Summary
The retrotrapezoid nucleus (RTN), located at the ventral surface of the medulla oblongata, contains glutamatergic Phox2b-expressing interneurons that have central respiratory chemoreceptor properties. RTN also operates as a relay for hypothalamic pathways that regulate breathing, one of which probably originates from the orexinergic neurons (Dias et al., J. Physiol. 587:2059–2067, 2009). The present study explores this hypothesis at the cellular level. Using immunohistochemistry in adult Phox2b-eGFP transgenic mice, we demonstrate the presence of numerous close appositions between orexin-containing axonal varicosities and RTN chemoreceptor neurons. Using electrophysiological recordings in slices from neonatal (P6–P10) Phox2b-eGFP mice, we show that orexin A produces a robust dose-dependent excitation of the acid-sensitive RTN neurons (ED50 ~250 nM). These data support the idea that RTN neurons are a point of convergence for several groups of CNS neurons that contribute to respiratory chemoreflexes, now including serotonergic and orexinergic neurons.
Keywords: Central chemoreceptors, chemoreflexes, Phox2b, sleep
1. Introduction
Within the brain, orexins are produced exclusively by hypothalamic neurons (Kukkonen et al., 2002). These neurons regulate reward processes, arousal and various stress responses (Harris and Aston-Jones, 2006; Adamantidis et al., 2007; Kuwaki et al., 2008). The descending projections of orexin neurons target brainstem and spinal cord regions involved in feeding, sleep, respiration and autonomic functions (Peyron et al., 1998; Kukkonen et al., 2002). Consistent with their general wake-promoting role, orexinergic neurons contribute to breathing predominantly during the waking state (Kuwaki, 2008; Kuwaki et al., 2008; Dias et al., 2009a; Dias et al., 2009b; Li and Nattie, 2010).
The retrotrapezoid nucleus located on the ventral medullary surface contains Phox2b-expressing glutamatergic neurons that play a pivotal role in central respiratory chemoreflexes (Guyenet et al., 2010; Marina et al., 2010). Increases in arterial PCO2 activate these neurons via a process that seems partially cell autonomous and partially dependent on the release of gliotransmitters by a specialized form of pH-sensitive glia (Abbott et al., 2009; Gourine et al., 2010; Guyenet et al., 2010). Phox2b mutations cause major deficits in respiratory chemoreception in humans (Amiel et al., 2009). Based on genetic models in mice, this congenital deficit seems to result from a profound and perhaps selective reduction in the number of RTN neurons (Amiel et al., 2009). The importance of the RTN neurons to respiratory chemoreflexes may have other explanations besides their well-documented central chemoreceptor properties (Guyenet et al., 2010; Marina et al., 2010). For example, these chemosensitive neurons also receive powerful excitatory inputs from the carotid bodies (Takakura et al., 2006; Marina et al., 2010). In addition, RTN neurons may be a convergence point for other CNS neurons that may also have chemoreceptor properties. A projection from serotonergic neurons on RTN neurons and an excitatory effect of 5-HT is already demonstrated (Mulkey et al., 2007). The possibility that RTN neurons receive an orexinergic input is suggested by the fact that microdialysis of an orexin antagonist into the RTN attenuates the chemoreflex (Dias et al., 2009b). The purpose of the present study was to ascertain whether RTN neurons actually receive an orexinergic input. Taking advantage of Phox2b-eGFP transgenic mice in which the RTN chemoreceptor neurons selectively express enhanced green fluorescent protein (Lazarenko et al., 2009) we used immunohistochemistry to show that RTN neurons are innervated by orexin-immunoreactive nerve terminals and electrophysiology to demonstrate that orexin has excitatory effects on these neurons.
