135.6. [Google Scholar]
- Rodriguez JS, Phelix CP, Martinez JL., Jr Local Oxytocin Antagonist Pretreament Decreases Intra-Accumbens Methamphetamine Self-Administration. Abstr: Soc Neurosci Prog. 2005:681.10. [Google Scholar]
- Rodriguez JS, Boctor SY, Phelix CF, Martinez JL., Jr Differences in performance between Sprague-Dawley and Fischer 344 rats in positive reinforcement tasks. Pharmacol Biochem Be. 2008;89:17–22. doi: 10.1016/j.pbb.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Gilman JP, Justinova Z, Vemuri VK, Makriyannis A, Goldberg SR. Effects of cannabinoid receptor antagonists on maintenance and reinstatement of methamphetamine self-administration in rhesus monkeys. Eur J Pharmacol. 2010;633(1-3):44–9. doi: 10.1016/j.ejphar.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–77. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- Sharkey KA, Cristino L, Oland LD, Van Sickle MD, Starowicz K, Pittman QJ, Guglielmotti V, Davison JS, Di Marzo V. Arvanil, anandamide and N-arachidonoyl-dopamine (NADA) inhibit emesis through cannabinoid CB1 and vanilloid TRPV1 receptors in the ferret. Eur J Neurosc. 2007;25(9):2773–2782. doi: 10.1111/j.1460-9568.2007.05521.x. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol. 2003;15(2):91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- Stelten BM, Noblesse LH, Ackermans L, Temel Y, Visser-Vandewalle V. The neurosurgical treatment of addiction. Neurosurg Focus. 2008;25(1):E5. doi: 10.3171/FOC/2008/25/7/E5. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Clarke CL, Pickel VM. Localization of the delta-opioid receptor and dopamine transporter in the nucleus accumbens shell: implications for opiate and psychostimulant cross-sensitization. Synapse: 1999;34(1):1–10. doi: 10.1002/(SICI)1098-2396(199910)34:1<1::AID-SYN1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Thiemann G, Di Marzo V, Molleman A, Hasenohrl RU. The CB (1) cannabinoid receptor antagonist AM251 attenuates amphetamine-induced behavioural sensitization while causing monoamine changes in nucleus accumbens and hippocampus. Pharmacol Biochem Behav. 2008;89:384–91. doi: 10.1016/j.pbb.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–6. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Oland LD, Ho W, Hillard CJ, Mackie K, Davison JS, Sharkey KA. Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology. 2001;121(4):767–774. doi: 10.1053/gast.2001.28466. [DOI] [PubMed] [Google Scholar]
- Vinklerova J, Novakova J, Sulcova A. Inhibition of methamphetamine self-administration rats by cannabinoid receptor antagonist AM251. J Psychopharmacol. 2002;16:139–143. doi: 10.1177/026988110201600204. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin R, Fowler JS, Franceschi D, Franceschi M, Logan J, Gatley SJ, Wong C, Ding YS, Hitzemann R, Pappas N. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci. 2000;67(12):1507–15. doi: 10.1016/s0024-3205(00)00731-1. [DOI] [PubMed] [Google Scholar]