Summary
Increased phosphorylation of Akt substrate of 160 kDa (AS160) is essential to trigger the full increase in insulin-stimulated glucose transport in skeletal muscle. The primary aim of this study was to characterize the timecourse for reversal of insulin-stimulated AS160 phosphorylation in rat skeletal muscle after insulin removal. The timecourses for reversal of insulin’s effects both upstream (Akt phosphorylation) and downstream (glucose uptake) of AS160 were also determined. Epitrochlearis muscles were incubated in vitro using 3 protocols which differed with regard to insulin exposure: No Insulin (never exposed to insulin), Transient Insulin (30 min with 1.8 nmol/l insulin, then incubation without insulin for 10, 20 or 40 min), or Sustained Insulin (continuously incubated with 1.8 nmol/l insulin). After removal of muscles from insulin, Akt and AS160 phosphorylation reversed rapidly, each with a half-time of <10 min and essentially full reversal by 20 min. Glucose uptake reversed more slowly (half time between 10 and 20 min with essentially full reversal by 40 min). Removal of muscles from insulin resulted in a rapid reversal of the increase in AS160 phosphorylation which preceded the reversal of the increase in glucose uptake, consistent with AS160 phosphorylation being essential for maintenance of insulin-stimulated glucose uptake.
Keywords: Akt, timecourse, exercise, glucose transport
Introduction
Insulin stimulates phosphorylation and activation of the Ser/Thr kinase known as Akt or protein kinase B, which has been shown to regulate several important processes including glucose transport (Czech and Corvera 1999, Saltiel and Kahn 2001). For many years, signaling steps intermediate to insulin’s activation of Akt and subsequent GLUT4 glucose transporter protein translocation remained unknown. In 2002, Lienhard and colleagues identified TBC1D4 as a substrate of Akt which in 3T3-L1 adipocytes, becomes phosphorylated upon insulin stimulation, and renamed this protein Akt substrate of 160 kDa (AS160) (Kane et al. 2002). They and others have provided compelling evidence that increased phosphorylation of AS160 is essential for the full effect of insulin on triggering GLUT4 translocation and glucose transport, apparently by modulating AS160’s Rab GTPase activity (Eguez et al. 2005, Gonzalez and McGraw 2006, Miinea et al. 2005, Sano et al. 2003).
Insulin and contractile activity are the most important stimuli for increasing glucose uptake into skeletal muscle (Cartee and Wojtaszewski 2007, Zorzano et al. 2005). Bruss et al. (Bruss et al. 2005) demonstrated that phosphorylation of AS160 in rat skeletal muscle is increased in response to either insulin or contractile activity. Arias et al. (Arias et al. 2007) recently found that AS160 phosphorylation in rat epitrochlearis muscle remained increased for 3 h post-exercise, by which time the exercise effect on glucose uptake had reversed. In light of these unexpected findings, it seemed important to determine if prior insulin stimulation, upon insulin's removal, also results in a sustained increase in phosphorylation of AS160 that persists after glucose uptake returns to basal levels, or if the loss of insulin's stimulation of AS160 phosphorylation precedes the reversal of insulin's effect on glucose transport. In contrast to the abundance of research on the sequence of events leading to the activation of insulin-stimulated glucose transport, the time-course of the reversal of insulin signaling and insulin-stimulated glucose transport has been remarkably understudied.
Therefore, the primary aim of the present investigation was to evaluate the transient effect of insulin on Akt phosphorylation, AS160 phosphorylation, and glucose uptake in isolated rat skeletal muscle. We hypothesized that unlike after exercise, prior exposure to insulin would not induce a long-lasting increase in AS160 phosphorylation in skeletal muscle after removal of insulin. In addition, we hypothesized that insulin removal, would lead to dephosphorylation of Akt and AS160, followed by a return of glucose uptake to basal levels.
Methods
Materials
Unless otherwise noted, all chemicals were purchased from Sigma Chemical (St. Louis, MO) or Fisher Scientific (Hanover Park, IL). Human recombinant insulin was obtained from Eli Lilly (Indianapolis, IN). Reagents and apparatus for SDS-PAGE and immunoblotting were from Bio-Rad Laboratories (Hercules, CA). Anti-phospho AktThr308 (pAkt; #9275), anti-phospho-(Ser/Thr) Akt substrate (anti-PAS; #9611), and anti-rabbit IgG horseradish peroxidase (#7074) were from Cell Signaling Technology (Danvers, MA). Anti-AS160 (−741) was from Millipore (Billerica, MA). 2-Deoxy-[3H]glucose ([3H]2-DG) and [14C]mannitol were from Perkin Elmer (Boston, MA).