2. Methods
2.1. Animals
Animal use was in accordance with National Institutes of Health Animal Care and Use Guidelines and approved by the Animal Care and Use Committee of the University of Virginia. We used two lines of BAC transgenic mice (B/G, N=15, and JX99, N=6) in which enhanced green fluorescent protein (eGFP) expression is directed selectively to Phox2b-expressing neurons. The derivation and properties of these mice have been described previously (Lazarenko et al., 2009). In the ventral medulla oblongata of the B/G mouse, GFP expression is limited to a subset of RTN neurons (approximately half) all of which are pH-responsive (Lazarenko et al., 2009). In the JX99 mouse, GFP is expressed by virtually every Phox2b-expressing brainstem neuron (Lazarenko et al., 2009). Within the rostral ventrolateral medulla, this population includes all RTN Phox2b-expressing neurons, the overlying facial motor nucleus and the nearby C1 catecholaminergic neurons (Lazarenko et al., 2009). In slices from JX99 mouse, RTN neurons were identified by a combination of three criteria: anatomical location under the facial motor nucleus, small elongated cell bodies with primary dendrites typically extending parallel to the ventral medullary surface and sensitive responses to changes in bath pH (Lazarenko et al., 2009). The first two criteria distinguish RTN neurons from the overlying facial motor neurons. The third distinguishes RTN neurons from neighboring C1 neurons which are unresponsive to pH variations under in vitro conditions (Lazarenko et al., 2009). To the best of our knowledge no other Phox2b-expressing cell type resides in the region of interest and the properties of RTN neurons in both mouse strains are identical (Lazarenko et al., 2009).
2.2. Brainstem slice preparation and cell recordings
Transverse slices were prepared from brainstems of neonatal (P6–P10) mice of either sex as described previously (Lazarenko et al., 2009; 2010). Briefly, mice were anesthetized with ketamine (375 mg/kg, i.m) and xylazine (25 mg/kg, i.m.) and brainstems were removed after rapid decapitation. Slices (300 µm) were cut with a microslicer (DSK 1500E; Dosaka) in an ice-cold substituted Ringer’s solution consisting of (in mM): 260 sucrose, 3 KCl, 5 MgCl2, 1 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, and 1 kynurenic acid. Slices were incubated for 30 min at 37°C and subsequently at room temperature in a normal Ringer’s solution containing (in mM): 130 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 glucose. Both substituted and normal Ringer’s solutions were bubbled with 95% O2 and 5% CO2.
Green fluorescent RTN neurons that were active at pH 7.3 (1–3 Hz) were recorded using the loose patch method. The slices were superfused continuously (~2 ml/min) with a solution composed of (mM): 140 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 10 HEPES, 10 glucose; the pH of the bath solution was adjusted between 7.0 and 8.0 by addition of HCl or NaOH. We added a mixture of blockers (10 µm CNQX, 10 µm bicuculline, and 30 µm strychnine) to inhibit fast excitatory (glutamate) and inhibitory neurotransmission (GABA, glycine). Orexin A (hypocretin-1, Sigma) was prepared as a 0.5 mM stock and stored at −20°C until added directly to the recording chamber at the indicated concentrations under stop-flow conditions. All recordings were performed at room temperature. Patch electrodes had a DC resistance of 3 – 6 MΩ when filled with internal solution containing (mM): 120 KCH3SO3, 4 NaCl, 1 MgCl2, 0.5 CaCl2, 10 HEPES, 10 EGTA, 3Mg-ATP, 0.3 GTP-Tris pH 7.2; electrode tips were coated with Sylgard 184 (Dow Corning).
Recordings were obtained using pClamp 9.0 to drive an Axopatch 200B amplifier via a Digidata 1322A analog-to-digital converter (all from Molecular Devices, Foster City, CA). Firing rate histograms were generated by integrating action potential discharge in 10-s bins and plotted using Spike 5.0 software (Cambridge Electronics). Results are shown as mean ± SEM. Dose-response data were analyzed statistically in GraphPad Prism 5.0 and fitted with a logistic equation to estimate slope and EC50 values. Two-way ANOVA was used to determine statistical significance. Differences in mean values were considered significant if P < 0.05.
