Abstract
We have created hyaluronic acid (HA)-based, cell-adhesive hydrogels that direct the initial attachment and the subsequent differentiation of human mesenchymal stem cells (MSCs) into pre-osteoblasts without osteogenic supplements. HA-based hydrogel particles (HGPs) with an average diameter of 5-6 μm containing an estimated 2.2 wt% gelatin (gHGPs) were synthesized by covalent immobilization of gelatin to HA HGPs prepared via an inverse emulsion polymerization technique. Separately, a photocrosslinkable HA macromer (HAGMA) was synthesized by chemical modification of HA with glycidyl methacrylate (GMA). Doubly crosslinked networks (DXNs) were engineered by embedding gHGPs in a secondary network established by HAGMA at a particle concentration of 2.5 wt%. The resultant composite gels, designated as HA-gHGP, have an average compressive modulus of 21 kPa, and are non-toxic to the cultured MSCs. MSCs readily attached to these gels, exhibiting an early stage of stress fibers assembly 3 h post seeding. By day 7, stellated-shaped cells with extended filopodia were found on HA-gHGP gels. Moreover, cells had migrated deep into the matrix, forming a three dimensional, branched and interconnected cell community. Conversely, MSCs on the control gels lacking gelatin moieties formed isolated spheroids with rounded cell morphology. After 28 days of culture on HA-gHGP, Type I collagen production and mineral deposition were detected in the absence of osteogenic supplements, suggesting induction of osteogenic differentiation. In contrast, cells on the control gels expressed markers for adipogenesis. Overall, the HA-gHGP composite matrix has great promise for directing the osteogenic differentiation of MSCs by providing an adaptable environment through the spatial presentation of cell adhesive modules.
Keywords: Hyaluronic acid, gelatin, hydrogel particles, doubly crosslinked networks, mesenchymal stem cells, adhesion, differentiation, osteogenesis
1. Introduction
Tissue engineering aims to develop functional substitutes for damaged tissues by applying engineering principles to developmental cell biology [1, 2]. Successful engineering of functional tissues in vitro relies on the strategic combination of synthetic scaffolds, viable cells and physiologically relevant biological cues and biophysical stimulations [3-5]. While primary cells isolated from the patients represent an optimal cell source, the inaccessibility of many cell types and their relatively short replicative life span post significant challenges for using these cells in tissue engineering [6]. On the other hand, multipotent human mesenchymal stem cells (MSCs) can be obtained from a variety of adult and fetal issues; they can be expanded for more than 50 cell doublings without signs of senescence and be differentiated into osteoblasts, chondrocytes, adipocytes and nerve cells under defined in vitro culture conditions [7-9]. MSCs are naturally sensitive to their environment, responding to chemical, physical and mechanical features of their matrices or substrates, as well as the spatial/temporal presentation of biochemical cues [10, 11]. Cellular behaviors, such as adhesion, proliferation, differentiation and migration, can be influenced by custom-designed, synthetic scaffolds that essentially recapitulate the native stem cell niche [12].
Hydrogels are widely employed as artificial matrices for tissue engineering due to their biocompatibility, high porosity and tissue-like elasticity [13-15]. The current investigation aims at developing hyaluronic acid (HA)-based hydrogels that are hierarchically structured, mechanically robust and chemically defined, suitable for use as conducive substrates for the controlled differentiation of bone-marrow-derived human MSCs. HA is a particularly attractive starting material for the fabrication of synthetic matrices due to its inherent bioactivity, biocompatibility, and biodegradability. Found primarily in the ECM of connective tissues (including bone marrow), HA functions in tissue support, lubrication, and modulation of tissue viscoelasticity [16]. More importantly, HA interacts with its cell surface receptors CD44 and RHAMM to activate various signaling pathways that direct a wide spectrum of cell functions [17, 18].
Our group has created a novel HA hydrogel system, referred to as the doubly crosslinked networks (DXNs), comprised of densely crosslinked HA hydrogel particles (HGPs) physically embedded in or covalently connected to a loosely crosslinked secondary network that is also HA-based [19-22]. While the HGPs exhibit inherent mesh size in the order of 5-10 [21] or 10-20 nm [23] depending on the particular chemistry employed for particle synthesis, the surrounding secondary matrix contains pores of hundreds of nanometers. The mechanical properties and the enzymatic stability of the HA DXNs can be separately tuned by altering the particle size as well as intra- and inter- particle crosslinking [20-22]. Although primary bovine chondrocytes were able to adapt to the three dimensional (3D) microenvironment and synthesize cartilage-specific glycosaminoglycan, the lack of cell-adhesive motifs in these HA DXNs limits their utility in long term culture of anchorage-dependent cells. Because MSC differentiation and subsequent neotissue formation is directly influenced by cell adhesion to their underlying biomaterials [24], imparting cell-adhesive properties to HA DXNs will significantly expand their applicability in regenerative medicine.
The ultimate goal of this study was to develop a hybrid HA matrix that can be employed to mediate cell adhesion and to direct the fate of MSCs by simple manipulation of the hydrogel structure and composition. To this end, HA microparticles containing residual aldehyde groups [20, 23] were utilized for gelatin immobilization. Gelatin-conjugated HA HGPs (gHGPs) were successfully synthesized by a reductive amination reaction between the lysine amines on gelatin and the aldedhyde groups on HA HGPs. Subsequently, gHGPs were physically embedded in a photocrosslinked secondary matrix derived from methacrylated HA [25]. Human MSCs were cultured on the composite gels in the growth media for up to 28 days. The biocompatibility of the composite matrix was assessed by live/dead staining and cell proliferation was quantified by alamar blue assay. Cell adhesion and cell morphology were characterized by standard immunohistochemical analyses. Finally, cell differentiation towards osteoblasts, chondrocytes and adipocytes was evaluated by assessing the respective differentiation markers expressed by the cells.
2. Materials and Methods
2.1. Materials
Hyaluronic acid (HA, sodium salt, 1.0 MDa and 500 KDa) was generously donated by Genzyme Corporation (Cambridge, MA). Gelatin type B (from bovine skin), alcian blue 8GX, sodium cyanoborohydride (NaBH3CN), 4-(dimethylamino) pyridine (DMAP), tetrabutylammonium bromide (TBAB), glycidyl methacrylate (GMA), 2,2-dimethoxy-2-phenylacetophenone (DMPA), fluorescamine, silver nitrate, sodium thiosulfate, and 1-vinyl-2-pyrrolidinone (NVP) were purchased from Aldrich (Milwaukee, WI). Acetone, hexane, isoproponal, hydrochloric acid, methyl red, sodium hydroxide, perchloric acid, ammonium hydroxide, sodium chloride, chloramine-T, p-dimethylaminobenzaldehyde (p-DAB), and ethanol were obtained from Thermo Fisher Scientific (Waltham. MA). Paraformaldehyde (16% in H2O) was obtained from Electron Microscopy Sciences (Hatfield, PA). Propidium iodide and Syto 13 were purchased from Genway Biotech, Inc (San Diego, CA). Mouse monoclonal anti-vinculin antibody and tetramethyl rhodamine isothiocyanate (TRITC)-conjugated phalloidin were purchased from Millipore (Billerica, MA). Mouse monoclonal anti-STRO-1 antibody, Alexa fluor 568-labeled secondary antibody (goat anti-rabbit IgG), Alexa fluor 488-labeled secondary antibody (goat anti-mouse IgG) were obtained from Invitrogen (Carlsbad, CA). Draq-5 was purchased from Axxora LLC (San Diego, CA). Rabbit monoclonal anti-CD44 antibody, mouse polyclonal anti-collagen type I antibody, and mouse monoclonal anti-α5β1 antibody were obtained from Abcam (Cambridge, MA). All antibodies were diluted in PBS containing 3% bovine serum albumin (BSA) (Jackson Immunoresearch, West Grove, PA).
