Abstract
The critical brain areas and molecular mechanisms involved in drug abuse and dependence have been extensively studied. Drug-induced persistent behaviors such as sensitization, tolerance or relapse, however, far outlast any previously reported mechanisms. A challenge in the field of addiction, therefore, has been to identify drug-induced changes in brain circuitry that may subserve long-lasting changes in behavior. The present study examined behavioral changes and electron microscopic evidence of altered synaptic connectivity within the nucleus accumbens (NAc) following repeated administration of cocaine or morphine. The unbiased quantitative stereological physical disector method was used to estimate the number of synapses per neuron. Increases in synapse to neuron ratio were found in the NAc shell of cocaine-treated (49.1%) and morphine-treated (55.1%) rats and in the NAc core of cocaine-treated animals (49.1%). This study provides direct ultrastructural evidence of drug-induced synaptic plasticity and identifies synaptic remodeling as a potential neural substrate underlying drug-induced behavioral sensitization.
Keywords: addiction, basal ganglia, sensitization, electron microscopy, ultrastructure
INTRODUCTION
The critical brain areas and molecular mechanisms that underlie drug abuse and dependence have been well documented. Drug-induced persistent behaviors, which can last for several years even after drug cessation, far outlast any previously reported molecular mechanisms. A major challenge in the field of addiction, therefore, has been to identify long-lasting changes in the brain that may underlie such drug-induced persistent behaviors as sensitization, tolerance and relapse, which reportedly contribute to the development of addiction (Hyman et al, 2006; Kalivas and Volkow, 2005; Robinson and Berridge, 2003; Russo et al., 2010).
Long-term treatment with cocaine and morphine has previously been reported to induce changes in dendritic morphology in both the nucleus accumbens (NAc) and prefrontal cortex (PFC) of behaviorally sensitized animals. The NAc and PFC are two primary targets of the dopamine (DA) mesocorticolimbic pathway that have been implicated in the cognitive, hedonic and motor effects of drugs of abuse and transition to addiction. Golgi-Cox staining of these brain areas has revealed an increase in dendritic branching and spine density in the NAc shell following repeated intraperitoneal (i.p.) treatment with cocaine (Robinson and Kolb, 1999a) and a decrease in dendritic branching and spine density in the NAc shell following repeated i.p. treatment with morphine (Robinson and Kolb, 1999b). Increases in spine density in the NAc core have further been associated with the expression of cocaine-induced behavioral sensitization, a phenomenon whereby increased drug effects such as enhancement of locomotor activity or stereotypic behavior occur with repeated drug administration (Li et al., 2004). The present study, hence, examined ultrastructural synaptic changes, presumably concomitant with dendritic branching and spine density changes, in animals exhibiting drug-induced behavioral sensitization.
Changes in dendritic morphology and drug-induced persistent behaviors resulting from repeated treatment of cocaine and morphine suggest that changes in synaptic connections are also occurring. Direct evidence of synaptic plasticity and the precise pattern of synaptic restructuring can be obtained with electron microscopy (EM). Although a large body of literature supports the link between synaptic plasticity and experience or learning (Anderson et al., 1996; Black et al., 1990; Kleim et al., 1996; Turner and Greenough, 1985;), only recently have studies begun to examine changes at the ultrastructural level to explain the relationship between altered connectivity and drug treatment (Morrow et al., 2007). The present study employed EM and unbiased quantitative stereology to test the hypothesis that changes in synapses per neuron occur in the NAc following repeated cocaine and morphine treatment. The synapse-to-neuron ratio, calculated using the physical dissector method, has been reported to accurately reflect changes in synapse number when neuron number is stable as was the case in the present study. This ratio accurately reflects changes in synapse number irrespective of any changes in tissue volume and is not affected by tissue shrinkage that may occur during tissue processing. We further speculate that changes in synaptic connectivity underlie such persistent drug-induced sequela as behavioral sensitization.
MATERIALS AND METHODS
Subjects
Twenty-four adult female Sprague-Dawley rats were used in this study. Female rats were used in this study since they show more robust sensitization than male rats (Robinson, 1984), and because earlier published work reporting cocaine- and morphine-induced alterations in the morphology of dendrites and dendritic spines in the NAc used female rats (Robinson and Kolb, 1999a,b). Rats were randomly assigned to 3 groups, Cocaine (n=7), Morphine (n=7), and Saline (n=8). Two animals were excluded from this study due to the inadvertent administration of the wrong drug to one animal and observed tissue pathology in a second animal. All efforts were made to minimize animal suffering and the number of animals used. Experimental procedures conformed to National Institutes of Health guidelines and were carried out under an institutionally reviewed and approved research protocol.
Drug administration and behavioral procedures
Morphine sulfate (10 mg/kg) and cocaine hydrochloride (15 mg/kg) were dissolved in 0.9% saline and injected i.p. at 1 mL/kg of body weight. Drugs were administered in the testing room and animals were immediately placed into test cages (45 × 23.5 × 20.75 cm clear plastic cages) in the testing room following injections. On the first day of drug testing, rats were removed from their home cage and given an i.p. injection of cocaine, morphine, or saline followed by placement in the test cage. Behaviors in the test cage were recorded with a video camera for 60 minutes immediately following the injection. Animals were then returned to their home cage following the testing session. These procedures were repeated 5 days a week, followed by 2 consecutive drug-free days over a total of 4 weeks. After the last testing day, animals were left undisturbed in their home cage for an additional 21 days before their brains were removed. The additional 3 week drug-free period was included in the experimental design in order to measure persistent drug-induced synaptic changes rather than possible transient morphological changes (Robinson and Kolb (1999a,b).
Behavioral analysis
Behavioral videotapes were coded and viewed by observers blind to group assignment. Behaviors quantified from the video footage for Days 1 and 28 included: line crosses, rearing, head bobbing, sniffing, immobility, chewing/gnawing, grooming and sleeping. Grid lines were made on the outside of the bottom of the cage dividing the cage into 6 quadrants. The lines were used to score line crosses following drug administration. Line crosses were counted when both forepaws crossed a line as the rat moved in the forward direction. Rearing activity was measured when the rat stood on its hind legs, either supported by the cage or unsupported. Head bobs were counted as head movements in the vertical direction (up to down), while stationary with all paws on the ground. Sniffing was timed while the rat was in a rearing position (sniffing up) or a standing position with all four paws on the ground (sniffing down). Grooming behavior was timed for only grooming of the face and head. Immobility was characterized by freezing behavior in the absence of head movement. Chewing/gnawing behavior was recorded when animals chewed either their claws (forepaws or hind paws) or the walls of the cage. Grooming, sniffing, immobility, and chewing/gnawing were measured and recorded in the number of bouts and in the number of seconds.