2.3. Immunohistochemistry
To determine whether RTN neurons receive orexinergic projections, 4 adult Phox2b-eGFP B/G transgenic mice were deeply anesthetized with an overdose of pentobarbital (125 mg/kg) and perfused transcardially, first with 5–10 ml of heparinized phosphate buffered saline (pH 7.4) followed by 80 ml of 4% phosphate-buffered (0.1 M; pH 7.4) paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA). The brains were removed and stored in the perfusion fixative for 24–48 hours at 4°C. A series of coronal sections (30 µm) from the brain were cut using a vibrating microtome and stored in cryoprotectant solution at −20° (20% glycerol plus 30% ethylene glycol in 50 mM phosphate buffer, pH 7.4) awaiting histological processing. All histochemical procedures were done with free-floating sections. Orexin A was detected with a goat antibody (1:5000, Santa Cruz Biotechnology (sc-8070), Santa Cruz, CA), Phox2b with a rabbit antibody (1:800, gift from J.-F. Brunet, Ecole Normale Supérieure, Paris, France) and eGFP with a chicken anti-GFP antibody (1:1000; Aves Labs Inc., Tigard, OR). These primary antibodies were detected by incubation with appropriate secondary antibodies tagged with fluorescent reporters to reveal orexin (1:200 donkey anti-goat Cy3, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), Phox2b (1:200 donkey anti-rabbit Cy3, Jackson) or eGFP (1:200 donkey anti-chicken Cy2, Jackson). The selectivity of the three primary antibodies used has been rigorously characterized before (for details and references, see the Journal of Comparative Neurology antibody database at http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1096-9861/homepage/jcn_antibody_database.htm). Sections were mounted on glass slides, dehydrated through a graded series of alcohols and xylenes and covered with DPX mounting medium.
2.4. Statistics
The effect of pH on RTN neuron activity in the absence of orexin and the effect of orexin of RTN neuronal activity at pH 7.3 were analyzed using Kruskal-Wallis one-way ANOVA on ranks (SigmaStat version 3.10). All pair-wise multiple comparisons procedures (Dunn’s method) were then used to assess the statistical significance between the various groups.
3. Results
3.1. Orexin terminals make close appositions with eGFP-labeled RTN neurons
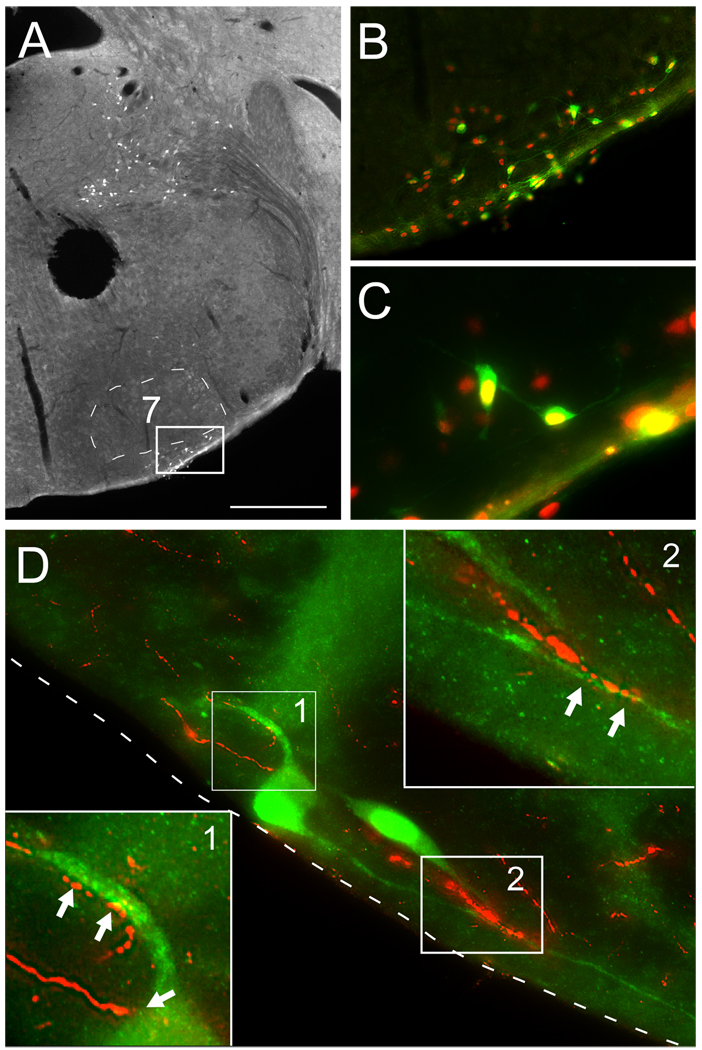
The anatomical proximity between orexin A-immunoreactive axons and RTN neurons was examined in the B/G mouse (N = 4) in which eGFP is exclusively expressed by RTN Phox2b-expressing neurons (Lazarenko et al., 2009)(Fig. 1A–C). Close appositions between orexinergic varicosities and the dendrites of eGFP-labeled RTN neurons were commonly observed suggesting the probable existence of synapses (Fig. 1D).