Animal Care
The animal protocol for this study was approved by the University of Michigan Committee on Use and Care of Animals. Male Wistar rats (~120–140 g; Harlan, Indianapolis, IN) were provided with standard rat chow (Lab Diet; PMI Nutritional International, Brentwood, MO) and water ad libitum until 17:00 prior to the day of the terminal surgery, at which time food was removed from their cages. The next day, rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg body wt; Ovation Pharmaceuticals, Deerfield, IL). Upon loss of pedal reflexes, epitrochlearis muscles were quickly excised, rinsed in warmed Krebs Henseleit buffer (KHB), and transferred into vials containing the appropriate incubation media.
Muscle incubation
The sequence, duration and insulin concentration for muscle incubation steps are summarized in Table 1. After excision, all muscles were incubated in vitro without insulin for 30 min. Thereafter, muscles underwent either two or three additional incubation steps using one of the following protocols which differed with regard to insulin exposure: No Insulin (never exposed to insulin), Transient Insulin (30 min with 1.8 nmol/l insulin in step 2, then removal of insulin for the remainder of the experiment, which was either 1 or 2 more incubation steps that totaled 10, 20, or 40 additional min), or Sustained Insulin (continuously incubated with 1.8 nmol/l insulin after step 1). During all incubation steps, muscles were placed in vials, warmed to 37°C in a heated water bath with shaking at 80 revolutions per min, and continuous gassing with 95% O2/5% CO2. During Step 1, all muscles were incubated in vials containing 2 ml KHB supplemented with 0.1% bovine serum albumin (BSA), 2 mmol/l sodium pyruvate, and 6 mmol/l mannitol for 30 min (Solution 1). All muscles were then transferred to a second vial containing either Solution 1 or Solution 1 supplemented with 1.8 nmol/l insulin for 30 min. Subsequently, the muscles that underwent only 3 incubation steps were transferred into vials containing 2-DG and treated as described below, whereas the muscles that underwent 4 incubation steps were transferred to a third vial containing Solution 1 with or without insulin and incubated for an additional 10 or 30 min. During the final incubation step, all muscles were placed in vials containing 2 ml KHB supplemented with 0.1% BSA 1 mmol/l 2-DG (including a final concentration of 2.25 mCi/mmol [3H]-2-DG), 9 mmol/l mannitol (including a final concentration of 0.022 mCi/mmol [14C]-mannitol), and 1.8 nmol/l insulin for the Sustained Insulin muscles only. After incubation for 10 min in 2-DG-containing media, muscles were rapidly blotted on filter paper moistened with ice-cold KHB, trimmed, freeze-clamped using aluminum tongs cooled in liquid nitrogen, and stored at −80°C for later processing and analysis.
Table 1. Sequence, Duration and Insulin Concentration (nmol/l) during Muscle Incubation Steps.
Summary of in vitro muscle incubation protocol. An incubation duration of 0 min for Step 3 indicates muscles were transferred directly from Step 2 to the [3H]-2-deoxyglucose (2-DG) Incubation Step.
| Groups | Incubation Step 1 (30min) |
Incubation Step 2 (30min) |
Variable Incubation Step 3 (0, 10 or 30min) |
[3H]-2DG Incubation Step (10min) |
|---|---|---|---|---|
| No Insulin | 0 nmol/l | 0 nmol/l | 0 nmol/l | 0 nmol/l |
| Transient Insulin | 0 nmol/l | 1.8 nmol/l | 0 nmol/l | 0 nmol/l |
| Sustained Insulin | 0 nmol/l | 1.8 nmol/l | 1.8 nmol/l | 1.8 nmol/l |
Muscle lysate preparation
Frozen muscles were weighed, and transferred to pre-chilled glass tissue grinding tubes (Kontes, Vineland, NJ), and homogenized in ice-cold lysis buffer (1 ml/muscle) using a glass pestle attached to a motorized homogenizer. The lysis buffer contained 20 mmol/l Tris-HCl, pH 7.4, 150 mmol/l NaCl, 1% Triton X-100 (vol/vol), 1 mmol/l EDTA, 1 mmol/l EGTA, 2.5 mmol/l sodium pyrophosphate, 1 mmol/l sodium vanadate, 1 mmol/l β-glycerophosphate, 1 µg/ml leupeptin, and 1 mmol/l phenylmethylsulfonyl fluoride. Homogenates were transferred to microfuge tubes, rotated for 1 h at 4°C, and then centrifuged at 15,000 g for 15 min at 4°C to remove insoluble material. Protein concentration was measured using the bicinchoninic acid method (Pierce Biotechnology, Rockford, IL).
