Abstract
Isolated Malpighian tubules of the yellow fever mosquito secrete NaCl and KCl from the peritubular bath to the tubule lumen via active transport of Na+ and K+ by principal cells. Lumen-positive transepithelial voltages are the result. The counter-ion Cl− follows passively by electrodiffusion through the paracellular pathway. Water follows by osmosis, but specific routes for water across the epithelium are unknown. Remarkably, the transepithelial secretion of NaCl, KCl and water is driven by a H+ V-ATPase located in the apical brush border membrane of principal cells and not the canonical Na/K ATPase. A hypothetical cation/H+ exchanger moves Na+ and K+ from the cytoplasm to the tubule lumen. Also remarkable is the dynamic regulation of the paracellular permeability with switch-like speed which mediates in part the post-blood-meal diuresis in mosquitoes. For example, the blood meal the female mosquito takes to nourish her eggs triggers the release of kinin diuretic peptides that 1) increases the Cl− conductance of the paracellular pathway, and 2) assembles V1 and V0 complexes to activate the H+ V-ATPase and cation/H+ exchange close by. Thus, transcellular and paracellular pathways are both stimulated to quickly rid the mosquito of the unwanted salts and water of the blood meal. Stellate cells of the tubule appear to serve a metabolic support role, exporting the HCO3− generated during stimulated transport activity. Septate junctions define the properties of the paracellular pathway in Malpighian tubules, but the proteins responsible for the permselectivity and barrier functions of the septate junction are unknown.
Keywords: proton pump, H+ V-ATPase, cation/H+ exchange, Cl/HCO3 exchange, septate junction, diuretic peptides
INTRODUCTION
The goal of this review is to give homage to the great experimental physiologist August Krogh. We will do this by summarizing our current state of knowledge on the molecular physiology of transepithelial ion secretion by renal (Malpighian) tubules of adult female mosquitoes. We focus on adult females, because they engorge on vertebrate blood and exhibit a wide range of Malpighian tubule function that is controlled with switch-like speed. The yellow fever mosquito Aedes aegypti will receive most of our attention given our laboratory’s interest in this species for more than 30 years. Many of the transport mechanisms that we have elucidated in Aedes Malpighian tubules are likely to apply to other mosquito genera (e.g., Anopheles and Culex) and possibly to other insects. Where no comparable data exist for mosquitoes, we will touch on the important insights provided by functional genetic studies of Drosophila melanogaster Malpighian tubules. However, over 250 million years of evolution have separated mosquitoes from fruit flies (Sieglaff et al., 2009), and fruit flies may not require the ‘extreme’ renal physiology displayed by female mosquitoes. Thus, data from Drosophila and Aedes Malpighian tubules may not always be exchangeable.
Malpighian tubules and Krogh
Although Krogh does not appear to have studied Malpighian tubules, the themes and concepts of Krogh’s earliest research on the buoyancy of aquatic insect larvae and his later research on the active uptake of ions across the gills and integument of aquatic organisms are highly relevant to our current understanding of salt and water transport by Malpighian tubules (Krogh, 1911, Krogh, 1939). For example, Krogh hypothesized that the ‘pumping’ of fluid - and not air - via ‘secretory’ or ‘osmotic’ mechanisms into or out of the air bladders of the midge Corethra serves the larva to maintain its vertical position in the water column (Krogh, 1911). Though the movement of fluid across the air bladder epithelium has not yet been studied in insects, it is probably mediated by similar mechanisms of fluid transport that have been elucidated in Malpighian tubules.
From Krogh’s studies of salt and water transport in a wide variety of aquatic invertebrates and vertebrates he established the concept of independent transport mechanisms for cations and anions (Krogh, 1939, Krogh, 1946). His statement “There must be a transport of both Cl− and Na+, and only one of them need be active” now has the quality of a paradigm because the separation of active and passive transport pathways has been confirmed at every level of investigating the transport of electrolytes across membranes and epithelial tissues (Krogh, 1946).
Krogh’s view of osmoregulation in aquatic animals mediated by the uptake of Na+ from fresh water in exchange for NH4+ and the uptake of Cl− in exchange for HCO3− must now be considered visionary because today cation/H+ antiporters and Cl−/HCO3− exchangers are extensively studied not only in all kinds of absorptive and secretory epithelia but also in cell volume regulation (Krogh, 1939). As will be described in the following pages, separate but integrated mechanisms of cation and anion transport play key roles in the secretion of electrolytes and water by Malpighian tubules. Thus, our present understanding of Malpighian tubule physiology can be traced back to the founding principles of membrane and epithelial transport that the expansive mind of Krogh had conceived.
Lastly, our own studies of mosquito Malpighian tubules have confirmed the ‘Krogh Principle’ which advises the careful selection of the best subject organism on which to undertake mechanistic physiological research (Bennett, 2003, Krebs, 1975). But this sound advice was not the reason why the graduate student James Williams first brought the yellow fever mosquito into our laboratory. He wanted to find out how mosquitoes produce large volumes of urine without glomerular filtration (Williams and Beyenbach, 1983, Williams and Beyenbach, 1984, Williams et al., 1983). Following up on his pioneering work, we have discovered that the yellow fever mosquito is a Krogh model organism on two accounts. Its Malpighian tubules 1) energize transepithelial ion transport by the H+ V-ATPase and not by the Na/K ATPase, and 2) use the paracellular pathway to mount a strong diuresis.
Beyond Krogh
The aim of understanding the functions of mosquito Malpighian tubules at the molecular level takes on added significance when one considers the recent emergence or reemergence of mosquito-borne tropical diseases. Viral diseases such as chikungunya, dengue, West Nile, and yellow fevers as well as protozoan diseases such as malaria are transmitted among humans and mammals when infected female mosquitoes feed on their blood. Accordingly, the elucidation of the molecular mechanisms of transepithelial transport across Malpighian tubules and their regulation by extra- and intracellular signaling molecules may provide new and specific strategies for controlling hematophagous insects. Krogh would be delighted by such prospects, knowing all the good that has come from his interest in the production of insulin and its wide distribution for no personal profit (Rehberg, 1951, Schmidt-Nielsen, 1995).