Figure 1. Orexin innervation of retrotrapezoid nucleus (RTN) neurons in the B/G mouse.
A. Coronal hemi-section of B/G mouse showing eGFP neurons limited to either the ventral surface (RTN, outlined by white rectangle) or dorsal structures very distant from RTN (vestibular nuclei). 7, facial motor nucleus.
B. Inset of RTN (outlined with white rectangle in panel A) showing eGFP (Cy2, green fluorescence) and Phox2b immunoreactivity (Cy3, red fluorescence).
C. Same field as B with higher magnification. Note that all the eGFP-positive neurons express Phox2b.
D. Orexin terminals (Cy3, red fluorescence) in RTN making close appositions with eGFP-expressing RTN neurons and dendrites (Cy2, green fluorescence). Two small areas (1 and 2) are shown at higher magnification at the upper right and lower left corners to highlight the putative synapses (arrowheads). The ventral surface of the medulla oblongata is indicated by the dotted line. Scale bar in A = 500 µm, B = 125 µm, C = 40 µm, D (main panel only, not applicable to the two inserts) = 20 µm.
3.2. Orexin excites RTN neurons in vitro
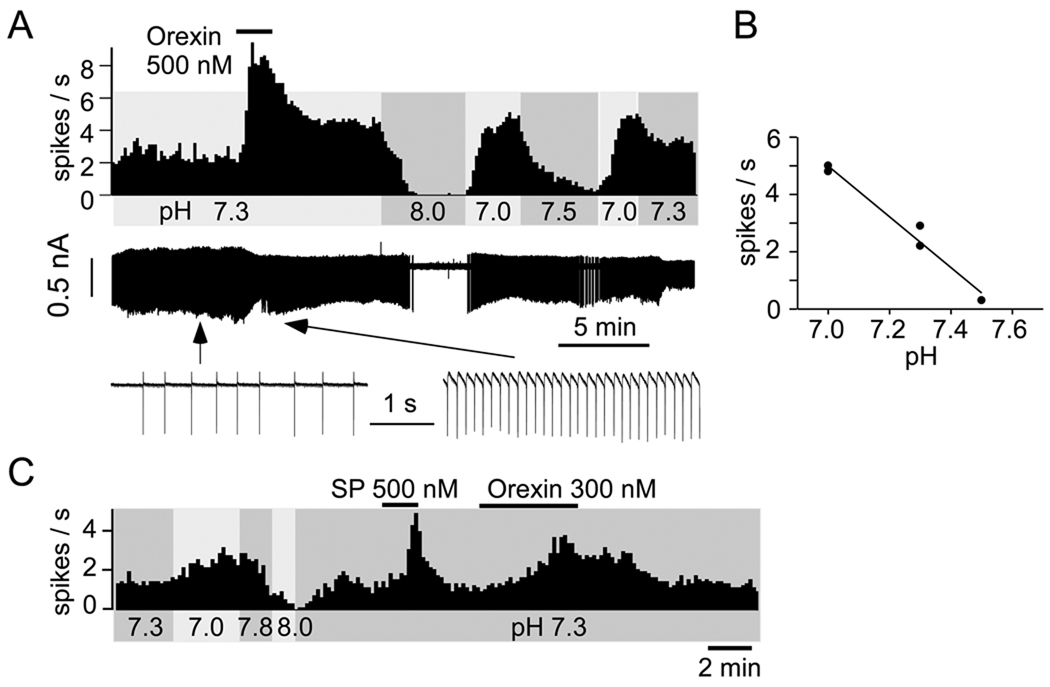
Bath applied orexin A excited eGFP-expressing RTN neurons in a dose-related manner regardless of the mouse strain used. The representative neuron shown in Figure 2 was pH-sensitive as expected (Lazarenko et al., 2009), showing increased activity upon bath acidification, decreased firing with alkalization and ~5 Hz dynamic range of response between pH 7.0 and 7.5. The inset depicts the relationship between bath pH and the steady-state activity of the neuron in the absence of orexin. Three pH levels are represented in this graph, two of which were repeated once to ascertain the stability of the cell’s response. Of interest here, the pH-sensitive neuron responded to bath application of 500 nM orexin with a robust increase in discharge (to ~8 Hz).