2-Deoxyglucose uptake measurement
Aliquots (200 µl) of the supernatants were combined in a vial with 10 ml of scintillation cocktail (Research Products International, Mount Prospect, IL) and a scintillation counter (Perkin Elmer, Waltman, MA) was used to determine 3H and 14C disintegrations per min. These values were used to determine [3H]-2-DG uptake as previously described (Hansen et al. 1994).
Immunoprecipitation
For immunoprecipitation, 200 µg of protein from each sample was combined with 2 µl of PAS antibody and rotated for 1 h at 4°C. Protein G-agarose beads (Upstate, Lake Placid, NY) were washed 3 times with lysis buffer. After initial antibody incubation, 100 µl of 50% slurry mix of protein G-agarose beads were added to the lysate/antibody mix and rotated 1 h at 4°C. Protein G-agarose beads were isolated by centrifugation (4000 rpm at 4°C for 1 min) and washed three times in lysis buffer. Antigens were eluted from the beads with 45 µl of 2x SDS loading buffer and were boiled for 5 min before SDS-polyacrylamide gel electrophoresis as described below.
Immunoblotting
Fifty µg of protein of each sample was combined with sample buffer, boiled for 5 min and loaded on a 7% SDS-polyacrylamide gel, before being transferred to nitrocellulose membranes. Membranes were blocked in 5% milk in TBST (0.1% Tween-20 in Tris-buffered saline, pH 7.5) for 1 h at room temperature and transferred to 5% BSA-TBST with primary (antiphospho AktThr308 or anti-AS160 for PAS-AS160) for 3 h at room temperature or overnight at 4°C. Membranes were then washed 3 times for 5 min/wash in TBST and incubated in anti-rabbit IgG horseradish peroxidase (1:20,000) for 1 h at room temperature. Blots were washed 3 times for 5 min/wash in TBST, then washed 2 times for 5 min/wash in TBS, and finally subjected to enhanced chemiluminescence (West Dura Extended Duration Substrate; Pierce, Rockford, IL) to visualize protein bands. Immunoreactive proteins were quantified by densitometry (Alpha Ease FC software; Alpha Innotech, San Leandro, CA). All values were expressed relative to an aliquot from a non-stimulated muscle lysate control that was loaded in one lane of each gel.
Statistical Analysis