Light and electron microscopy tissue preparation
Following drug treatment and behavioral testing, animals were anesthetized with pentobarbital (100 mg/kg) and perfused transcardially with 4% paraformadehyde/0.6% glutaraldehyde in 0.1M phosphate buffered saline, pH 7.4. The brains were removed, post-fixed in 4% paraformaldehyde for 2 hours, and sectioned with a vibratome (100 μm-thick). Sections were then processed for EM. Polymerized and osmicated EM processed tissue sections were initially inspected under a light microscope to locate the shell and core compartments of the NAc using the Paxinos and Watson Stereotaxic Coordinates for the Rat Brain (1998). Excised regions from the core and shell compartments for each animal (Fig. 1) were then attached to an epoxy block and trimmed into a small pyramid of tissue. For each trimmed tissue block, twenty semithin sections (~500 nm-thick) and sixteen ultrathin sections (~70 nm-thick) were cut on an ultramicrotome (Leica Ultracut UCT) using a diamond knife (Ted Pella). After the first five semithin sections were collected, 4 silver-gray serial sections (~ 70 um think) were taken using a diamond knife and sectioning continued alternating between semithin and ultrathin sections. All semithin sections were dried onto glass slides and all ultrathin sections were collected onto formvar-coated slot grids.
Figure 1. Coronal Section of the Rat Brain.
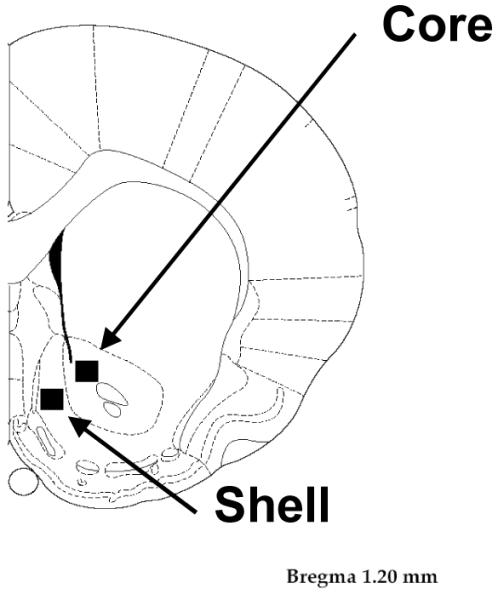
Schematic representation of a coronal section (Bregma 1.20 mm) of the rat brain atlas (Paxinos and Watson, 1998). The boxes indicate the sample areas where neuron density and synapse density quantifications were obtained from the shell and core compartments of the NAc for each animal.
Unbiased quantitative stereology procedures
The present study used unbiased quantitative stereology to examine changes in the synapse-to-neuron ratio, a method described in 1979 (Mayhew) for measuring synaptic changes in brain tissue that has been widely used (Black et al., 1990; Braendgaard and Gundersen, 1986; Cooke and Woolley, 2005; Geinisman et al., 1996a,b; Gundersen, 1986; Gundersen et al., 1988; Kleim et al., 1996; Poe et al., 2001; Turner and Greenough, 1983). The physical disector method was used to estimate the synapse-to-neuron ratio calculated from neuronal density and synapse density (Gundersen et. al., 1988; Sterio, 1984). Previous experiments have reported that experience can lead to an increase in the number of synapses as well as tissue volume where those synapse changes are occurring (Rosenzweig et al., 1962; Turner and Greenough, 1985). Synapse density alone, therefore, does not always accurately reflect changes in synapse number because of potential changes in tissue volume. The synapse-to-neuron ratio, however, accurately reflects changes in the number of synapses (made onto any part of the neuron) when neuron number is stable (Anker and Cragg, 1974). Thus, when using the ratio of synapses per neuron, volume is removed from the equation (Poe et al., 2001). Furthermore, variable tissue shrinkage can result from tissue processing. The synapse-to-neuron ratio overcomes this problem since it is unaffected by tissue shrinkage when both neuron density and synapse density are calculated from the same block of tissue, as done in this study (Geinisman et al., 1996a). The synapse to neuron ratio was therefore the most valid way to test the hypothesis that the number of synapses change in the NAc with drug treatment.
Neuron Density
Semithin sections were stained with toluidine blue and used to calculate neuronal density using a 40X lens on a computer-assisted light microscope. Neuronal density was calculated by dividing the number of cells counted by the volume of the tissue which involved comparing two serial sections in a series of ten sections (Gundersen, et al., 1988; Sterio, 1984). The first section is called the Reference section and the second is the Lookup section. Then the second section became the Reference section for the third section and so on, using a total of ten semithin sections. When a cell nucleus was found in the Reference section, but not the Lookup section, the nucleus was counted. Within an unbiased counting frame (Aframe) of a known area (54,600 μm2 at 40x for semithin sections) the number of nuclei that were present in the Reference section and not in the Lookup section was counted (Q-neuron). Next the volume of tissue through which the cells were counted (Vdis) was calculated using the following formula: Vdis=Aframe × H, where H is section thickness (1μm) multiplied by the number of sections. The neuronal density, Nvneuron, was then determined by dividing the number of cells counted by the volume of the tissue using the following formula: Nvneuron = Q-neuron/Vdis (using semithin sections).
Synapse Density
Ultrathin sections were collected on formvar-coated copper slot grids and stained with 2% aqueous uranyl acetate. Sections were then examined and photographed using a Philips EM208 transmission electron microscope (TEM) (FEI Company) at 80kv. Images, 1 Mb in size, were collected with an AMT Advantage HT camera. All electron photomicrographs were taken at a magnification of 18000X. Images were stored and analyzed by an observer blind to group assignment. Twenty electron photomicrographs were taken from two adjacent sections from the same positions within each section. Landmarks used to identify the correct location in each section typically consisted of cross-sections of small myelinated fibers and clusters of mitochondria transversing the section. A total of 40 micrographs were collected per NAc compartment per animal (twenty disector pairs), using one slot grid. Synapses were categorized as asymmetric, symmetric or undefined. Asymmetric synapses were identified by the presence of at least three round or spherical shaped synaptic (neurotransmitter) vesicles in the presynaptic terminal, a thickened postsynaptic density and a synaptic cleft. Symmetric synapses were identified by the presence of at least three presynaptic synaptic vesicles with predominantly flattened or elongated appearance (i.e. pleomorphic), a synaptic cleft and two closely apposing parallel membranes with no prominent postsynaptic density. The postsynaptic target was identified and recorded for each synapse (i.e. soma, dendritic shaft or spine, and axon) whenever possible. For example, the dendritic shaft was identified by microfilaments and mitochondria visible in dendritic profiles, whereas dendritic spines were identified by their characteristic size, “fluffy” or “flocculent” material, absence of mitochondria and/or presence of spine apparatus (Peters et al., 1991; Peters and Palay, 1996).
The observers counted all synapses in the shell and core compartments of the NAc and the physical disector method was again used to calculate the synapse density, for which the number of synapses present in the reference section and not the look-up section were counted (Q-synapse). The total number of synapses within an unbiased counting frame of a known area (Aframe) was counted (25.8μm2 at 18000x for the ultrathin sections). Again, the dissector volume of tissue (Vdis) is calculated using the formula from above, where H is section thickness (70 nm) multiplied by the number of sections. Synapse density, Nvsynapse, was calculated using the following formula: Nvsynapse=Q-synapse/Vdis (using ultrathin sections).
Synapse-to-Neuron Ratio
The number of synapses per neuron was determined by dividing the density of synapses per cubic millimeter by the neuronal density per cubic millimeter: Nvneuron/Nvsynapse (synapse to neuron ratio). Section thickness of ultrathin sections was verified using “Small-Fold’s” technique (Weibel, 1979). For this calculation an image of a small fold was captured from an ultrathin section and the width of the fold was estimated using the image scale bar. The following formula was then used: 2t=l, where l is the width of the small fold (using the scale bar) and t is the estimated thickness of the ultrathin section.