Figure 2. Effect of pH and orexin on RTN neurons: representative examples.
A. Top trace: discharge rate of one RTN neuron (integrated rate histogram, 10 s bins, JX99 mouse). Middle trace: raw recording of action currents recorded in loose-patch mode. Lower trace: action currents shown at higher resolution before and during application of orexin. B: steady-state relationship between discharge rate and pH in the absence of orexin for the neuron shown in A. C. discharge of one RTN neuron in a B/G Phox2b-eGFP mouse (integrated rate histogram, 10 s bins). Substance P (SP; 500 nM) was also applied to this neuron, producing the expected vigorous activation (Mulkey et al., 2007).
Incomplete return to baseline after application of orexin, such as shown in Fig. 2A, was not a uniform finding (3 out of 8 exposures to 300 or 500 nM orexin). A second example (Fig. 2C) illustrates a case in which complete recovery from orexin was observed. Our observations were not sufficiently extensive to ascertain whether the incomplete recovery from orexin seen in Fig. 2A and a few other cases was caused by a drift in the basal activity of the cells or denoted a long-lasting, possibly dose-dependent, effect of the peptide. Figure 2C also illustrates the excitatory response of the RTN neuron to substance P, consistent with prior evidence (Mulkey et al., 2007).
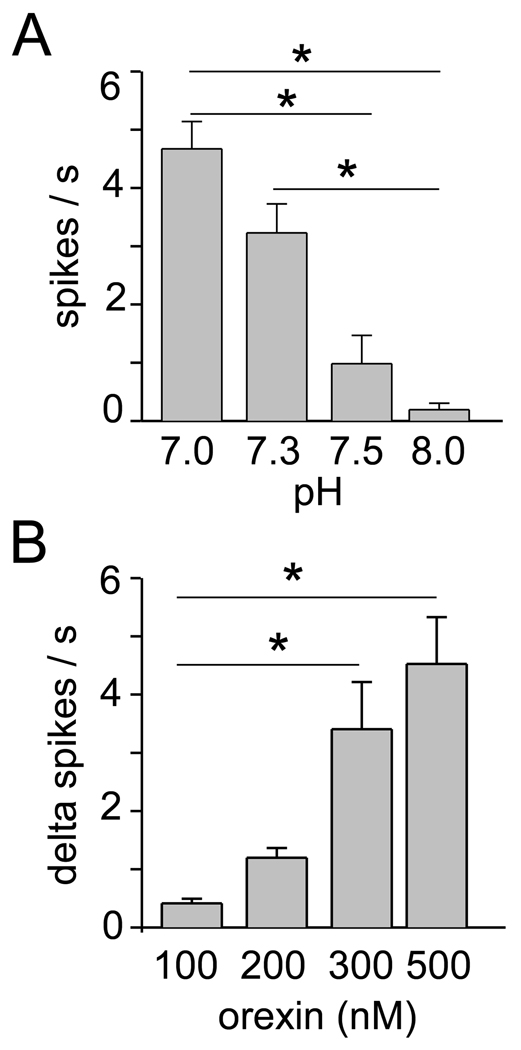
The group data shown in Figure 3A,B were obtained from 14 RTN neurons (6 from JX99 mice and 8 from B/G mice). As previously reported, the discharge rate of these neurons was highly–dependent on bath pH (P<0.001, Kruskall-Wallis ANOVA on ranks; Fig. 3A). Orexin produced a dose-dependent increase in the discharge rate of these neurons (P=0.002, Kruskall-Wallis ANOVA on ranks; Fig. 3B). Only one neuron was recorded per slice and, except in two cases in which a single neuron was exposed successively to 100 and 200 nM of the peptide, each neuron received a single application of the peptide. As indicated in the methods, in order to reduce costs, flow through the chamber had to be temporarily stopped when orexin was introduced and, for the same reason, the highest concentration tested was 500 nM. The momentary interruption of the perfusion did not change the discharge rate as evidenced by the fact that the response to orexin was dose-dependent and that the response was negligible at a concentration of 100 nM. Assuming near saturation of the effect of orexin at the dose of 500 nM, an EC50 of about 250 nM could be estimated.