One-way analysis of variance (ANOVA) was used to determine significant main effects and interactions. Bonferroni t-tests were used for post hoc analysis to identify the source of significant variance (SigmaStat; SPSS, Chicago, IL). Data are presented as mean ± SEM. A p value of ≤ 0.05 was accepted as statistically significant. In addition, to assess insulin’s effects above baseline, delta values for pAkt, PAS-AS160, and glucose uptake were determined by subtracting the mean value for the No Insulin group from the values for each muscle in the insulin-stimulated groups (Transient Insulin or Sustained Insulin) at each time (Cartee et al. 1989).
Results
Phospho-AktThr308
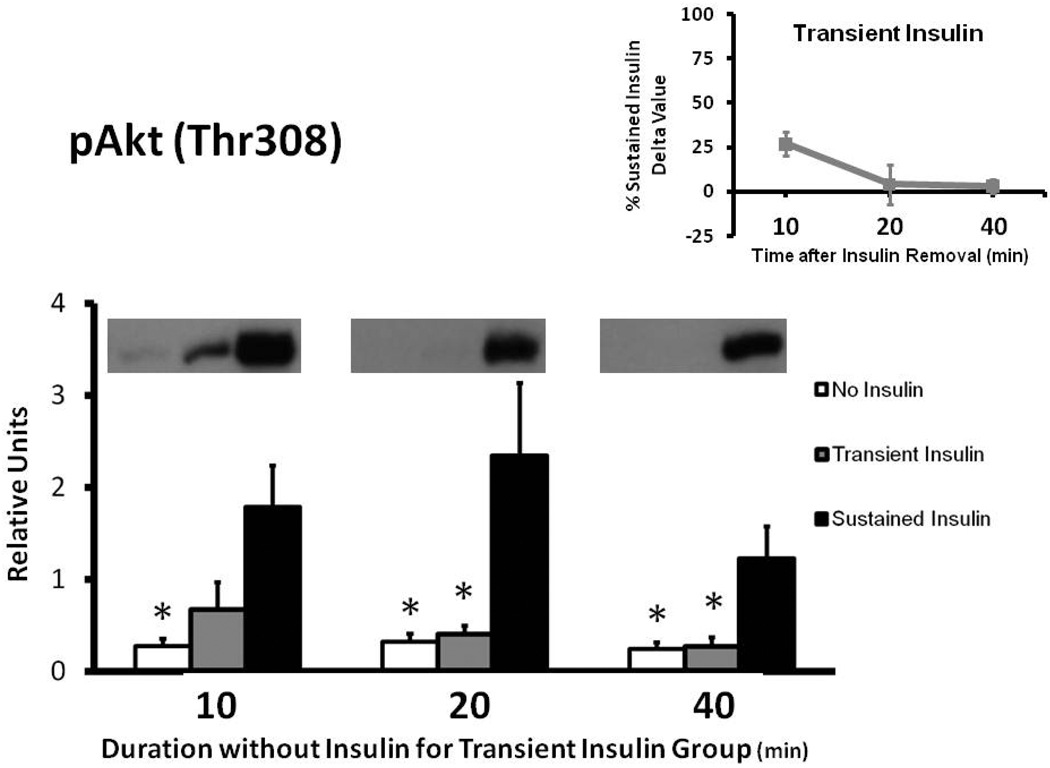
Muscles in the Sustained Insulin vs. No Insulin group had greater pAkt at each time measured (Fig. 1). The Transient Insulin and No Insulin groups were not significantly different at any of the times evaluated. After insulin removal, pAkt values for the Transient Insulin compared to the Sustained Insulin group tended to be reduced at 10 min (p = 0.059), and the decrement became significant (p < 0.05) at 20 and 40 min of insulin removal. The delta pAkt values for the Transient Insulin group were 27%, 4%, and 3% of the Sustained Insulin group at 10, 20, and 40 min after insulin removal, respectively (Fig. 1 inset).
Figure 1.
Phosphorylation of AktThr308 (pAkt) in rat epitrochlearis samples determined by immunoblotting. *p < 0.05 versus Sustained Insulin group. Data are means ± SEM. n=6–8 muscles per treatment at each time. The values on the x-axis refer to the duration (min) after muscles in the Transient Insulin group were removed from insulin prior to being freeze-clamped. Inset: Delta pAkt for the Transient Insulin Group expressed as a percentage of the Sustained Insulin Group for each time point examined. Delta pAkt values were determined by subtracting the mean value for the No Insulin group from the values for each muscle in the insulin-stimulated groups (Transient Insulin or Sustained Insulin) at each time.
PAS-AS160
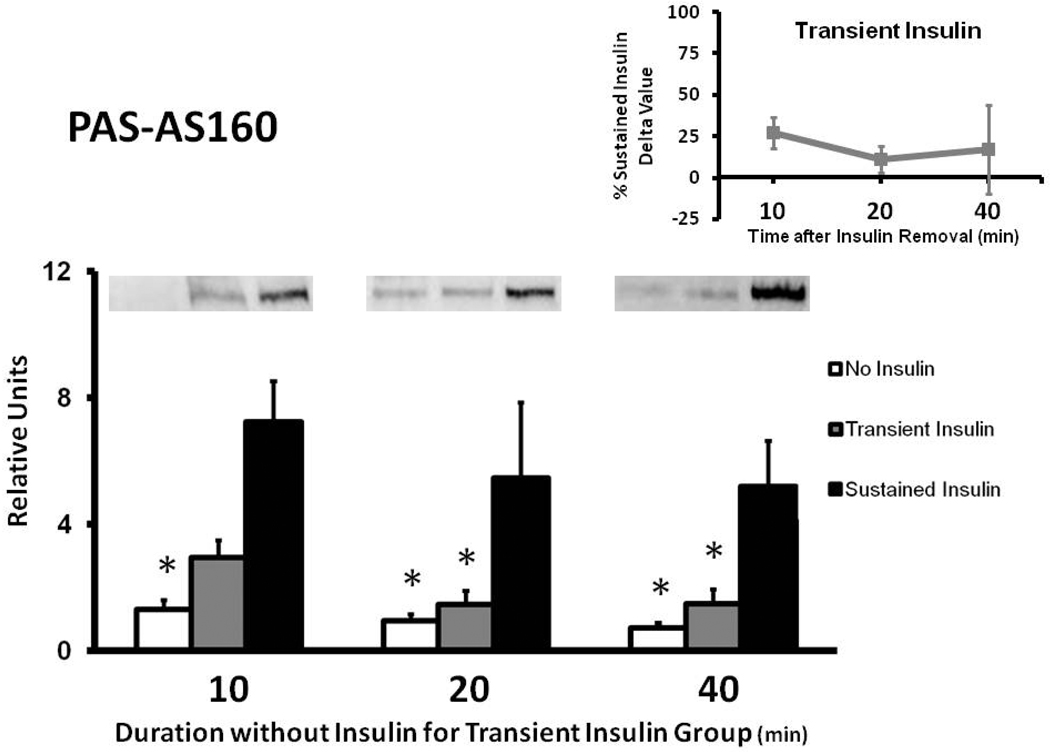
Muscles in the Sustained Insulin compared to the No Insulin group had greater PASAS160 values at each time measured (Fig. 2). The Transient Insulin and No Insulin groups were not significantly different at any of the times studied. At 10 min, PAS-AS160 for the Transient Insulin compared to the Sustained Insulin group tended to be reduced (p = 0.066). PAS-AS160 was significantly lower (p < 0.05) for Transient Insulin vs. Sustained Insulin at 20 and 40 min. The delta PAS-AS160 values for the Transient Insulin group were 27%, 11%, and 17% of the Sustained Insulin group at 10, 20, and 40 min after insulin removal, respectively (Fig. 2 inset).