Malpighian tubules and osmoregulation in adult mosquitoes
In general, Malpighian tubules are considered the ‘kidneys’ of insects (Ramsay, 1951, Maddrell, 1971). As with most renal epithelia, Malpighian tubules are a multifunctional tissue involved in osmoregulation, the renal excretion of nitrogenous wastes, the elimination of xenobiotics, and immunity (Beyenbach et al., 2010, Dow and Davies, 2006, O'Donnel, 2009, Melton et al., 1999, Morillo et al., 1999). As shown in Fig. 1a, mosquitoes possess five Malpighian tubules, which at least in Aedes aegypti are identical to one another in form and function (Beyenbach et al., 1993). However, morphological differences between male and female Malpighian tubules are profound which most likely reflect secretory activities and capacities in the female far greater than those in the male (Fig. 1b). Each tubule begins with a blind end at its distal extreme (relative to the gut) and connects with the digestive tract at the junction of the midgut and hindgut (Fig. 1a). The distal portions of the tubule secrete fluid into the lumen to form a urinary compartment into which can be dumped nitrogenous wastes, toxins, and other solutes. In proximal portions of the tubule and in the hindgut and rectum, life-essential solutes and water may be reabsorbed before the final urine is ejected from the rectum. In the desiccating terrestrial environment, off-host mosquitoes are primarily concerned with the threat of evaporative water loss and their Malpighian tubules secrete fluid at low rates (Benoit and Denlinger, 2010, Beyenbach, 2003b, Williams et al., 1983). In fact, we have never observed mosquitoes void urine in our environmental control chamber where the colony is maintained on a liquid diet of 3% sucrose.
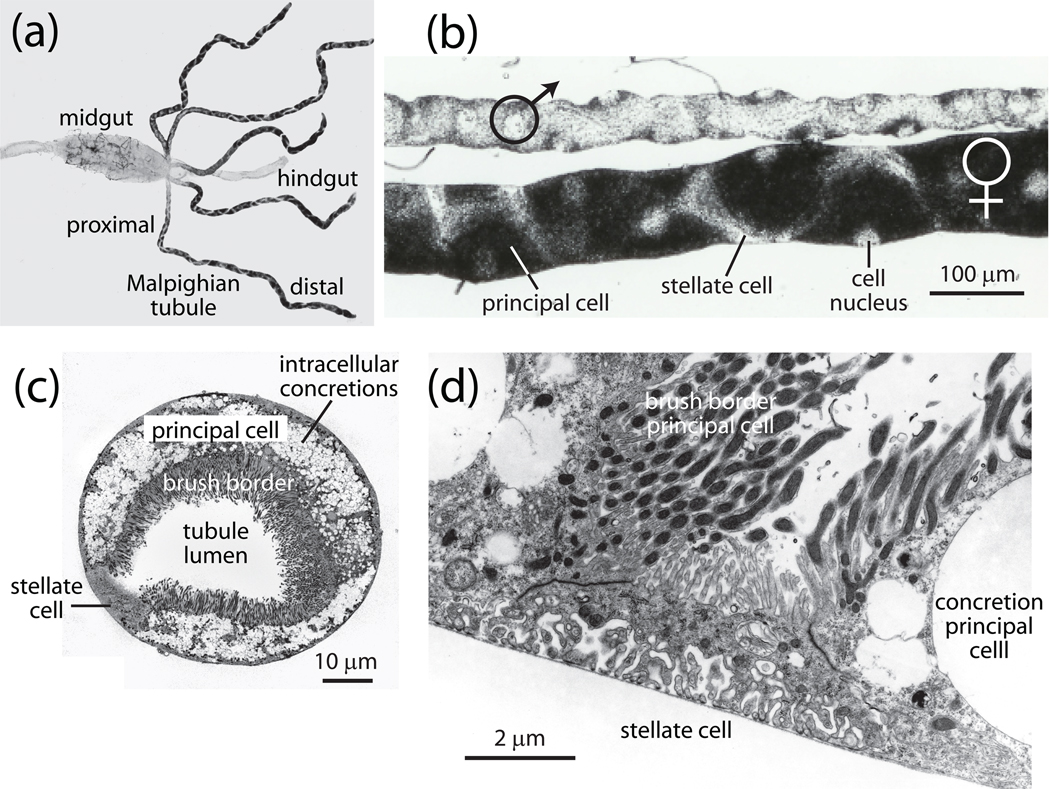
Fig. 1.
Malpighian tubules of the yellow fever mosquito Aedes aegypti. A, five Malpighian tubules empty their secretions into the gut at the junction of the midgut and hindgut of an adult female mosquito (modified from (Yu and Beyenbach, 2004). B, sexual dimorphism of adult Malpighian tubules in the yellow fever mosquito. Principal cells are large and opaque on account of the density of intracellular concretions, and stellate cells are small and transparent (modified from (Plawner et al., 1991). C, transverse section through a Malpighian tubule of a female yellow fever mosquito. Note the tall, mitochondrion-rich brush border and the intracellular concretions in principal cells, and the small size of the stellate cell (modified from(Beyenbach and Piermarini, 2009). D, stellate cell with extensive infoldings of the basal membrane, a sparse cytoplasm and a short brush border. Note the brush border of neighboring principal cells where each microvillus is home to a mitochondrion (modified from (Beyenbach and Piermarini, 2009).
