Abstract
Kawasaki disease (KD) is the most common cause of acquired cardiac disease and acute vasculitis in children in the developed world. Injection of a cell wall extract isolated from Lactobacillus casei (LCCWE) into mice causes a focal coronary arteritis that histopathologically mimics the coronary lesions observed in KD patients. In this study we used this model to investigate the participation of T cells, B cells and dendritic cells (DCs) in the development of coronary arteritis.
Results
RAG1−/−, B cell-null, and wild type (WT) mice were injected with a single dose of LCCWE (500 μg/mouse i.p.). None of the RAG1−/− mice developed coronary arteritis, while 70% of WT and 100% of B cell-null mice developed coronary lesions, indicating that T cells were required for lesion formation. When splenocytes isolated from LCCWE-treated mice were restimulated with LCCWE, we observed significant IFN-γ secretion in WT but not in RAG1−/− mice. Immunohistochemical staining showed F4/80+ macrophages, activated MIDC8+ myeloid DCs (mDCs), plasmacytoid DCs (pDCs), and colocalization of CD3+ T cells with mDCs in coronary artery lesions, suggesting an antigen-driven process.
Conclusions
T cells but not B cells, are required for LCCWE-induced coronary arteritis. Similar to human lesions, the coronary lesions contain macrophages, activated mDCs, and pDCs all in close proximity to T cells, further strengthening the relevance of this mouse model to the immunopathology of coronary disease in KD. These studies are consistent with the interpretation that macrophages and DCs may collaborate with T cells in the pathologic mechanisms of coronary arteritis.
INTRODUCTION
Kawasaki disease (KD) (1) is an acute vasculitis of unknown etiology that predominantly afflicts children under the age of 5. Already the leading cause of acquired heart disease among children in the United States (2–6), recent data report that the incidence of KD is steadily increasing (7). The coronary arteries are a specific target, and the resultant coronary arteritis is characterized histologically by inflammatory cell infiltration and destruction of the arterial media and coronary artery aneurysm formation. Coronary artery aneurysms develop in as many as 25% of untreated children with KD, leading to ischemic heart disease, myocardial infarction and even death (8).
The precise cause of KD is unknown, and is the subject of considerable debate (9). KD is believed to be caused by one or more infectious agents, and for unknown reasons, some individuals seem particularly predisposed to developing the disease (10, 11). The evidence that KD is caused by an infectious agent is primarily derived from epidemiologic studies and clinical observations. First, the illness has a sudden, acute onset, but is self-limited. Second, young children constitute the vast majority of cases, but KD occurs only rarely in children under 6 months of age and virtually never in adulthood. Additionally, epidemiologic studies have identified clear geographical and temporal clustering of cases (11, 12). Partly because of similarities to toxic shock syndrome and related superantigen-driven disorders, it has been proposed that KD also is caused by an as yet unidentified superantigen (12–14). However, this proposal is very controversial, and results from our laboratory (15, 16) and others (17, 18) are most consistent with the interpretation that a conventional antigen is the most likely cause of KD. Supporting this notion, Rowley et al. have recently identified a previously unrecognized, ubiquitous RNA virus in the lungs of fatal KD patients but not controls (19, 20). These investigators demonstrated that the virus forms intracytoplasmic inclusion bodies and can result in persistent infection in ciliated bronchial epithelium and macrophages in lung tissues from late-stage KD fatalities (19, 20) Although therapeutic strategies to control inflammation with intravenous immunoglobulin (IVIG) have reduced morbidity and mortality associated with KD (3, 8, 21, 22), lack of an etiologic agent and incomplete understanding of the molecular mechanisms mediating either the pathologic changes of KD or the mechanism of action of IVIG have hampered the development of targeted and more effective treatment options (10). Other impediments to understanding the etiology of KD include difficulty in studying mechanisms in patients afflicted with the disease and a scarcity of clinical samples available for analyses.
However, a mouse model of coronary arteritis is available that closely mimics the important histological features of the coronary artery lesions seen in patients with KD (23, 24). Almost twenty-five years ago, Lehman et al. reported that a single intraperitoneal injection of a cell wall extract isolated from Lactobacillus casei (LCCWE), reproducibly leads to the development of proximal coronary arteritis that is histopathologically very similar to the coronary arteritis observed in human KD (24, 25). Moreover, as in children with KD (8, 21, 22), coronary arteritis in LCCWE-treated mice responds to therapy with IVIG (25, 26) and is suppressed by treatment with antibodies against tumor necrosis factor-α (27, 28).
We (15) and others (26, 28–32) have utilized this model to test potential mechanisms that might cause KD. Our studies have identified innate immune signalling pathways that are required for the development of LCCWE-induced arteritis (15). Specifically, intact innate immune signalling via TLR2 and MyD88 was necessary for mice to develop coronary arteritis. Acute KD coronary arteritis is characterized by transmural infiltration of CD4 and CD8 T lymphocytes and macrophages and notable absence of B cells (33). In a recent study, we demonstrated that coronary artery lesions of KD patients contain increased numbers of mature and activated myeloid DCs with high HLA-DR expression and frequent T cell contacts detected immunohistochemically, suggesting that mDCs might be activating T cells in situ (16). These findings support the concept that both innate and adaptive immunity participate in the pathophysiology of this vasculitis.
Here we tested the hypothesis that adaptive immunity is also required for the development of LCCWE-induced coronary arteritis. We also wished to determine whether macrophages, DCs and T cells are also present in the histological lesions observed in this mouse model similar to findings as we and others have described in KD patients (16). Our results indicate that T cells are essential for development of arteritis, but B cells are not. Furthermore, similar to findings in KD patients (16, 33), we show infiltration of mouse coronary lesions with T cells, mDCs, pDCs, and macrophages, all of which participate in innate and adaptive immunity. These observations further strengthen the relevance of this LCCWE-induced KD mouse model in mimicking the histopathological findings of the coronary lesions seen in KD patients, and provide important new data suggesting the type of immune cell interactions in the coronary artery lesions (16, 33). However, B cells were dispensable, suggesting that an antibody-mediated response was not importantly involved. Furthermore, when splenocytes isolated from LCCWE-treated mice were subsequently treated with LCCWE in vitro, we observed higher levels of IFN-γ production in WT and B cell null mice, but not in RAG1−/− mice, suggesting a major role for T cells in this model. Since DCs and macrophages that infiltrate the lesion and are in close proximity to T cells in the coronary lesions, our results here together with our previous work (15, 16) indicate that both innate and adaptive immune mechanisms are critical in LCCWE-induced coronary arteritis, and thus suggest that both arms of the immune system may similarly participate in the pathophysiology of the mouse model of KD as well as clinical KD.
MATERIALS AND METHODS
Preparation of the Lactobacillus casei cell Wall Extract (LCCWE)
Group B L. casei (ATCC 11578) were grown in Lactobacillus MRS broth (DIFCO, Detroit, MI), harvested by centrifugation during the exponential growth phase, and washed with phosphate buffered saline (PBS) at pH 7.4. The bacteria were then disrupted by overnight incubation at room temperature in 4% sodium dodecylsulfate (SDS) in twice their packed volume, then washed 8 times with PBS to remove any residual SDS and the cell wall extract prepared as described earlier (15). Briefly, the cell wall fragment preparation was then sonicated for 2 hours using a Heat Systems Ultrasonic W 373 sonicator with a ¾″ horn and a garnet tip at maximum power. During sonication, the cell wall fragments were maintained near 4°C by cooling with dry ice and ethanol. After sonication, the cell wall fragments were spun for 1 hour at 20,000g at 4°C and the supernatant was retained. All procedures were carried out using sterile technique. The L. casei cell wall content of the supernatant was determined by colorimetric phenol-sulfuric acid extraction technique and expressed as mg of total rhamnose/ml of PBS (34). The endotoxin concentration of this preparation was < 2.7 pg/ml as determined by the Limulus amebocyte lysate assay (Associates of Cape Cod, Falmouth, MA).
