Abstract
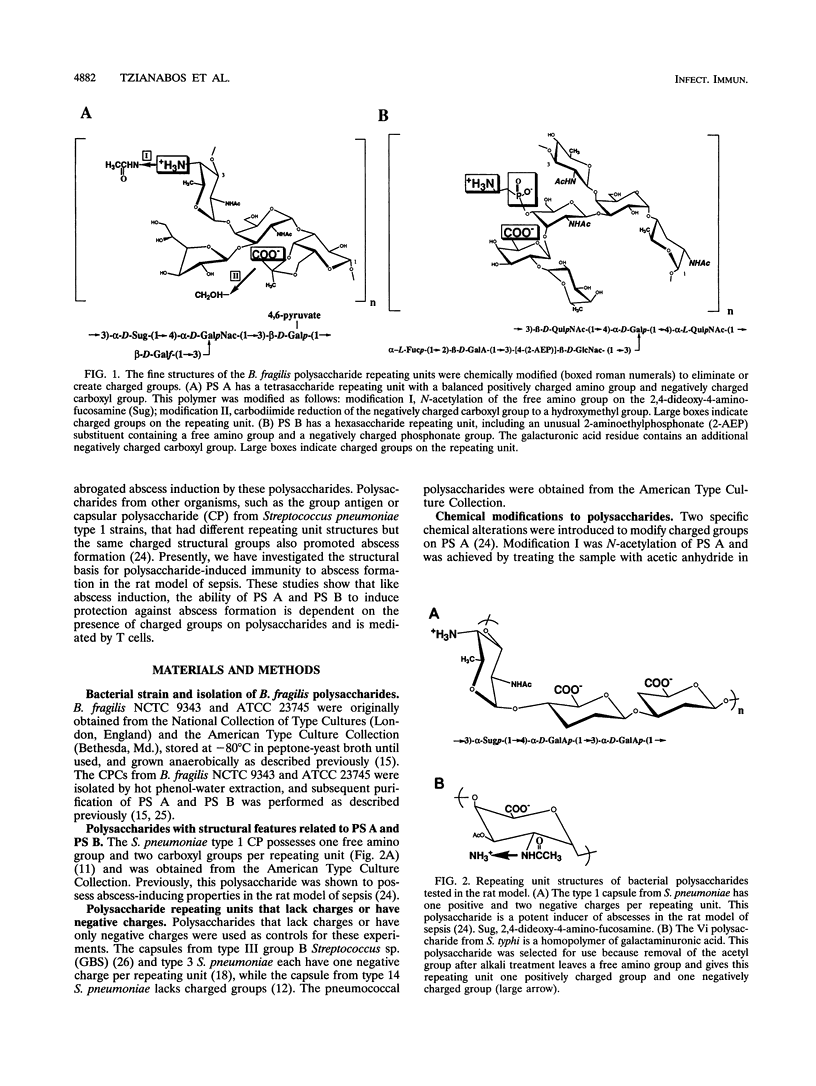
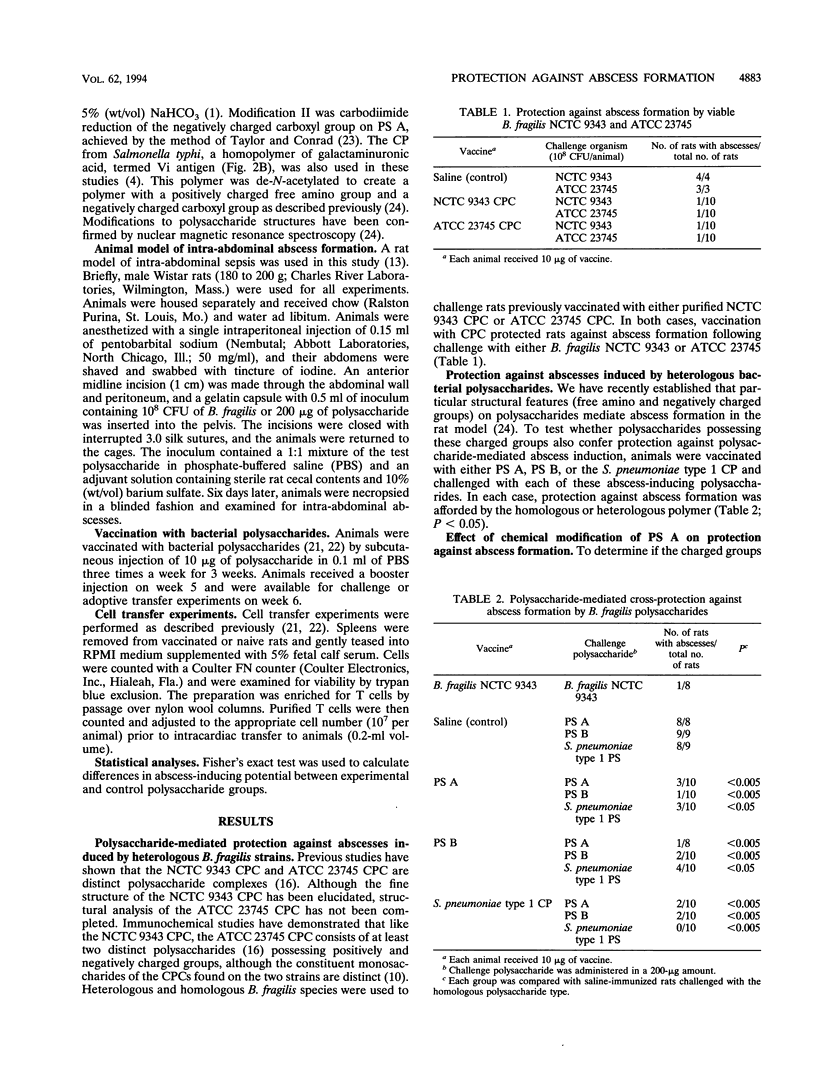
Bacteroides fragilis is the anaerobe most commonly isolated from clinical cases of intra-abdominal sepsis. In a rodent model of this disease process, intraperitoneal injection of the capsular polysaccharide complex (CPC) from B. fragilis provokes abscess formation, while subcutaneous administration of this complex confers protection against B. fragilis-induced intra-abdominal abscesses. The CPC consists of two discrete polysaccharides, polysaccharides A and B (PS A and PS B), each possessing oppositely charged structural groups critical to the ability of these carbohydrates to induce the formation of abscesses. Other bacterial polysaccharides that possess oppositely charged groups (such as the group antigen or capsular polysaccharide from Streptococcus pneumoniae type 1 strains) also exhibited potent abscess-inducing capabilities. We report here that positively and negatively charged groups on polysaccharides are also essential for inducing protection against abscess formation. Vaccination of rats with B. fragilis PS A, PS B, or the S. pneumoniae type 1 capsule protected against intra-abdominal abscesses subsequent to intraperitoneal challenge with each of these polysaccharides. Chemical conversion of the free amino or carboxyl groups on PS A to uncharged N-acetyl or hydroxymethyl groups, respectively, abrogated the ability of this polymer to confer protection against polysaccharide-mediated abscess formation. Adoptive transfer of splenic T cells from polysaccharide-vaccinated rats to naive animals demonstrated that T cells mediated this protective activity. T cells transferred from animals vaccinated with a polysaccharide repeating unit (Salmonella typhi Vi antigen) that normally contains one carboxyl group but was chemically converted to a polymer that possesses both free amino and carboxyl groups (accomplished by de-N-acetylating the Vi antigen) protected naive T-cell recipients against polysaccharide-induced abscesses. These results demonstrate that a distinct structural motif associated with the B. fragilis polysaccharides is necessary for induction of protective immunity against abscess formation associated with intra-abdominal sepsis. However, protection is not antigen specific in a traditional sense. Rather, the protective ability of these structurally dissimilar polysaccharides is conferred by, and perhaps specific for, a motif of oppositely charged groups.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann H., Tzianabos A. O., Brisson J. R., Kasper D. L., Jennings H. J. Structural elucidation of two capsular polysaccharides from one strain of Bacteroides fragilis using high-resolution NMR spectroscopy. Biochemistry. 1992 Apr 28;31(16):4081–4089. doi: 10.1021/bi00131a026. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L., Bartlett J. G. Anaerobic infections. 