As adults, nectar-sipping male mosquitoes are likely to never encounter a situation where the rapid enhancement of Malpighian tubule function is required. Accordingly, male mosquitoes do not have the renal mass of female mosquitoes (Fig. 1b). However, the blood-feeding females present a different story. In a remarkable example of ‘extreme’ biology, the female mosquito consumes a volume of blood nearly twice her own body weight as part of the reproductive cycle (Williams et al., 1983). Although the nutrient- and protein-rich blood meal is essential for her to initiate vitellogenesis and the maturation of her eggs, the blood meal introduces acute physiological and ecological risks (Klowden, 1995). In particular, the engorged mosquito faces the immediate threat of an extracellular-fluid volume expansion. It is a hypo-osmotic expansion of her hemolymph because mammalian plasma is hypo-osmotic to mosquito hemolymph by about 60 mOsm (Williams et al., 1983). Since mammalian plasma consists largely of NaCl and water, the hypo-osmotic volume expansion still loads the hemolymph with Na+ and Cl−. Moreover, the weight of the blood meal substantially increases the ‘flight payload’ of the mosquito, which reduces lift and maneuverability and makes her more vulnerable to predation (Beyenbach, 2003b, Roitberg et al., 2003).
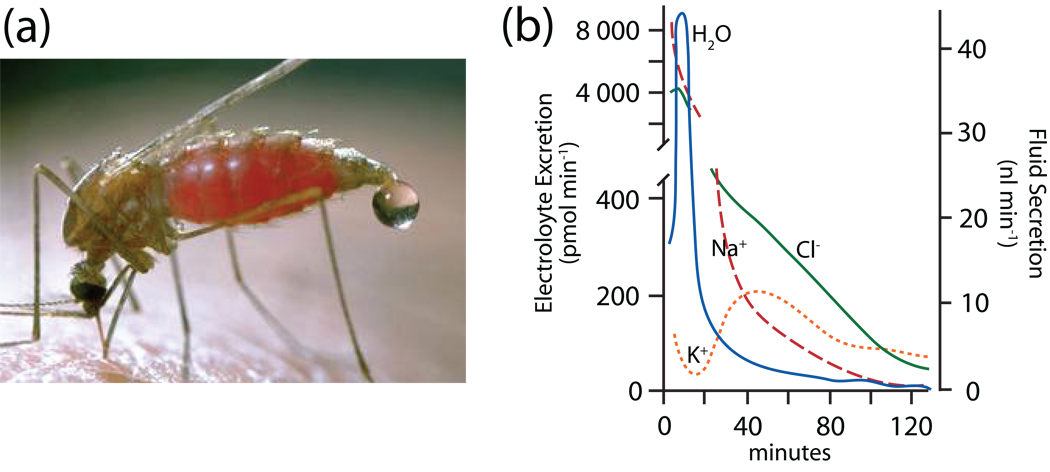
To meet the triple challenge of the blood meal (weight, water and NaCl), the mosquito mounts a strong diuresis of NaCl and water even before she has finished her meal (Fig. 2). We have measured the composition of the urine droplets (10–12 nl) that are ejected from her rectum at high rates after engorging (Williams et al., 1983). Urine produced during the first 20 min of the diuresis contains primarily Na+, Cl− and water (and probably also HCO3−) that rid the mosquito of unwanted parts of the blood meal (Fig. 2b). Within 1 hour of the blood meal the mosquito commences to excrete excess K+ that is absorbed from the digestion of the ingested blood cells (Fig. 2b).
Fig. 2.
The blood meal diuresis in two mosquitoes. A, the diuresis in the mosquito Anopheles freeborni (courtesy of Jack Kelly Clark, University of California). Note the onset of the diuresis before the blood meal has been finished. B, in vivo time course of the blood meal diuresis in the yellow fever mosquito Aedes aegypti. The droplets ejected from the rectum were analyzed for the composition of Na+, K+ and Cl−. The blood meal took place in the first two minutes. Data redrawn from (Williams et al., 1983).
The diuresis triggered by the blood meal must rev up transport and metabolism alike. The metabolic support of transport has never received much attention in any epithelium, and studies of Malpighian tubules are no exception. Suffice to say here, that the metabolic demands for mounting a diuresis that processes a volume equal to the entire extracellular fluid volume of the mosquito in under 7 min must be high (Beyenbach, 2003a). For this reason, Malpighian tubules are richly endowed with mitochondria strategically located in microvilli of principal cells in close proximity to sites of ATP-consuming active transport steps of transepithelial electrolyte secretion (Fig. 1c,d). As to revving up transport, we will focus on the mechanism by which one family of diuretic hormones, the kinins, stimulate both transcellular and paracellular transport pathways to mount the potent blood meal diuresis. In particular, the kinins increase transcellular cation transport by increasing 1) the activity of the H+ V-ATPase in the apical brush border of principal cells of the tubule, and 2) paracellular anion transport. Together, the stimulation and integration of transcellular active and paracellular passive transport mechanisms are the first responses of Malpighian tubules to deal with the onslaughts of salt and water of the blood meal. Before considering active and passive transepithelial transport pathways in detail, it is instructive to review the anatomy of the mosquito Malpighian tubule.
THE ANATOMY OF MALPIGHIAN TUBULES IN MOSQUITOES
In mosquitoes, the five Malpighian tubules are each composed of two distinct morphological segments: the distal and the proximal segments of the tubule (Fig. 1a). The distal blind-ended segment represents ~66–80% of the total tubule length (Patrick et al., 2006, Satmary and Bradley, 1984). It is composed primarily of principal cells intercalated sporadically with stellate cells. The remaining ~20–33% of the total tubule length represents the proximal segment, which opens into the digestive tract at the midgut/hindgut junction (Patrick et al., 2006, Satmary and Bradley, 1984). It is composed exclusively of principal cells without interruption by stellate cells.
