Abstract
Cadmium (Cd), a highly toxic environmental pollutant, induces neurodegenerative diseases. Recently we have demonstrated that Cd induces neuronal apoptosis in part through activation of the mammalian target of rapamycin (mTOR) pathway. However, the underlying mechanism is unknown. Here we show that Cd induced generation of reactive oxygen species (ROS) by upregulating expression of NADPH oxidase 2 (NOX2) and its regulatory proteins (p22phox, p67phox, p40phox, p47phox and Rac1) in PC12 and SH-SY5Y cells. Cd induction of ROS contributed to activation of mTOR signaling, as pretreatment with N-acetyl-L-cysteine (NAC), a ROS scavenger, prevented this event. Further studies reveal that Cd induction of ROS increased phosphorylation of type I insulin-like growth factor receptor β subunit (IGFRβ), which was abrogated by NAC. Wortmannin, a phosphoinositide 3′-kinase (PI3K) inhibitor, partially attenuated Cd-induced phosphorylation of Akt, p70 S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4E-BP1), as well as apoptosis of the neuronal cells. In addition, overexpression of wild-type phosphatase and tensin homologue deleted on chromosome 10 (PTEN) or pretreatment with aminoimidazole carboxamide ribonucleotide (AICAR), an AMP-activated protein kinase (AMPK) activator, partially prevented Cd-induced ROS and activation of mTOR pathway, as well as cell death. The results indicate that Cd induction of ROS activates mTOR signaling, leading to neuronal cell death, in part by activating the positive regulators IGFR/PI3K, and by inhibiting the negative regulators PTEN/AMPK. The findings suggest that the inhibitors of PI3K and mTOR, activators of AMPK, or antioxidants may be exploited for prevention of Cd-induced neurodegenerative diseases.
Keywords: Cadmium, Apoptosis, Reactive oxygen species, Mammalian target of rapamycin, Phosphatase and tensin homologue deleted on chromosome 10, AMP-activated kinase
Introduction
Cadmium (Cd), a highly toxic heavy metal, is mainly released from the smelting, burning of fossil fuels and municipal wastes, and refining of metals and cigarette smoking, resulting in the pollution of water, air and soil. Exposure of human to Cd-contaminated environment or food chain may be implicated in some human disorders related to hyperactivity and increased aggressiveness [1], for example, neurological disorders such as learning disabilities and hyperactivity in children [2, 3], olfactory dysfunction and neurobehavioral defects in attention, psychomotor speed, and memory in workers [4, 5]. Therefore, Cd intoxication has been considered and studied as a possible etiological factor of neurodegenerative diseases.
Oxidative stress is a prominent feature of many neurodegenerative disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS). Cd-induced oxidative stress, e.g. reactive oxygen species (ROS), is closely associated with PD and AD [6–10]. Accumulating data show that elevation of intracellular ROS may increase permeability of the blood-brain barrier, tubulin alterations, and perturbation in synaptic transmission [5]. Under pathological conditions, excessive amounts of ROS induced by Cd can modify proteins, lipids and DNA, alter their functions, and activate related signaling pathways [4, 7, 11–14]. The results from the laboratory and others indicate that Cd activates MAPK pathway via induction of ROS and inhibition of protein phosphatases 2A and 5, thereby leading to neuronal apoptosis [15, 16]. However, how Cd induces ROS in neuronal cells remains to be refined. Also, whether Cd induction of ROS targets other signaling pathways remains elusive.
NADPH oxidases (NOXs), including NOX1, NOX3, NOX4, NOX5, DUOX1, and DUOX2, are a family of transmembrane enzymes that share the capacity to transport electrons across the plasma membrane and to generate ROS [17]. Among the NOX isoforms, NOX2, also known as gp91phox, is the most widely distributed in the diverse tissues and cells, including neurons [17,18]. NOX2 constitutively associates with a non-catalytic subunit, p22phox, and is activated through a series of interactions with other regulatory proteins, such as p40phox, p47phox, p67phox and the small GTPase Rac1 [17]. Once activated, there is a fusion of NOX2-containing vesicles with the plasma membrane or the phagosomal membrane. The active enzyme complex transports electrons from cytoplasmic NADPH to extracellular or phagosomal oxygen to generate ROS [17].
The signaling pathway composed of type I insulin-like growth factor receptor (IGFR), phosphatidylinositol 3′-kinase (PI3K), protein kinaseB (PKB/Akt) and mammalian target of rapamycin (mTOR), designated the IGFR-PI3K-Akt-mTOR signaling pathway, is crucial for cell growth, proliferation and survival [19]. In response to ligand binding, IGFR is activated via auto-phosphorylation of multiple tyrosine residues. Activated IGFR in turn phosphorylates the insulin receptor substrates 1–4 (IRS1-4) and src- and collagen-homology (SHC) adaptor proteins, which can trigger multiple downstream signal transduction pathways, such as the mitogen-activated protein kinase (MAPK) and PI3K pathways [20]. Phosphorylated IRS recruits the p85 subunit of PI3K and signals to the p110 catalytic subunit of PI3K, resulting in activation of PI3K. Activated PI3K catalyzes the conversion of phosphatidylinositol (4,5)-bisphosphate (PIP2) to phosphatidylinositol-3,4,5-trisphosphate (PIP3). This pathway is negatively regulated by PTEN (phosphatase and tensin homolog on chromosome ten), a dual-specificity protein and lipid phosphatase. Increased PIP3 binds to the pleckstrin homology (PH) domain of Akt and, in combination with additional Ser/Thr phosphorylation of Akt by phosphoinositide-dependent kinase 1 (PDK1) and PDK2 (or mTORC2), results in full activation of Akt. Subsequently, activated PI3K or Akt may positively regulate mTOR, leading to increased phosphorylation of ribosomal p70 S6 kinase (S6K1) and eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4E-BP1), the two best-characterized downstream effector molecules of mTOR [19,20]. Studies have placed tuberous sclerosis complex (TSC) 1/2 as a modulator between PI3K/Akt and mTOR [21–23]. The TSC1/2 complex acts as a repressor of mTOR function [21–23]. TSC2 has GTPase-activating protein (GAP) activity towards the Ras family small GTPase Rheb (Ras homolog enriched in brain), and TSC1/2 antagonizes the mTOR signaling pathway via stimulation of GTP hydrolysis of Rheb [24–28]. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38 [29], though this remains controversial [30]. The TSC can also be activated by energy depletion or oxidative stress through the activation of AMP-activated kinase (AMPK) [31]. This, in turn, activates the TSC, which catalyzes the conversion of Rheb-GTP to Rheb-GDP and thus inhibits mTOR [31]. Since Cd potently induces apoptosis of neuronal cells [4, 32, 33], we originally speculated that Cd might suppress mTOR signaling. However, to our surprise, exposure of PC12 and SH-SY5Y cells to Cd increased phosphorylation of mTOR and its downstream effector molecules, such as S6K1 and 4E-BP1, and rapamycin prevented Cd-induced apoptosis of the cells [34], indicating that Cd causes neuronal apoptosis by activation of mTOR pathway. However, how Cd activates mTOR pathway remains largely unknown.