2.2. Synthesis of gelatin-conjugated HA HGPs (gHGP)
HA HGPs were synthesized by an inverse emulsion polymerization technique employing HA derivatives containing hydrazide (HAADH) and aldehyde (HAALD) functional groups, as previously reported. [20, 23] After overnight reaction at 40°C, the hydrogel particles were collected by precipitation and centrifugation. Particles were subjected to thorough washing with hexane, isopropanol and acetone before being dried under vacuum overnight at ambient temperature. Vacuum-dried HGPs (20 mg) were dispersed in a PBS solution (10 mL) containing 10 wt% gelatin at pH 7.4 and the reaction mixture was agitated at 37-40°C for 4 h, after which NaBH3CN (5.8 mg) was added and the reaction was allowed to proceed for an additional 4 h at 37-40°C. After exhaustive washing and vacuum drying, the gelatin modified particles (50 mg) were allowed to react with glycine (18 mg) in DI H2O (5 mL) for 2 h at 37°C under constant stirring. The final product, gHGP, was purified by extensive washing with water, isopropanol and acetone and before being dried under vacuum. Particles blocked with glycine without gelatin conjugation (bHGP) were also prepared.
2.3. Synthesis of HA DXNs
Photocrosslinkable HA macromer (HAGMA) was synthesized by chemical modification of HA with glycidyl methacrylate (GMA) in an aqueous solution in the presence of DMAP and TBAB following previously reported procedures.[22] HAGMA was isolated from the reaction mixture by repeated precipitation in acetone, followed by extensive dialysis against DI H2O in the dark before being lyophilized. Hybrid hydrogels were prepared by entrapping gHGPs in a photcrosslinked HAGMA matrix. Specifically, dry glHGPs were dispersed in a 2 wt% HAGMA (in DI H2O) at a particle concentration of 2.5 wt%. To this suspension was added 1.5 μL of the initiator solution (30 wt% DMPA in NVP). The mixture was thoroughly mixed and exposed to a long wavelength UV lamp (Model 100AP, Blak-Ray) for 20 min for complete gelation; the resultant gels were referred to as HA-gHGP. Two types of control gels were also prepared. The first type of control gels (HA-bHGP) was prepared following the aforementioned procedure using bHGPs instead of gHGPs, whereas the second type of control gels were prepared using HAGMA without any particles. The as-synthesized gel disks were imaged with a Nikon phase contrast optical microscope (Nikon, Kanagawa, Japan) with a Coolpix digital camera.
2.4. Evaluation of gelatin conjugation
Gelatin conjugation was qualitatively confirmed by fluorescamine staining and quantitatively assessed by hydroxyproline assay. For fluorescamine assay, HGPs were allowed to react with acetaldehyde prior to gelatin coupling and acetaldehyde-treated HGPs (aHGPs) without gelatin conjugation were included as the negative controls. Briefly, HGPs (1 mg) were reacted with acetaldehyde (10 mg) in the presence of NaBH3CN (0.8 mg) in DI H2O (1 mL) at 37°C under constant stirring for 2 h. Particles were collected by centrifugation, washed with DI H2O, isopropanol and acetone (three times each) and vacuum-dried. For staining purposes, gHGPs or aHGPs were suspended in 100 μL of DI H2O, to which 2 μL of fluorescamine solution (5 mg/mL in acetone) was added. The reaction mixture was stirred at room temperature for 5 min. Stained particles were collected by centrifugation and were imaged with a Zeiss 5 Live Duo Laser Scanning Microscope (LSM, Carl Zeiss Inc. Thornwood, New York).
For hydroxyproline assay [26], gHGPs (1 mg) were hydrolyzed in 1 mL HCl (6N) in a glass vial fitted with a Teflon-lined cap at 110°C for 18 h. Methyl red (2 μL) was added as a pH indicator. The solution was neutralized with 2.5 N NaOH and 0.5 N HCl and brought to a total volume of 1 mL with DI H2O. Separately, chloramine-T was dissolved in a citrate buffer (pH 6)/isopropanol mixture (8/1, v/v) at a concentration 15.7 mg/mL, and the p-DAB solution was prepared by dissolving p-DAB in isopropanol (30 mL) and perchloric acid (60%, 13 mL) at a concentration 174 mg/mL. One hundred microliters of the neutralized solution were mixed with 50 μL of chloramines-T solution in a 96-well plate for 15 min at room temperature. Fifty microliters of p-DAB solution were then added, and the plate was incubated at 37°C for 30 min. The absorbance of the final solution was detected at 550 nm using a PerkinElmer plate reader (Universal Microplate Analyzer, PerkinElmer, Waltham, MA) and the relative absorbance from each sample was calculated by subtracting the background absorption of blank particles from the sample reading. Standard curves were constructed from serially diluted gelatin solutions at concentrations ranging from 0 to 100 μg/mL.
2.5. Particle size and size distribution
The average size and size distribution of HGPs and gHGPs were analyzed using a Coulter Counter Multisizer 3 (Beckman Coulter, Fullerton, CA) with a 2-70 μm orifice. Prior to the measurement, HGPs were diluted to concentration 10 μg/mL using isotonic solution.
2.6. Mechanical Testing
Gel disks (height: 3.0 mm and diameter: 6.3 mm) were prepared in cell culture inserts (Millipore, Billerica, MA) and the compression tests were performed on the as-synthesized gels using a dynamic mechanical analyzer (RSA III instrument, TA Instruments, New Castle, DE) with a parallel plate geometry. Deformation of hydrogel disks was measured as a function of normal stress, and the corresponding stress vs strain curve was plotted. All samples were compressed at a rate of 20%/min until fracture. The compressive modulus was calculated using the initial linear portion of the stress-strain curve. The compression tests were performed in triplicate for all samples.
2.7. MSC culture and cell viability
Human mesenchymal stem cells (Lonza, Walkersville, MD) were cultured in MSC growth media (MSCGM™, Lonza Walkersville, MD) at 37°C in a humidified atmosphere with 5% CO2. Hydrogels were prepared as described above in cell culture inserts and were equilibrated in PBS at 37°C for overnight. The gel disks were washed three times with PBS, soaked in 0.4 mL of media for another 4 h at 37°C and sterilized by UV irradiation for 30 min. Confluent cells (passage 5-6) were trypsinized, re-dispersed in the media and dropped on top of the gel disks at a density of 15,000 cells per insert. The cell-seeded hydrogels were incubated for up to 28 days and the medium was refreshed every three days. Cell viability was assessed by staining the cells with propidium iodide (1:2000 dilution) and Syto 13 (1:1000 dilution) after 3, 7 and 14 days of culture. Images were acquired using a Zeiss 5 Live Duo LSM with a Zeiss 40X C-Apochromat (numerical aperture 1.2) water immersion objective lens.
2.8. Cell morphology and focal adhesion
At a predetermined time, the cell/gel constructs were washed with PBS three times and fixed with a 4% paraformaldehyde solution at room temperature for 15 min. After washing with PBS (3 times), cells were permeabilized with a Triton X-100 solution (0.1% in PBS) at room temperature for 5 min. After a 30-min treatment with the blocking buffer (3% BSA in PBS), samples were incubated with anti-vinculin monoclonal primary antibody (1:200 dilution) at 4°C overnight. Upon removal of the unbound antibody, cells were incubated with the Alexa Fluor 488-labeled secondary antibody (1:100 dilution) and TRITC-conjugated phalloidin (1:200 dilution) in the dark for another hour at room temperature. Finally, cell nuclei were stained with Draq-5 (1:1000 dilution) for 5 min at room temperature. Confocal images were acquired using a Zeiss 5 Live Duo LSM.
2.9. Inhibition for cell adhesion
Suspensions of MSCs in serum free media were incubated with mouse monoclonal anti-α5β1 antibody at a concentration of 100 μg/mL for 15 min at 37°C. Cells were then plated on selected gel disks and were cultured at 37°C for 3 h. Unattached cells, collected from the hydrogel substrates by gentle rinsing with media 3 times, were transferred to another culture disk and were incubated for another 3 h at 37°C. The number of cells attached to the gels and the culture dish were separately determined using the colorimetric alamar blue assay following the manufacturer's instructions. In short, medium containing 10% alamar blue was added to the cell culture and the cells were incubated for 4 h. Aliquot (100 μL) of the medium was then withdrawn and the solution absorbance was measured at 550 nm using a plate reader. Medium containing 10% alamar blue without the cells was used as the blank, and the calibration curve was constructed by incubating different numbers of cells (50-80,000) on tissue culture polystyrene (TCPS) with 10% alamar blue. Control experiments were conducted similarly without the antibody treatment. Cell attachment is reported as a percentage of cells attached to the hydrogels relative to the hydrogel adherent and plastic adherent cells combined.