Principal Cells
Principal cells represent at least 80% of the cell population of mosquito Malpighian tubules and likely exceed more than 90% of the total tubule mass given their extensive size compared to stellate cells (Beyenbach et al., 2009, Beyenbach et al., 2010, Satmary and Bradley, 1984, Isnard-Bagnis et al., 2003, Bradley et al., 1982). In adult female Aedes aegypti, the principal cells are fusiform in shape and curl upon themselves to form a tubule lumen (Fig. 1b,c). Principal cells are between 80–120 µm long and ~30 µm thick at the center (Yu and Beyenbach, 2004). Their large size suits them for two-electrode voltage clamp studies in the intact epithelium which has allowed us to 1) quantify the relative ionic conductances of the basolateral membrane of principal cells, 2) examine the role of ATP in maintaining these conductances, and 3) discover intercellular coupling by gap junctions (Masia et al., 2000, Beyenbach and Masia, 2002, Wu and Beyenbach, 2003, Weng et al., 2008).
Besides their large size, principal cells exhibit distinct ultrastructural features. First, their luminal side consists of a dense array of elongated microvilli that form a brush border (Fig. 1c,d). Within each microvillus is a mitochondrion (Fig. 1d), in convenient proximity to the consumer of ATP, the H+ V-ATPase (see also Fig. 3). Second, the cytoplasm of principal cells is filled with membrane-bound mineralized concretions (Fig. 1c,d) that serve as storage sites for ions, metals and uric acid (Beyenbach and Piermarini, 2009, Wessing and Zierold, 1999, Wessing et al., 1992, Wessing et al., 1988). The histochemical and immunochemical reactivity for carbonic anhydrase in mosquito Malpighian tubules appears to be closely associated with these concretions (Palatroni et al., 1981, Smith et al., 2007).
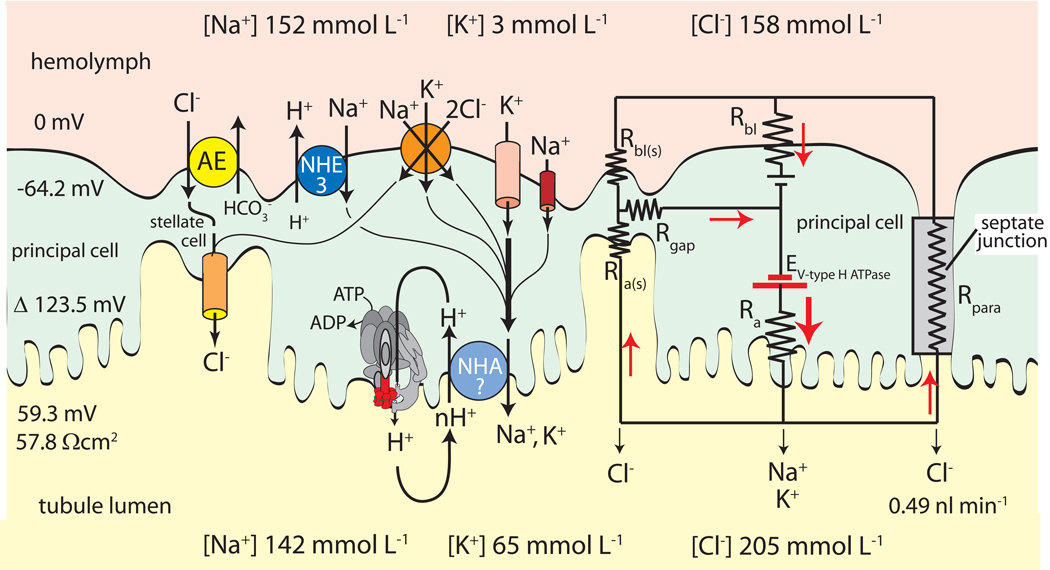
Fig. 3.
Minimal model of transepithelial NaCl and KCl secretion by Malpighian tubules of Aedes aegypti. Principal cells mediate active transcellular secretion of Na+ and K+ via the cooperation of the H+ V-ATPase and the cation/H+ exchanger (NHA?) located in apical brush border membrane. Stellate cells may mediate the passive transcellular secretion of Cl− via the anion exchanger (AE) located in basal membranes and Cl− channels located in apical membranes. Principal and stellate cells shown to be connected by gap junctions. An equivalent electrical circuit of the active transport pathway through a principal cell in parallel to the passive transport pathways through stellate cells and the paracellular pathway illustrates how the H+ V-ATPase drives 1) the transepithelial secretion of Cl− through the apical membrane of stellate cells and/or the paracellular pathway, and 2) the entry of cations from the hemolymph to the cytoplasm of principal cells. Red arrows indicate the movement of positive charge. Positive charge moving in one direction is equivalent to negatively charged ions moving in the opposite direction (modified from (Wieczorek et al., 2009).
We are unaware of ultrastructural differences between principal cells of the distal and proximal segments, but principal cells of the proximal segments are known to express histochemical activity for alkaline phosphatase and immunochemical activity for the α-subunit of the Na,K-ATPase, whereas those of the distal segments do not (Cabrero et al., 2004, Patrick et al., 2006). These findings are consistent with distinct genetic and/or functional ‘domains’ along the length of mosquito Malpighian tubules as in Drosophila Malpighian tubules (Sozen et al., 1997).
Stellate cells
Stellate cells are found sporadically along the distal segment where they intercalate between principal cells (Fig. 1). Stellate cells have a small, thin cell body from which 3 or 4 arm-like projections branch, giving the cell a star-like or ‘stellate’ appearance (Figs. 1B, 5D). Although the length of a stellate cell can extend over 100 µm (from arm to arm), the cells are very thin (Fig. 1c,d) compared to principal cells and are usually less than 5 µm thick (Yu and Beyenbach, 2004). These small dimensions preclude electrophysiological studies with intracellular microelectrodes (Weng et al., 2008).