Here, for the first time, we show that Cd induces ROS generation, which is correlated to elevated expression of NOX2 and its regulatory proteins in PC12 and SH-SY5Y cells. Furthermore, Cd induction of ROS activates Akt/mTOR pathway by activating the positive regulators (IGFR and PI3K) and inhibiting negative regulators (PTEN and AMPK), leading to neuronal apoptosis.
Materials and Methods
Materials
Cadmium chloride (Sigma, St. Louis, MO) was dissolved in sterile distilled water to prepare the stock solutions (0–40 mM), aliquoted and stored at room temperature. Dulbecco’s Modified Eagle Medium (DMEM) was purchased from Mediatech (Herndon, VA). Horse serum and fetal bovine serum (FBS) were supplied by Hyclone (Logan, UT), whereas 0.05% Trypsin-EDTA was from Invitrogen (Grand Island, NY). Enhanced chemiluminescence solution was from Pierce (Rockford, IL). CellTiter 96® AQueous One Solution Cell Proliferation Assay kit was from Promega (Madison, WI). The AMPK activator, 5-amino-4-imidazolecarboxamide ribose (AICAR) was obtained from Cell Signaling Technology (Beverly, MA). The PI3K inhibitor, wortmannin was purchased from MP Biomedicals (Solon, OH). The following antibodies were used: phospho-AMPKα (Thr172), phospho-acetyl-CoA carboxylase (ACC, Ser79), ACC, phospho-TSC2 (Ser1462), phospho-mTOR (Ser2448), phospho-mTOR (Ser2481), phospho-S6 ribosomal protein (Ser235/236), S6 ribosomal protein (all from Cell Signaling Technology, Beverly, MA); IGF-1R β subunit (IGFRβ, p85), p22phox, p40phox, p47phox, p67phox, NOX2, PTEN, AMPKα, phospho-S6K1 (Thr389), S6K1, phospho-Akt (Thr308), phospho-Akt (Ser473), Akt, TSC2 and mTOR (all from Santa Cruz Biotechnology, Santa Cruz, CA); Rac1 (Cytoskeleton, Denver, CO), 4E-BP1 (Zymed Laboratories, South San Francisco, CA); phosphotyrosine (p-Tyr; BD Biosciences, San Jose, CA); β-tubulin (Sigma), Goat anti-rabbit IgG-horseradish peroxidase (HRP), goat anti-mouse IgG-HRP, and rabbit anti-goat IgG-HRP (Pierce). 5-(and-6)-chloromethyl-2′, 7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) were from MP Biomedicals (Solon, OH). N-acetyl-L-cysteine (NAC), Poly-D-lysine (PDL) and all the other chemicals were purchased from Sigma.
Cell culture
Rat pheochromocytoma (PC12) and human neuroblastoma (SH-SY5Y) cell lines were from American Type Culture Collection (ATCC) (Manassas, VA). PC12 cells were grown in antibiotic-free DMEM supplemented with 10% horse serum and 5% FBS, whereas SH-SY5Y cells were grown in antibiotic-free DMEM supplemented with 10% FBS. Cells were maintained in a humid incubator (37°C, 5% CO2).
Recombinant adenoviral constructs and infection of cells
The recombinant adenovirus encoding wild-type human PTEN (Ad-PTEN) and the control virus encoding the green fluorescence protein (GFP) (Ad-GFP) were generated, amplified, and titrated as described [35,36]. For experiments, PC12 and SH-SY5Y cells were grown in the growth medium, and infected with the individual adenovirus for 24 h at 5 of multiplicity of infection (MOI = 5). Subsequently, cells were used for experiments. Ad-GFP served as a control. PTEN expression was determined by Western blot with antibodies to PTEN (Santa Cruz Biotechnology, Santa Cruz, CA).
Cell viability evaluation and morphological analysis
Cells were seeded at a density of 1 × 104 cells/well in a flat-bottomed 96-well plate, pre-coated with (for PC12) or without (for SH-SY5Y) PDL (0.2 μg/ml). Next day, cells were treated with/without Cd (10 μM) for 24 h following pretreatment with/without AICAR (2 mM) for 1 h with 6 replicates of each treatment. In some cases, after infection with Ad-PTEN or Ad-GFP, cells were exposed to Cd (10 and 20 μM) for 24 h. Subsequently, each well was added 20 μl of one solution reagent (Promega, Madison, WI) and incubated for 3 h. Cell viability was determined by measuring the optical density (OD) at 490 nm using a Wallac 1420 Multilabel Counter (PerkinElmer Life Sciences, Wellesley, MA).
Additionally, PC12 cells were seeded at a density of 5 × 105 cells/well in a PDL-coated 6-well plate. Next day, cells were exposed to Cd (10 and 20 μM) following pretreatment with/without AICAR (2 mM) or wortmannin (0.5 μM) for 1 h. After incubation for 24 h, images were taken with an Olympus inverted phase-contrast microscope (Olympus Optical Co., Melville, NY) (200 ×) equipped with the Quick Imaging system.
ROS assay
The level of ROS was measured as described previously [15]. Briefly, cells were seeded at a density of 1 × 104 cells/well in 96-well plate, pre-coated with (for PC12) or without (for SH-SY5Y) PDL (0.2 μg/ml). The next day, cells were loaded with CM-H2DCFDA (10 μM) per manufacturer’s protocol, incubated in the presence of various concentrations of Cd (0–20 μM) for 24 h with 6 replicates of each treatment. In some cases, cells were pre-incubated with NAC (5 mM) for 1 h, and then treated with/without CdCl2 (10 and 20 μM) for 24 h, followed by loading with CM-H2DCFDA for 40 min. For experiments with the mTOR inhibitor rapamycin and the AMPK activator AICAR, cells were pre-incubated with Rapa (200 ng/ml) for 48 h and AICAR (2 mM) for 1 h, and then treated with 10 and 20 μM Cd for 24 h followed by loading with CM-H2DCFDA. Additionally, cells infected with Ad-PTEN or Ad-GFP were treated with Cd (10 and 20 μM) in the presence of CM-H2DCFDA for 24 h. Fluorescent intensity was recorded by excitation at 485 nm and emission at 535 nm using a Wallac 1420 Multilabel Counter (PerkinElmer Life Sciences, Wellesley, MA).