Data Analysis
The statistical analyses of behavioral and anatomical data were designed to test whether changes in the synapse per neuron ratio in the NAc shell and core were significantly different in the drug-treated animals relative to control animals and whether rats showed behavioral sensitization to repeated drug treatment by comparing drug effect on day 1 to day 28. Statistical analyses of behavioral data were performed using SPSS (SPSS, Inc.) repeated-measures analysis of variance (ANOVAs) for the effects of Groups, Days, and Group by Day interaction followed by pairwise comparisons using Bonferroni’s correction. Significant changes in the synapse per neuron ratio were determined by a one-way ANOVA followed by pairwise comparisons using Bonferroni’s correction. Significance level was set at p < .05.
RESULTS
Cocaine- and morphine-induced behavior
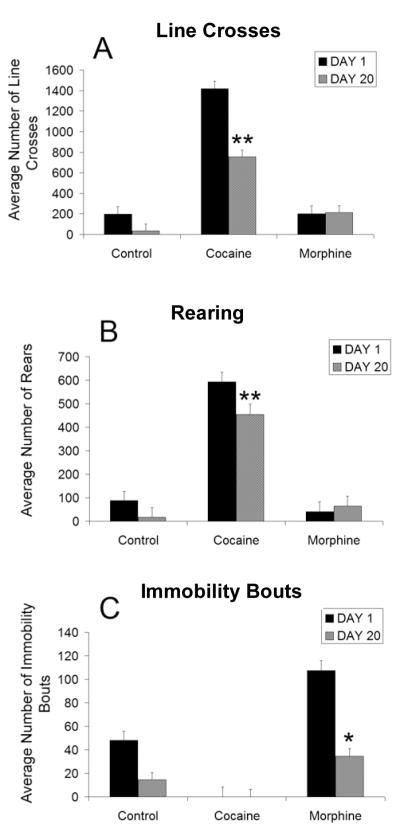
Cocaine-treated animals showed significantly more locomotor activity (line crosses and rearing) relative to control animals both on days 1 and 28 (Fig. 2A, B). A significant decrease in the total number of line crosses (Fig. 2A) and rears (Fig. 2B) was observed in cocaine-treated animals by day 28. In contrast to cocaine, morphine administration on day 1 did not produce significant increases in locomotor activity relative to controls (Fig. 2A, B). Instead, morphine administration on day 1 produced motor immobility (opiate catalepsy) (Fig. 2C). Furthermore this drug-induced immobility was significantly higher on day 1 relative to day 28, suggesting evidence of motor tolerance.
Figure 2. Non-stereotypic behaviors.
The average (±SEM) number of line crosses (A) rears (B) and immobility bouts (C) during the 1-hour testing session, as a function of treatment and day. Significant decreases in line crosses and rears were observed in the cocaine treated animals compared to controls. A significant decrease in those behaviors, were observed in the cocaine group on day 28 compared to day 1. Morphine produced motor immobility and it was significantly higher on day 1 relative to day 28 indicative of motor tolerance. (** indicates statistical significance (p < .05) relative to control group and day 1; * indicates statistical significance (p < .05) relative to day 1).
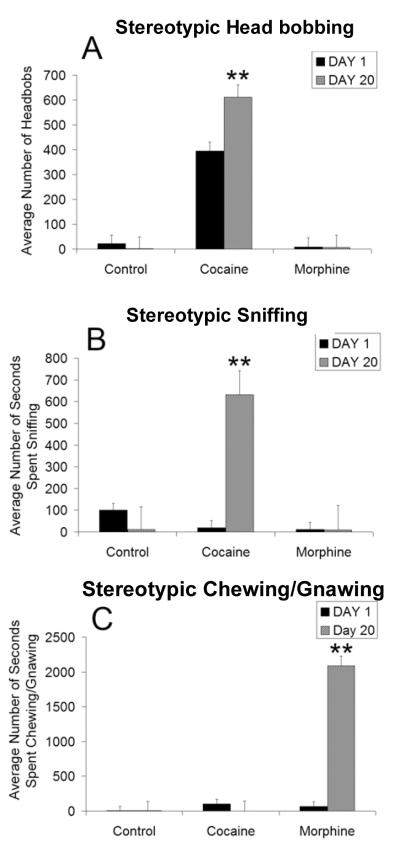
A significant increase in the average number of stereotypic head bobs (Fig. 3A) and stereotypic sniffing (Fig. 3B), two reliable measures of cocaine-induced behavioral sensitization, was found in cocaine-treated animals by day 28, relative to controls. Morphine-treated rats did not exhibit significant behavioral levels of stereotypic head bobbing; however, a pronounced morphine-induced sensitization of stereotypic chewing/gnawing behavior was evident on day 28 (Fig. 3C).
Figure 3. Stereotypic behaviors.
The average (±SEM) number of head bobs (A) seconds spent sniffing (B) and seconds spent chewing/gnawing (C) during the 1-hour testing session, as a function of treatment and day. Sensitization of head bobbing and sniffing was evident in the cocaine group by day 28 relative to controls, whereas the morphine treated animals displayed sensitization of chewing and gnawing behavior by day 28 (** indicates statistical significance (p < .05) relative to control group and day 1; * indicates statistical significance (p < .05) relative to day 1).
Analysis of sleeping behavior revealed that control animals spent significantly more time sleeping on day 28 relative to the first day of testing. Drug-treated animals did not exhibit sleeping behavior during the 1 hour testing session. Therefore, all behavioral measures were adjusted for sleeping behavior observed in the control animals and re-evaluated (data not shown). All statistically significant behaviors reported in the present study, remained significant. Additionally, when grooming behavior was adjusted for the time animals were asleep on day 28, instead of the total 60 minute video, a significant increase in grooming bout duration (i.e. the number of seconds spent grooming per bout) and the total number of grooming bouts was found when day 1 was compared with day 28 (data not shown).
Altered synaptic connectivity measured by electron microscopy
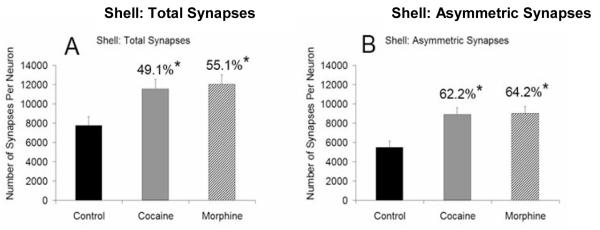
A significant increase in the total number of synapses per neuron in the NAc shell of cocaine- (49.1%, p < .05) and morphine- (55.1%, p < .05, F = 6.205) treated animals was found, relative to controls (Fig. 4A). Neuron density was stable (i.e. not statistically different) in all three groups as indicated by the mean and standard deviation (SD): cocaine = 244,113 (SD 25,124) neurons per mm3; morphine = 265,175 (SD 54,740.3) neurons per mm3; and control = 279,215 (SD 51,940.2) neurons per mm3. Thus, the calculated number of synapses per neuron reflects a change in the number of synapses. Specifically, there was a significant increase in the number of asymmetric (presumably glutamatergic) synapses onto cells in the NAc shell in cocaine-treated animals (62.2%, p < .05) and morphine-treated animals (64.2%, p < .05, F = 9.220) (Fig. 4B).