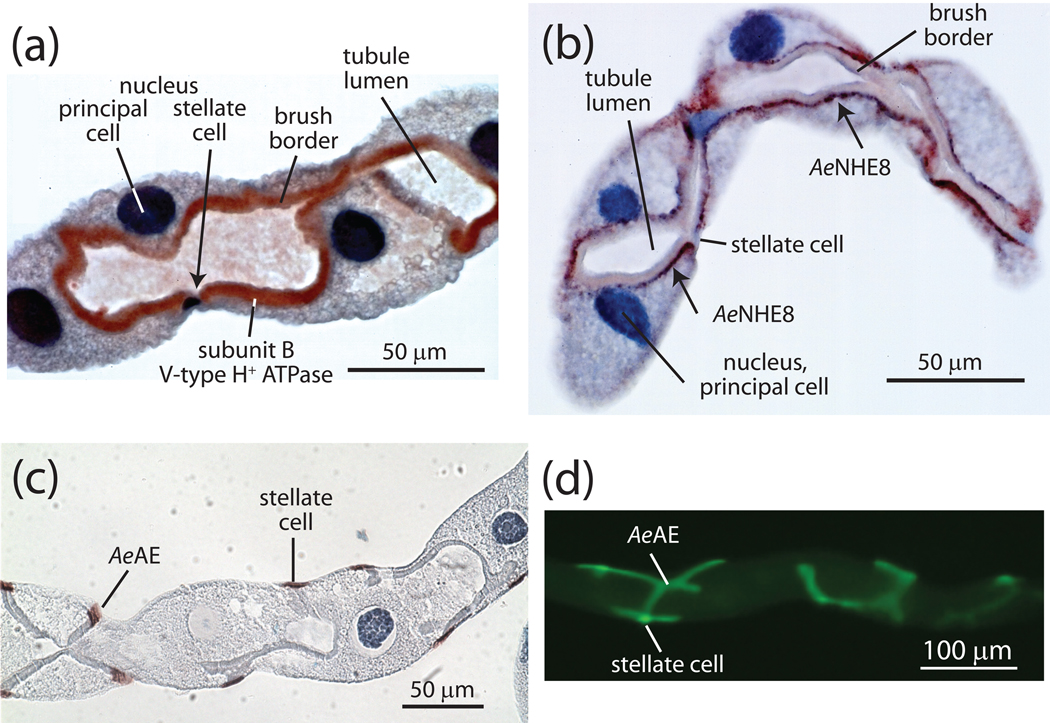
Fig. 5.
Immunolocalization of transporters in distal segments of Malpighian tubules of Aedes aegypti. A, subunit B of the catalytic complex of the H+ V-ATPase is richly expressed in the brush border membrane of principal cells but not stellate cells (modified from (Beyenbach et al., 2009). B, the Aedes Na+/H+ exchanger NHE8 localizes to subapical membrane vesicles in principal cells but not stellate cells (modified from (Beyenbach et al., 2009). C,D, the Aedes Cl−/HCO3− exchanger is present in stellate cells but not principal cells (unpublished observations).
In addition to their smaller size, stellate cells contrast from principal cells in their ultrastructure. As shown in Fig. 1c,d the apical microvilli of stellate cells are not nearly as long or as densely packed as those of principal cells, and they do not contain mitochondria (Beyenbach, 2003b, Bradley et al., 1982, Satmary and Bradley, 1984, Yu and Beyenbach, 2004). Furthermore, cytoplasmic concretions are absent in stellate cells (Fig. 1). Lastly, the basal membranes of stellate cells are characterized by extensive infoldings (Fig. 1d) that appear to form a labyrinth (Beyenbach, 2003b, Bradley et al., 1982, Satmary and Bradley, 1984, Yu and Beyenbach, 2004).
ACTIVE TRANSCELLULAR CATION SECRETION BY PRINCIPAL CELLS
Blind-ended distal segments of mosquito Malpighian tubules generate an isosmotic tubular fluid by the active electrogenic secretion of cations (primarily K+ and Na+) from the hemolymph to the tubule lumen. The secretion of cations creates a lumen-positive transepithelial voltage which serves as the driving force for the passive transepithelial secretion of Cl−. Luminal NaCl and KCl concentrations then provide the osmotic pressure for water to follow the movement of salt.
Principal cells are the exclusive sites of active transepithelial K+ and Na+ secretion in mosquito Malpighian tubules (Beyenbach and Wieczorek, 2006, Williams and Beyenbach, 1983, Williams and Beyenbach, 1984). For such secretion to occur, principal cells require the following: 1) a pump and an associated energy source to move cations from the hemolymph to the tubule lumen against their electrochemical potentials, 2) entry mechanisms for the uptake of K+ and Na+ from the hemolymph into the cytoplasm, and 3) exit mechanisms for the export of K+ and Na+ from the cytoplasm to the tubule lumen (Fig. 3).
The pump and energy source
In distal segments of mosquito Malpighian tubules, epithelial transport ‘begins and ends’ with the V H+ V-ATPase. As shown in Fig. 3 and also 5A, the H+ V-ATPase localizes to the apical brush border membrane of principal cells in Aedes Malpighian tubules in close proximity to the mitochondria that deliver a steady supply of ATP (Beyenbach, 2001, Patrick et al., 2006, Weng et al., 2003). Although principal cells of the proximal segment and stellate cells of the distal segment express immunoreactivity for the α-subunit of the Na,K-ATPase, the ouabain- and vanadate-sensitive ATPase activity represents a nominal fraction of the total ATPase activity (Patrick et al., 2006, Weng et al., 2003). In contrast, the major ATPase activity is bafilomycin and NO3− sensitive, indicating the dominance of the H+ V-ATPase in distal segments of Aedes Malpighian tubules (Weng et al., 2003).