2.10. Cell proliferation
A total of 15,000 cells were seeded on each hydrogel substrate in a cell culture insert at day 0. At a pre-determined time, medium containing 10% alamar blue was added to the cell culture and the cell number was determined using the alamar blue assay as described above.
2.11. Immunofluorescence staining for CD44 and STRO-1
For CD44 staining, cells were fixed and permeabilized as described above. The cell/gel constructs were subsequently incubated with anti-CD44 monoclonal primary antibody (1:50 dilution), followed by staining with Alexa Fluor 568-labeled secondary antibody (1:200). For STRO-1 staining, cells were fixed with cold methanol at −20°C and were subsequently incubated with mouse monoclonal anti-STRO-1 primary antibody (1:50 dilution) at 4°C overnight, followed by a 1-h treatment with Alexa Fluor 488-labeled secondary antibody (1:100 dilution) at room temperature. Confocal images were taken using a Zeiss 5 Live Duo LSM.
2.12. Cell differentiation
Cell differentiation was assessed after 28 days of culture on various hydrogel substrates and TCPS.
2.12.1. Osteogenesis
Mineral production was collectively assessed by Alizarin Red and von Kossa staining. Prior to staining, Alizarin Red S was dissolved in DI H2O at a concentration of 2 wt% and the solution pH was adjusted to 4 using 10% ammonium hydroxide. Cells were fixed in 4% paraformaldehyde for 15 min at room temperature. After PBS washing, gel disks were stained with Alizarin Red S solution (400 μL each) for 15 min at ambient temperature. After washing with DI H2O, samples were imaged with a Nikon optical microscope. For von Kossa staining, cells were fixed with paraformaldehyde, and the gel disks were incubated in the silver nitrate solution (400 μL, 5% in DI H2O) in the presence of UV irradiation for 30 min. The reaction was terminated by exposing the samples to a sodium thiosulfate (5% in DI H2O) solution for 5 min at room temperature. Finally gel disks were washed with DI H2O and imaged using a Nikon optical microscope. For Collagen type I staining, cells were fixed with paraformaldehyde and permeabilized with Triton X-100, as described above. After BSA blocking, samples were incubated with mouse polyclonal anti-collagen type I primary antibody (1:1000 dilution) at 4°C overnight. After washing with PBS, gel disks were incubated with Alexa Fluor 488-labeled secondary antibody (1:100 dilution) for 1 h. Finally, cell nuclei were stained with Draq-5 (1:1000) for 5 min at room temperature. Confocal images were acquired using a Zeiss 5 Live Duo LSM.
2.12.2. Chondrogenesis
Gel disks were washed with PBS, fixed in 4% (v/v) paraformaldehyde and stained for glycosaminoglycan (GAG) with Alcian blue (0.5 wt% in 3% glacial acid) at pH 1.0 overnight at ambient temperature. Samples were washed with DI H2O before being imaged with a Nikon microscope.
2.12.3. Adipogenesis
The ability of cells to produce lipid was characterized by Oil Red staining. After washing with PBS, cells were fixed in 4% paraformaldehyde for 15 min at room temperature. Gel disks were washed with 60% isopropanol and then incubated with Oil Red O (0.21% w/v in isopropanol) for 10 min at room temperature. Finally, samples were washed with 60% isopropanol and subsequently with DI H2O before being imaged with a Coolpix Nikon microscope.
2.13. Statistical analysis
All quantitative measurements were performed at least in 3 replicates. All values are expressed as means ± standard deviations (SD). Statistical significance was determined using a two-tailed student t-test. A p value of less than 0.05 was considered to be statistically different.
3. Results
3.1. Synthesis of HA-gHGP
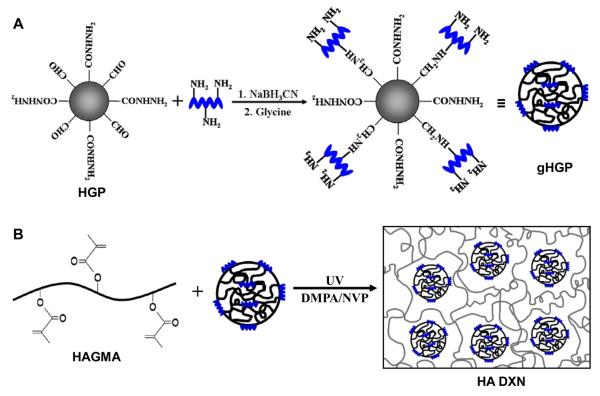
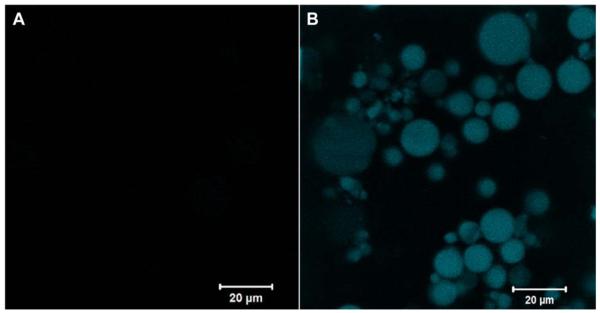
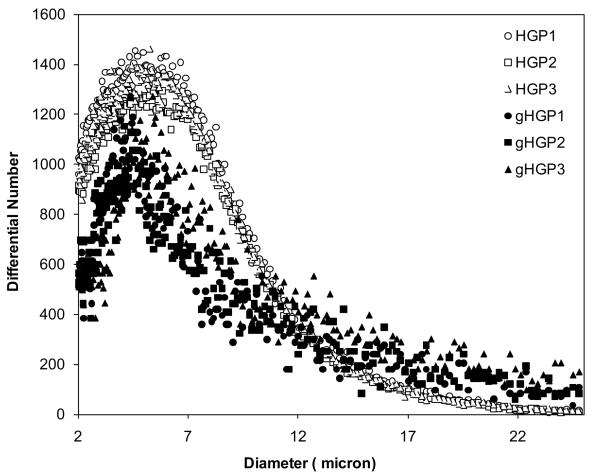
HA HGPs were synthesized by chemical crosslinking of HAALD and HAADH within the water droplets dispersed in mineral oil and stabilized by Span 80 [20]. The spherical particles contain residual aldehyde and hydrazide groups that can be used as reactive handles for bioconjugation purposes [20, 23]. Thus, gelatin was immobilized to HA HGPs via the coupling reaction between the lysine amines on gelatin and the aldehyde groups on HGPs, forming a stable C-N bond at the interface. (Scheme 1A). The excess aldehyde groups were passivated using glycine [23]. Fluorescamine staining was employed for the qualitative assessment of gelatin immobilization, owing to the ability of the unreacted lysine amines on the conjugated gelatin to convert the non-fluorescent fluorescamine into particle-immobilized pyrolinone that is fluorescently active [27]. Both HA HGPs and gHGPs were treated with acetaldehyde prior to the assay to eliminate the interference from the residual hydrazide groups [20] in the particles. The efficacy of hydrazide deactivation was confirmed by the absence of blue fluorescence from aHGPs (Figure 1A). The reappearance of the blue fluorescence from gHGPs (Figure 1B) confirmed the presence of gelatin throughout the particles. Prolonged incubation (1 month) of gHPGs in a saline solution did not lead to a significant reduction in fluorescence intensity from the stained particles (data not shown), suggesting that gelatin was stably conjugated to HA HGPs. Using a standard curve constructed from solutions of known gelatin concentration, hydroxyproline assay revealed that gHGPs contain an average 22.45 ± 1.36 μg gelatin per milligram particles. Particle size analysis showed that both HGPs and gHGPs had an average particle diameter of 5-6 μm under isotonic conditions (Figure 2); gelatin conjugation did not alter the average size and size distribution of the particles.