Figure 2.
Phosphorylation of AS160 in rat epitrochlearis samples that were immunoprecipitated with phospho-Akt substrate (PAS) antibody prior to immunoblotting with the anti-AS160 antibody. *p < 0.05 versus Sustained Insulin group. Data are means ± SEM. n=6–8 muscles per treatment at each time. The values on the x-axis refer to the duration (min) after muscles in the Transient Insulin group were removed from insulin prior to being freeze-clamped. Inset: Delta PAS-AS160 for the Transient Insulin Group expressed as a percentage of the Sustained Insulin Group (see Figure 1).
2-Deoxyglucose Uptake
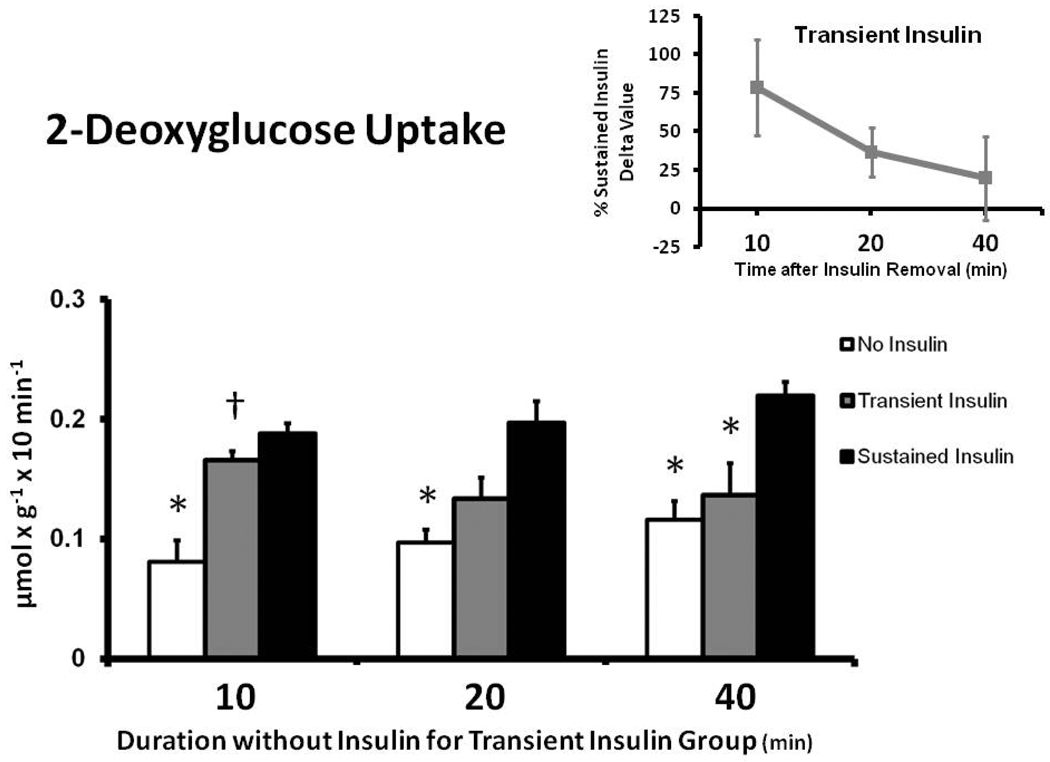
Muscles in the Sustained Insulin compared to the No Insulin group had greater 2-DG uptake at each time measured (Fig. 3). For Transient Insulin vs. No Insulin muscle, the 2-DG uptake was greater (p < 0.05) at 10 min only, with no significant difference at 20 or 40 min. Muscles in the Transient Insulin vs. Sustained Insulin group had similar 2-DG uptake at 10 min, with a trend (p = 0.093) to be reduced at 20 min and a significant decrement (p < 0.05) at 40 min. The delta 2-DG uptake values for the Transient Insulin group were 79%, 37% and 20% of the Sustained Insulin group at 10, 20, and 40 min after insulin removal, respectively (Fig. 3 inset).