1. N Engl J Med. 1974 May 23;290(21):1177–1184. doi: 10.1056/NEJM197405232902106. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Roof R. W., Allen P. M., Unanue E. R. Effects of pH and polysaccharides on peptide binding to class II major histocompatibility complex molecules. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2740–2744. doi: 10.1073/pnas.88.7.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishioka G. Y., Lamont A. G., Thomson D., Bulbow N., Gaeta F. C., Sette A., Grey H. M. MHC interaction and T cell recognition of carbohydrates and glycopeptides. J Immunol. 1992 Apr 15;148(8):2446–2451. [PubMed] [Google Scholar]
- Kasper D. L., Finberg R. F., Crabb J., Onderdonk A. B. Immune mechanisms in the prevention of intra-abdominal abscess formation. Scand J Infect Dis Suppl. 1989;62:29–34. [PubMed] [Google Scholar]
- Kasper D. L., Hayes M. E., Reinap B. G., Craft F. O., Onderdonk A. B., Polk B. F. Isolation and identification of encapsulated strains of Bacteroides fragilis. J Infect Dis. 1977 Jul;136(1):75–81. doi: 10.1093/infdis/136.1.75. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Onderdonk A. B., Crabb J., Bartlett J. G. Protective efficacy of immunization with capsular antigen against experimental infection with Bacteroides fragilis. J Infect Dis. 1979 Nov;140(5):724–731. doi: 10.1093/infdis/140.5.724. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Seiler M. W. Immunochemical characterization of the outer membrane complex of Bacteroides fragilis subspecies fragilis. J Infect Dis. 1975 Oct;132(4):440–450. doi: 10.1093/infdis/132.4.440. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Weintraub A., Lindberg A. A., Lönngren J. Capsular polysaccharides and lipopolysaccharides from two Bacteroides fragilis reference strains: chemical and immunochemical characterization. J Bacteriol. 1983 Feb;153(2):991–997. doi: 10.1128/jb.153.2.991-997.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg B., Lindqvist B., Lönngren J., Powell D. A. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 1. Carbohydr Res. 1980 Jan 1;78(1):111–117. doi: 10.1016/s0008-6215(00)83664-2. [DOI] [PubMed] [Google Scholar]
- Lindberg B., Lönngren J., Powell D. A. Structural studies on the specific type-14 pneumococcal polysaccharide. Carbohydr Res. 1977 Sep;58(1):177–186. doi: 10.1016/s0008-6215(00)83413-8. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Kasper D. L., Cisneros R. L., Bartlett J. G. The capsular polysaccharide of Bacteroides fragilis as a virulence factor: comparison of the pathogenic potential of encapsulated and unencapsulated strains. J Infect Dis. 1977 Jul;136(1):82–89. doi: 10.1093/infdis/136.1.82. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Markham R. B., Zaleznik D. F., Cisneros R. L., Kasper D. L. Evidence for T cell-dependent immunity to Bacteroides fragilis in an intraabdominal abscess model. J Clin Invest. 