Scheme 1.
Synthetic procedures for the production of gelatin conjugated HA HGPs (gHGPs, A) and the gelatin-containing, doubly crosslinked networks (B). Gelatin was coupled to HA HGPs via the reductive amination reaction between the lysine amines on gelatin and the aldehyde groups on HA HGPs. The excess aldehyde groups were passivated by glycine. HA DXNs were prepared by entrapping gHGPs in a photocrosslinked HAGMA secondary matrix.
Figure 1.
Monitoring the presence of gelatin on gHGPs by fluorescamine staining. Particles were treated with acetaldehyde prior to gelatin conjugation. Acetaldehyde-treated HGPs without gelatin immobilization (aHGPs) were included as the negative controls. Particles (aHGPs and gHGPs) were allowed to react with fluorescamine in H2O/acetone for 5 min before being imaged with a Zeiss 5 Live Duo LSM. (A) Acetaldehyde treated HGPs (aHGP); (B) Acetaldehyde treated, gelatin-conjugated HGPs (gHGP).
Figure 2.
Particle size and size distribution determined by Coulter Counter Multisizer 3. Water-swollen particles were diluted in isotonic solution at a concentration of 10 mg/mL prior to the analysis. Three separate runs were carried out on each type of particles. Open symbols: HGP; filled symbols: gHGP.
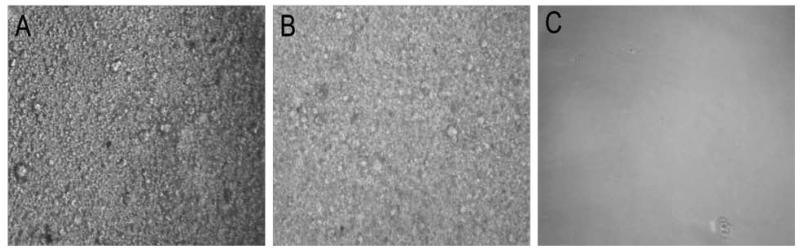
HA-based DXNs were synthesized by in situ encapsulation of gelatin-decorated HA HGPs in a secondary HA matrix derived from glycidyl methacrylate (GMA)-modified HA (HAGMA) [22], and the resultant hybrid hydrogels are designated as HA-gHGP (Scheme 1B). Two types of control gels were included in the current investigation. In the first type of control gels, gHGPs were replaced with glycine-passivated HGPs (HA-bHGP) and the second type of control gels were HAGMA bulk gels lacking the particulate fillers. While the HAGMA gels were optically transparent, both HA-gHGP and HA-bHGP were semi-opaque with a yellowish tint. Examination of HA-gHGP and HA-bHGP gels under an optical microscope revealed the presence of spherical particles homogenously distributed in a secondary HA matrix (Figure 3).
Figure 3.
Typical phase contrast images of as-synthesized HA-gHGP (A), HA-bHGP (B) and HAGMA (A). Magnification: x10.
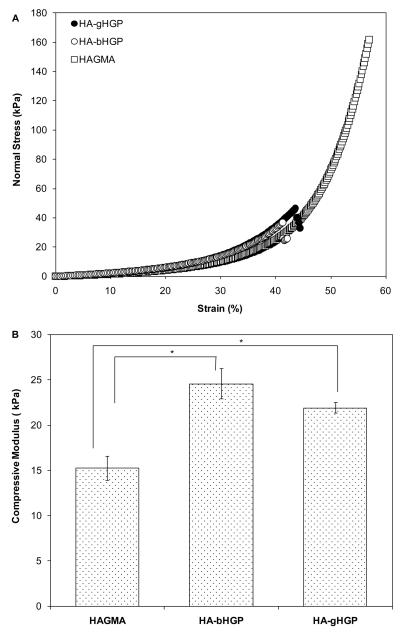
The stress-stain curves of the hydrogels were obtained by compressing the gel disks at 20%/min until fracture. While HAGMA gels failed at ~57% strain, gHGP-containing gels are more brittle, failing at a maximum strain of approximately 42% (Figure 4A). The compressive modulus, inferred from the slope of the linear region of the stress-strain curves, were 15.2 ± 1.3 kPa, 24.6 ± 1.6 kPa and 21.3 ± 0.6 kPa, for HAGMA, HA-bHGP and HA-gHGP, respectively (Figure 4B). Although both HA-bHGP and HA-gHGP are stiffer than HAGMA (p<0.05), no statistical difference was observed between HA-bHGP and HA-gHGP in terms of their compressive moduli.
Figure 4.
Mechanical properties of various HA-based hydrogels. (A): Representative stress-strain curves for HA-gHGP (●), HA-bHGP (○) and HAGMA (□) gels. (B): Average compressive modulus for various HA networks. *: The compressive moduli for HA-gHGP and HA-bHGP are significantly different from that for HAGMA, p<0.05.
3.2. MSC viability and morphology
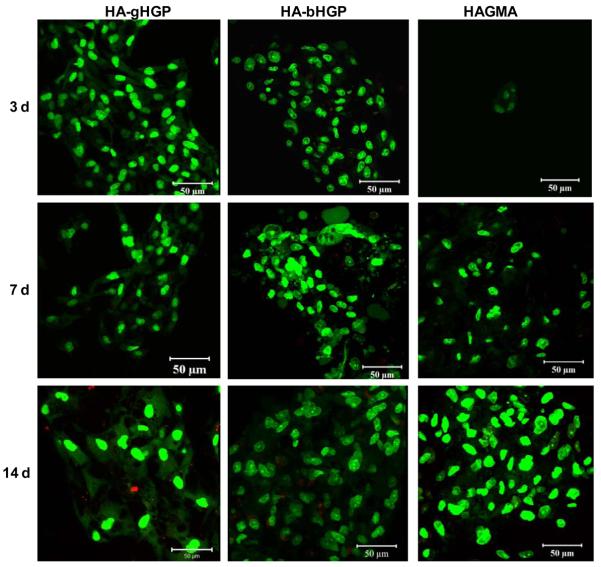
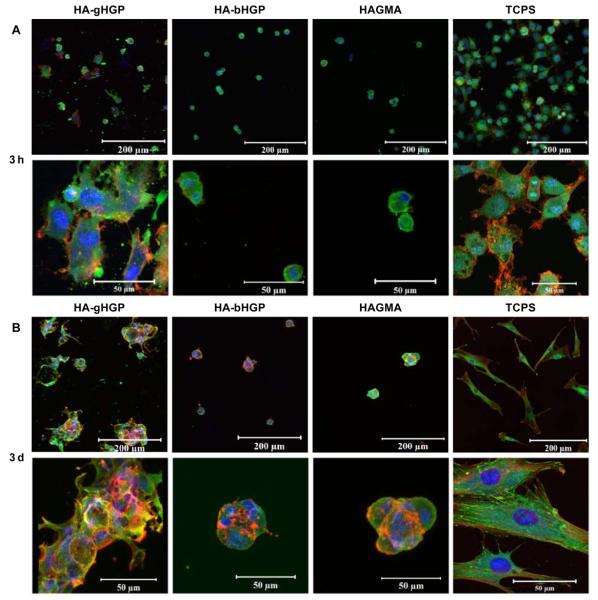
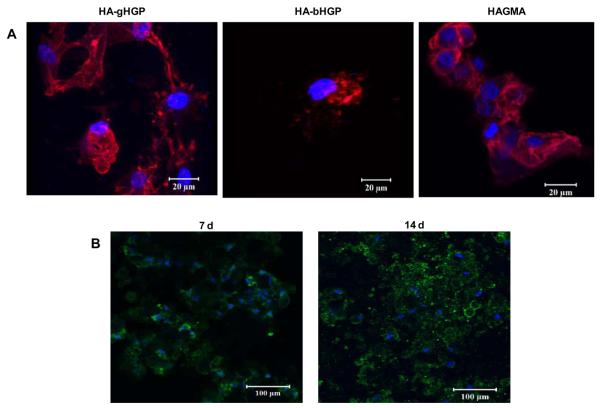
Live/dead assay revealed that MSCs attached to various HA gels were viable, irrespective of the chemical composition of the gels and the cultivation time (Figure 5). Occasionally, isolated red staining, possibly due to the non-specific association of the dye with the matrix, was detected. Of note, cells detached from the substrates during staining were not detected by this assay. Cell aggregates with varying sizes were present, especially on HA-bHGP and HAGMA gels. Information regarding cell morphology and the degree of cell spreading was gleaned from vinculin/F-actin staining at various culture times (Figure 6). Three hours post seeding, MSCs on TCPS appeared spheroidal, with many cells starting to develop F-actin stress fibers, as evidenced by the intense, mesh-like red staining extended from the cell perimeter (Figure 6A). Similarly, spheroidal cell were observed on HA-gHGP gels. Although mature F-actin stress fibers have not yet developed, some red spikes at the cell periphery are already visible. By day 3, MSCs became fully attached to TCSP, assuming a flat, spindle-shaped, polarized morphology, with well-developed stress fibers. Intense green spots, representative of focal adhesion complexes, were readily detected at the cell termini along the long axis of the cell body. The well spread-out morphology seen on TCPS was absent from cells cultured on HAgHGP gels on day 3. Instead, cells formed irregular clusters with overlapping cell processes. F-actin stress fibers are visible both within and at the edge of the cell clusters. Focal adhesion staining was not as pronounced as those in cells attached to TCPS.