Figure 3.
2-Deoxyglucose (2-DG) uptake in rat epitrochlearis muscles. *p < 0.05 versus Sustained Insulin group, †p < 0.05 versus No Insulin group. Data are means ± SEM. n=6–8 muscles per treatment at each time. The values on the x-axis refer to the duration (min) after muscles in the Transient Insulin group were removed from insulin prior to being freeze-clamped. Inset: Delta 2-DG for the Transient Insulin Group expressed as a percentage of the Sustained Insulin Group (see Figure 1).
Discussion and Conclusions
The most important new findings from the present study were that after removal of incubated rat skeletal muscle from insulin, phosphorylation of Akt and AS160 were rapidly reversed (each with a half-time of <10 min and approaching full reversal by 20 min), which preceded the reversal of glucose uptake (half-time of between 10 and 20 min and not approaching full reversal until 40 min). These results are significant because very little has been reported about the timecourses for reversal of insulin signaling or glucose uptake after removing insulin from skeletal muscle, the major site for insulin-stimulated clearance of blood glucose. Rapid reversal of insulin signaling and glucose uptake in skeletal muscle has physiological importance because slow reversal of insulin signaling, after the removal of insulin, would promote excessive glucose uptake and favor the development of hypoglycemia, a potentially life-threatening condition.
The present study is apparently the first to report the timecourse for reversal of Akt and AS160 phosphorylation with the removal of insulin. The loss of Akt and AS160 phosphorylation were quite rapid, with neither significantly different from basal values at 10 min. The levels of Akt and AS160 phosphorylation depend on the balance between kinases and phosphatases (Brady and Saltiel 2001, Kahn and Goldfine 1993, Meyerovitch et al. 1992). The removal of muscle from insulin results in the loss of insulin's stimulation of kinases. The rapid dephosphorylation of Akt and AS160 suggests that, in addition to the loss of activation of their upstream kinases, these proteins were also readily accessible to active phosphatases.
In both isolated rat adipocytes (Garvey et al. 1985, Karnieli et al. 1981) and L6 muscle cells (Dugani et al. 2008), upon removal from insulin, glucose uptake reversed to 50% of maximal stimulation at approximately 10 min, with complete reversal after ~30–60 min. Unlike a monolayer of cells, there are diffusion limitations in an intact muscle, even in the very flat and thin epitrochlearis [only ~20 fibers thick (Wallberg-Henriksson 1987)], that delay insulin's removal when the muscle is transferred to insulin-free solution. Accordingly, using intact muscle underestimates the rate of reversal at the cellular level. Nonetheless, the loss of Akt and AS160 phosphorylation were quite rapid, with neither significantly different from basal values at 10 minutes. Previous research indicates that AS160 phosphorylation participates in the initiation of glucose transport (Arias et al. 2007, Bruss et al. 2005, Kramer et al. 2006, Sano et al. 2003). Our novel observation that reversal of glucose uptake occurs subsequent to AS160 dephosphorylation suggests that AS160 phosphorylation may also be involved in the maintenance of insulin-stimulated glucose transport. Recently, another Rab-GTPase activating protein known as TBC1D1 has been shown to be expressed in skeletal muscle and to become phosphorylated in response to insulin-stimulation (Chavez JBC 2008; Taylor 2008). Based on data from L6 myotubes and 3T3-L1 adipocytes, it has been suggested that TBC1D1 may modulate glucose transport by an insulin-independent mechanism (Chavez et al. 2008, Ishikura and Klip 2008). However, the role of Tbc1d1 in insulin-stimulated glucose uptake by skeletal muscle tissue is unclear, as is its potential role in the reversal of glucose transport after removal of insulin.