Figure 3. Effect of pH and orexin on RTN neurons: group data.
A. Effect of bath pH on the steady-state discharge of 14 RTN neurons. There was a highly significant effect of pH on the discharge rate (P<0.001 by Kruskall-Wallis ANOVA on ranks). B. Increase in discharge rate elicited by four concentrations of orexin in the same 14 neurons recorded at pH 7.3 (up to 2 concentrations tested per neuron, 4 determinations for the 100, 300, and 500nM levels, 6 for the 200 nM concentration). There was a highly significant dose-effect relationship (P=0.002 by Kruskall-Wallis ANOVA on ranks). Asterisks denote statistically significant pair-wise comparisons. The pairs are identified by the end of the horizontal lines.
4. Discussion
The present results confirm and extend prior evidence that orexin neurons facilitate breathing in part by recruiting RTN neurons (Dias et al., 2009b; Li and Nattie, 2010). The original evidence was based on orexin microdialysis experiments. They provided an essential functional link between the endogenous release orexin in the ventrolateral medulla and respiration but they did not permit precise determination of the targeted neurons given the neuronal complexity or the medulla oblongata. We show here that the acid-sensitive Phox2b-expressing RTN neurons are indeed targeted by orexinergic terminals and are excited by orexin. Since selective activation of these Phox2b-expressing neurons enhances breathing (Abbott et al., 2009; Kanbar et al., 2010), the reduction in breathing caused by infusing an orexin antagonist into the RTN is indeed likely to result partly from the removal of the tonic excitatory effect of orexin on these neurons (Dias et al., 2009b). However, the orexinergic input to the ventrolateral medulla is not restricted to the RTN (Peyron et al., 1998) and a large fraction of randomly sampled neurons located in the ventrolateral medulla respond to orexin in vitro (Huang et al., 2010). RTN neurons are therefore unlikely to be the only respiration-related ventral medullary neurons regulated by orexin. The orexinergic neurons are wake-promoting neurons that control many physiological functions besides breathing. In addition, the role of orexinergic neurons role in breathing regulation is not limited to chemoreflex modulation but includes breathing responses to a variety of stressors (Zhang et al., 2006; Adamantidis et al., 2007; Kuwaki et al., 2008).
Hypercapnia increases slightly the proportion of orexin neurons that express c-Fos (Sunanaga et al., 2009) and orexin neurons are activated by acidification in slices (Gonzalez et al., 2009). Therefore orexin neurons, or a subset thereof, may also have central respiratory chemoreceptor properties (Kuwaki, 2008; Dias et al., 2009a; Dias et al., 2009b).
Subsets of RTN neurons with distinctive biochemistry or discharge characteristics have been characterized to various extents in more intact preparations (for a detailed discussion see (Guyenet and Mulkey, 2010). We selected for study eGFP-positive neurons that had a stable ongoing activity (1–3Hz) at pH 7.3 in slices. To the best of our knowledge every RTN neuron with this characteristic is acid sensitive and the pH sensitivity of these neurons is the same in both mouse strains (Lazarenko et al., 2009). However, the RTN could conceivably contain a second type of Phox2b-positive cells that is silent at pH 7.3. Such neurons would never have been recorded from in the present study given our selection criteria.