The H+ V-ATPase is an electrogenic proton pump because it translocates cytosolic H+ to the tubule lumen without the cotransport or exchange transport of another charged solute (Fig. 3). Estimates of the electromotive force of this proton pump yield at least 146.1 mV to account for the substantial membrane potential across the apical membrane (123.5 mV, Fig. 3) (Beyenbach, 2001). From an electrical view, the electrogenic transport of H+ ions from the cytoplasm to the tubule lumen constitutes a current that must return to the cytoplasmic face of the proton pump, otherwise the transport activity of the pump will stop as the apical membrane voltage reaches the electromotive force of the proton pump. As shown in Fig. 3, pump current may be carried by 1) Cl− across the apical membrane of stellate cells in series with gap junctions to principal cells, and/or Cl− through paracellular septate junctions in series with K+ and Na+ channels in the basolateral membrane of principal cells. The membrane voltage across the basolateral membrane (−64.2 mV) of principal cells is thus largely the product of current and resistance (Beyenbach et al., 2000). It follows that both apical and basolateral membrane voltages go to zero when the H+ V-ATPase at the apical membrane is pharmacologically inhibited by bafilomycin or by the removal of ATP (Beyenbach et al., 2000, Weng et al., 2003, Pannabecker et al., 1992, Wu and Beyenbach, 2003). Both maneuvers halt the transepithelial secretion of all solute and water.
One would expect the acidification of the luminal fluid in view of the strong expression of the H+ V-ATPase in the apical membrane of principal cells. However, the pH of secreted fluid is 7.2 in Aedes Malpighian tubules (Petzel et al., 1999). In fact, the proton concentration in the tubule lumen is less than that in the cytoplasm of principal cells. It raises the question how the H+ V-ATPase energizes the extrusion of Na+ and K+ across the apical membrane. One hypothesis conceives a low pH microenvironment in the extracellular matrix of the tall brush border which could provide the inward proton driving force to power electroneutral H+/K+ and H+/Na+ exchange across the apical membrane (Beyenbach et al., 2009). Another hypothesis postulates that instead of an inward chemical proton gradient, the high membrane voltage across the apical membrane is the principal driving force for H+/K+ and H+/Na+ exchange. As illustrated in Fig. 3, the hypothesis requires that the carrier(s) operate with a stoichiometry of nH+ for each K+ or Na+ moved from cytoplasm to tubule lumen (Wieczorek et al., 2009, Beyenbach and Wieczorek, 2006).
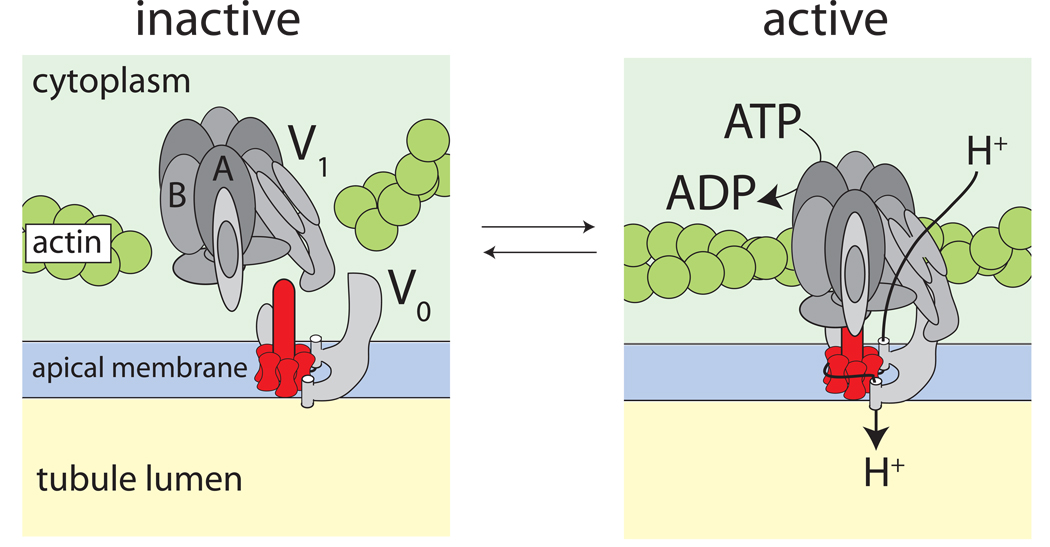
Structurally, the H+ V-ATPase consists of two large multi-subunit complexes that associate to form a functional ‘holoenzyme’ (Fig. 4). The holoenzyme is thought to function like a rotary motor (Beyenbach and Piermarini, 2009, Beyenbach and Wieczorek, 2006, Inoue et al., 2005, Murata et al., 2005, Wieczorek et al., 2009). The cytosolic V1 complex is the catalytic site of ATP hydrolysis and it is the stator that anchors the holoenzyme to the cytoskeletal infrastructure (e.g., actin) of principal cells (Fig. 4). The membrane-bound V0 complex is a rotor that translocates H+ across the apical membrane (Fig. 4). The association/dissociation of the V1 and V0 complexes is known to be a rapid and reversible mechanism for regulating the activity of the H+ V-ATPase in a variety of cells (Beyenbach and Piermarini, 2009, Beyenbach and Wieczorek, 2006, Dames et al., 2006, Kane, 1995, Kane and Parra, 2000, Sautin et al., 2005, Wieczorek et al., 1991).
Fig. 4.
Model of the reversible assembly of the H+ V-ATPase. Association of the cytoplasmic, catalytic V1 complex and the membrane-embedded V0 complex activates proton pumping as it connects the source of energy (ATP hydrolysis) with the site of proton translocation. The extrusion of protons from the cytoplasm to the extracellular side has been modeled after the Na+-transporting H+ V-ATPase of the bacterium Enterococcus hirae where a rotor transfers Na+ ions from an inner half-channel to an outer half-channel (Murata et al., 2005).