Figure 4. Total and asymmetric synaptic quantification in the NAc shell.
The average (±SEM) number of total synapses (A) and asymmetric synapses (B), as a function of treatment in the NAc shell. A significant increase in the total number of synapses per neuron and asymmetric synapses per neuron in the NAc were found in the cocaine and morphine treated animals (* indicates statistical significance (p < .05) relative to control group).
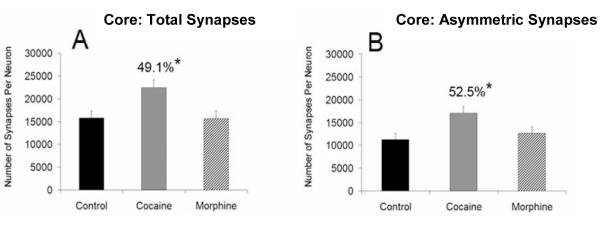
A significant increase in the total number of synapses per neuron was also found in the NAc core of cocaine-treated animals (49.1%, p < .05, F = 6.019), but not morphine-treated animals, relative to controls (Fig. 5A). Again, a stable neuron density: cocaine = 208,647 (SD 47,076.2) neurons per mm3; morphine = 209,167 (SD 30,166.9) neurons per mm3; control = 203,962.13 (SD 30,711.5) neurons per mm3, reveals that this effect is driven by a change in synapse number. In the NAc core, the number of asymmetric synapses increased by 52.5% (p < .05, F = 4.531) relative to the control group (Fig. 5B). The percentage of asymmetric and symmetric synapses made onto dendritic shafts and spines (Fig. 6) are reported in Table 1. Although symmetric synapses were quantified in both the NAc shell and core, the number of symmetric synapses counted was not large enough to draw any definitive conclusions about changes related specifically to this category of synapses.
Figure 5. Total and asymmetric synaptic quantification in the NAc core.
The average (±SEM) number of total synapses (A) and asymmetric synapses (B), as a function of treatment in the NAc core. A significant increase in the total number of synapses per neuron and asymmetric synapses per neuron in the NAc was found in the cocaine group (* indicates statistical significance (p < .05) relative to control group).
Figure 6. Electron micrograph of synapses in the NAc.
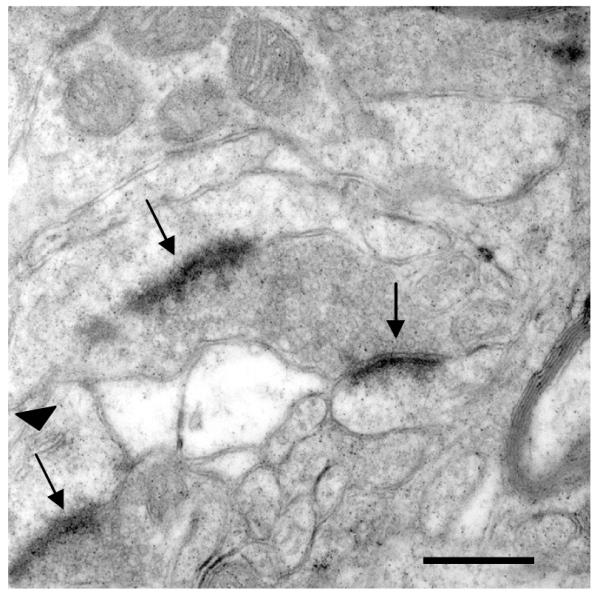
Electron micrograph (18,000X) taken of asymmetric synapses (arrows) made onto spines in the NAc. A Spine apparatus can be seen in the lower left spine (arrowhead). Scale bar = 500 nm.
Table 1. Percentage of asymmetric and symmetric synapses onto dendritic spines and dendritic shafts in the nucleus accumbens shell and core.
The values listed reflect the total group means and standard errors for each condition (Control, Cocaine, Morphine) for the percent of total synapses categorized as asymmetric and symmetric that made contact with either dendritic spines or dendritic shafts. Significant increases in the total number of synapses per neuron were found in the NAc shell of cocaine-treated and morphine-treated and in the NAc core of cocaine-treated animals.
| Control | Cocaine | Morphine | |
|---|---|---|---|
| Shell Total Number of Synapses per Neuron |
7759 ± 1286 | 11567 ± 2862* | 12036 ±3363* |
| Dendritic Spine | 42.1% ± 4.4% | 34.0% ± 4.4% | 41.7 % ± 4.2% |
| Dendritic Shaft | 44.3% ± 4.5% | 53.5% ± 4.5% | 42.8% ± 4.2% |
| Asymmetric | 71.1% ± 2.9% | 77.5% ± 3.1% | 75.9% ± 3.1% |
| Dendritic Spine | 54.1% ± 5.3% | 49.5 % ± 5.7% | 43.3% ± 5.7% |
| Dendritic Shaft | 33.7% ± 4.8% | 39.0% ± 5.2% | 45.9% ± 5.2% |
| Symmetric | 23.5% ± 2.5% | 18.5% ± 2.6% | 20.8% ± 2.6% |
| Dendritic Spine | 9.9% ± 5.1 % | 18.4% ± 5.5% | 9.8% ± 5.5% |
| Dendritic Shaft | 80.6% ± 5.2% | 74.7% ± 5.5% | 86.8% ± 5.5% |
| Core Total Number of Synapses per Neuron |
15781 ± 3982 | 22477 ± 5877* | 15645 ± 2083 |
| Dendritic Spine | 43.2% ± 3.1% | 39.3% ± 3.1% | 42.8% ± 2.9% |
| Dendritic Shaft | 26.8% ± 2.9% | 32.4% ± 2.9% | 29.1% ± 2.7% |
| Asymmetric | 71.2% ± 3.5% | 72.1% ± 3.7% | 73.9% ± 3.7% |
| Dendritic Spine | 53.8% ± 3.2% | 55.3% ± 3.4% | 48.7% ± 3.4% |
| Dendritic Shaft | 22.8% ± 1.9% | 20.2% ± 2.0% | 25.8% ± 2.0% |
| Symmetric | 24.2% ± 3.5% | 21.8% ± 3.7% | 20.1% ± 3.7% |
| Dendritic Spine | 11.3% ± 3.2% | 11.5% ± 3.2% | 18.1% ± 3.0% |
| Dendritic Shaft | 56.4% ± 6.8% | 67.7% ± 6.8% | 50.5% ± 6.4% |
(indicates significance at p < .05).
DISCUSSION
The present study provides ultrastructural evidence of drug-induced synaptic plasticity in the shell and core compartments of the NAc of animals. Because the drug-induced behaviors reported in the present study replicate previous work (Robinson and Kolb, 1999a,b), the behavioral data indicate that the new synaptic findings are occurring in a behaviorally validated context. Structural changes were observed following 4 weeks of drug treatment and a subsequent 3-week drug-free period. The NAc in addition to the amygdala, dorsal striatum, and PFC have been reported to play critical roles in the cognitive, hedonic and motor aspects of addiction. Functionally distinct subregions within those brain areas are further associated with specific drug effects. For example, the shell NAc plays a critical role in the reinforcing effects of drugs (DiChiara et al., 2004; Pontieri et al., 1995) whereas the core is more closely associated with drug-induced behavioral sensitization (Cadoni and DiChiara, 1999; Ferrario et al., 2005; Li et al., 2004) and drug-seeking behavior (Kalivas and Volkow, 2005). Based on this evidence, we speculate that an increase in the number of synapses per neuron observed in the NAc shell may represent a neural substrate underlying the reinforcing effects of cocaine and morphine; whereas altered synaptic connectivity in the NAc core may underlie cocaine-induced behavioral sensitization.