Western blot analysis
After treatment, cells were briefly washed with cold PBS. On ice, cells were lysed in RIPA buffer [50 mM Tris, pH 7.2; 150 mM NaCl; 1% sodium deoxycholate; 0.1% SDS; 1% Triton-X 100; 10 mM NaF; 1 mM Na3VO4; protease inhibitor cocktail (1:1000, Sigma). Lysates were sonicated for 10 s and centrifuged at 14,000 rpm for 10 min at 4°C. Protein concentration was determined by bicinchoninic acid assay with bovine serum albumin as standard (Pierce, Rockford, IL). Equivalent amounts of protein were separated on 7.5%–12% SDS-polyacrylamide gel and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). Membranes were incubated with PBS containing 0.05% Tween 20 and 5% nonfat dry milk to block nonspecific binding and were incubated with primary antibodies, then with appropriate secondary antibodies conjugated to horseradish peroxidase. Immunoreactive bands were visualized by using enhanced chemiluminescence solution (Pierce). To check the amount of protein loaded, the immunoblots were treated with stripping solution (62.5 mM Tris buffer, pH 6.7, containing 2% SDS and 100 mM β-mercaptoethanol) for 30 min at 50°C and incubated with mouse monoclonal anti-β-tubulin antibody (Sigma) followed by horseradish peroxidase-coupled goat anti-mouse IgG (Pierce).
Detection of phosphorylation of IGFR
PC12 cells (5×105/ml) were seeded in 100-mm dishes, pre-coated with PDL (0.2 μg/ml). The next day, cells were pretreated with or without NAC (5mM) for 1 h, and then exposed to Cd (10 and 20 μM) for 4 h. Cells were washed once in ice-cold 1×PBS, followed by lysis in RIPA buffer. After clearing via centrifugation (14000 rpm, 15 min, 4°C), supernatants (500 μl) of cell lysates were collected and incubated overnight at 4°C with 1 μg of antibody to IGFRβ and 30 μl of protein A/G-agarose beads (Santa Cruz Biotechnology). Immunoprecipitates were washed with RIPA buffer two times and twice with ice-cold PBS, followed by immunoblotting with antibodies to p-Tyr and IGFRβ, respectively. IgG-heavy chain and light chain from each immunoprecipitated sample are shown as loading control.
Statistical analysis
Results were expressed as mean values ± standard error (mean ± S.E.). Statistical analysis was performed by Student’s t-test (STATISTICA, Statsoft Inc, Tulsa, OK). A level of P < 0.05 was considered to be significant.
Results
Cd induces ROS generation by upregulating NOX2 and its regulatory proteins in neuronal cells
Recently we have shown that Cd induces apoptosis of PC12 and SH-SY5Y cells by induction of ROS [15]. To address whether Cd induction of ROS is associated with activation of ROS-generating enzymes, PC12 and SH-SY5Y cells were exposed to Cd (0–20 μM) for 24 h. By Western blot analysis, we found that Cd upregulated expression of ROS-generating NOX family members including NOX2, p22phox, p40phox, p47phox, p67phox, and Rac1 in PC12 (Fig. 1A) and SH-SY5Y cells (data not shown) in a concentration dependent manner. Concomitantly, Cd induced ROS production in PC12 and SH-SY5Y cells (Fig. 1B), which closely corresponds to decreased cell viability observed in our previous studies [15, 34]. The data suggest that Cd induces ROS generation at least by activation of NOX2, contributing to apoptosis of neuronal cells.
Fig. 1. Cd induces ROS generation by upregulating NOX2 and its regulatory proteins in neuronal cells.
Indicated cells were treated with Cd (0–20 μM) for 24 h (A, B), with Cd (10 μM) for 0–24 h (C, D), or pretreated with/without NAC (5 mM) for 1 h and then exposed to Cd (0–20 μM) for 24 h (E, F), followed by Western blotting using indicated antibodies (A, D, F), or ROS assay (B, C, E). For (A, D, F), β-tubulin served as a loading control. Similar results were observed in at least three independent experiments. For (B, C, E), results are presented as mean ± S.E. n=6. aP<0.05, bP<0.01, difference vs. control group; cP<0.01, difference vs. 10 μM Cd group; dP<0.01, difference vs. 20 μM Cd group.
To unravel the relationship between ROS induction and increased expression of NOX2 family members, we performed time course studies, and found that treatment of PC12 cells with Cd (10 μM) increased ROS level significantly within 2 h (Fig. 1C), but did not elevate NOX2 protein expression obviously until 4–6 h (Fig. 1D). The results suggest that Cd induced ROS generation initially coming from non-NOX systems in the cells that secondarily upregulated the expression of the ROS generating enzyme NOX2 and its regulatory subunits. To substantiate this finding, PC12 and SH-SY5Y cells were pretreated for 1 h with a ROS scavenger, NAC (5 mM), and then exposed to Cd (0–20 μM) for 24 h. We observed that NAC slightly reduced the basal level of ROS in the cells, but dramatically abolished Cd-induced ROS generation (Fig. 1E). In consistence, NAC modestly downregulated the basal levels of Rac1, p40phox, p47phox, and p67phox, despite no effect on the basal levels of NOX2 and p22phox (Fig. 1F). However, NAC strikingly blocked Cd-stimulated expression of NOX2 and its regulatory proteins in the cells (Fig. 1D). The finding implies that Cd-induced ROS initially through non-NOX systems in the cells may upregulate expression of NOX2 family members through a positive feedback mechanism.
Cd induction of ROS activates mTOR in part by upregulating the activities of IGFR and PI3K in neuronal cells
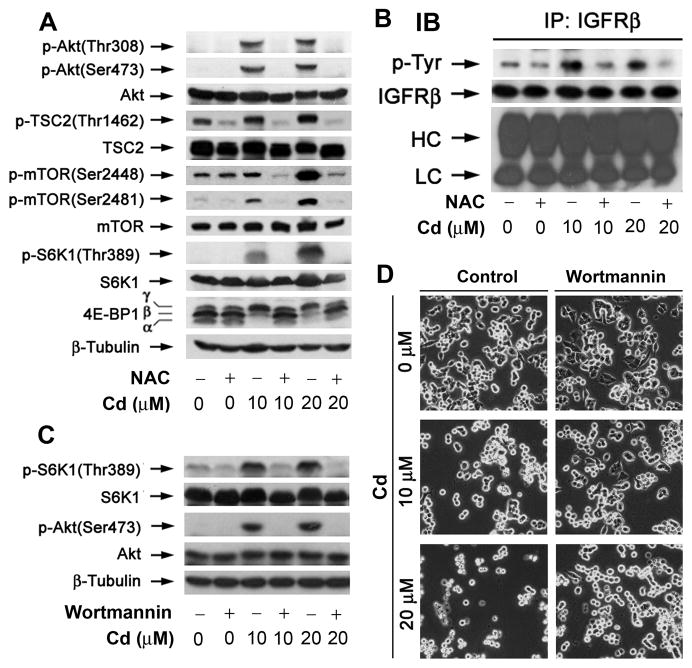
Our recent studies have shown that Cd induces phosphorylation of mTOR and its downstream effector molecules S6K1 and 4E-BP1 [34]. To determine whether this is attributed to Cd induction of ROS, PC12 cells were pretreated with NAC (5 mM) for 1 h, and then exposed to Cd (0–20 μM) for 24 h, followed by Western blot analysis. Our results showed that Cd-induced phosphorylation of mTOR, S6K1 and 4E-BP1 was almost completely blocked by NAC (Fig. 2A). Consistently, we noticed that Cd-activated Akt was also abolished by NAC (Fig. 2A). In response to growth factors or nutrients, Akt is activated and phosphoryaltes TSC2 (Thr1462), which disrupts TSC1/2 complex stability, and de-represses Rheb-mTOR signaling [21–28]. In the studies, we also observed that Cd increased TSC2 phosphorylation (Thr1462), which was obviously blunted by NAC (Fig. 2A). Collectively, the findings indicate that Cd activated Akt/mTOR pathway by induction of ROS in the neuronal cells.