We have observed proteomic evidence consistent with the reversible assembly of the H+ V-ATPase in Aedes Malpighian tubules treated with the diuretic peptide aedeskinin for only 1 min (Beyenbach et al., 2009). The abundances of subunits A and B of the catalytic V1 complex significantly decrease in the cytosol as this complex joins the V0 complex in support of diuresis. In other words, the coupling of the V1 complex to the V0 complex adds subunits A and B to the membrane fraction of the cell thereby removing them from the cytosolic fraction of the cell (Beyenbach et al., 2009). The joining of V1 and V0 complexes activates the H+ V-ATPase by coupling the hydrolysis of ATP to the extrusion of H+ across the apical membrane (Kane, 1995, Sumner et al., 1995, Zimmermann et al., 2003). The laboratory of Dow has observed subtle shifts in the localization of the SFD subunit of the V1 complex from the cytosol towards the brush border of principal cells in Malpighian tubules of Drosophila after treatment with the diuretic peptide Capa-1. However, this shift is only a minor part of the full response of the tubule to Capa-1 (Terhzaz et al., 2006).
The effect of blood feeding (or diuretic peptides and hormones) on the immunolocalization of the V1 complex has not yet been examined in mosquito Malpighian tubules. However, the laboratory of Petzel has observed tantalizing effects on the localization of β-actin in principal cells of Aedes Malpighian tubules. That is, the immunoreactivity of β-actin in principal cells redistributes from the cytoplasm to the brush border within 5 min after blood feeding or within 10 min after treating isolated tubules with dibutyryl (db)-cAMP (Karas et al., 2005). The blood meal and the stimulation of isolated Malpighian tubules with cAMP are known to rapidly increase fluid secretion by Malpighian tubules, and in blowfly salivary glands, cAMP is known to trigger the assembly of V1 and V0 complexes via the activation of protein kinase A in support of heightened salivary secretion (Wheelock et al., 1988, Williams and Beyenbach, 1983, Williams et al., 1983, Voss et al., 2008, Voss et al., 2007). Moreover, incubating isolated tubules with known disrupters of actin polymerization (e.g., cytochalasin) not only prevents the redistribution of actin, but also blocks the diuresis stimulated by cAMP (Karas et al., 2005). Given the well-known association of the V1-sector with elements of the actin cytoskeleton, it is enticing to presume that the redistribution of actin in Aedes Malpighian tubules reflects the association of cytosolic V1 sectors with their membrane-bound V0 sectors in the brush border (Beyenbach and Wieczorek, 2006, Vitavska et al., 2005, Wieczorek et al., 2009) (see Fig. 4).
The formation of the H+ V-ATPase holoenzyme is without much use in the absence of an adequate source of ATP. As such, when the intracellular concentrations of ATP in principal cells of Aedes Malpighian tubules are diminished—by inhibiting the mitochondrial synthesis of ATP with cyanide and/or dinitrophenol—the electrophysiological correlates of H+ V-ATPase activity are substantially reduced (Masia et al., 2000, Pannabecker et al., 1992, Wu and Beyenbach, 2003). However, the pump resumes its activity when intracellular ATP returns to normal levels after washout of the inhibitors (Wu and Beyenbach, 2003). Thus, one would expect principal cells to possess mechanisms for maintaining high levels of ATP during periods of enhanced H+ V-ATPase activity to prevent the pump from depleting its fuel source.
Mechanisms to enhance mitochondrial ATP production during periods of diuresis have not been studied in mosquito Malpighian tubules. However, in isolated Malpighian tubules of Drosophila, the laboratory of Dow has observed that within 10 minutes of exposure to the diuretic peptide Capa-1, the mitochondria in the brush border of principal cells elevate production of ATP (Terhzaz et al., 2006). This apical source of ATP is probably earmarked for consumption by the H+ V-ATPase close by (Figs. 1,3).
Entry across the basolateral membrane
K+-channels. Our laboratory has shown that barium-sensitive K+-channels provide the primary conductive mechanism for the entry of K+ into principal cells from the peritubular medium of Aedes Malpighian tubules (Beyenbach and Masia, 2002, Masia et al., 2000, Scott et al., 2004). The channels afford a route for positive charge (current) to flow from the extracellular fluid into the cytoplasm of principal cells (Fig. 3). In unstimulated Aedes Malpighian tubules, peritubular barium blocks 1) nearly 65% of the total basolateral membrane conductance, 2) ~60% of the spontaneous fluid secretion rates, and 3) ~80% of the transepithelial secretion of K+ (Beyenbach and Masia, 2002, Scott et al., 2004). In addition, it blocks ~50% of transepithelial Na+ secretion, suggesting the presence of barium-sensitive Na+ channels (Scott et al., 2004). Alternatively, barium-sensitive K+-channels may permit the passage of Na+, or they may be functionally coupled to Na+-transporters via a third player.
The basolateral membrane K+-channels of principal cells (Fig. 3) appear to be sensitive to intracellular concentrations of ATP, because the depletion of ATP closes these channels (Wu and Beyenbach, 2003). The shutdown of cationic entry may serve as a protective mechanism to the principal cells, allowing them to remain in steady-state when cations cannot be extruded from them in the absence of ATP (Wu and Beyenbach, 2003).
The molecular identity of the barium-sensitive channels that mediate the above transport in mosquito principal cells is unknown, but their physiological activity is consistent with that of inward rectifying K+-channels (Hibino et al., 2010). Molecular transcriptomic and RT-PCR studies in Drosophila Malpighian tubules have revealed the enriched expression of 3 genes encoding putative inward rectifying K+-channels: ir, irk2, and irk3 (Evans et al., 2005, Wang et al., 2004). Moreover, in situ hybridization experiments in Drosophila Malpighian tubules demonstrate the expression of all three transcripts in principal cells of the secretory segment, which makes them promising candidates for mediating the uptake of K+ across the basolateral membrane (Evans et al., 2005). Orthologs of these putative inward rectifiers are present in the genomes of the mosquitoes Aedes aegypti, Anopheles gambiae, and Culex quinquefasciatus (personal observations).