Although the rapid loss of AS160 phosphorylation after insulin removal might seem to be predictable, this expectation was less certain in the context of our earlier observation that, after a bout of exercise, AS160 phosphorylation in rat epitrochlearis can remain increased for at least 3–4 h (Arias et al. 2007). The persistent effect of exercise on AS160 has been confirmed by others who reported that AS160 phosphorylation in human skeletal muscle remained increased at 2.5 (Sriwijitkamol et al. 2007) and 14 h (Frosig et al. 2007) post-exercise compared to resting controls. It is unclear if the differing results after insulin and exercise are because of differences inherent to the two stimuli, or if other differences in the experimental designs of the current study compared to these earlier studies are important. For example, the insulin stimulation was performed in vitro whereas in vivo exercise was used in the earlier studies. Regardless, ultimately the differences in the persistence of elevated AS160 phosphorylation are indicative of differences in the balance of kinases and phosphatases. Although no data are available with regard to the phosphatases, information is available regarding the kinases. Phosphorylation of Akt, the kinase that phosphorylates AS160 with insulin stimulation, was also rapidly reversed with insulin removal. Akt phosphorylation was not increased immediately after exercise by rats (Arias et al. 2007), suggesting that another kinase phosphorylated AS160. AMP-activated protein kinase (AMPK), which can also phosphorylate AS160 (Bruss et al. 2005, Chen et al. 2008, Kramer et al. 2006, Treebak et al. 2007, Treebak et al. 2006), was increased immediately post-exercise, but it had reversed to basal levels at 3–4 h post-exercise at which time AS160 phosphorylation remained elevated (Arias et al. 2007). Because there is no evidence for persistent kinase activation after cessation of insulin stimulation or exercise, our working hypothesis is that differences in the accessibility of the protein phosphatase(s) that dephosphorylate AS160 may explain the differences in the reversal of AS160 phosphorylation after insulin compared to exercise.
In summary, removal of rat epitrochlearis muscle from insulin results in rapid dephosphorylation of Akt and AS160, followed by reversal of insulin's effect on glucose uptake. These results suggest that increased AS160 phosphorylation may be important for maintenance, in addition to initiation, of insulin-stimulated glucose uptake. The observation that the reversal of insulin-stimulated signaling and glucose uptake occurs relatively rapidly in skeletal muscle, the main target for glucose disposal (DeFronzo et al. 1981), has physiological significance for glucose homeostasis. It would be useful for future studies to determine the timecourses for reversal of increased AS160 phosphorylation when the insulin stimulation occurs in vivo. Additionally, it would be interesting to determine if predominantly slow-twitch muscles compared to the predominantly fast-twitch epitrochlearis exhibit similar reversal rates for AS160 phosphorylation after insulin removal. The effects of insulin resistance and/or hyperglycemia on the rate of reversal of insulin signaling upon removal of insulin are also unknown, but may be relevant for glucoregulation. Finally, elucidation of the role of protein phosphatases in the different regulation of AS160 phosphorylation after insulin stimulation and post-exercise would be valuable.
Acknowledgements
This research was supported by National Institutes of Health grants AG-10026 and DK-071771 (to G.D. Cartee)
References
- Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab. 292;2007:E1191–E1200. doi: 10.1152/ajpendo.00602.2006. [DOI] [PubMed] [Google Scholar]
- Brady MJ, Saltiel AR. The role of protein phosphatase-1 in insulin action. Recent Prog Horm Res. 2001;56:157–173. doi: 10.1210/rp.56.1.157. [DOI] [PubMed] [Google Scholar]
- Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes. 2005;54:41–50. doi: 10.2337/diabetes.54.1.41. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab. 2007;32:557–566. doi: 10.1139/H07-026. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy JO. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol. 1989;256:E494–E499. doi: 10.1152/ajpendo.1989.256.4.E494. [DOI] [PubMed] [Google Scholar]
- Chavez JA, Roach WG, Keller SR, Lane WS, Lienhard GE. Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase-activating protein abundant in skeletal muscle, is partially relieved by AMP-activated protein kinase activation. J Biol Chem. 2008;283:9187–9195. doi: 10.1074/jbc.M708934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, Mackintosh C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J. 2008;409:449–459. doi: 10.1042/BJ20071114. [DOI] [PubMed] [Google Scholar]