Fig. 2. Cd induction of ROS activates mTOR in part by upregulating the activities of IGFR and PI3K in neuronal cells.
PC12 cells, pretreated with/without NAC (5 mM) for 1 h, were exposed to Cd (0–20 μM) for 24 h (A) or 4 h (B), followed by Western blotting using indicated antibodies (A), or immunoprecipitation with antibodies to IGFRβ plus A/G-agarose, and immunoblotting with antibodies to phosphotyrosine (p-Tyr) and IGFRβ, respectively (B). β-tubulin served as a loading control for (A), whereas the light chain (LC) and the heavy chain (HC) of IgG as loading control for (B). (C) PC12 cells, pretreated with/without wortmannin (500 nM) for 1 h, were exposed to Cd (0–20 μM) for 4 h (C) or 24 h (D), followed by Western blotting using indicated antibodies (C), or cell morphological analysis (D). Similar results were observed in at least three independent experiments for (A–D).
IGFR and PI3K are the upstream kinases that positively regulate Akt/mTOR pathway [19,20]. We postulated that Cd-induction of ROS stimulates Akt/mTOR signaling in part by activating these kinases. To assess whether IGFR is involved, PC12 cells were pretreated with NAC (5 mM) for 1 h, and then exposed to Cd (0–20 μM) for 4 h, followed by immnoprecipitation with antibodies to IGFR β-subunit (IGFRβ), and immunoblotting with antibodies to phosphotyrosine (p-Tyr) and IGFRβ. As shown in Fig. 2B, Cd-induced ROS increased tyrosine phosphorylation of IGFRβ, which was prevented by NAC.
To examine whether PI3K is activated, PC12 cells were exposed to Cd (0–20 μM) for 4 h after pretreatment with the specific PI3K inhibitor wortmannin for 1 h, followed by Western blotting. We observed that Cd-induced phosphorylation of Akt and S6K1 was completely blocked by wortmannin (500 nM) (Fig. 2C). In addition, we noticed that pretreatment with wortmannin (500 nM) for 1 h also partially prevented apoptosis induced by exposure to Cd (10–20 μM) for 24 h (Fig. 2D). The findings suggest that Cd induction of ROS activates Akt/mTOR pathway at least partially by activating IGFR and PI3K, leading to apoptosis in neuronal cells.
Cd induction of ROS activates mTOR in part by downregulating expression of PTEN and phosphorylation of AMPKα
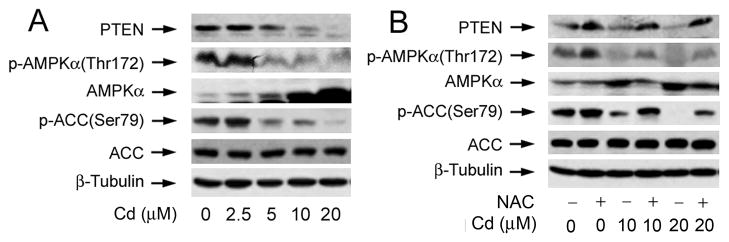
PTEN and AMPK are the two negative regulators of Akt/mTOR pathway [19, 31, 37]. We hypothesized that Cd activates Akt/mTOR signaling partially via downregulation of PTEN and AMPKα. To this end, PC12 cells were exposed with Cd (0–20 μM) for 24 h, followed by Western blotting. We observed that Cd markedly reduced cellular protein levels of PTEN (Fig. 3A). Of interest, Cd increased cellular protein levels of AMPKα (Fig. 3A), but decreased phosphorylation of AMPKα (Thr172) and its substrate acetyl-CoA carboxylase (ACC) (Ser79) in a concentration-dependent manner (Fig. 3A), indicating inhibition of AMPK activity. Similar data were seen in SH-SY5Y cells (data not shown).
Fig. 3. Cd-induction of ROS downregulates protein expression of PTEN and phosphorylation of AMPKα.
PC12 cells were treated with Cd (0–20 μM) for 24 h (A), or pretreated with/without NAC (5 mM) for 1 h and then exposed to Cd (0–20 μM) 24 h (B), followed by Western blotting using indicated antibodies. β-tubulin served as a loading control. Similar results were observed in at least three independent experiments.
Next we examined whether Cd downregulation of PTEN protein expression and AMPKα phosphorylation is due to ROS induction. Our Western blot analysis showed that pretreatment with NAC (5 mM) for 1 h obviously attenuated Cd downregulation of PTEN and phospho-AMPKα levels in PC12 cells (Fig. 3B) and SH-SY5Y cells (data not shown). The data suggest that Cd induction of ROS activates Akt/mTOR pathway partially through downregulation of PTEN protein expression and AMPKα phosphorylation.
Rapamycin attenuates Cd-induced NOX2 upregulation, ROS generation, PTEN downregulation and AMPK inactivation
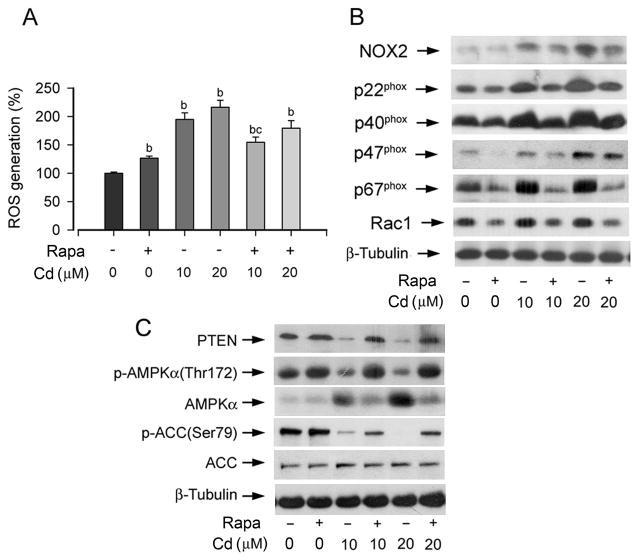
In the previous studies, we noticed that inhibition of mTOR by rapamycin prevented Cd-induced neuronal apoptosis [34]. As Cd induction of ROS causes the neuronal cell death [15], we asked whether rapamycin affects ROS generation. To this end, PC12 cells were pretreated with 200 ng/ml rapamycin for 48 h, and then exposed to Cd (0–20 μM) for 24 h, followed by ROS detection. We found that pretreatment with rapamycin alone increased ROS levels slightly (by ~1.2 fold), while exposure to Cd (10 and 20 μM) alone for 24 h significantly elevated ROS levels (by about 2–2.2 fold) in PC12 cells (Fig. 4A). Interestingly, pretreatment with rapamycin significantly attenuated Cd induction of ROS (Fig. 4A).