LCCWE-induced coronary arteritis model
Wild type control C57BL/6, B6.129S7-RAG1 tm1Mom/J (RAG1−/−) and B6.129S2-Igh-6tm1Cgn/J (B-null) mice (Jackson Labs, Bar Harbor, ME) (35) were injected with 500 μg of LCCWE (total rhamnose amount as determined above) in phosphate-buffered saline (PBS) or PBS alone (controls). Mice were sacrificed twenty-one days later; hearts were removed, perfused with PBS, and embedded in optimal cutting temperature (OCT) compound. Coronary arteries were identified in serial sections (6 μm) stained with haematoxylin and eosin. We analyzed 6–10 sections for each mice showing the aortic root and coronary sections. Blinded assessment of the histopathology of the coronary arteries and aortic root was performed as described earlier (15), with particular emphasis on the region in proximity to the ostium of the coronary arteries. It should be noted that no coronary aneurysms were observed in these mice as they were sacrificed prior to aneurysm development.
Splenocytes Harvest and ex-vivo Restimulation experiments
Seven days after LCCWE injection, mice were sacrificed, two spleens per treatment group (injected or not injected) were harvested, and isolated splenocytes were pooled. Spleens were manually crushed between frosted ends of sterile glass slides (VWR International), the resulting suspensions were filtered through a 70 μm nylon cell strainer (BD Falcon), and red blood cells were lysed using red blood cell lysis buffer (eBioscience). Splenocytes were isolated, cultured (106 cells/ml) in a 96-well plate, and restimulated with LCCWE (20ng-5μg/ml) or medium (RPMI, 10% FBS) alone. Supernatant was recovered 72 hours later to assess IFN-γ release by ELISA (eBioscience).
Assessment of Cytokines Release
Splenocytes (0.5 × 106) were seeded into 96-well U-bottom culture plates (BD Falcon) at a final volume of 200 μl/well. Cells were stimulated with LCCWE (5 μg/ml, 1 μg/ml, 200 ng/ml or 40 ng/ml) or control media for 72 h. IFN-γ secreted into supernatants was analyzed by ELISA (eBioscience) using the manufacturer’s suggested protocol.
Assessment of DCs, Macrophages and T cells in the Coronary Arteritis Sections in LCCWE-injected mice
Frozen heart sections were immunohistochemically analyzed for DC, macrophage, and T cell infiltration. Rat anti-mouse MIDC-8 antibody (Serotec), specific for mature DCs identification (36, 37), anti-mouse PDCA-1 specific for pDCs (38), anti-mouse F4/80 (Serotec) specific marker for macrophages (39), and anti-mouse CD3, a T cell marker, were used as described earlier (17). IgG2a and IgG2b were the isotype controls (Serotec).
Computer-assisted image analysis
Digital images were taken at a magnification of 300× with a CCD-camera (Nikon DXM 1200, Düsseldorf, Germany) of three consecutive sections of the aortic root and coronary lesion. DCs, macrophages and T cells in lesions were quantified by computer-assisted histomorphometry (Image-Pro Plus, Media Cybernetics, Silver Spring, MD). For each analysis, the color threshold for immunostained cells was manually adjusted in the images until the computerized detection matched visual interpretation. The numbers of immunostained cells were digitally counted in the defined area (0.25 μm2) of the aortic root/coronary artery section. For each cell type, the mean cell number was calculated (n=3) out of the corresponding three sections for each animal (3 animals/group). Microscopic analyses were performed independently by two different investigators, and intra- and inter-observer coefficients of variabilities were less than 10%.
GM-CSF expression in whole heart cells stimulated with LCCWE ex-vivo
The whole heart was harvested, minced and digested to a single cell suspension. Hearts were harvested from WT, TLR2−/−, MyD88−/− mice and were stimulated with 20 μg/ml LCCWE or 10ng/ml LPS in-vitro. Six hours later, total RNA from heart cells were isolated using RNeasy mini kit (Qiagen) and probed using mouse cardiovascular disease gene array II (atherosclerosis cDNA gene array) following the manufacturer’s recommendations(SuperArray Bioscience). Analysis of the images and quantification was performed by the GE array expression analysis suite software(SuperArray Bioscience), and genes of interest were normalized to the housekeeping gene GAPDH.
GM-CSF induction by LCCWE in murine endothelial cells (ECs)
Primary murine aortic ECs were isolated and purified to >95% purity from wild-type, MyD88−/−, TLR2−/−, and TLR4−/−mice as we previously described (40, 41) ECs were grown to 80% confluency and stimulated overnight with LCCWE (10, 20 μg/ml ) or LPS (10ng/ml). GM-CSF release into the cell-free supernatant was determined after 24 h of treatment by ELISA (eBioscience) as described (42).
Statistical Analysis
Data are expressed as mean ± SEM. Statistical significance was determined by Student’s t test and/or one-way ANOVA for multiple comparisons. A p value less than 0.05 was considered statistically significant.
RESULTS
T cells are present in LCCWE-induced coronary arteritis
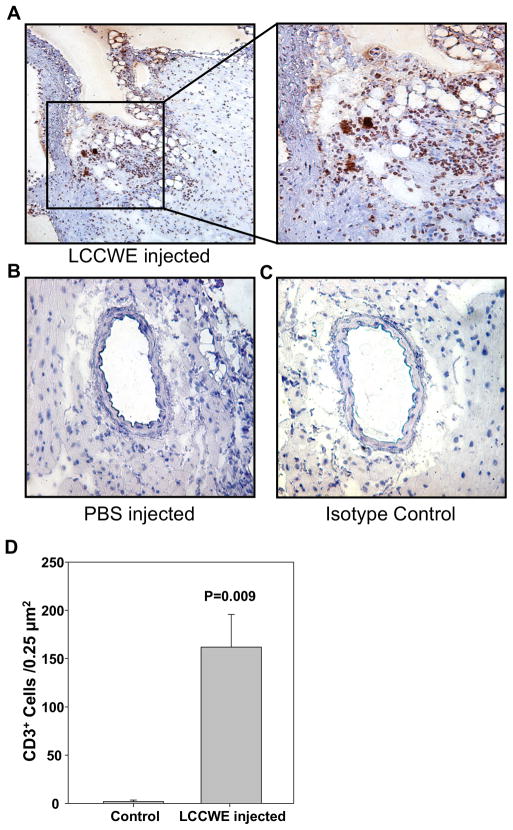
In patients, acute KD coronary arteritis is characterized by infiltration of T lymphocytes (33). To assess the presence of T cells infiltrating the coronary lesions in this mouse model we performed immunohistochemical staining for CD3+ cells in LCCWE-injected mice. As shown in Fig. 1A and 1D, LCCWE-injected wild type mice showed a significantly increased presence of CD3+ cells in the proximity of the aorta and coronary artery lesions. Isotype Control (IgG2a) did not show any positive staining (Figure 1C). No CD3+ T cells were observed in coronary arteries of PBS injected control mice (Figure 1B).