- Czech MP, Corvera S. Signaling mechanisms that regulate glucose transport. J Biol Chem. 1999;274:1865–1868. doi: 10.1074/jbc.274.4.1865. [DOI] [PubMed] [Google Scholar]
- Defronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- Dugani CB, Randhawa VK, Cheng AW, Patel N, Klip A. Selective regulation of the perinuclear distribution of glucose transporter 4 (GLUT4) by insulin signals in muscle cells. Eur J Cell Biol. 2008;87:337–351. doi: 10.1016/j.ejcb.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Eguez L, Lee A, Chavez JA, Miinea CP, Kane S, Lienhard GE, McGraw TE. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2005;2:263–272. doi: 10.1016/j.cmet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Frosig C, Rose AJ, Treebak JT, Kiens B, Richter EA, Wojtaszewski JF. Effects of endurance exercise training on insulin signaling in human skeletal muscle: interactions at the level of phosphatidylinositol 3-kinase, Akt, and AS160. Diabetes. 2007;56:2093–2102. doi: 10.2337/db06-1698. [DOI] [PubMed] [Google Scholar]
- Garvey WT, Olefsky JM, Marshall S. Insulin receptor down-regulation is linked to an insulin-induced postreceptor defect in the glucose transport system in rat adipocytes. J Clin Invest. 1985;76:22–30. doi: 10.1172/JCI111950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E, McGraw TE. Insulin signaling diverges into Akt-dependent and - independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol Biol Cell. 2006;17:4484–4493. doi: 10.1091/mbc.E06-07-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol. 1994;76:979–985. doi: 10.1152/jappl.1994.76.2.979. [DOI] [PubMed] [Google Scholar]
- Ishikura S, Klip A. Muscle cells engage Rab8A and myosin Vb in insulin-dependent GLUT4 translocation. Am J Physiol Cell Physiol. 2008;295:C1016–C1025. doi: 10.1152/ajpcell.00277.2008. [DOI] [PubMed] [Google Scholar]
- Kahn CR, Goldfine AB. Molecular determinants of insulin action. J Diabetes Complications. 1993;7:92–105. doi: 10.1016/1056-8727(93)90034-v. [DOI] [PubMed] [Google Scholar]
- Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem. 2002;277:22115–22118. doi: 10.1074/jbc.C200198200. [DOI] [PubMed] [Google Scholar]
- Karnieli E, Zarnowski MJ, Hissin PJ, Simpson IA, Salans LB, Cushman SW. Insulin-stimulated translocation of glucose transport systems in the isolated rat adipose cell. Time course, reversal, insulin concentration dependency, and relationship to glucose transport activity. J Biol Chem. 1981;256:4772–4777. [PubMed] [Google Scholar]
- King KL, Okere IC, Sharma N, Dyck JR, Reszko AE, McElfresh TA, Kerner J, Chandler MP, Lopaschuk GD, Stanley WC. Regulation of cardiac malonyl-CoA content and fatty acid oxidation during increased cardiac power. Am J Physiol Heart Circ Physiol. 2005;289:H1033–H1037. doi: 10.1152/ajpheart.00210.2005. [DOI] [PubMed] [Google Scholar]
- Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes. 2006;55:2067–2076. doi: 10.2337/db06-0150. [DOI] [PubMed] [Google Scholar]
- Meyerovitch J, Backer JM, Csermely P, Shoelson SE, Kahn CR. Insulin differentially regulates protein phosphotyrosine phosphatase activity in rat hepatoma cells. Biochemistry. 1992;31:10338–10344. doi: 10.1021/bi00157a023. [DOI] [PubMed] [Google Scholar]
- Miinea CP, Sano H, Kane S, Sano E, Fukuda M, Peranen J, Lane WS, Lienhard GE. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J. 2005;391:87–93. doi: 10.1042/BJ20050887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, Defronzo RA, Sakamoto K, Musi N. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes. 2007;56:836–848. doi: 10.2337/db06-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treebak JT, Birk JB, Rose AJ, Kiens B, Richter EA, Wojtaszewski JF. AS160 phosphorylation is associated with activation of alpha2beta2gamma1- but not alpha2beta2gamma3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab. 2007;292:E715–E722. doi: 10.1152/ajpendo.00380.2006. [DOI] [PubMed] [Google Scholar]
- Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jorgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes. 2006;55:2051–2058. doi: 10.2337/db06-0175. [DOI] [PubMed] [Google Scholar]
- Wallberg-Henriksson H. Glucose transport into skeletal muscle. Influence of contractile activity, insulin, catecholamines and diabetes mellitus. Acta Physiol Scand Suppl. 1987;564:1–80. [PubMed] [Google Scholar]
- Zorzano A, Palacin M, Guma A. Mechanisms regulating GLUT4 glucose transporter expression and glucose transport in skeletal muscle. Acta Physiol Scand. 2005;183:43–58. doi: 10.1111/j.1365-201X.2004.01380.x. [DOI] [PubMed] [Google Scholar]