Fig. 4. Rapamycin attenuates Cd-induced NOX2 upregulation, ROS generation, PTEN downregulation and AMPK inactivation.
PC12 cells, pretreated with/without rapamycin (Rapa; 200 ng/ml) for 48 h, were exposed to Cd (0–20 μM) for 24 h, followed by ROS assay (A) or Western blotting using indicated antibodies (B, C). For (A), results are presented as mean ± S.E. n=6. bP<0.01, difference vs. control group; cP<0.01, difference vs. 10 μM Cd group. For (B) and (C), β-tubulin served as a loading control. Similar results were observed in at least three independent experiments.
As Cd induction of ROS is closely associated with increased expression of NOX2 family members (Fig. 1), we reasoned that rapamycin might inhibit expression of these ROS generating proteins. Our Western blotting results revealed that pretreatment with rapamycin for 48 h did not obviously affect expression of NOX2, but markedly inhibited expression of Rac1, p47phox, and p67phox in PC12 cells. Interestingly, rapamycin strikingly attenuated 24 h Cd-induced expression of NOX2 and all regulatory proteins in the cells (Fig. 4B).
Since Cd induction of ROS downregulated expression of PTEN and phospho-AMPKα (Fig. 3), we also investigated whether rapamycin alters expression of these proteins. It appears that pretreatment with rapamycin for 48 h alone did not affect protein expression of PTEN as well as phosphorylation of AMPK and ACC, but substantially blocked Cd downregulation of PTEN protein expression and AMPKα/ACC phosphorylation (Fig. 4C). The results suggest that rapamycin inhibits mTOR pathway, not only by directly inhibiting mTOR function, but also by indirectly restoring the activities of PTEN and AMPK suppressed by Cd-induced ROS.
Overexpression of PTEN partially prevents Cd-induced ROS and activation of Akt/mTOR pathway, as well as cell death
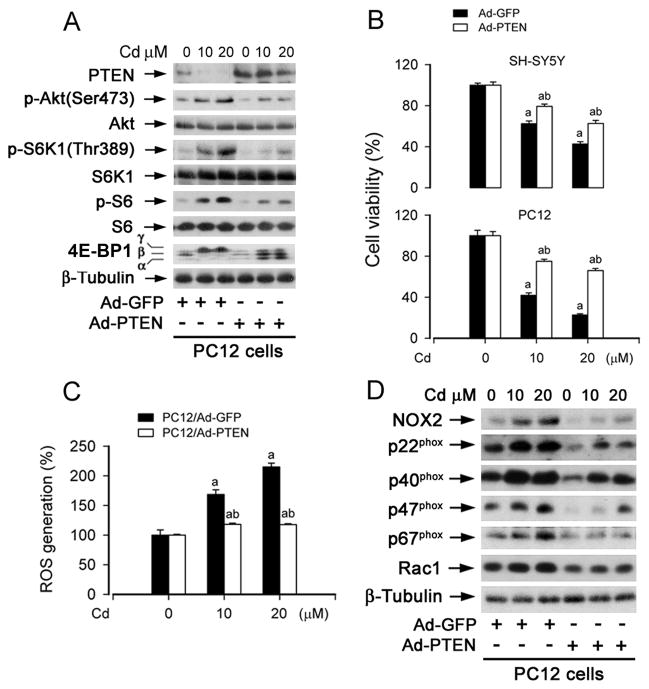
To validate the role of PTEN in Cd-induced activation of mTOR and neuronal apoptosis, SH-SY5Y cells were infected with recombinant adenoviral vector (Ad-PTEN) encoding wild type human PTEN and Ad-GFP (as control), respectively, and then exposed to Cd (0–20 μM) for 4 h, followed by Western blot analysis. We observed that infection with Ad-PTEN increased expression of PTEN by ~4 fold, and slightly inhibited the basal levels of phosphorylation of Akt and S6K1, comparing with infection with Ad-GFP (Fig. 5A). However, overexpression of PTEN remarkably suppressed Cd-induced phosphorylation of Akt, S6K1, S6 and 4E-BP1 (Fig. 5A). Of importance, overexpression of PTEN also in part attenuated the toxic effect of Cd (24 h) on PC12 and SH-SY5Y cells (Fig. 5B). Taken together, these results support a viewpoint that PTEN acts as a negative regulator, inhibiting Cd-induced activation of Akt/mTOR pathway thereby preventing neuronal apoptosis.
Fig. 5. Overexpression of PTEN partially prevents Cd-induced ROS and activation of Akt/mTOR pathway, as well as cell death.
Indicated cells, infected with Ad-PTEN or Ad-GFP (as control), were exposed with Cd (0–20 μM) for 24 h (A–D), followed by Western blotting using indicated antibodies (A, D), cell viability evaluation using one solution assay (B), or ROS assay (C). For (A) and (D), β-tubulin served as a loading control. Similar results were observed in at least three independent experiments. For (B) and (C), results are presented as mean ± S.E. n=6. aP<0.05, bP<0.01, difference vs. control group.
Recent evidence has implicated PTEN as a crucial mediator of ROS generation and neurodegenerative diseases [38, 39]. This prompted us to examine whether increase of PTEN expression affects Cd-induced ROS generation. As shown in Fig. 5C, ectopic overexpression of PTEN potently prevented Cd-induced ROS generation in PC12 cells. Furthermore, overexpression of PTEN also inhibited Cd enhanced expression of ROS-generating enzymes, including NOX2, p22phox, p40phox, p47phox, p67phox and Rac1 in the cells (Fig. 5D).