We recorded RTN neurons in the presence of a cocktail of substances that block ionotropic glutamate, glycine and GABA receptors. The use of this drug mixture reduces the likelihood that the effect of orexin and pH on RTN neurons could have resulted from activation or inhibition of other types of neurons than the Phox2b-expressing ones. This interpretation is also consistent with the presence of presumptive synapses (close appositions) between orexin terminals and the dendrites of the RTN neurons. The existence of these synapses needs ultrastructural confirmation. In addition, we have not excluded a primary effect of orexin on the local glial cells that probably contribute to the chemosensitivity of the RTN neurons (Erlichman et al., 1998; Gourine et al., 2010) because the receptor antagonist mixture that we used does not block the effects of ATP nor the actions of glutamate mediated by metabotropic receptors.
The excitatory effect of a probably near-saturating concentration of orexin was large compared to the dynamic range of response of the RTN neurons to pH. However, the relevance of such a comparison must be tempered by recalling that the pH sensitivity of RTN neurons recorded in neonate rodent slices at room temperature (delta discharge rate per pH unit) is only about one sixth of the value observed in the adult in vivo when arterial pH is used as the reference (Guyenet et al., 2005). About half of this discrepancy can be simply explained by the temperature sensitivity of the response of neonatal RTN neurons to pH (Guyenet et al., 2005). The rest may be related to the later development of a glial cell-dependent mechanism or to the specific contribution of molecular CO2 to the hypercapnia response (Gourine et al., 2010; Huckstepp et al., 2010). In theory, the CO2-sensitivity of RTN neurons could also be enhanced in vivo by the regulated release of transmitters that modify their intrinsic properties or that of the surrounding glia. No such substance has yet been described. The best studied, serotonin, does not appear to work this way since its effects on RTN neurons and those of acidification are simply additive in slices (Mulkey et al., 2007). The possibility that orexin and acid could have supra-additive effects on RTN neurons has not been specifically tested in the present experiments but it is not supported by prior in vivo experiments either. The orexin input presumably contributes to the activation of RTN neurons elicited by stimulating the perifornical hypothalamus of anesthetized animals (Fortuna et al., 2009). This activation is independent of the level of arterial PCO2 which suggests that orexin, like serotonin, may activate RTN neurons by a cellular mechanism that is additive with but independent of the effect of arterial PCO2 on these neurons (Mulkey et al., 2007).
The present study was not designed to determine the relative contribution of RTN vs. other neurons to the breathing stimulation elicited by activating the orexinergic system. This question would be difficult to answer because the orexinergic neurons target vast regions of the midbrain, brainstem and spinal cord (e.g. ventrolateral medulla, locus coeruleus, serotonergic system and phrenic motor neurons), each of which has the potential to contribute to the breathing stimulation and chemoreflex facilitation caused by activating the orexinergic system (Peyron et al., 1998; Adamantidis et al., 2007; Nattie and Li, 2009; Li and Nattie, 2010).
In summary, RTN neurons are probably directly innervated by orexinergic neurons and respond to application of orexin in vitro by an excitation. The orexinergic neurons contribute to the increased breathing associated with the waking state and this regulation is partially mediated via activation of the RTN neurons.