Addiction, also known as substance dependence, is defined by the current American Psychiatric Association (APA), Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) as a cluster of cognitive, behavioral and physiological symptoms. It includes a pattern of repeated self-administration that can result in compulsive drug use (APA, DSM-IV, 2000). Compulsive drug-intake behavior has been hypothesized to be an important transition to addiction and can be studied through the phenomenon of behavioral and neural sensitization. The development of psychomotor sensitization has in fact been directly related to increases in cocaine-taking behavior and relapse (De Vries et al 1998, 2002; Zhao and Becker, 2009).
Cocaine sensitization is typically measured following a single high dose of cocaine followed by a challenge dose of cocaine 24 h later (Caster et al., 2007) or following 1 week of cocaine administration with effects that can last up to 3 weeks (Boudreau et al., 2007; Boudreau and Wolf, 2005; Pierce et al., 1996). A more extended cocaine sensitization paradigm (15mg/kg for 20 days), however, was reported by Robinson and colleagues to induce persistent structural changes in the NAc (Robinson and Kolb, 1999a,b) and more recently by Zhao and Becker (2009) to promote cocaine self-administration. The present study used the paradigm previously reported by Robinson and Kolb (1999a,b) and similarly used female rats. Extension of the present results to other findings in the literature is possible given that several recent studies investigating cocaine sensitization and cocaine self-administration also used female rats (Hu et al., 2004; Jackson et al., 2006; Nephew and Febo, 2010; Yang et al., 2007; Zhao and Becker, 2009). Altogether, the findings from these studies, which used female rats, are valuable since sex differences in drug abuse have been reported to exist in humans and animal models (for review see Becker and Hu, 2008).
Structural changes in the core and shell NAc of animals given limited access to cocaine self-administration (1 hour/day) and extended access to cocaine self-administration (6 hours/day) have been reported by Robinson and colleagues (Ferrario et al., 2005). Extended administration of cocaine followed by a cocaine challenge 1 month after the last self-administration test session, marked escalation in drug consumption, enhanced drug-seeking behavior, robust behavioral sensitization and increased density of dendritic spines on medium spiny neurons, specifically in the core of the NAc. Furthermore, the changes were specific to the NAc core since they were not observed in the shell or PFC. These findings suggest that the structural changes in the NAc core are associated with cocaine sensitization and not due to the pharmacological effects of high doses of cocaine. Consistent with these and other findings (Ferrario et al., 2005; Li et al., 2004), the changes in the number of synapses observed in the NAc core in the present study are therefore likely to be specific to cocaine sensitization.
The synaptic changes observed in the NAc in the present study may represent a neural substrate for cocaine-induced stereotypic behaviors. In general, cocaine-sensitized rats exhibit enhanced exploratory behaviors such as locomotor activity and rearing, as well as stereotyped sniffing and head bobbing minutes after drug administration (Broderick, 1991; O’Dell et al., 1996; Tzechentk and Schmidt, 1998). In the present study, a categorical shift occurred from locomotor activity to stereotypic behaviors with repeated administration of cocaine. Such changes in drug-induced behaviors with repeated administration of drugs have been previously described as the inverted U-shaped function (Dews and Wenger, 1977; Lyon and Robbins, 1975). This theory describes a shift in the replacement of drug-induced motor and exploratory behaviors with the emergence of repetitive, stereotypic behaviors, presumably involving distinct neural circuits. The shift to stereotypic behaviors may represent the emergence of a critical and persistent component (i.e. compulsive drug-seeking state) of addiction. The NAc and dorsal striatum have previously been identified as neural correlates for psychostimulant sniffing and head bobbing (Broderick, 1991; Sharp et al., 1987; White et al., 1998).
Morphine-induced immobility (opiate catalepsy) was significantly higher on day 1 relative to day 28, suggestive of motor tolerance. The exact mechanism underlying opiate-induced catalepsy is not known, although it can be significantly prevented or reversed by phenytoin (antiepileptic) and naloxone (opioid receptor antagonist) (Cookson and Mann, 1980), and the development of tolerance to morphine-induced catalepsy can be delayed by lesioning the ventral tegmental area (VTA) (Hand and Franklin, 1985).
The synaptic changes observed in the NAc shell, in the present study, may underlie not only the reinforcing effects of drugs of abuse but also morphine-induced behavioral sensitization. Morphine-induced sensitization of stereotypic chewing/gnawing behavior was evident in the present study on day 28. Although this behavior has received some attention (Cadoni and Di Chiara, 1999; Kornetsky, 2004; Pollock and Kornetsky, 1989), the neurochemical mechanisms underlying morphine-induced oral stereotypy are not yet clear. MK-801(NMDA receptor antagonist), Fluoxetine (selective serotonin reuptake inhibitor), and SCH23390 (dopamine D1 receptor antagonist) all block the expression of this behavior, whereas only pretreatment with MK-801 blocks the development of sensitization to this oral stereotypy (Livezey et al., 1995; Wennemer and Kornetsky, 1999). Cerebral metabolic rates for glucose were measured in rats exhibiting morphine-induced oral stereotypy and the NAc was significantly more metabolically active in behaviorally sensitized animals than in controls (Kraus and Kornetsky, 2000). Furthermore, the metabolic changes occurred in the NAc shell in the presence or absence of a morphine-associated cue, but in the NAc core only when morphine was accompanied by a cue.
The increase in the number of synapses per neuron observed in the NAc shell in both cocaine and morphine-treated animals were due primarily to an increase in the number of asymmetric synapses. Asymmetric synapses in the accumbens are characteristic of glutamatergic synapses formed by inputs from the PFC, amygdala, hippocampus, or thalamus (Gerfen, 1988; Johnson et al., 1994; Meredith and Wouterlood, 1990; Pinto et al., 2003; Sesack and Pickel, 1990; Smith and Bolam, 1990; Torterdell and Smith, 1989). A proposed role for glutamatergic synaptic remodeling in the present study is consistent with the reported role of glutamate (GLU) in drug reinforcement (Todtenkopf et al., 2006) and cocaine and morphine-induced sensitization (Livezey et al., 1995; McFarland et al., 2003). In the present study, an overall increase in the number of asymmetric synapses per neuron was observed in the NAc shell of morphine-treated animals. Interestingly, this is in contrast to a reported reduction in spine density reported by Robinson and Kolb (1999b). One possible explanation is that the decrease in spine density shifts to an increase in the number of synapses made onto dendritic shafts due to spine retraction. Such a trend was observed in the present study (see Table 1). This notion is supported by a recent study using quantitative time lapse confocal imaging of cultured neurons, which reported that 40% of pruned spines kept their presynaptic innervation, which were transformed into shaft synapses (Ovtscharoff et al., 2008). These findings suggest that opiates and psychostimulants differentially affect neural inputs and synaptic connectivity within the shell and core regions of the NAc. Overall, the magnitude of synaptic changes reported in the present study was greater than the spine density changes previously reported by Robinson and colleagues (Li et al., 2004; Robinson and Kolb, 1999a,b). A probable reason may be that a substantial proportion of asymmetric synapses as well as most symmetric synapses do not terminate on clearly identifiable spines and therefore would not have been identified by spine density counts. Future EM studies may shed light on the unique alterations in region and cell specific synaptic circuitry that mediate a variety of behavioral changes associated with distinct drug classes.