Figure 5.
Representative live/dead staining results of MSCs cultured on HA-gHGP, HA-bHGP and HAGMA after 3, 7 and 14 days of culture in MSC growth media. Live (green) and dead (red) cells were stained with Syto 13 and propidium iodide, respectively.
Figure 6.
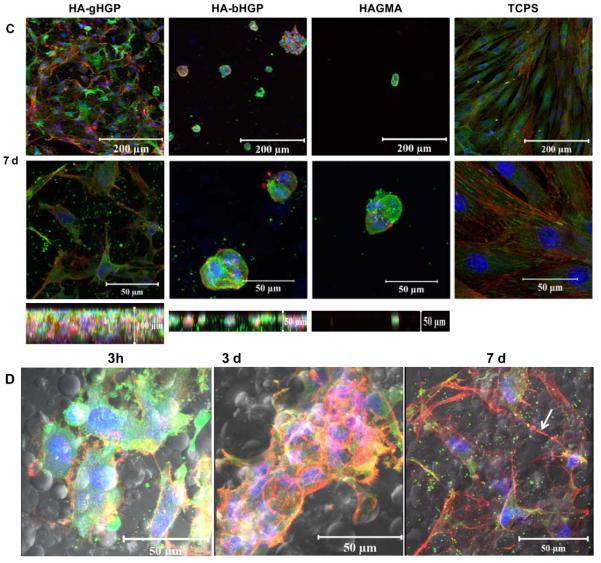
Characterization of MSC adhesion to various substrates including HA-gHGP, HA-bHGP, HAGMA and TCPS. F-actin filaments were stained red by TRITC-phalloidin, focal adhesion sites were stained green by anti-vinculin and FITC-labeled secondary antibody, and cell nuclei were counterstained blue by Draq 5. Representative confocal images (with different magnifications) of MSCs after 3 h (A), 3 days (B), and 7 days (C) of cultivation. The bottom panels in C reveal the thickness of the respective 3D, Z-stack images at day 7. (D) Representative confocal images overlapped with the phase contrast images showing MSCs attached to HA-gHGP at various times.
By day 7, a confluent monolayer of MSCs was observed on TCPS. Cells exhibited an extended fibroblastic morphology with actin stress fibers traversing the length of the cell. Intense vinculin clusters along the fibers are abundant. Interestingly, MSCs on HA-gHGP gels aggregated to form branched and interconnected multicellular networks that cover the entire surface of the gel. Individual cells exhibit a stellate morphology with no preferential cell orientation. The co-localization of F-actin and vinculin was not as straightforward as in the case of TCPS. Strikingly, MSCs seeded on top of the gels had migrated 100 μm into the gels and established a 3D community (bottom panel, Figure 6C). When the phase channel of the confocal microscope is open, the location of the cells relative to the particles can be inferred (Figure 6D). MSCs seem to cluster locally around the particles initially, and extend the processes to interrogate their surroundings later on. Elongated cell processes bridging to neighboring cells are apparent, especially on day 7 (Figure 6D, white arrow). Overall, the extent of F-actin stress fiber formation increased with the culturing time for cells on HA-gHGP and TCPS.
Conversely, MSCs plated on both HAGMA and HA-bHGP control gels remained rounded and stationary, lacking noticeable filopodia extensions observed on TCSP or HA-gHGP. This response did not depend on the culture time. Compared to cells on TCPS, individual cells are much smaller in size and the F-actin and vinculin staining are diffuse and co-localized around the nuclei. The low affinity of MSCs to these substrates was manifested by the formation of spheroids that contain several aggregated cells. Individual cell aggregates remain isolated throughout the culture. Repeated washing during the staining inevitably led to the loss of loosely anchored cells or spheroids. Limited cell penetration into the matrix was detected (Figure 6C).
3.4. Antibody inhibition and cell proliferation
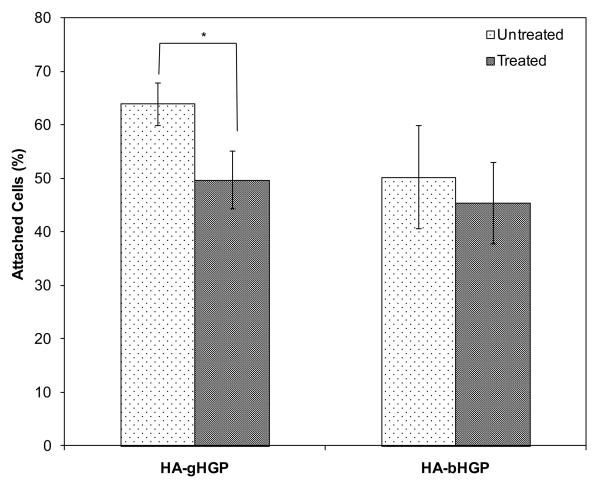
Integrin blocking experiments were performed in order to determine the role of α5β1 integrin in mediating the early attachment of MSCs to HA-based substrates. Pre-incubation of MSCs with the antibody in suspension led to the equilibrium binding of the antibody with the integrin receptors [28]. Loosely attached cells dislodged from the hydrogel substrates were re-seeded on a second culture dish so that the cell number can be inferred by alamar blue assay. The number of hydrogel-adherent and plastic-adherent cells combined was in good agreement with the number of cells initially seeded on the hydrogels. Without the antibody treatment, approximately 64% of cells were attached to HA-gHGP within the first 3 h of incubation (Figure 7). The antibody reduced the attachment of MSCs on HA-gHGP by ~22% (p<0.05). Only 50% of cells seeded were anchored to HA-bHGP, and the antibody treatment did not result in any significant changes in terms of the cell attachment.
Figure 7.
Inhibition of MSC attachment with anti-integrin antibody. Suspended cells were treated with anti-integrin α5β1 antibody (100 μg/mL) and then allowed to attach to HA-gHGP or HA-bHGP. Cell attachment was expressed as the percentage of cells attached relative to the total numbers of cells initially seeded.