Fig 1.
CD3+ cells are found in LCCWE induced coronary arteritis lesions. 21 days after i. p. injection with LCCWE, WT mice were sacrificed and the hearts were frozen in OCT. IHC was performed using anti-CD3. A) LCCWE injected wt mice (40X and 150X). B) PBS injected control mice (40X). C) Isotype control rat IgG2a (40X). D) Quantification of CD3+ cells in the LCCWE induced coronary lesion (n= 3 mice/group).
RAG1−/− mice do not develop LCCWE-induced coronary arteritis
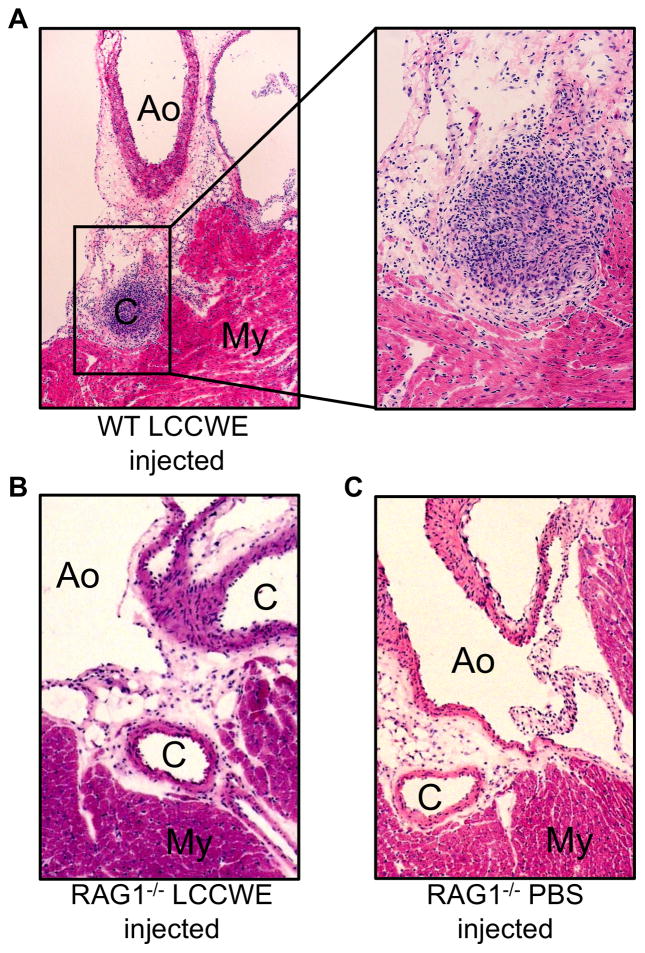
To better elucidate the role of the adaptive immunity in the development of coronary arteritis in the mouse model, we utilized RAG1−/− mice, which do not possess mature T cells or B cells owing to faulty VDJ recombination (43). Injection of LCCWE induced severe focal acute coronary arteritis in 7 out of 10 wild type mice (Table 1 and Figure 2A). Histological results obtained from WT mice 21 days post LCCWE injection appeared qualitatively similar to those we reported previously in WT mice (15, 24). The coronary lesions consisted of a localized, nodular infiltrate of acute and chronic inflammatory cells localized to the most proximal portion of the coronary artery. Often, the combination of inflammation and intimal proliferation resulted in complete occlusion of the arterial lumen (Fig. 2A). In contrast, we observed a complete absence of inflammatory infiltrates in the coronary arteries of the LCCWE-injected RAG1−/− mice (Table 1 and Figure 2B), which appeared histologically indistinguishable from the PBS injected wild type mice (Figure 2C). These observations indicate that adaptive immune system (T and/or B cells) is required in LCCWE-induced coronary lesion formation.
Table I.
| WT | RAG1−/− | B null | |
|---|---|---|---|
| (+) Coronary Arteritis | 7/10 (70%) | 0/8 (0%) *** | 8/11 (66.67%) |
p<0.001
LCCWE-induced coronary arteritis in RAG1−/− and B cell null mice.
Fig 2.
T-cells are required for LCCWE induced coronary arteritis. Wt and RAG1−/− mice were injected with LCCWE and sacrificed 21 days later. Hematoxylin and eosin staining was performed on frozen sections of the heart. A) LCCWE injected wild type (40X and 150X). B) RAG1−/− mice injected with LCCWE (40X). C) PBS injected RAG1−/− mice (40x). (C= coronary artery, Ao= aorta, My=myocardium)
B cells are not required for LCCWE-induced coronary arteritis
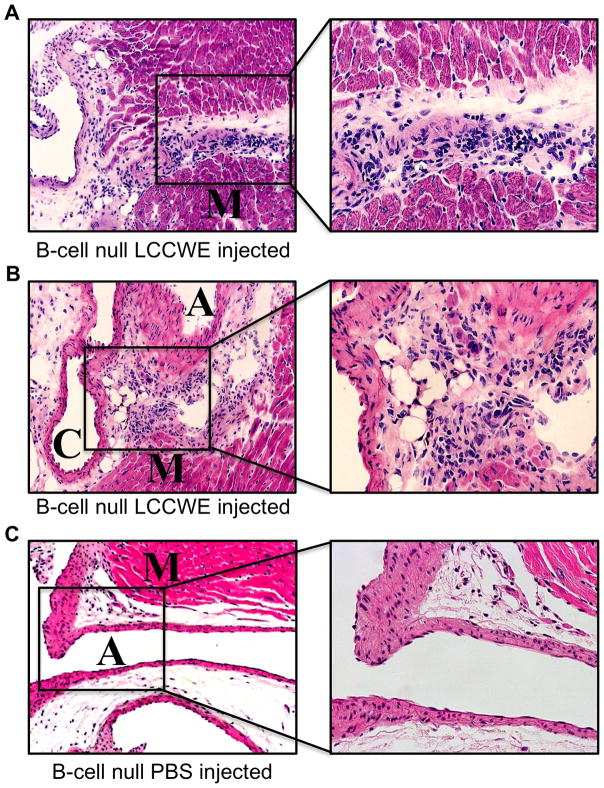
To determine whether T cells, B cells, or both are required to trigger coronary arteritis, we injected B cell-deficient mice (35) with LCCWE or PBS vehicle only (3C). These B cell-null mice have a mutation that blocks B cell development at the pro-B stage (35). Almost 70% of B cell-null mice developed coronary lesions. Histological analysis revealed a severe inflammatory cell influx into coronary artery lesions (Figures 3A, 3B). Intimal proliferation and massive infiltration of inflammatory cells was observed and tended to occlude the artery lumen (Figures 3A and 3B). B null LCCWE-injected mice showed histopathology similar to that of wild type LCCWE-injected mice. Increased inflammatory cells were also detected at the level of the myocardium immediately adjacent to the coronary artery (Figure 3A). These results indicate that T cells are required to produce coronary arteritis after LCCWE injection, but B cells are unnecessary. A summary of the coronary lesion development can be found in Table 1.
Fig 3.