Activation of AMPK partially blocks Cd-induced ROS and activation of mTOR pathway, as well as cell death
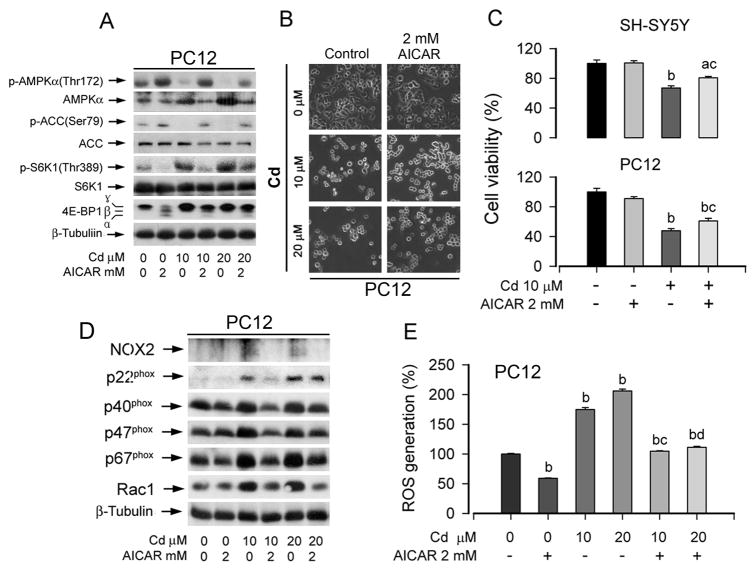
To confirm the role of AMPK in Cd activation mTOR pathway, PC12 cells were exposed to Cd (0–20 μM) for 24 h after pretreatment with AICAR, an AMPK activator, for 1 h. Consistent with our observation in Fig. 3, Cd downregulated phosphorylation of AMPKα or ACC, which was profoundly attenuated by AICAR (2 mM) (Fig. 6A). Activation of AMPK by AICAR further inhibited Cd-induced phosphorylation of S6K1 and 4E-BP1. Consistently, when PC12 cells were pretreated with AICAR for 1 h, and then exposed to Cd (0–20 μM) for 24 h, AICAR itself did not apparently alter cell morphology, but in part attenuated the toxicity of Cd in PC12 and SH-SY5Y cells (Fig. 6B and C). Furthermore, activation of AMPKα with AICAR also markedly suppressed Cd-induced expression of NOX2 family members (Fig. 6D) as well as ROS production (Fig. 6E).
Fig. 6. Activation of AMPK partially blocks Cd-induced ROS and activation of mTOR pathway, as well as cell death.
Indicated cells, pretreated with/without AICAR (2 mM) for 1 h, were exposed to Cd (0–20 μM) for 4 h (A) or 24 h (B–E), followed by Western blotting using indicated antibodies (A, D), morphological analysis (B), cell viability evaluation using one solution assay (C), or ROS assay (E). For (A), (B), and (D), similar results were observed in at least three independent experiments. For (C) and (E), results are presented as mean ± S.E. n=6. aP<0.05, bP<0.01, difference vs. control group; cP<0.01, difference vs. 10 μM Cd group; dP<0.01, difference vs. 20 μM Cd group.
Discussion
Recently we have demonstrated that Cd induces apoptosis of neuronal cells in part by activation of Akt/mTOR signaling pathway [34]. However, the underlying mechanism remains enigmatic. Here we present evidence that Cd induced ROS generation, resulting in activation of Akt/mTOR in PC12 and SH-SY5Y cells. This is strongly supported by the finding that NAC, a ROS scavenger, almost completely blocked this event. Furthermore, in this study, we found that Cd induction of ROS was linked to increased tyrosine phosphorylation of IGFRβ. Wortmannin, a PI3K inhibitor, also blocked Cd activation of Akt/mTOR pathway and partially prevented Cd-induced apoptosis of the neuronal cells. In addition, overexpression of PTEN was able to partially inhibit Cd-induced activation of Akt/mTOR, and apoptosis of the cells. Furthermore, AICAR, an AMPK activator, could attenuate Cd-induced activation of mTOR signaling, as well as cell death. Collectively, our findings suggest that Cd induction of ROS activates Akt/mTOR pathway, at least in part by activating the positive regulators IGFR and PI3K [19,20], and concurrently by inhibiting the negative regulators PTEN and AMPKα [31,37], leading to apoptosis of the neuronal cells.
In addition to Cd, other oxidative agents, such as diamide, phenylarsine oxide, have also been found to activate mTOR signaling [40]. However, it seems that not all oxidative agents activate mTOR. For example, hydrogen peroxide (H2O2), a well-known oxidant, was found to inhibit mTOR signaling [41,42]. Probably, this is related to the kinds of ROS generated by the individual oxidative agents. Current knowledge indicates that ROS contains oxygen radicals, including superoxide (O2•−), hydroxyl (•OH), peroxyl (RO2•), alkoxyl (RO•), and certain nonradicals that are either oxidizing agents and/or are readily converted into radicals, such as hypochlorous acid (HOCl), ozone (O3), singlet oxygen (1O2), and hydrogen peroxide (H2O2) [17]. We do not know what kinds of ROS induced by Cd. Since H2O2 has been found to inhibit mTOR [41,42], we can tentatively deduce that the main component of Cd-induced ROS should not be H2O2. Clearly, more studies are needed to figure out the identity of ROS induced by Cd. Identifying what kind of ROS that activates mTOR may shed a novel insight into the mechanism by which certain oxidative stress contributes to tumorigenesis or neurodegeneration.
It is known that the prototype phagocytic NADPH oxidases, composed of membrane-bound NOX2 and p22phox as well as cytosolic subunits such as p40phox, p47phox, p67phox and the small GTPase Rac1, are associated with ROS generation in phagocytes and in numerous non-phagocytic cells, including neurons [17, 18, 43]. ROS generation by NOX enzymes has been implicated in a variety of neurological disorders, including ischemic stroke, AD and PD [17]. Cd induces ROS in neuronal cells [15], yet the underlying mechanism is poorly defined. Here, for the first time, we show that on one hand, Cd induction of ROS generation was closely related to upregulating expression of the ROS-generating enzyme NOX2 and its regulatory subunits (Fig. 1A,B); on the other hand, Cd-induced ROS stimulated expression of NOX2 family members, because treatment with NAC abolished Cd-induced ROS and also negeted Cd-induced expression of NOX2, p22phox, p67phox, p40phox, p47phox and Rac1 in the cells (Fig. 1E,F). The results suggest a positive feedback loop involved in the regulation of the ROS generation and the NOX2 activity. mTOR is a master kinase to control protein synthesis [19,20]. Likely, Cd induction of ROS initially through non-NOX systems activates mTOR pathway, increasing protein synthesis of NOX2 family members; subsequently, upregulated NOX2, in turn, generates more ROS, which further stimulate mTOR, resulting in more NOX2 expression. This is implicated in our findings that inhibition of mTOR by rapamycin profoundly reduced Cd-induced expression of NOX2 family members and ROS generation. Recently mTOR has also been found to control globular adiponectin-induced generation of ROS in murine macrophage cell line RAW 264 as well [44]. In cells, ROS homeostasis is tightly controlled by the ROS generating and eliminating systems [45]. Superoxide dismutases, glutathione peroxidase, peroxiredoxins, glutaredoxin, thioredoxin and catalase play crucial roles for elimination of ROS [45]. Whether Cd induces ROS also in part by downregulation of any of these ROS-scavenging enzymes remains to be defined.