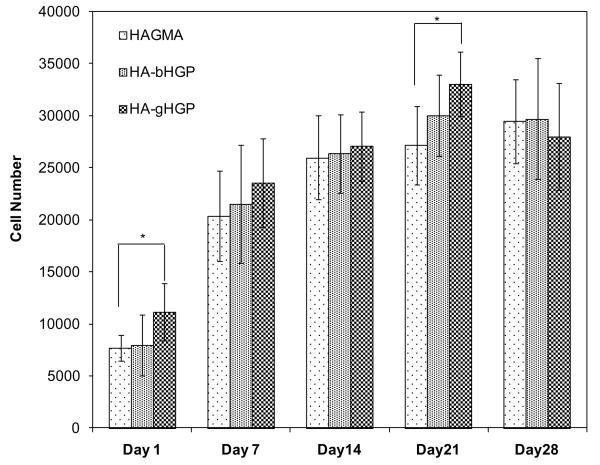
Alamar blue assay was again utilized to determine the proliferation of MSCs on various hydrogels up to 28 days (Figure 8). Although the same number (15,000) of cells were initially seeded on all three substrates with the same surface area, more metabolically cells were found on HA-gHGP than on HAGMA at day 1 (p<0.05). The number of cells on HA-gHGP gels increased steadily with the culture time, reaching a maximum value of approximately 33,000 on day 21. HA-gHGP gels were substantially (p<0.05) more populated by MSCs than HAGMA gels on day 21. The total number of metabolically active cells on HA-gHGPs is not significantly different on days 21 and 28. Interestingly, the number of cells more than doubled during the first week of culture, after which cells proliferated at a much slower rate. Overall, cells exhibit similar proliferation patterns on all three types of gels throughout the culture.
Figure 8.
Cell proliferation on HAGMA, HA-bHGP and HA-gHGP as measured by the Alamar Blue assay. A total of 15,000 cells were seeded on each gel at day 0. *: p<0.05.
3.5. MSC phenotype and differentiation
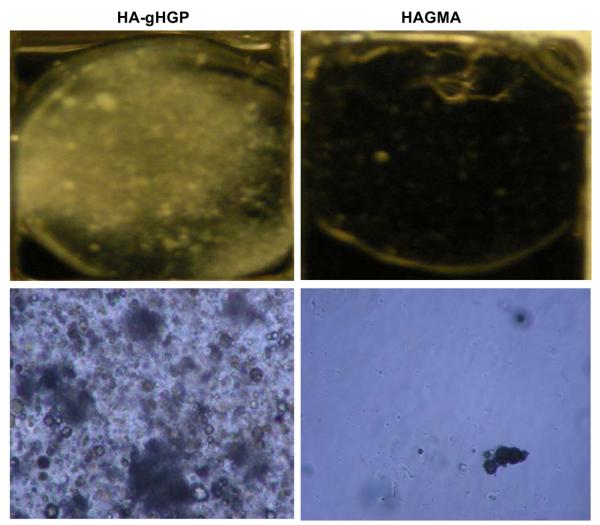
Figure 9A shows the immunohistochemical analysis for CD44, a cell surface receptor for HA and an MSC marker [7], after 7 days of culture. Cells were stained positive for CD44 irrespective of the hydrogel composition. Cells cultured on HA-gHGP also exhibited a positive STRO-1 staining at day 7 and day 14 (Figure 9B). These results suggest MSCs maintained their stemness during the early stages of culture although partial differentiation cannot be ruled out. By day 28, HA-gHGP gels became opalescent; mineral deposition was visible to the naked eyes. Meanwhile, HAGMA gels remained transparent throughout the culture (Figure 10). Examination of the HA-gHGP samples under a phase contrast microscope revealed the presence of irregular-shaped dark aggregates along with smaller, spherical objects with dark rings, in sharp contrast to the featureless HAGMA gels (Figure 10).
Figure 9.
(A): Immunostaining of CD44 after 7 days of MSC culture on HA-gHGP, HA-bHGP and HAGMA gels. (B): Representative STRO-1 staining of MSCs after 7 and 14 days of culture on HA-gHGP gels. CD44 was stained red, STRO-1 was stained green and cell nuclei were stained blue.
Figure 10.
Digital (top) and optical (bottom, magnification: x10) images of MSC-populated HA-gHGP (left) and HAGMA (right) gels.
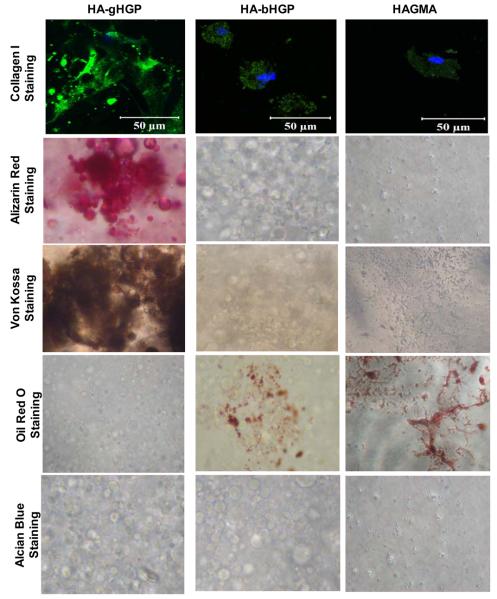
More in-depth characterizations were carried out to assess the differentiation potential of HA gels (Figure 11). MSCs on HA-gHGP gels were stained strongly positive for collagen I, and the newly synthesized protein was distributed in a random pattern in the extracellular space (Figure 11). Collagen I antibody did not recognize the immobilized gelatin, as confirmed by the negative staining of the cell-free substrates (data not shown). Strong pinkish-red and brown-colored nodules were detected by Alizarin Red and Von Kossa staining, respectively, indicating the ability of MSCs to deposit minerals on HA-gHGP. Both stainings seem to be preferentially localized around the embedded gHGPs. Of note, cells were cultivated in MSC growth media, not osteogenic media. Oil Red and Alcian Blue were employed to assess the ability of MSCs to secret lipid or to synthesize GAG, respectively. MSCs on HA-gHGP gels did not differentiate along the adipocyte or chondrocyte lineages, as evidenced by the negative Oil Red and Alcian Blue staining. On the other hand, MSCs on both types of control gels, HA-bHGP and HAGMA, exhibit intracellular collagen I staining that is weak and diffuse. While positive Oil Red staining was detected, Alizarin Red, von Kossa and Alcian blue staining were at the level similar to the cell-free gels. Collectively, HA-gHGP is conducive to osteogenic differentiation, while HA-bHGP and HAGMA gels appear to facilitate adipogenesis of MSCs. The spindle-shaped MSCs cultured on TCPS in the growth media were stained negative by Alizarin Red, Oil Red and Alcian Blue although faint and diffuse intracellular collagen I staining could be detected (Figure S1). The faint background staining from von Kossa assay may be a result of intracellular carbonate or phosphate ions being displaced by silver ions. [29, 30] The lack of identifiable mineralization nodules confirms the absence of osteogenesis.
Figure 11.
Characterization of MSC differentiation by histochemical analyses at day 28. Osteogenesis was characterized by histochemical staining of collagen I (green, nuclei stained blue by Draq 5) and calcium (by alizarin red and von Kossa). Adipogenesis was revealed by Oil Red staining while chondrogenesis was assessed by alcian blue staining.
4. Discussion
In the native tissue, MSCs reside in a defined microenvironment that regulates stem cell survival, self-renewal, and differentiation through growth factors, cell-cell contacts and cell-matrix adhesions [11]. Although soluble factors are potent regulators of stem cell differentiation, recent discoveries underscore the importance of physical and chemical characteristics of the matrices on stem cell fate determination [12]. In order to exploit the full potential of stem cells in regenerative medicine, their adhesion and differentiation must be tightly controlled. To improve the cell-adhesive properties of HA DXNs, the embedded HGPs, rather than the secondary HAGMA network, were chosen as the targets for gelatin immobilization. With their large surface areas and spherical geometry, HGPs are likely to provide the physical anchorage points that facilitate intergrin-mediated MSC attachment to HA DXNs. In addition to its biocompatibility, biodegradability and commercial availability, gelatin exhibits readily accessible cell adhesive motifs and matrix metalloproteinase (MMP)-sensitive degradation sites that are essential for cell-matrix interactions. The presence of positively charge residues in gelatin, such as lysine and arginine, allows for the complexation of various growth factors and ECM components [31-34]
Reductive amination reaction was employed for gelatin conjugation through the lysine amines on gelatin via the residual aldehyde groups of HGPs under physiological conditions. This chemistry has been successfully applied for the covalent conjugation of a basement membrane protein to the same types of particles without compromising the biological activities of the protein [23]. The excess aldehyde groups on the particles were passivated by glycine so as to avoid non-specific reaction with proteins in subsequent cell culture studies. Gelatin conjugation did not alter the particle size or the size distribution, nor did it impair the dispensability of the particles. This observation, combined with gelatin's high solubility [35] and overall negative charge under the experimental conditions employed [36], confirms the molecular level, covalent coupling of gelatin to HA HGPs. HA DXNs containing gelatin modified HGPs were prepared by UV irradiation of a HAGMA macromer solution containing dispersed gHGPs. The presence of micron-sized hydrogel particles did not compromise the crosslinkability of HAGMA. The addition of bHGPs and gHGPs to HAGMA matrix led to a moderate increase in the gel stiffness. This is expected because the HGPs are significantly more crosslinked than the HAGMA networks [21]. The lack of covalent connection between the particles and the secondary matrix inevitably compromised the ultimate strength of the composite matrices [22].