B-cells are not required for LCCWE induced coronary arteritis. B-cell null mice were injected with LCCWE and sacrificed 21 days later. Hematoxylin and eosin staining was performed on frozen sections of the heart. A, B) LCCWE injected B-cell null mice (40X, 200X). C) PBS injected B-cell null mice (40X). (A=aorta, C-coronary artery, and M-myocardium).
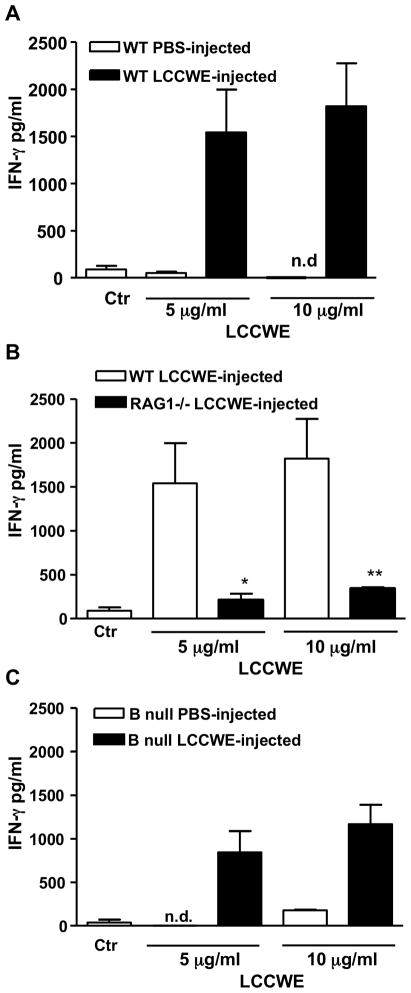
In-vitro restimulation with LCCWE induces IFN-γ secretion by splenocytes isolated from LCCWE-injected wild type but not RAG1−/− mice
Since IFN-γ expression is a hallmark of a Th1 response (44), we wished to determine antigen-specific recall responses in WT and RAG1−/− mice by measuring IFN-γ release from splenocytes following LCCWE restimulation ex-vivo. We harvested splenocytes 7 days after injection of LCCWE into WT, RAG1−/−, or B null mice. We re-stimulated these cells with LCCWE (5 μg/ml, 1 μg/ml, 200 ng/ml, or 40 ng/ml). WT and B null splenocytes showed a significant increase in IFN-γ release after LCCWE stimulation (p < 0.01; Figures 4A–C). However, RAG1−/− splenocytes produced very low levels of IFN-γ compared to the medium-treated wild type-derived splenocytes (Figure 4B). In control experiments, IFN-γ secretion was not detectable from splenocytes, obtained from PBS-treated WT mice and stimulated with LCCWE (Figure 4A). These results indicate that T cells are required to produce a specific response to LCCWE, which in turn suggests that the adaptive immune system is involved in the pathologic mechanism mediating coronary arteritis. It should be noted that while B-cell null splenocytes restimulated with LCCWE did still produce a significant amount of IFN-γ, the level was slightly reduced compared to WT splenocytes. This was probably due to the fact that B-cells can act as weak antigen presenting cells. While these results suggest that B cells are not essential in LCCWE-induced arteritis, it does not rule out any potential role B cells may play in this model.
Fig. 4.
Restimulation of splenocytes with LCCWE. Splenocytes harvested 7 days post injection with LCCWE and stimulated in vitro with LCCWE for 72 hours. Supernatants were tested by ELISA for IFN-γ production. A) WT splenocytes harvested from mice injected with either LCCWE or PBS control. B) IFN-γ production inRAG1−/− and WT splenocytes. C) B-cell null splenocytes harvested from mice injected with either LCCWE or PBS control. Data are shown as mean ±SEM n=3. Statistical differences were determined by means of Student’s t test and are represented as * and ** for p<0.05 and p<0.01.
Both activated myeloid DCs (mDCs) and plasmacytoid DCs (pDCs) as well as macrophages are present in LCCWE-induced coronary artery lesion
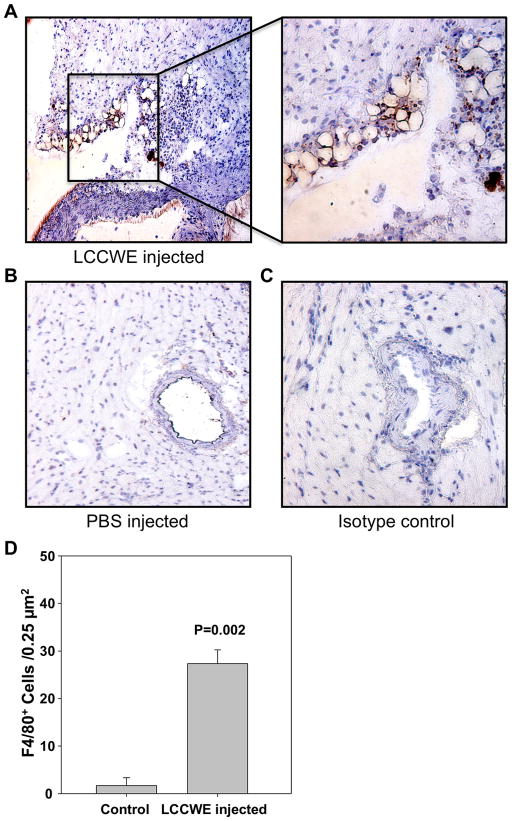
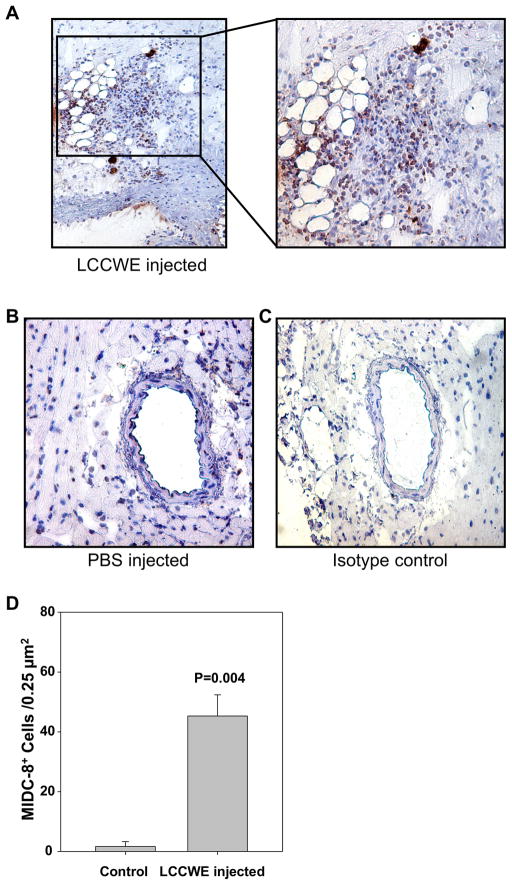
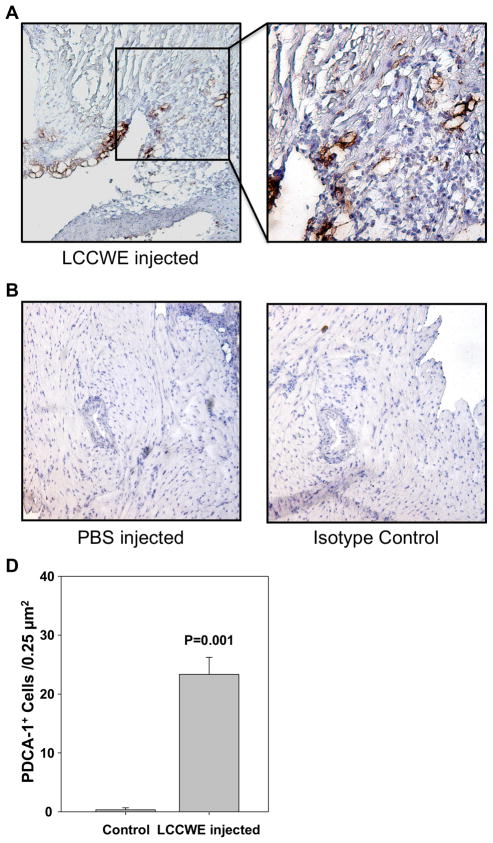
Significant numbers of mDCs accumulate in coronary lesions in patients with KD, particularly in the intimal layer of affected arteries as we have recently reported (16). To determine if this also occurs in the LCCWE-induced coronary arteritis mouse model that mimics the human KD lesions, we performed immunohistochemical analyses using antibodies against MICD-8 to identify mDCs (36, 37) PDCA-1 antibody to identify pDCs and F4/80 to identify macrophages in the mouse coronary lesions (39). As shown in Figure 5A and D, macrophages were significantly more numerous in the proximal coronary arteries of LCCWE-injected mice compared to PBS-injected controls (Figure 5B). Similarly, significantly increased infiltration of myeloid MIDC-8 positive DCs (mDCs) was observed in LCCWE-induced coronary lesions (Figure 6A and D) but not in PBS-treated control mice (Figure 6B). Isotype controls for macrophage and mDC staining did not show any positive staining (Figures 5C and 6C). Interestingly, PDCA-1 immunostaining also showed significantly increased numbers of infiltrating pDCs in coronary artery lesions after LCCWE injection in WT mice compared to PBS injected control mice (Figure 7A and D), and isotype controls were negative (Figures 7B, C). These data suggest that, like human coronary artery lesions obtained from KD patients, the LCCWE-induced coronary arteritis mouse model also mimics the mDC, pDC and macrophages infiltration in close proximity to T cells, further validating this mouse model. The results also imply antigen presentation and an ongoing antigen-specific inflammatory response to LCCWE in the coronary artery lesions.