1982 Jan;69(1):9–16. doi: 10.1172/JCI110445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantosti A., Tzianabos A. O., Onderdonk A. B., Kasper D. L. Immunochemical characterization of two surface polysaccharides of Bacteroides fragilis. Infect Immun. 1991 Jun;59(6):2075–2082. doi: 10.1128/iai.59.6.2075-2082.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantosti A., Tzianabos A. O., Reinap B. G., Onderdonk A. B., Kasper D. L. Bacteroides fragilis strains express multiple capsular polysaccharides. J Clin Microbiol. 1993 Jul;31(7):1850–1855. doi: 10.1128/jcm.31.7.1850-1855.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk B. F., Kasper D. L. Bacteroides fragilis subspecies in clinical isolates. Ann Intern Med. 1977 May;86(5):569–571. doi: 10.7326/0003-4819-86-5-569. [DOI] [PubMed] [Google Scholar]
- Rhodes J. Erythrocyte rosettes provide an analogue for Schiff base formation in specific T cell activation. J Immunol. 1990 Jul 15;145(2):463–469. [PubMed] [Google Scholar]
- Rhodes J. Evidence for an intercellular covalent reaction essential in antigen-specific T cell activation. J Immunol. 1989 Sep 1;143(5):1482–1489. [PubMed] [Google Scholar]
- Shapiro M. E., Kasper D. L., Zaleznik D. F., Spriggs S., Onderdonk A. B., Finberg R. W. Cellular control of abscess formation: role of T cells in the regulation of abscesses formed in response to Bacteroides fragilis. J Immunol. 1986 Jul 1;137(1):341–346. [PubMed] [Google Scholar]
- Shapiro M. E., Onderdonk A. B., Kasper D. L., Finberg R. W. Cellular immunity to Bacteroides fragilis capsular polysaccharide. J Exp Med. 1982 Apr 1;155(4):1188–1197. doi: 10.1084/jem.155.4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- Tzianabos A. O., Onderdonk A. B., Rosner B., Cisneros R. L., Kasper D. L. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 1993 Oct 15;262(5132):416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- Tzianabos A. O., Onderdonk A. B., Smith R. S., Kasper D. L. Structure-function relationships for polysaccharide-induced intra-abdominal abscesses. Infect Immun. 1994 Aug;62(8):3590–3593. doi: 10.1128/iai.62.8.3590-3593.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzianabos A. O., Pantosti A., Baumann H., Brisson J. R., Jennings H. J., Kasper D. L. The capsular polysaccharide of Bacteroides fragilis comprises two ionically linked polysaccharides. J Biol Chem. 1992 Sep 5;267(25):18230–18235. [PubMed] [Google Scholar]
- Wessels M. R., Pozsgay V., Kasper D. L., Jennings H. J. Structure and immunochemistry of an oligosaccharide repeating unit of the capsular polysaccharide of type III group B Streptococcus. A revised structure for the type III group B streptococcal polysaccharide antigen. J Biol Chem. 1987 Jun 15;262(17):8262–8267. [PubMed] [Google Scholar]
- Zheng B., Brett S. J., Tite J. P., Lifely M. R., Brodie T. A., Rhodes J. Galactose oxidation in the design of immunogenic vaccines. Science. 1992 Jun 12;256(5063):1560–1563. doi: 10.1126/science.1598588. [DOI] [PubMed] [Google Scholar]