In this preliminary investigation, we evaluated the cell-matrix interactions using a two dimensional (2D) culture protocol by plating MSCs directly on top of the nanoporous, microstructured gel disks. Unlike the traditional cell culture system where cells are grown on 2D, stiff tissue culture polystyrene surfaces, the soft and elastic HA DXNs present localized adhesive motifs and hierarchical features that resemble the dynamic microenvironments cells experience in the natural ECM more closely [37]. Experimental parameters optimized from the 2D culture studies can be readily adapted to our future 3D culture studies. Our long term goal is to investigate the matrix-mediated, controlled differentiation of MSCs to various cell lineages, including fibroblasts, chondrocytes and osteoblasts.
Because cell attachment to polymeric substrates regulates cell morphology and differentiations, we conducted systematic investigations on MSC attachment to various HA-based hydrogels, including HA-gHGP, HA-bHGP and HAGMA, as well as the TCPS. MSCs readily adhered to TCPS, assembled their cytoskeleton, developed focal adhesion complexes and adopted a conventional spread-out morphology. When MSCs were plated on the soft, composite HA-gHGP hydrogel, focal adhesions become smaller and less pronounced. These observations agree with previous reports highlighting the importance of the dimensionality and substrate pliability on cell morphology and focal adhesion [38, 39]. As with TCPS, cells started to assemble their stress fibers as early as 3 h (the earliest time point investigated). The actin stress fibers of MSCs spread preferentially around the particles because MSC recognized gelatin on the particles in HA-gHGP gels. The elongated cell filopodia extensions bridging neighboring HGPs further confirm the preferential interaction of MSCs with gelatin. Most strikingly, MSCs on HA-gHGPs form 3D hierarchical structures, suggesting intimate cell-cell contacts and cell-matrix interactions that were not seen when cells were cultured on TCPS. The 3D structure developed from a 2D culture can be attributed to the presence of topographical features in the gels and the ability of MSCs to remodel the substrates. It is worth emphasizing that MSC attachment was observed only when the gHGP concentration is ≥2.5wt%, confirming that cell attachment is dependent on the density of the adhesive ligands. Blocking assays showed that anti-α5β1 antibody partially inhibited MSC adhesion to HA-gHGP. Thus, MSC's attachment to HA-gHGP was mediated, in part, by α5β1 integrin. The antibody treatment did not completely abolish the MSC attachment to HA-gHGP gels. This is reasonable since many integrins are involved in cell adhesion to gelatin. [40]
Conversely, cells on HAGMA and HA-bHGP assumed a rounded morphology and lacked organized actin stress fibers. This response is independent of the culture time, and the percentage of cells adhered to HA-bHGP was not altered by the anti-integrin antibody treatment. These observations can be attributed to the absence of biochemical signals for integrin activation and focal adhesion formation, as well as the ability of the gelatin-null HA gels to prevent adsorption of natural ECM proteins secreted by the cells or otherwise present in the media. We show that HA-gHGP and HA-bHGP exhibited similar mechanical properties. Therefore, the distinctly different cell morphology detected on these substrates is not a consequence of cellular response to the substrate stiffness. In the absence of the immobilized gelatin, individual cell clusters may be loosely associated with the HA substrates through HA-CD44 interaction [41, 42]. Interestingly, while MSCs exhibit distinctly different morphologies on HA-gHGPs vs the control gels, cell proliferation, indirectly assessed by measuring their metabolic activity, follows a similar trend at various times examined. Cells were relatively more proliferative during the initial week of culture, but became increasingly engaged in differentiation and matrix synthesis later on, especially during the last week of culture.
HA DXNs containing cell-adhesive motifs localized on the dispersed microparticles effectively stimulate MSCs to produce bone minerals after 28 days of in vitro culture, in the absence of osteogenic supplements. The substrate-induced stem cell differentiation can be attributed to the ability of the cells to form focal adhesions through gelatin. The co-localization of calcium with HGPs confirms the stimulatory effects of gelatin. The osteogenic effects of collagen [43] and gelatin [40] have been well documented. In fact, the denaturation of collagen I leads to the exposure of the cryptic RGD motif that induces faster cell attachment and enhances osteogenic differentiation [40]. The unique mesh-like multicellular community established on HA-gHGP may further enhance the osteogenic differentiation, possibly due to the improved cell-cell communications. The inability of traditional tissue culture polystyrene to induce osteogenesis in the absence of osteogenic supplements underscores the importance of the chemical and structural characteristics of the hydrogel substrates in controlling the osteogenic differentiation of MSCs. MSCs deprived of the adhesive moieties exhibited a round shape and differentiated into adipocytes, as in the case of HA-bHGPs. Although HA-CD44 interaction has been shown to facilitate osteogenic differentiation [44, 45], in the absence of osterogenic supplements, this interaction alone is not sufficient to induce detectable osteogenesis. The different differentiation patterns is not due to the difference in cell density [7, 46], because all three substrates supported similar numbers of cells at day 28.
Our results convincingly show that MSC adhesion and phenotype can be readily modulated by varying the chemical composition and structural organization of the hydrogel substrates. Particularly, MSCs cultured on DXNs lacking the gelatin signals assumed a rounded morphology and acquired an adipogenic fate. MSCs attached to gelatin-containing DXNs formed complex 3D cellular structures and differentiate preferentially towards the preosteoblasts. HA-gHGP not only facilitated integrin-mediated cell attachment during the early stage of culture but also potentially promoted the immobilization and deposition of ECM components and cytokines secreted by the attached cells, enhancing and propagating the continued differentiation over time. Overall, the HA-gHGP system provides the natural ECM environment with a complex mechanical and biochemical interplay that is conducive to osteoblastic differentiation of MSCs. Taken together, the HA-gHGP DXN system has the potential as a differentiation tool to directly guide MSCs into osteoblast-like cells without the osteogenic media.