Fig 5.
Macrophages are found in LCCWE induced coronary arteritis lesions. 21 days after i. p. injection with LCCWE, wt mice were sacrificed and the hearts were frozen in OCT. IHC was performed using anti-F4/80. A) LCCWE injected wt mice (40X and 150X). B) PBS injected control mice (40X). C) Isotype control rat IgG2b (40X). D) Quantification of F4/80+ cells in the LCCWE induced coronary lesion (n= 3 mice/group).
Fig 6.
Activated myeloid dendritic cells are found in LCCWE induced coronary arteritis lesions. 21 days after i. p. injection with LCCWE, wt mice were sacrificed and the hearts were frozen in OCT. IHC was performed using anti-MIDC-8. A) LCCWE injected wt mice (40X and 150X). B) PBS injected control mice (40X). C) Isotype control rat IgG2a (40X). D) Quantification of MIDC8+ cells in the LCCWE induced coronary lesion (n= 3 mice/group).
Fig 7.
Plasmacytoid dendritic cells are found in LCCWE induced coronary arteritis lesions. 21 days after i. p. injection with LCCWE, wt mice were sacrificed and the hearts were frozen in OCT. IHC was performed using anti-PDCA-1. A) LCCWE injected wt mice (40X and 150X). B) PBS injected control mice (40X). C) Isotype control rat IgG2a (40X). D) Quantification of PDCA-1+ cells in the LCCWE induced coronary lesion (n= 3 mice/group).
LCCWE induces GM-CSF gene and protein expression in mouse aortic Endothelial Cells in a TLR2- and MyD88-dependent manner
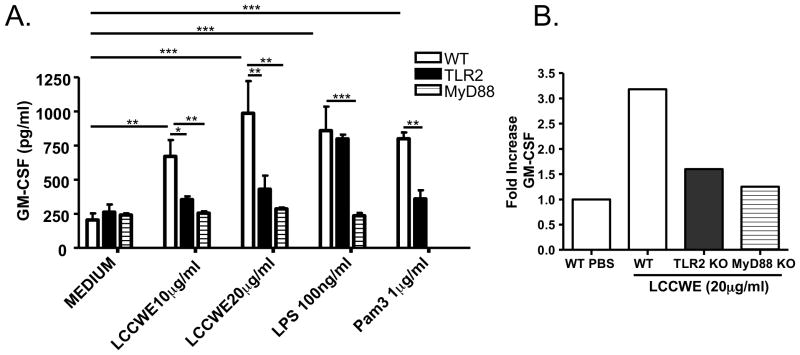
We have previously reported that LCCWE-induced coronary lesions are absent in TLR2−/− or MyD88−/− mice, suggesting the critical role of the TLR2/MyD88 innate immune signalling pathway in development of lesions (15). A recent study reported that GM-CSF plays a key role in DC migration into atherosclerotic lesions and that lack of GM-CSF in hypercholesterolemic mice resulted in smaller atherosclerotic lesions and a dramatic decrease in the numbers of DCs accumulating in these atherosclerotic plaques (45). Considering atherosclerosis is another inflammatory condition of the arteries, including the coronaries, we wished to investigate whether LCCWE could stimulate expression of GM-CSF in endothelial cells, which in turn might lead to increased recruitment of DCs to coronary artery lesions in our mouse model amplifying the inflammatory response. To test this possibility we isolated primary mouse aortic ECs (MAECs) from wild-type or TLR2, TLR4, or MyD88−/− mice, and stimulated these cells with LCCWE (or PBS), and assessed GM-CSF secretion. LCCWE significantly increased GM-CSF release in wild type MAECs, while there was a significantly reduced GM-CSF release from ECs obtained from TLR2−/− or MyD88−/− mice (Figure 8A). Furthermore, we also observed significantly increased GM-CSF gene expression (over 3-fold increase) in whole heart cells from mice treated with LCCWE ex vivo when compared with PBS treated controls, and the GM-CSF gene expression was significantly decreased in hearts obtained from TLR2−/− and MyD88−/− mice, which are resistant in LCCWE-induced coronary lesions (Figure 8B). These results suggest that the differential induction of LCCWE-induced GM-CSF production may play a role in the recruiting and/or retaining activated DCs in the coronary lesions of LCCWE-injected mice.
Figure 8.
LCCWE induces GM-CSF gene and protein expression. A) Primary murine aortic endothelial cells from WT, TLR2−/−, and MyD88−/− mice were stimulated with LCCWE, and GM-CSF production was measured by ELISA. Data are presented as mean values ± SEM; n = 5–7. Statistically significant differences are denoted by *, **, and ***, which indicate p < 0.05, p < 0.01, and p < 0.001, respectively (Student’s t test and one-way ANOVA). B) GM-CSF expression levels were measured in whole heart cells obtained from WT, TLR2−/−, and MyD88−/− mice after LCCWE stimulation ex-vivo, and measured by cDNA gene array. Data were normalized to GAPDH expression and fold increase was determined relative to PBS controls.