An increase in the number of asymmetric synapses observed in both the NAc shell and core in the present study supports the proposed role of glutamatergic signaling in behavioral plasticity to cocaine. Several studies have implicated GLU signaling and receptor plasticity in cocaine-related phenomena including cocaine administration, cocaine-seeking behavior, behavioral sensitization, withdrawal, reinstatement, craving and relapse. Although each of the GLU ionotropic and metabotropic receptors have been implicated in these phenomena, the majority of the work has shown the ionotropic glutamate AMPA receptor subunits GluR1 and GluR2 in the NAc, to be important in these cocaine-mediated processes (Anderson et al., 2008; Boudreau et al., 2007; Conrad et al., 2008; Kalivas and McFarland 2003; Kourrich et al., 2007; McFarland et al., 2003; Ping et al., 2008; Self et al., 2004; Sutton et al., 2003) including behavioral sensitization (Bachtell et al., 2008; Boudreau and Wolf, 2005; Ferrario et al., 2005; Zhang et al., 2007). Additionally, pretreatment with MK-801 prevents the induction of behavioral sensitization implicating the role of glutamatergic NMDA receptors in the induction of cocaine-induced sensitization (Wolf and Jeziorski, 1993). Moreover, application of this NMDA receptor antagonist also prevents molecular neuroadaptations associated with cocaine sensitization, such as DA autoreceptor subsensitivity and DA D1 receptor supersensitivity (Li et al., 1999). The activation of dopamine receptors, however, is not required for the development of behavioral sensitization to cocaine (White et al., 1998). Importantly, pharmacological and electrophysiological studies examining the effects of cocaine on behavioral sensitization consistently show involvement of the PFC. For example, repeated electrical stimulation of the PFC produces behavioral sensitization (Schenk and Snow, 1994), whereas lesions of the PFC block the development of this behavior (Tzschentke and Schmidt, 1998). Further work, however, is necessary to determine whether the changes in the number of asymmetric synapses per neuron, observed in the present study, involve glutamatergic prefronto-accumbal projections or inputs from other areas such as the amygdala, or hippocampus.
The precise mechanisms involved in mediating drug-induced synaptic remodeling will be of major interest to investigate in future studies. Glutamate NMDA receptor activation, for example, is known to mediate the effects of second messenger cascades and transcription factors such as cAMP response element binding protein (CREB) (Huang et al., 2008). CREB in the NAc is associated with tolerance and dependence and ΔFosB, which exerts opposite effects is associated with behavioral sensitization (Carlezon et al., 1998; McClung and Nestler, 2003; Nestler 2001, 2004; Self et al., 1998).
These transcription factors in turn affect gene expression of proteins that regulate drug-related neurotransmission. These molecular mechanisms, however, do not last as long as drug-induced behaviors, but instead are likely to trigger synaptic plasticity that underlies drug-induced persistent behaviors. ΔFosB, for example, accumulates and persists after repeated exposure to cocaine, for weeks or months even after the drug has been withdrawn (Nestler, 2001). Cyclin-dependent kinase 5 (Cdk5), a down-stream target of ΔFosB, in turn has been implicated in dendritic plasticity and synaptic restructuring (Hawasli et al., 2007; Hawasli and Bibb, 2007; Lai and Ip, 2009). Furthermore, site-specific administration of roscovitine, a Cdk5 blocker, has been shown to attenuate cocaine-induced spine outgrowth in the NAc core and shell (Norrholm et al., 2003). Roscovitine further augments the incentive-motivational effects of cocaine and the development and expression of cocaine-induced locomotor sensitization (Bibb et al., 2001; Taylor et al., 2007). Additional studies are further necessary to demonstrate direct causal links between the mechanisms that underlie drug-induced synaptic plasticity and corresponding behavioral changes. Altogether, previous work together with our present findings provide the framework for future studies aimed at identifying the necessary and sufficient mechanisms that mediate such drug-related behaviors as sensitization or tolerance.
In conclusion, altered synaptic connectivity involving asymmetric synapses, observed in the present study, supports the proposed role of glutamatergic PFC and limbic inputs to the NAc in mediating long-lasting drug-induced behavioral changes such as sensitization that may contribute to the transition to addiction. Further work is necessary to identify the precise neuronal cell types within the NAc, such as medium spiny projection neurons or aspiny cholinergic interneurons previously reported to be affected by drug treatment (Berlanga et al., 2003; Camp et al., 2006), that contribute to this synaptic remodeling. The key intracellular signaling mechanisms involved in synaptic reorganization within these accumbal neuronal networks and other brain areas such as the dorsal striatum or PFC will also be important to define. Converging DA/GLU synapses made onto region and cell specific networks will provide further insight into the underlying neural basis of learning-associated compulsive drug intake, habit learning, and stress- or cue-induced relapse. Furthermore, identifying the interplay between prefrontal and subcortical circuits involved at different stages of drug treatment will be useful in the development of improved genetic, pharmacotherapeutic and behavioral treatments for addiction. Particular focus should be made on identifying novel treatments designed to strengthen the appropriate region and cell specific synaptic connections and alternatively weaken or eliminate maladaptive synapses that underlie sensitization, tolerance, compulsive drug intake, craving or relapse that may contribute to the switch to addiction.
ACKNOWLEDGEMENT
The authors thank Dr. Terry Robinson for consultation on the drug administration regimen, Dr. Timothy Schallert for his expertise with the behavioral analysis, Dr. Theresa Jones and Dr. William Greenough for their expertise on stereology, Julia Wibskov for assistance with the data collection, Susanna Douglas for computer technical assistance, and Marguerite Camp for valuable comments on the manuscript.