In this study, we found that Cd induction of ROS inhibited cellular protein expression of PTEN, suggesting inhibiting the activity of the phosphatase. It has been described that the activity of PTEN could be almost completely abolished by H2O2 even at 10 μM in vitro, and by treatment of Swiss 3T3 fibroblasts with 1 mM H2O2 for 10 min in vivo [46]. In RAW264.7 macrophages, lipopolysaccharide (LPS) and phorbol 12-myristate 13-acetate (PMA) stimulates ROS production, which increases oxidation and inactivates PTEN as well [46]. However, in contrast to Cd, H2O2, LPS or PMA induced certain ROS that only oxidizes PTEN but does not alter the total cellular protein levels of PTEN [42, 46]. This further suggests that Cd-induced ROS is unique and different from that induced by H2O2, LPS or PMA. Our findings that Cd inactivation of PTEN results in activation of mTOR signaling and apoptosis of neuronal cells also imply that loss of PTEN function due to deletion or mutations of PTEN gene may promote development of neurodegenerative disorders.
Another question that arises from the current work is how Cd downregulates PTEN protein expression. Recently, it has been described that zinc decreases PTEN protein level in primary cortical neurons and neuroblastoma N2a cells through E3 ubiquitin ligase NEDD4-1-mediated PTEN ubiquitination [47]. Whether Cd reduces PTEN expression in PC12 and SH-SY5Y cells by a similar mechanism remains to be determined.
AMPK is activated by a number of pathological stresses, including hypoxia, oxidative stress, glucose deprivation, exercise and dietary hormones, such as leptin and adiponectin [48]. AMPK can protect neurons against metabolic and excitotoxic insults relevant to the pathogenesis of several different neurodegenerative conditions [49]. For example, the AMPK-activating agent AICAR protected hippocampal neurons against death induced by glucose deprivation, chemical hypoxia, and exposure to glutamate and amyloid beta-peptide, implying that AMPK activation may play a protective role against stress in neuronal cells [50]. Consistent with the above findings, in the present study, we noticed that Cd induction of ROS upregulated cellular protein expression of AMPKα through an unknown mechanism, but very potently inhibited the activity of AMPK, leading to activation of mTOR signaling and neuronal cell death; and activation of AMPK with AICAR attenuated the neurotoxicity of Cd. Our findings strongly support the notion that dietary restriction, which activates AMPK and inhibits mTOR, may have preventive effects against neurodegeneration.
In summary, here we have shown that Cd activates Akt/mTOR pathway by induction of ROS, which is closely associated with upregulating expression of the ROS generating enzyme NOX2 and its regulatory proteins. Furthermore, we have identified that Cd induction of ROS not only activates the upstream kinases of Akt/mTOR, such as IGF-1R and PI3K, but also inhibits the negative regulators, PTEN and AMPK, leading to activation of Akt/mTOR pathway as well as apoptosis of neuronal cells. Our findings suggest that the inhibitors of PI3K and mTOR, activators of AMPK, or antioxidants (e.g. NAC), may be exploited for prevention of Cd-induced neurodegenerative diseases.
Acknowledgments
This work was supported in part by NIH grant CA115414 (S. H.), American Cancer Society (RSG-08-135-01-CNE) (S. H.), Louisiana Board of Regents grant NSF(2009)-PFUND-144 (S.H.), and National Natural Science Foundation of China grant No.30971486 (L.C.).
Abbreviations
- 4E-BP1
eukaryotic initiation factor 4E binding protein 1
- ACC
acetyl-CoA carboxylase
- AD
Alzheimer’s disease
- AICAR
5-amino-4-imidazolecarboxamide ribose
- Akt
protein kinase B (PKB)
- ALS
amyotrophic lateral sclerosis
- AMPK
AMP-activated kinase
- Cd
cadmium
- CM-H2DCFDA
5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
- DMEM
Dulbecco’s Modified Eagle Medium
- FBS
fetal bovine serum
- H2O2
hydrogen peroxide
- IGFRβ
insulin-like growth factor-1 receptor β subunit
- mTOR
mammalian target of rapamyicn
- MAPK
mitogen-activated protein kinase
- NAC
N-acetyl-L-cysteine
- OD
optical density
- PBS
phosphate buffered saline
- PDL
Poly-D-lysine
- PD
Parkinson’s disease
- PI3K
phosphoinositide 3′-kinase
- PTEN
phosphatase and tensin homologue deleted on chromosome 10
- ROS
reactive oxygen species
- S6K1
S6 kinase 1
- SDS
sodium dodecyl sulfate
- TSC1/2
tuberous sclerosis complex 1/2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lopez E, Arce C, Oset-Gasque MJ, Canadas S, Gonzalez MP. Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radic Biol Med. 2006;40:940–951. doi: 10.1016/j.freeradbiomed.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 2.Marlowe M, Cossairt A, Moon C, Errera J, MacNeel A, Peak R, Ray J, Schroeder C. Main and interaction effects of metallic toxins on classroom behavior. J Abnorm Child Psychol. 1985;13:185–198. doi: 10.1007/BF00910641. [DOI] [PubMed] [Google Scholar]
- 3.Pihl R, Parkes M. Hair element content in learning disabled children. Science. 1977;198:204–206. doi: 10.1126/science.905825. [DOI] [PubMed] [Google Scholar]
- 4.Kim S, Moon C, Eun S, Ryu P, Jo S. Identification of ASK1, MKK4, JNK, c-Jun, and caspase-3 as a signaling cascade involved in cadmium-induced neuronal cell apoptosis. Biochem Biophys Res Commun. 2005;328:326–334. doi: 10.1016/j.bbrc.2004.11.173. [DOI] [PubMed] [Google Scholar]
- 5.Baxter LC, Sparks DL, Johnson SC, Lenoski B, Lopez JE, Connor DJ, Sabbagh MN. Relationship of cognitive measures and gray and white matter in Alzheimer’s disease. J Alzheimers Dis. 2006;9:253–260. doi: 10.3233/jad-2006-9304. [DOI] [PubMed] [Google Scholar]
- 6.Johnson S. Gradual micronutrient accumulation and depletion in Alzheimer’s disease. Med hypotheses. 2001;56:595–597. doi: 10.1054/mehy.2000.1301. [DOI] [PubMed] [Google Scholar]
- 7.Li Z, Arnaud L, Rockwell P, Figueiredo-Pereira ME. A single amino acid substitution in a proteasome subunit triggers aggregation of ubiquitinated proteins in stressed neuronal cells. J Neurochem. 2004;90:19–28. doi: 10.1111/j.1471-4159.2004.02456.x. [DOI] [PubMed] [Google Scholar]
- 8.Monroe RK, Halvorsen SW. Cadmium blocks receptor-mediated Jak/STAT signaling in neurons by oxidative stress. Free Radic Biol Med. 2006;41:493–502. doi: 10.1016/j.freeradbiomed.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Okuda B, Iwamoto Y, Tachibana H, Sugita M. Parkinsonism after acute cadmium poisoning. Clin Neurol Neurosurg. 1997;99:263–265. doi: 10.1016/s0303-8467(97)00090-5. [DOI] [PubMed] [Google Scholar]