DISCUSSION
KD is now recognized as the leading cause of acquired heart disease in children in the United States and the developed world (1, 3). The underlying etiology and mechanisms leading to medium and small vessel inflammatory response and coronary artery lesions and aneurysms that are the hallmarks of KD are largely unknown. Furthermore, it is unclear how and why acute systemic immune activation leads to chronic inflammatory arterial damage that localizes to the coronary arteries. The activation of the immune system in KD seems to encompass both the innate and adaptive immunity. Both human studies and animal models indicate the presence of various activated immune cells in coronary artery lesions (15, 16, 23–28, 30, 31, 46, 47). Intact innate immune signaling pathways are essential for such lesions to develop (15). Neutrophils are present in the early phase of the inflammatory pattern, rapidly followed by macrophages, dendritic cells (DCs), plasma cells and T cells (33, 48). Over the years, considerable evidence has accumulated to suggest that T cells play a significant role in the pathogenesis of KD, and several studies have documented the presence of infiltrating T lymphocytes in the human coronary artery lesions (33, 49). We have also shown that human coronary lesions obtained from children with fatal KD have significant numbers of mature, activated mDCs, pDCs and macrophages in close contact with CD3+ T cells, suggesting and antigen-driven adaptive immune process (16).
There are striking similarities in the histopathology and kinetics of disease between human KD and LCCWE-induced coronary arteritis mouse model (23–25, 47, 50). Initially, we analyzed the specific innate and adaptive immune responses and infiltrating cell types between the human and mouse model of coronary artery lesions. We have shown that LCCWE-induced coronary arteritis in mice requires intact signaling via TLR2 and MyD88, both of which are key participants in innate immunity (15). However, those studies did not address the potential participation of acquired immune system in the mouse model of coronary arteritis.
Here we report direct evidence indicating that acquired immune mechanisms participate in the development of LCCWE-induced coronary arteritis in mice. We show that T cells but not B cells are required for development of arteritis. Coronary artery lesions in LCCWE-injected mice showed positive CD3 immunostaining, but more importantly, no RAG1−/− mice develop coronary lesions after LCCWE treatment. In contrast, B cell-deficient mice developed arteritis at the same frequency and severity as wild type mice. Since RAG1−/− mice have no T cells or B cells, these results indicate that T cells are essential for coronary arteritis to develop, but B cells are not. The fact that B cells are dispensable in turn indicates that an antibody-mediated response either did not occur, or was a non-essential contributor to the pathophysiology of the disease. Our results therefore strongly implicate a major role for T cells in this murine model of coronary arteritis, and suggest that the same is likely true for patients with KD, since in both cases the lesions contain subpopulations of the same lymphocytes (16, 33).
Splenocytes from LCCWE-treated RAG1−/− mice failed to produce IFN-γ when re-stimulated ex vivo. LCCWE causes increased local expression of IFN-γ in affected arteries in a biphasic manner, with an initial spike 3 to 7 days after LCCWE, then a late spike at 28 to 42 days (51). However, IFN-γ expression in the arterial lesion does not appear to be essential for development of lesions, since LCCWE can still induce coronary lesions in IFN-γ-deficient mice (51). Nevertheless, our ex vivo results where restimulation of splenocytes with LCCWE elicited IFN-γ production clearly support the interpretation that direct activation of antigen-specific T cells occurs in response to LCCWE treatment and invariably accompanies development of arteritis. Because T cells produce a variety of cytokines and chemokines, it seems reasonable to surmise that IFN-γ is redundant, since even when T cells are genetically incapable of producing IFN-γ, they can still promote focal arteritis after LCCWE treatment (51). Consistent with this concept, another study reported that both TNF-α receptor knockout mice and wild type mice treated with the TNF-α blocking agent etanercept were protected from development of coronary lesions (31). These results are consistent with the interpretation that TNF-α is necessary for induction of coronary artery inflammation and aneurysm formation in the LCCWE-induced coronary arteritis mouse model. Of interest, TNF-α blocking agents have also been successfully used clinically in KD patients refractory to standard treatment with intravenous immune globulin and aspirin (52), suggesting that TNF-α expression may similarly be required for the development and/or progression of coronary lesions in clinical KD.
In addition to T cells, we also observed that mDCs, pDCs, and macrophages accumulate in the coronary artery lesions in LCCWE-injected mice. All of these cell types are key contributors to innate and adaptive immunity. These findings are very similar to observations we described on human coronary artery tissues obtained from KD patients (16). Collectively, these previous studies (15, 16) and results reported here strongly implicate the involvement of both innate and adaptive immune mechanisms in LCCWE-induced coronary arteritis, and thus suggest that both arms of the immune system also participate in the pathophysiology of clinical KD. It is interesting that we not only found pDCs in the coronary lesions in both coronary artery lesions of KD patients (16) and LCCWE-injected mice but also that in both cases pDCs were co-localized with mDCs, suggesting that activation of mDCs could lead to exacerbation of the coronary lesions. Previous studies investigating the role of pDCs in human atherosclerosis, another example of focal arterial inflammatory disease (53, 54), similarly found clustering of pDCs with mDCs in atherosclerotic plaques leading to enhanced cytotoxic T cell responses. Furthermore, production of IFN-α by pDCs increased expression of TNF-α in atherosclerotic plaques, thus exacerbating the arterial inflammation (55). Therefore, it is tempting to speculate that in both atherosclerotic coronary artery plaques and in KD lesions, pDCs may amplify focal inflammation and may contribute to the development and progression of these vascular lesions. However, this possibility requires further study.
Recently, Shaposhnik et al. showed that GM-CSF plays a key role in DC migration into atherosclerotic lesions in hypercholesterolemic mouse models of atherosclerosis and that mice deficient in GM-CSF exhibit both diminished lesion size and significantly decreased DC accumulation in the atherosclerotic plaques (42, 45). Indeed, we recently reported that DC accumulation in Chlamydia pneumoniae-mediated acceleration of atherosclerotic lesions in the aortic root of ApoE-null mice were related to bacteria-induced GM-CSF production (42). Similarly, here we observed that LCCWE induced a dose-dependent expression and release of GM-CSF in murine primary aortic ECs in a TLR2- and MyD88-dependent manner. Therefore, it is tempting to propose that the LCCWE-induced GM-CSF production may play a role in the migration and accumulation of DCs into the coronary artery lesions that we observed in this mouse model of arteritis.
In conclusion, we demonstrate that both innate and adaptive immunity are essential for development of coronary lesions in the LCCWE mouse model of coronary arteritis, and that while T cells are essential, B cells are dispensable. Additionally, we highlight the histological similarities between this mouse model of coronary arteritis and human coronary artery lesions seen in patients with KD, providing further validation for the use of this model to study the immunopathology of coronary lesions of KD. These findings expand our understanding of the cellular mechanisms regulating immune activation and localized inflammation in the coronary arteries, which may potentially lead to improved treatments and to minimize the long-term morbidity and mortality in children with KD.