REFERENCES
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. American Psychiatric Association; Washington DC: 2000. Text Revision. [Google Scholar]
- Anderson BJ, Alcantara AA, Greenough WT. Motor skill learning: changes in synaptic organization of the rat cerebellar cortex. Neurobiol Learn Mem. 1996;66:221–229. doi: 10.1006/nlme.1996.0062. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vkili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. 2008. [DOI] [PubMed]
- Anker RL, Cragg BG. Estimation of the number of synapses in a volume of nervous tissue from counts in thin sections by electron microscopy. J Neurocytol. 1974;3:725–735. doi: 10.1007/BF01097194. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Choi KH, Simmons DL, Falcon E, Monteggia LM, Neve RL, Self DW. Role of GluR1 expression in nucleus accumbens neurons in cocaine sensitization and cocaine-seeking behavior. Eur J Neurosci. 2008;27:2229–22240. doi: 10.1111/j.1460-9568.2008.06199.x. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlanga ML, Olsen CM, Chen V, Ikegami A, Herring BE, Duvauchelle CL, Alcantara AA. Cholinergic interneurons of the nucleus accumbens and dorsal striatum are activated by the self-administration of cocaine. Neuroscience. 2003;120:1149–1156. doi: 10.1016/s0306-4522(03)00378-6. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn A, Nestler EJ, Greengard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braendgaard H, Gundersen HJG. The impact of recent stereological advances on quantitative studies of the nervous system. J Neurosci Meth. 1986;18:39–78. doi: 10.1016/0165-0270(86)90112-3. [DOI] [PubMed] [Google Scholar]
- Broderick PA. Cocaine: on-line analysis of an accumbens amine neural basis for psychomotor behavior. Pharmacol Biochem Behav. 1991;40:959–968. doi: 10.1016/0091-3057(91)90112-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G. Reciprocal changes in dopamine responsiveness in the nucleus accumbens shell and core and in the dorsal caudate-putamen in rats sensitized to morphine. Neuroscience. 1999;90:447–455. doi: 10.1016/s0306-4522(98)00466-7. [DOI] [PubMed] [Google Scholar]
- Camp MC, Mayfield RD, McCracken M, McCracken L, Alcantara AA. Neuroadaptations of Cdk5 in cholinergic interneurons of the nucleus accumbens and prefrontal cortex of inbred alcohol-preferring rats following voluntary alcohol drinking. Alcohol Clin Exp Res. 2006;30:1322–1335. doi: 10.1111/j.1530-0277.2006.00160.x. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Olson TJ, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. A single high dose of cocaine induces differential sensitization to specific behaviors across adolescence. Psychopharm. 2007;193:247–260. doi: 10.1007/s00213-007-0764-5. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Sexually dimorphic synaptic organization of the medial amygdala. J Neurosci. 2005;25:10759–10767. doi: 10.1523/JNEUROSCI.2919-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson SL, Mann JD. Phenytoin reversal and prevention of morphine-induced catalepsy in the rat. Pharmacol Biochem Behav. 1980;12:743–746. doi: 10.1016/0091-3057(80)90160-4. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Mulder AH, Vanderschuren LJ. Drug-induced reinstatement of heroin- and cocaine-seeking behavior following long-term extinction is associated with expression of behavioral sensitization. Eur J Neurosci. 1998;10:3565–3571. doi: 10.1046/j.1460-9568.1998.00368.x. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Raaso H, Vanderschuren LJ. Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharm. 2002;26:18–26. doi: 10.1016/S0893-133X(01)00293-7. [DOI] [PubMed] [Google Scholar]
- Dews PB, Wenger GR. In: Advances in Behavioral Pharmacology. Thompson T, Dews PB, editors. Academic Press; NY: 1977. pp. 167–227. [Google Scholar]
- DiChiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacol. 2004;47:227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Combag HS, Li Y, Kolb B, Robinson TE. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Detoledo-Morrell L, Morrell F, Persina IS, Beatty MA. Synapse restructuring associated with the maintenance phase of hippocampal long-term potentiation. J Comp Neurol. 1996a;368:413–423. doi: 10.1002/(SICI)1096-9861(19960506)368:3<413::AID-CNE7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Gundersen HJ, van der Zee E, West MJ. Unbiased stereological estimation of the total number of synapses in a brain region. J Neurocytol. 1996b;25:805–819. doi: 10.1007/BF02284843. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Synaptic organization of the striatum. J Electron Microsc Tech. 1988;10:265–281. doi: 10.1002/jemt.1060100305. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J Microsc (Lond) 1986;143:3–45. [PubMed] [Google Scholar]
- Gundersen HJG, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ. The new stereological tools: Disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Hand TH, Franklin KB. Lesions of ventral tegmental dopamine neurons delay the development of tolerance to morphine catalepsy. Neurosci Lett. 1985;55:367–370. doi: 10.1016/0304-3940(85)90463-x. [DOI] [PubMed] [Google Scholar]
- Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Cambon P, Greengard P, Powell CM, Cooper DC, Bibb JA. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawasli AH, Bibb JA. Alternative roles for Cdk5 in learning and synaptic plasticity. Biotechnol J. 2007;2:941–948. doi: 10.1002/biot.200700093. [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharm. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Huang YH, Lin Y, Brown TE, Han MH, Saal DB, Neve RL, Zukin RS, Sorg BA, Nestler EJ, Malenka RC, Dong Y. CREB modulates the functional output of nucleus accumbens neurons: a critical role of N-methyl-D-aspartate glutamate receptor (NMDAR) receptors. J Biol Chem. 2008;283:2751–2760. doi: 10.1074/jbc.M706578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharm. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Aylward RL, Hussain Z, Totterdell S. Input from the amygdala to the rat nucleus accumbens: its relationship with tyrosine hydroxylase immunoreactivity and identified neurons. Neurosci. 1994;61:851–865. doi: 10.1016/0306-4522(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharm. 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and FOS expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornetsky C. Brain-stimulation reward, morphine-induced oral stereotypy, and sensitization: implications for abuse. Neurosci Biobehav Rev. 2004;27:777–786. doi: 10.1016/j.neubiorev.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MA, Kornetsky C. Cue-induced changes in basal local cerebral glucose utilization 13 days after morphine sensitization in the Fischer 344 rat: relevance for drug craving. Brain Res. 2000;865:194–201. doi: 10.1016/s0006-8993(00)02214-9. [DOI] [PubMed] [Google Scholar]
- Lai KO, IP NY. Recent advances in understanding the roles of Cdk5 in synaptic plasticity. Biochim Biophys Acta. 2009;1792:741–745. doi: 10.1016/j.bbadis.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioral sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Hu X-T, Berney TG, Vartanian AJ, Stine CD, Wolf ME, White FJ. Both glutamate receptor antagonists and prefrontal cortex lesions prevent induction of cocaine sensitization and associated neuroadaptations. Synapse. 1999;34:169–180. doi: 10.1002/(SICI)1098-2396(19991201)34:3<169::AID-SYN1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Livezey RT, Pearce LB, Kornetsky C. The effect of MK-801 and SCH23390 on the expression and sensitization of morphine-induced oral stereotypy. Brain Res. 1995;692:93–98. doi: 10.1016/0006-8993(95)00627-3. [DOI] [PubMed] [Google Scholar]
- Lyon M, Robbins TW. The action of central nervous system stimulant drugs: a general theory concerning amphetamine effects. In: Essman WB, Valzelli L, editors. Current Developments in Psychopharmacology. Spectrum Publications; New York: 1975. pp. 79–163. [Google Scholar]