Acknowledgments
This work was supported by the following grants from the National Institutes of Health (HL74011 to PGG, NS33583 to DAB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbott SBG, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus Phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J. Neurosci. 2009;29:5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, De Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiel J, Dubreuil V, Ramanantsoa N, Fortin G, Gallego J, Brunet JF, Goridis C. PHOX2B in respiratory control: lessons from congenital central hypoventilation syndrome and its mouse models. Respir. Physiol. Neurobiol. 2009;168:125–132. doi: 10.1016/j.resp.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie E. The orexin receptor 1 (OX(1)R) in the rostral medullary raphe contributes to the hypercapnic chemoreflex in wakefulness, during the active period of the diurnal cycle. Respir. Physiol. Neurobiol. 2009a;170:96–102. doi: 10.1016/j.resp.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie EE. Antagonism of orexin receptor 1 (OX1R) in the retrotrapezoid nucleus (RTN) inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J. Physiol. 2009b;587:2059–2067. doi: 10.1113/jphysiol.2008.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlichman JS, Li A, Nattie EE. Ventilatory effects of glial dysfunction in a rat brain stem chemoreceptor region. J. Appl. Physiol. 1998;85:1599–1604. doi: 10.1152/jappl.1998.85.5.1599. [DOI] [PubMed] [Google Scholar]
- Fortuna MG, Stornetta RL, West GH, Guyenet PG. Activation of the retrotrapezoid nucleus by posterior hypothalamic stimulation. J. Physiol. 2009;587:5121–5138. doi: 10.1113/jphysiol.2009.176875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JA, Jensen LT, Doyle SE, Miranda-Anaya M, Menaker M, Fugger L, Bayliss DA, Burdakov D. Deletion of TASK1 and TASK3 channels disrupts intrinsic excitability but does not abolish glucose or pH responses of orexin/hypocretin neurons. Eur. J. Neurosci. 2009;30:57–64. doi: 10.1111/j.1460-9568.2009.06789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes Control Breathing Through pH-Dependent Release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J. Neurosci. 2005;25:8938–8947. doi: 10.1523/JNEUROSCI.2415-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J. Comp. Neurol. 2010;518:3883–3906. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Huang SC, Dai YW, Lee YH, Chiou LC, Hwang LL. Orexins depolarize rostral ventrolateral medulla neurons and increase arterial pressure and heart rate in rats mainly via orexin 2 receptors. J Pharmacol. Exp Ther. 2010;334:522–529. doi: 10.1124/jpet.110.167791. [DOI] [PubMed] [Google Scholar]
- Huckstepp RT, Id BR, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J. Physiol. 2010;588:3901–3920. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbar R, Stornetta RL, Cash DR, Lewis SJ, Guyenet PG. Photostimulation of Phox2b medullary neurons activates cardiorespiratory function in conscious rats. Am. J. Respir. Crit Care Med. 2010;182:1184–1194. doi: 10.1164/rccm.201001-0047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukkonen JP, Holmqvist T, Ammoun S, Åkerman KEO. Functions of the orexinergic/hypocretinergic system. Am. J. Physiol. Cell Physiol. 2002;283:C1567–C1591. doi: 10.1152/ajpcell.00055.2002. [DOI] [PubMed] [Google Scholar]
- Kuwaki T. Orexinergic modulation of breathing across vigilance states. Respir. Physiol. Neurobiol. 2008;164:204–212. doi: 10.1016/j.resp.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Kuwaki T, Zhang W, Nakamura A, Deng BS. Emotional and state-dependent modification of cardiorespiratory function: role of orexinergic neurons. Auton. Neurosci. 2008;142:11–16. doi: 10.1016/j.autneu.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Lazarenko RM, Fortuna MG, Shi Y, Mulkey DK, Takakura AC, Moreira TS, Guyenet PG, Bayliss DA. Anesthetic activation of central respiratory chemoreceptor neurons involves inhibition of a THIK-1-like background K(+) current. J Neurosci. 2010;30:9324–9334. doi: 10.1523/JNEUROSCI.1956-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J. Comp. Neurol. 2009;517:69–86. doi: 10.1002/cne.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie E. Antagonism of rat orexin receptors by almorexant attenuates central chemoreception in wakefulness in the active period of the diurnal cycle. J. Physiol. 2010;588:2935–2944. doi: 10.1113/jphysiol.2010.191288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JF, Gourine AV. Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J. Neurosci. 2010;30:12466–12473. doi: 10.1523/JNEUROSCI.3141-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG. Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J. Neurosci. 2007;27:14128–14138. doi: 10.1523/JNEUROSCI.4167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E, Li A. Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol. 2009;106:1464–1466. doi: 10.1152/japplphysiol.00112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, Van den Pol AN, De Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunanaga J, Deng BS, Zhang W, Kanmura Y, Kuwaki T. CO(2) activates orexin-containing neurons in mice. Respir. Physiol. Neurobiol. 2009;166:184–186. doi: 10.1016/j.resp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J. Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Sakurai T, Fukuda Y, Kuwaki T. Orexin neuron-mediated skeletal muscle vasodilation and shift of baroreflex during defense response in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1654–R1663. doi: 10.1152/ajpregu.00704.2005. [DOI] [PubMed] [Google Scholar]