Acknowledgments
Supported by R01AI072726-01A to MA, 1 K08 A1070162-01 to DJS
References
- 1.Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974;54:271–276. [PubMed] [Google Scholar]
- 2.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 3.Burns JC, Glode MP. Kawasaki syndrome. Lancet. 2004;364:533–544. doi: 10.1016/S0140-6736(04)16814-1. [DOI] [PubMed] [Google Scholar]
- 4.Rowley AH, Shulman ST. Kawasaki syndrome. Clin Microbiol Rev. 1998;11:405–414. doi: 10.1128/cmr.11.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taubert KA, Rowley AH, Shulman ST. Seven-year national survey of Kawasaki disease and acute rheumatic fever. Pediatr Infect Dis J. 1994;13:704–708. doi: 10.1097/00006454-199408000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Curtis N, Levin M. Kawasaki disease thirty years on. Curr Opin Pediatr. 1998;10:24–33. doi: 10.1097/00008480-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura Y, Yashiro M, Uehara R, Oki I, Kayaba K, Yanagawa H. Increasing incidence of Kawasaki disease in Japan: nationwide survey. Pediatr Int. 2008;50:287–290. doi: 10.1111/j.1442-200X.2008.02572.x. [DOI] [PubMed] [Google Scholar]
- 8.Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, Glode MP, Mason WH, Reddy V, Sanders SP, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 9.Guzman-Cottrill JA, Shulman ST. Recent developments and controversies in Kawasaki disease. Minerva Pediatr. 2004;56:51–61. [PubMed] [Google Scholar]
- 10.Burns JC. The riddle of Kawasaki disease. N Engl J Med. 2007;356:659–661. doi: 10.1056/NEJMp068268. [DOI] [PubMed] [Google Scholar]
- 11.Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–2738. doi: 10.1161/CIRCULATIONAHA.106.179568. [DOI] [PubMed] [Google Scholar]
- 12.Matsubara K, Fukaya T. The role of superantigens of group A Streptococcus and Staphylococcus aureus in Kawasaki disease. Curr Opin Infect Dis. 2007;20:298–303. doi: 10.1097/QCO.0b013e3280964d8c. [DOI] [PubMed] [Google Scholar]
- 13.Gupta-Malhotra M, Viteri-Jackson A, Thomas W, Zabriskie JB. Antibodies to highly conserved peptide sequence of staphylococcal and streptococcal superantigens in Kawasaki disease. Exp Mol Pathol. 2004;76:117–121. doi: 10.1016/j.yexmp.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Nomura Y, Masuda K, Yoshinaga M, Takei S, Miyata K. Possible relationship between streptococcal pyrogenic exotoxin A and Kawasaki syndrome in patients older than six months of age. Pediatr Infect Dis J. 2003;22:794–798. doi: 10.1097/01.inf.0000083824.15218.de. [DOI] [PubMed] [Google Scholar]
- 15.Rosenkranz ME, Schulte DJ, Agle LM, Wong MH, Zhang W, Ivashkiv L, Doherty TM, Fishbein MC, Lehman TJ, Michelsen KS, Arditi M. TLR2 and MyD88 contribute to Lactobacillus casei extract-induced focal coronary arteritis in a mouse model of Kawasaki disease. Circulation. 2005;112:2966–2973. doi: 10.1161/CIRCULATIONAHA.105.537530. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz A, Rowley A, Schulte DJ, Doherty TM, Schroder NW, Fishbein MC, Kalelkar M, Cicha I, Schubert K, Daniel WG, Garlichs CD, Arditi M. Activated myeloid dendritic cells accumulate and co-localize with CD3+ T cells in coronary artery lesions in patients with Kawasaki disease. Exp Mol Pathol. 2007;83:93–103. doi: 10.1016/j.yexmp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Rowley AH, Baker SC, Shulman ST, Fox LM, Takahashi K, Garcia FL, Crawford SE, Chou P, Orenstein JM. Cytoplasmic inclusion bodies are detected by synthetic antibody in ciliated bronchial epithelium during acute Kawasaki disease. J Infect Dis. 2005;192:1757–1766. doi: 10.1086/497171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowley AH, Baker SC, Shulman ST, Garcia FL, Guzman-Cottrill JA, Chou P, Terai M, Kawasaki T, Kalelkar MB, Crawford SE. Detection of antigen in bronchial epithelium and macrophages in acute Kawasaki disease by use of synthetic antibody. J Infect Dis. 2004;190:856–865. doi: 10.1086/422648. [DOI] [PubMed] [Google Scholar]
- 19.Rowley AH, Baker SC, Shulman ST, Garcia FL, Fox LM, Kos IM, Crawford SE, Russo PA, Hammadeh R, Takahashi K, Orenstein JM. RNA-containing cytoplasmic inclusion bodies in ciliated bronchial epithelium months to years after acute Kawasaki disease. PLoS ONE. 2008;3:e1582. doi: 10.1371/journal.pone.0001582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowley AH, Baker SC, Orenstein JM, Shulman ST. Searching for the cause of Kawasaki disease--cytoplasmic inclusion bodies provide new insight. Nat Rev Microbiol. 2008;6:394–401. doi: 10.1038/nrmicro1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furusho K, Kamiya T, Nakano H, Kiyosawa N, Shinomiya K, Hayashidera T, Tamura T, Hirose O, Manabe Y, Yokoyama T, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1984;2:1055–1058. doi: 10.1016/s0140-6736(84)91504-6. [DOI] [PubMed] [Google Scholar]
- 22.Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, Colan SD, Duffy CE, Fulton DR, Glode MP, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633–1639. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 23.Lehman TJ, Warren R, Gietl D, Mahnovski V, Prescott M. Variable expression of Lactobacillus casei cell wall-induced coronary arteritis: an animal model of Kawasaki’s disease in selected inbred mouse strains. Clin Immunol Immunopathol. 1988;48:108–118. doi: 10.1016/0090-1229(88)90161-4. [DOI] [PubMed] [Google Scholar]
- 24.Lehman TJ, Walker SM, Mahnovski V, McCurdy D. Coronary arteritis in mice following the systemic injection of group B Lactobacillus casei cell walls in aqueous suspension. Arthritis Rheum. 1985;28:652–659. doi: 10.1002/art.1780280609. [DOI] [PubMed] [Google Scholar]
- 25.Lehman TJ. Can we prevent long term cardiac damage in Kawasaki disease? Lessons from Lactobacillus casei cell wall-induced arteritis in mice. Clin Exp Rheumatol. 1993;11(Suppl 9):S3–6. [PubMed] [Google Scholar]
- 26.Myones BL, Bathoria JM, Lehman TJA, Shulman ST. Lactobacillus casei-inducible coronary arteritis in a murine model. In: KH, editor. The 5th International Kawasaki Disease Symposium. Elsevier Science; Fukuoka, Japan: 1995. p. 252. [Google Scholar]
- 27.Lehman TJA, Sherry B, Gietl DM, Nguyen HT, Cerami A. Suppression of Lactobacillus casei cell wall-induced coronary arteritis in mice by antibody to murine tumor necrosis factor. Proceedings of the Third International Conference on Kawasai Disease; Tokyo, Japan. 1988. pp. 203–206. [Google Scholar]