- Mayhew TM. Stereological approach to the study of synapse morphometry with particular regard to estimating number in a volume and on a surface. J Neurocyt. 1979;8:121–138. doi: 10.1007/BF01175556. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kapish CC, Kalivas PW. Prefrontal glutamate release in the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Wouterlood FG. Hippocampal and midline thalamic fibers and terminals in relation to the choline acetyltransferase-immunoreactive neurons in nucleus accumbens of the rat: a light and electron microscopic study. J Comp Neurol. 1990;296:204–221. doi: 10.1002/cne.902960203. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Hajszan T, Leranth C, Elsworth JD, Roth RH. Prenatal exposure to cocaine is associated with increased number of spine synapses in rat prelimbic cortex. Synapse. 2007;61:862–865. doi: 10.1002/syn.20430. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Febo M. Effect of cocaine sensitization prior to pregnancy on maternal care and aggression in the rat. Psychopharm. 2010;209:127–135. doi: 10.1007/s00213-010-1777-z. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuropharm. 2004;47:24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Khroyan TV, Neisewander JL. Dose-dependent characterization of the rewarding and stimulant properties of cocaine following intraperitoneal and intravenous administration in rats. Psychopharmacol (Berl) 1996;123:144–153. doi: 10.1007/BF02246171. [DOI] [PubMed] [Google Scholar]
- Ovtscharoff W, Jr., Segal M, Goldin M, Helmeke C, Kreher U, Greenberger V, Herzog A, Michaelis B, Braun K. Electron microscopic 3D-reconstruction of dendritic spines in cultured hippocampal neurons undergoing synaptic plasticity. Dev Neurobiol. 2008;68:870–876. doi: 10.1002/dneu.20627. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Stereotaxic Coordinates for the Rat Brain. Academic Press; San Diego CA: 1998. [Google Scholar]
- Peters A, Palay SL. The morphology of synapses. J Neurocytol. 1996;25:687–700. doi: 10.1007/BF02284835. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HD. The fine structure of the nervous system: neurons and their supporting cells. 3rd Edition Oxford University Press; New York: 1991. [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas KW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping A, Xi J, Prasad BM, Wang MH, Kruzich PJ. Contributions of nucleus accumbens core and shell GluR1 containing AMPA receptors in AMPA- and cocaine-primed reinstatement of cocaine-seeking behavior. Brain Res. 2008;1215:173–182. doi: 10.1016/j.brainres.2008.03.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Jankowski M, Sesack SR. Projections from the paraventricular nucleus of the thalamus to the rat prefrontal cortex and nucleus accumbens shell: ultrstructural characteristics and spatial relationships with dopamine afferents. J Comp Neurol. 2003;459:142–155. doi: 10.1002/cne.10596. [DOI] [PubMed] [Google Scholar]
- Poe BH, Linville C, Brunso-Bechtold J. Age-related decline of presumptive inhibitory synapses in the sensorimotor cortex as revealed by the physical dissector. J Comp Neurol. 2001;439:65–72. doi: 10.1002/cne.1335. [DOI] [PubMed] [Google Scholar]
- Pollock J, Kornetsky C. Evidence for the role of dopamine D1 receptors in morphine induced stereotypic behavior. Neurosci Lett. 1989;102:291–296. doi: 10.1016/0304-3940(89)90094-3. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, DiChiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci USA. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE. Behavioral sensitization: characterization of enduring changes in rotational behavior produced by intermittent injections of amphetamine in male and female rats. Psychopharmacol. 1984;84:466–475. doi: 10.1007/BF00431451. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999a;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999b;33:160–162. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Krech D, Bennett EL, Diamond MC. Effects of environmental complexity and training on brain chemistry and anatomy: a replication and extension. J comp Physiol Psychol. 1962;55:429–437. doi: 10.1037/h0041137. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010 doi: 10.1016/j.tins.2010.02.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Snow S. Sensitization to cocaine’s motor activating properties produced by electrical kindling of the medial prefrontal cortex but not of the hippocampus. Brain Res. 1994;659:17–22. doi: 10.1016/0006-8993(94)90858-3. [DOI] [PubMed] [Google Scholar]
- Self DW, Choi KH, Simmons D, Walker JR, Smagula CS. Extinction training regulates neuroadaptive responses to withdrawal from cocaine self-administration. Learn Mem. 2004;11:648–657. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nester EJ. Involvement of camp-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J Neurosci. 1998;18:1848–1959. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in apposition to each other. Brain Res. 1990;527:266–279. doi: 10.1016/0006-8993(90)91146-8. [DOI] [PubMed] [Google Scholar]
- Sharp T, Zetterstrom T, Ljungberg T, Ungerstedt U. A direct comparison of amphetamine-induced behaviors and regional brain dopamine release in the rat using intracerebral dialysis. Brain Res. 1987;401:322–330. doi: 10.1016/0006-8993(87)91416-8. [DOI] [PubMed] [Google Scholar]
- Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurons. Trends Neurosci. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the dissector. J Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behavior. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Lynch WJ, Sanchez H, Olausson P, Nestler EJ, Bibb JA. Inhibition of Cdk5 in the nucleus accumbens the locomotor-activating and incentive-motivational effects of cocaine. Proc Natl Acad Sci USA. 2007;104:4147–4152. doi: 10.1073/pnas.0610288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Parsegian A, Naydenov A, Neve RL, Konradi C, Jr., Carlezon WA. Brain reward regulated by AMPA receptor subunits in nucleus accumbens shell. J Neurosci. 2006;26:11665–11669. doi: 10.1523/JNEUROSCI.3070-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totterdell S, Smith AD. Convergence of hippocampal and dopaminergic input onto identified neurons in the nucleus accumbens of the rat. J. Chem Neuroanat. 1989;2:285–298. [PubMed] [Google Scholar]
- Turner AM, Greenough WT. Synapses per neuron and synaptic dimensions in occipital cortex of rats reared in complex, social or isolation housing. Acta Stereol [Supp] 1983;2:239–244. [Google Scholar]
- Turner AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. I. Synaptic and neuronal density and synapses per neuron. Brain Res. 1985;329:195–203. doi: 10.1016/0006-8993(85)90525-6. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. The development of cocaine-induced behavioral sensitization is affected by discrete quinolinic acid lesions of the prelimbic medial prefrontal cortex. Brain Res. 1998;795:71–76. doi: 10.1016/s0006-8993(98)00254-6. [DOI] [PubMed] [Google Scholar]
- Weibel E. Stereological Methods. Volume 1, Practical Methods for Biological Morphometry. Academic Press; London: 1979. [Google Scholar]
- Wennemer HK, Kornetsky C. Fluoxetine blocks expression but not development of sensitization to morphine-induced oral stereotypy in rats. Psychopharmacol. 1999;146:19–23. doi: 10.1007/s002130051083. [DOI] [PubMed] [Google Scholar]
- White IM, Doubles L, Rebec GV. Cocaine-induced activation of striatal neurons during focused stereotypy in rats. Brain Res. 1998;810:146–152. doi: 10.1016/s0006-8993(98)00905-6. [DOI] [PubMed] [Google Scholar]
- White FJ, Joshi A, Koeltzow TE, HU XT. Dopamine receptor antagonists fail to prevent induction of cocaine sensitization. Neuropsychopharm. 1998;18:26–40. doi: 10.1016/S0893-133X(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Jeziorski M. Coadministration of MK-801 with amphetamine, cocaine or morphine prevents rather than transiently masks the development of behavioral sensitization. Brain Res. 1993;613:291–294. doi: 10.1016/0006-8993(93)90913-8. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhao W, Hu M, Becker JB. Interactions among ovarian hormones and time of testing on behavioral sensitization and cocaine self-administration. Behav Brain Res. 2007;184:174–184. doi: 10.1016/j.bbr.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lee TH, Davidson C, Lazarus C, Wetsel WC, Ellinwood EH. Reversal of cocaine-induced behavioral sensitization and associated phosphorylation of the NR2B and GluR1 subunits of the NMDA and AMPA receptors. Neuropsychopharm. 2007;32:377–387. doi: 10.1038/sj.npp.1301101. [DOI] [PubMed] [Google Scholar]
- Zhao W, Becker JB. Sensitization enhances acquisition of cocaine self-administration in female rats: Estradiol further enhances cocaine intake after acquisition. Horm Behav. 2009 doi: 10.1016/j.yhbeh.2009.09.005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]