5. Conclusion
We have successfully engineered a new class of HA-based, osteogenic hydrogels with inherent hierarchical structures and built-in biochemical cues. Cell-adhesive HA DXNs were prepared using gelatin-decorated, HA HGPs and a water-soluble, radically crosslinkable HA macromer as the building blocks. Cells cultivated on these hybrid and heterogeneous HA DXNs developed stress fibers and focal adhesions that are uniquely different from those observed from cells cultured on traditional 2D TCPS. The adaptable substrates also permit cell migration into the gels and the establishment of branched, multicellular 3D structures. MSCs were predominantly undifferentiated during the early phase of culture, but became increasingly differentiated towards the pre-osteoblastic phenotype by day 28 in the absence of osteogenic supplements. The controlled differentiation was attributed to the ability of HA-gHGP to foster the integrin-mediated attachment of MSCs and the subsequent sequestration of native ECM components and soluble cytokines that are conducive to osteogenesis. The HA-based DXNs, with the built-in topological features, variable mesh sizes and the ability to present biologically active molecules in a spatial manner, recapture the essential structural, morphological and functional characteristics of the native ECM. Our results provide critical insight into designing biomimetic hydrogel materials to modulate the behaviors of MSCs.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Drs. Michael Mackay and Eleftherios T. Papoutsakis for providing access to the Dynamic Mechanical Analyzer and Coulter Counter Multisizer 3, respectively. We thank Dr. Jeffrey Caplan for his assistance with confocal imaging and Genzyme for the generous gift of HA. This work was supported in part by NIH from grants R01 DC008965 (NIDCD) and P20 RR017716 (NCRR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–92. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 2.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 3.Freed LE, Vunjak-Novakovic G. Culture of organized cell communities. Adv Drug Deliv Rev. 1998;33:15–30. doi: 10.1016/s0169-409x(98)00017-9. [DOI] [PubMed] [Google Scholar]
- 4.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–70. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh K, Ingber DE. Micromechanical control of cell and tissue development: implications for tissue engineering. Adv Drug Deliv Rev. 2007;59:1306–18. doi: 10.1016/j.addr.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Thibeault SL. Novel isolation and biochemical characterization of immortalized fibroblasts for tissue engineering vocal fold lamina propria. Tissue Eng Part C-Methods. 2009;15:201–12. doi: 10.1089/ten.tec.2008.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 8.Jiang YH, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 9.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–7. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 10.Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng Part A. 2009;15:205–19. doi: 10.1089/ten.tea.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–41. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–79. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 14.Jia XQ, Kiick KL. Hybrid multicomponent hydrogels for tissue engineering. Macromol Biosci. 2009;9:140–56. doi: 10.1002/mabi.200800284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 16.Garg HG, Hales CA. Chemistry and biology of hyaluronan. 1st ed. Elsevier Ltd.; Oxford: 2004. [Google Scholar]
- 17.Turley EA, Noble PW, Bourguignon LYW. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–92. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 18.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–61. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 19.Sahiner N, Jha AK, Nguyen D, Jia XQ. Fabrication and characterization of cross-linkable hydrogel particles based on hyaluronic acid: potential application in vocal fold regeneration. J Biomater Sci-Polym Ed. 2008;19:223–43. doi: 10.1163/156856208783432462. [DOI] [PubMed] [Google Scholar]
- 20.Jia XQ, Yeo Y, Clifton RJ, Jiao T, Kohane DS, Kobler JB, et al. Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules. 2006;7:3336–44. doi: 10.1021/bm0604956. [DOI] [PubMed] [Google Scholar]
- 21.Jha AK, Hule RA, Jiao T, Teller SS, Clifton RJ, Duncan RL, et al. Structural analysis and mechanical characterization of hyaluronic acid-Based doubly cross-linked networks. Macromolecules. 2009;42:537–46. doi: 10.1021/ma8019442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jha AK, Malik MS, Farach-Carson MC, Duncan RL, Jia XQ. Hierarchically structured, hyaluronic acid-based hydrogel matrices via the covalent integration of microgels into macroscopic networks. Soft Matter. 2010;6:5045–55. doi: 10.1039/C0SM00101E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jha AK, Yang WD, Kirn-Safran CB, Farach-Carson MC, Jia XQ. Perlecan domain Iconjugated, hyaluronic acid-based hydrogel particles for enhanced chondrogenic differentiation via BMP-2 release. Biomaterials. 2009;30:6964–75. doi: 10.1016/j.biomaterials.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gumbiner BM. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–57. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 25.Jia XQ, Burdick JA, Kobler J, Clifton RJ, Rosowski JJ, Zeitels SM, et al. Synthesis and characterization of in situ cross-linkable hyaluronic acid-based hydrogels with potential application for vocal fold regeneration. Macromolecules. 2004;37:3239–48. [Google Scholar]
- 26.Farran AJE, Teller SS, Jha AK, Jiao T, Hule RA, Clifton RJ, et al. Effects of matrix composition, microstructure, and viscoelasticity on the behaviors of vocal fold fibroblasts cultured in three-dimensional hydrogel networks. Tissue Eng Part A. 2010;16:1247–61. doi: 10.1089/ten.tea.2009.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morse GA. Primary amine analysis in polymers. J Polym Sci A. 1984;22:3611–5. [Google Scholar]
- 28.Feng YZ, Mrksich M. The synergy peptide PHSRN and the adhesion peptide RGD mediate cell adhesion through a common mechanism. Biochemistry. 2004;43:15811–21. doi: 10.1021/bi049174+. [DOI] [PubMed] [Google Scholar]
- 29.Bancroft JD, Gamble M. Theory and practice of histological techniques. Harcourt Publishers Limited; London: 2002. [Google Scholar]
- 30.Rungby J, Kassem M, Eriksen EF, Danscher G. The vonKossa reaction for calcium deposits - silver lactate staining increases sensitivity and reduces background. Histochem J. 1993;25:446–51. doi: 10.1007/BF00157809. [DOI] [PubMed] [Google Scholar]
- 31.Lee SB, Jeon HW, Lee YW, Lee YM, Song KW, Park MH, et al. Bio-artificial skin composed of gelatin and (1 -> 3), (1 -> 6)-beta-glucan. Biomaterials. 2003;24:2503–11. doi: 10.1016/s0142-9612(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 32.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–44. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rathna GVN. Hydrogels of modified ethylenediaminetetraacetic dianhydride gelatin conjugated with poly(ethylene glycol) dialdehyde as a drug-release matrix. J Appl Polym Sci. 2004;91:1059–67. [Google Scholar]
- 34.Shu XZ, Liu YC, Palumbo F, Prestwich GD. Disulfide-crosslinked hyaluronan-gelatin hydrogel films: a covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials. 2003;24:3825–34. doi: 10.1016/s0142-9612(03)00267-9. [DOI] [PubMed] [Google Scholar]
- 35.Djabourov M, Papon P. Influence of termal treatments on the structure and stability of gelatin gels. Polymer. 1983;24:537–42. [Google Scholar]
- 36.Saxena A, Antony T, Bohidar HB. Dynamic light scattering study of gelatin-surfactant interactions. J Phys Chem B. 1998;102:5063–8. [Google Scholar]
- 37.Abbott A. Cell culture: biology's new dimension. Nature. 2003;424:870–2. doi: 10.1038/424870a. [DOI] [PubMed] [Google Scholar]
- 38.Fraley SI, Feng YF, Krishnamurthy R, Kim DH, Celedon A, Longmore GD, et al. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat Cell Biol. 2010;12:598–U169. doi: 10.1038/ncb2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Docheva D, Popov C, Mutschler W, Schieker M. Human mesenchymal stem cells in contact with their environment: surface characteristics and the integrin system. J Cell Mol Med. 2007;11:21–38. doi: 10.1111/j.1582-4934.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taubenberger AV, Woodruff MA, Bai HF, Muller DJ, Hutmacher DW. The effect of unlocking RGD-motifs in collagen I on pre-osteoblast adhesion and differentiation. Biomaterials. 2010;31:2827–35. doi: 10.1016/j.biomaterials.2009.12.051. [DOI] [PubMed] [Google Scholar]
- 41.Underhill C. CD44-the hyaluronan receptor. J Cell Sci. 1992;103:293–8. doi: 10.1242/jcs.103.2.293. [DOI] [PubMed] [Google Scholar]
- 42.Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, et al. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928–35. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 43.Tsai KS, Kao SY, Wang CY, Wang YJ, Wang JP, Hung SC. Type I collagen promotes proliferation and osteogenesis of human mesenchymal stem cells via activation of ERK and Akt pathways. J Biomed Mater Res Part A. 2010;94A:673–82. doi: 10.1002/jbm.a.32693. [DOI] [PubMed] [Google Scholar]
- 44.Cristino S, Grassi F, Toneguzzi S, Piacentini A, Grigolo B, Santi S, et al. Analysis of mesenchymal stem dimensional HYAFF 11 (R)-based cells grown on a prototype ligament scaffold. J Biomed Mater Res Part A. 2005;73A:275–83. doi: 10.1002/jbm.a.30261. [DOI] [PubMed] [Google Scholar]
- 45.Zou LJ, Zou XN, Chen L, Li HS, Mygind T, Kassem M, et al. Effect of hyaluronan on osteogenic differentiation of porcine bone marrow stromal cells in vitro. J Orthop Res. 2008;26:713–20. doi: 10.1002/jor.20539. [DOI] [PubMed] [Google Scholar]
- 46.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–95. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.