- 28.Brahn E, Lehman TJ, Peacock DJ, Tang C, Banquerigo ML. Suppression of coronary vasculitis in a murine model of Kawasaki disease using an angiogenesis inhibitor. Clin Immunol. 1999;90:147–151. doi: 10.1006/clim.1998.4645. [DOI] [PubMed] [Google Scholar]
- 29.Lau AC, Duong TT, Ito S, Yeung RS. Matrix metalloproteinase 9 activity leads to elastin breakdown in an animal model of Kawasaki disease. Arthritis Rheum. 2008;58:854–863. doi: 10.1002/art.23225. [DOI] [PubMed] [Google Scholar]
- 30.Duong TT, Silverman ED, Bissessar MV, Yeung RS. Superantigenic activity is responsible for induction of coronary arteritis in mice: an animal model of Kawasaki disease. Int Immunol. 2003;15:79–89. doi: 10.1093/intimm/dxg007. [DOI] [PubMed] [Google Scholar]
- 31.Hui-Yuen JS, Duong TT, Yeung RS. TNF-alpha is necessary for induction of coronary artery inflammation and aneurysm formation in an animal model of Kawasaki disease. J Immunol. 2006;176:6294–6301. doi: 10.4049/jimmunol.176.10.6294. [DOI] [PubMed] [Google Scholar]
- 32.Okitsu-Negishi S, Nakano I, Suzuki K, Hashira S, Abe T, Yoshino K. The induction of cardioangitis by Lactobacillus casei cell wall in mice. I. The cytokine production from murine macrophages by Lactobacillus casei cell wall extract. Clin Immunol Immunopathol. 1996;78:30–40. doi: 10.1006/clin.1996.0005. [DOI] [PubMed] [Google Scholar]
- 33.Brown TJ, Crawford SE, Cornwall ML, Garcia F, Shulman ST, Rowley AH. CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J Infect Dis. 2001;184:940–943. doi: 10.1086/323155. [DOI] [PubMed] [Google Scholar]
- 34.Dubois M, Giles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 35.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 36.Breel M, Mebius RE, Kraal G. Dendritic cells of the mouse recognized by two monoclonal antibodies. Eur J Immunol. 1987;17:1555–1559. doi: 10.1002/eji.1830171105. [DOI] [PubMed] [Google Scholar]
- 37.Wolenski M, Cramer SO, Ehrlich S, Steeg C, Grossschupff G, Tenner-Racz K, Racz P, Fleischer B, von Bonin A. Expression of CD83 in the murine immune system. Med Microbiol Immunol. 2003;192:189–192. doi: 10.1007/s00430-003-0179-9. [DOI] [PubMed] [Google Scholar]
- 38.Wendland M, Czeloth N, Mach N, Malissen B, Kremmer E, Pabst O, Forster R. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci U S A. 2007;104:6347–6352. doi: 10.1073/pnas.0609180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 40.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shuang C, Wong MH, Schulte DJ, Arditi M, Michelsen KS. Differential expression of Toll-like receptor 2 (TLR2) and responses to TLR2 ligands between human and murine vascular endothelial cells. J Endotoxin Res. 2007;13:281–296. doi: 10.1177/0968051907085096. [DOI] [PubMed] [Google Scholar]
- 42.Naiki Y, Sorrentino R, Wong MH, Michelsen KS, Shimada K, Chen S, Yilmaz A, Slepenkin A, Schroder NW, Crother TR, Bulut Y, Doherty TM, Bradley M, Shaposhnik Z, Peterson EM, Tontonoz P, Shah PK, Arditi M. TLR/MyD88 and liver X receptor alpha signaling pathways reciprocally control Chlamydia pneumoniae-induced acceleration of atherosclerosis. J Immunol. 2008;181:7176–7185. doi: 10.4049/jimmunol.181.10.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 44.Berenson LS, Ota N, Murphy KM. Issues in T-helper 1 development--resolved and unresolved. Immunol Rev. 2004;202:157–174. doi: 10.1111/j.0105-2896.2004.00208.x. [DOI] [PubMed] [Google Scholar]
- 45.Shaposhnik Z, Wang X, Weinstein M, Bennett BJ, Lusis AJ. Granulocyte macrophage colony-stimulating factor regulates dendritic cell content of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2007;27:621–627. doi: 10.1161/01.ATV.0000254673.55431.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeshita S, Nakatani K, Tsujimoto H, Kawamura Y, Kawase H, Sekine I. Increased levels of circulating soluble CD14 in Kawasaki disease. Clin Exp Immunol. 2000;119:376–381. doi: 10.1046/j.1365-2249.2000.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lehman TJ, Mahnovski V. Animal models of vasculitis. Lessons we can learn to improve our understanding of Kawasaki disease. Rheum Dis Clin North Am. 1988;14:479–487. [PubMed] [Google Scholar]
- 48.Ichiyama T, Yoshitomi T, Nishikawa M, Fujiwara M, Matsubara T, Hayashi T, Furukawa S. NF-kappaB activation in peripheral blood monocytes/macrophages and T cells during acute Kawasaki disease. Clin Immunol. 2001;99:373–377. doi: 10.1006/clim.2001.5026. [DOI] [PubMed] [Google Scholar]
- 49.Terai M, Kohno Y, Namba M, Umemiya T, Niwa K, Nakajima H, Mikata A. Class II major histocompatibility antigen expression on coronary arterial endothelium in a patient with Kawasaki disease. Hum Pathol. 1990;21:231–234. doi: 10.1016/0046-8177(90)90135-r. [DOI] [PubMed] [Google Scholar]
- 50.Lehman TJ, Sherry B, Gietl DM, Nguyen HT, Cerami A. Suppression of Lactobacillus casei cell wall-induced coronary arteritis in mice by antibody to murine tumor necrosis factor. Third International Conference on Kawasaki Disease; Tokyo, Japan. 1988. pp. 203–206. [Google Scholar]
- 51.Chan WC, Duong TT, Yeung RS. Presence of IFN-gamma does not indicate its necessity for induction of coronary arteritis in an animal model of Kawasaki disease. J Immunol. 2004;173:3492–3503. doi: 10.4049/jimmunol.173.5.3492. [DOI] [PubMed] [Google Scholar]
- 52.Burns JC, Best BM, Mejias A, Mahony L, Fixler DE, Jafri HS, Melish ME, Jackson MA, Asmar BI, Lang DJ, Connor JD, Capparelli EV, Keen ML, Mamun K, Keenan GF, Ramilo O. Infliximab Treatment of Intravenous Immunoglobulin-Resistant Kawasaki Disease. J Pediatr. 2008 doi: 10.1016/j.jpeds.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation. 2006;114:2482–2489. doi: 10.1161/CIRCULATIONAHA.106.642801. [DOI] [PubMed] [Google Scholar]
- 54.Niessner A, Shin MS, Pryshchep O, Goronzy JJ, Chaikof EL, Weyand CM. Synergistic proinflammatory effects of the antiviral cytokine interferon-alpha and Toll-like receptor 4 ligands in the atherosclerotic plaque. Circulation. 2007;116:2043–2052. doi: 10.1161/CIRCULATIONAHA.107.697789. [DOI] [PubMed] [Google Scholar]
- 55.Szabo G, Dolganiuc A. The role of plasmacytoid dendritic cell-derived IFN alpha in antiviral immunity. Crit Rev Immunol. 2008;28:61–94. doi: 10.1615/critrevimmunol.v28.i1.40. [DOI] [PubMed] [Google Scholar]
- 56.Xu C, Poirier B, Van Huyen JP, Lucchiari N, Michel O, Chevalier J, Kaveri S. Modulation of endothelial cell function by normal polyspecific human intravenous immunoglobulins: a possible mechanism of action in vascular diseases. Am J Pathol. 1998;153:1257–1266. doi: 10.1016/S0002-9440(10)65670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]