- 10.Panayi AE, Spyrou NM, Iversen BS, White MA, Part P. Determination of cadmium and zinc in Alzheimer’s brain tissue using inductively coupled plasma mass spectrometry. J Neurol Sci. 2002;195:1–10. doi: 10.1016/s0022-510x(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 11.Figueiredo-Pereira ME, Yakushin S, Cohen G. Disruption of the intracellular sulfhydryl homeostasis by cadmium-induced oxidative stress leads to protein thiolation and ubiquitination in neuronal cells. J Biol Chem. 1998;273:12703–12709. doi: 10.1074/jbc.273.21.12703. [DOI] [PubMed] [Google Scholar]
- 12.Stadtman E. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 13.Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 14.Green KN, Peers C. Divergent pathways account for two distinct effects of amyloid beta peptides on exocytosis and Ca(2+) currents: involvement of ROS and NF-kappaB. J Neurochem. 2002;81:1043–1051. doi: 10.1046/j.1471-4159.2002.00907.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Liu L, Huang S. Cadmium activates the mitogen-activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic Biol Med. 2008;45:1035–1044. doi: 10.1016/j.freeradbiomed.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Rockwell P, Martinez J, Papa L, Gomes E. Redox regulates COX-2 upregulation and cell death in the neuronal response to cadmium. Cell Signal. 2004;16:343–353. doi: 10.1016/j.cellsig.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 18.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polak P, Hall MN. mTOR and the control of whole body metabolism. Curr Opin Cell Biol. 2009;21:209–18. doi: 10.1016/j.ceb.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Huang S, Houghton PJ. Targeting mTOR signaling for cancer therapy. Curr Opin Pharmacol. 2003;3:371–377. doi: 10.1016/s1471-4892(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 21.Gao X, Zhang Y, Arrazola P, Hino O, Kobayashi T, Yeung RS, Ru B, Pan D. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat Cell Biol. 2002;4:699–704. doi: 10.1038/ncb847. [DOI] [PubMed] [Google Scholar]
- 22.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 23.Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA. 2002;99:13571–13576. doi: 10.1073/pnas.202476899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stocker H, Radimerski T, Schindelholz B, Wittwer F, Belawat P, Daram P, Breuer S, Thomas G, Hafen E. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol. 2002;5:559–565. doi: 10.1038/ncb995. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 26.Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, Kozma SC, Hafen E, Bos JL, Thomas G. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11:1457–1466. doi: 10.1016/s1097-2765(03)00220-x. [DOI] [PubMed] [Google Scholar]
- 27.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 29.Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y, Jiang Y. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007;318:977–980. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Fonseca BD, Tang H, Liu R, Elia A, Clemens MJ, Bommer UA, Proud CG. Re-evaluating the roles of proposed modulators of mammalian target of rapamycin complex 1 (mTORC1) signaling. J Biol Chem. 2008;283:30482–30492. doi: 10.1074/jbc.M803348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corradetti MN, Inoki K, Bardeesy N, DePinho RA, Guan KL. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 2004;18:1533–1538. doi: 10.1101/gad.1199104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau A, Zhang J, Chiu J. Acquired tolerance in cadmium-adapted lung epithelial cells: roles of the c-Jun N-terminal kinase signaling pathway and basal level of metallothionein. Toxicol Appl Pharmacol. 2006;215:1–8. doi: 10.1016/j.taap.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Lopez E, Figueroa S, Oset-Gasque M, MPG Apoptosis and necrosis: two distinct events induced by cadmium in cortical neurons in culture. Br J Pharmacol. 2003;138:901–911. doi: 10.1038/sj.bjp.0705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L, Liu L, Luo Y, Huang S. MAPK and mTOR pathways are involved in cadmium-induced neuronal apoptosis. J Neurochem. 2008;105:251–261. doi: 10.1111/j.1471-4159.2007.05133.x. [DOI] [PubMed] [Google Scholar]
- 35.Findley CM, Cudmore MJ, Ahmed A, Kontos CD. VEGF induces Tie2 shedding via a phosphoinositide 3-kinase/Akt dependent pathway to modulate Tie2 signaling. Arterioscler Thromb Vasc Biol. 2007;27:2619–2626. doi: 10.1161/ATVBAHA.107.150482. [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Chen L, Chung J, Huang S. Rapamycin inhibits F-actin reorganization and phosphorylation of focal adhesion proteins. Oncogene. 2008;27:4998–5010. doi: 10.1038/onc.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Y, Hoell P, Ahlemeyer B, Krieglstein J. PTEN: a crucial mediator of mitochondria-dependent apoptosis. Apoptosis. 2006;11:197–207. doi: 10.1007/s10495-006-3714-5. [DOI] [PubMed] [Google Scholar]
- 39.Zhu Y, Hoell P, Ahlemeyer B, Sure U, Bertalanffy H, Krieglstein J. Implication of PTEN in production of reactive oxygen species and neuronal death in in vitro models of stroke and Parkinson’s disease. Neurochem Int. 2007;50:507–516. doi: 10.1016/j.neuint.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Sarbassov DD, Sabatini DM. Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex. J Biol Chem. 2005;280:39505–39509. doi: 10.1074/jbc.M506096200. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Kimball SR, Jefferson LS, Shenberger JS. Hydrogen peroxide impairs insulin-stimulated assembly of mTORC1. Free Radic Biol Med. 2009;46:1500–1509. doi: 10.1016/j.freeradbiomed.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen L, Xu B, Liu L, Luo Y, Yin J, Zhou H, Chen W, Shen T, Han X, Huang S. Hydrogen peroxide inhibits mTOR signaling by activation of AMPKalpha leading to apoptosis of neuronal cells. Lab Invest. 2010;90:762–773. doi: 10.1038/labinvest.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frey RS, Ushio-Fukai M, Malik A. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal. 2009;11:791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujimoto A, Akifusa S, Kamio N, Hirofuji T, Nonaka K, Yamashita Y. Involvement of mTOR in globular adiponectin-induced generation of reactive oxygen species. Free Radic Res. 2010;44:128–134. doi: 10.3109/10715760903348328. [DOI] [PubMed] [Google Scholar]
- 45.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 46.Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwak YD, Wang B, Pan W, Xu H, Jiang X, Liao FF. Functional interaction of phosphatase and tensin homologue (PTEN) with the E3 ligase NEDD4-1 during neuronal response to zinc. J Biol Chem. 2010;285:9847–9857. doi: 10.1074/jbc.M109.091637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fogarty S, Hardie DG. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim Biophys Acta. 2010;1804:581–591. doi: 10.1016/j.bbapap.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17:45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- 50.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]