Na+-channels. As shown in Fig. 3, the Na+ conductance of the basolateral membrane of principal cells is small, only 14% of the total membrane conductance in unstimulated Aedes Malpighian tubules (Beyenbach and Masia, 2002). However, when isolated tubules are stimulated with db-cAMP, the Na+-conductance increases substantially (Beyenbach and Masia, 2002, Sawyer and Beyenbach, 1985, Williams and Beyenbach, 1983, Williams and Beyenbach, 1984). As a result, the increased entry of Na+ into the cell improves its competition for extrusion across the apical membrane, and Na+ replaces K+ as the dominant cation in the tubular fluid (Williams and Beyenbach, 1983, Williams and Beyenbach, 1984). Thus, the Na+-conductance likely plays a crucial role in the natriuresis triggered by the blood meal (Fig. 2b). Indeed, the mosquito natriuretic peptide that is released into the hemolymph during engorgement causes a rapid rise in intracellular concentrations of cAMP in Aedes Malpighian tubules (Petzel et al., 1987, Petzel et al., 1985, Petzel et al., 1986, Wheelock et al., 1988). The mosquito natriuretic peptide is now known to be the calcitonin-like peptide Anoga-DH31 (Coast et al., 2005).
The cAMP-activated Na+ conductance and fluid secretion rates are both blocked by peritubular amiloride, which suggests the involvement of degenerins/epithelial Na+-channels, e.g., ENaC (Beyenbach and Masia, 2002, Hegarty et al., 1992). However, at least in the Malpighian tubules of Drosophila, the closest orthologs of ENaC (i.e., pickpockets) are not highly expressed (Giannakou and Dow, 2001, Wang et al., 2004). Thus, either an unconventional amiloride-sensitive Na+-channel is responsible (e.g., a barium-sensitive non-selective cation channel), or the conductance is mediated by an electrogenic exchanger or carrier. In regards to the latter, one potential candidate may be a Na/H antiporter (NHA) with a stoichiometry of 2Na+:1H+ such as that found in epithelia of crustaceans (Ahearn et al., 2001).
Cation Coupled Cl− cotransporters. Pharmacological studies of the effects of bumetanide in isolated Aedes Malpighian tubules implicate a role for a cation-coupled Cl− cotransporter in the basolateral membrane of principal cells (Hegarty et al., 1991, Scott et al., 2004). We presume that this cotransporter is a Na/K/2Cl-cotransporter NKCC (Fig. 3). The addition of peritubular bumetanide alone has nominal effects on fluid secretion and electrophysiology under control conditions, but it decreases the concentration of K+ in secreted fluid by ~70% with a reciprocal increase in the concentration of Na+ (Hegarty et al., 1991). However, when peritubular bumetanide is added to isolated tubules together with barium, the secretory activities of the tubule shut down altogether, including Na+ secretion (Scott et al., 2004). Thus, the mechanisms of K+ and Na+ entry across the basolateral membrane are coupled in ways we have yet to elucidate.
The effects of bumetanide are more profound in tubules stimulated by cAMP, one of the second messengers of the mosquito natriuretic peptide. Here bumetanide prevents and reverses the cAMP-mediated increase of Na+-secretion and decreases K+-secretion even further (Hegarty et al., 1991). The heightened sensitivity to bumetanide in cAMP stimulated tubules may indicate the activation of NKCC via phosphorylation by protein kinase A (Yang et al., 2001), and the effect on K+ secretion reflects again the enigmatic coupling of K+ and Na+ entry mechanisms across the basolateral membrane.
The molecular identity of the bumetanide-sensitive transporter in mosquito Malpighian tubules is unknown, but it may be a member of the SLC12 family of cation-coupled chloride cotransporters that includes NKCCs, NCCs, and KCCs (Hebert et al., 2004). The laboratory of Gill has cloned a putative SLC12-like transporter from Aedes aegypti, but the expression of neither the cDNA nor the protein were detectable in Malpighian tubules (Filippov et al., 2003). In Drosophila, Sun and colleagues have heterologously characterized a SLC12-like transporter (CG4357) that exhibits bumetanide-sensitive NKCC activity (Sun et al., 2010). However, the expression of this gene and other SLC12 orthologs in Malpighian tubules of Drosophila do not appear to be highly enriched (Chintapalli et al., 2007, Filippov et al., 2003).
Na+/H+ exchangers. In unstimulated Malpighian tubules of Aedes aegypti, a Na/H exchanger (NHE) in the basolateral membrane of principal cells is considered the primary mechanism for the uptake of Na+ from the hemolymph (Fig. 3). Peritubular amiloride inhibits the spontaneous rates of fluid secretion by 60% (Hegarty et al., 1992, Petzel, 2000). The inhibition is electrically silent, and the NHE-specific amiloride analog EIPA is the most potent at blocking fluid secretion (Hegarty et al., 1992, Petzel, 2000). The laboratory of Petzel has provided functional evidence consistent with the presence of an NHE in the basolateral membrane of principal cells via measurements of intracellular pH (pHi). For example, the removal of peritubular Na+ or the addition of peritubular EIPA induces a pronounced acidification of pHi (Petzel, 2000). Furthermore, the recovery of principal cell pHi from an NH4Cl-induced acid load is Na+-dependent and EIPA-sensitive (Petzel, 2000).
The molecular identity of the amiloride/EIPA-sensitive NHE has not been resolved, but it is presumably a member of the SLC9 family of cation/H+ antiporters (CPAs). Among insects, the SLC9 family includes three genes that encode vertebrate-like NHEs and two genes that encode bacterial-like NHAs (Brett et al., 2005, Piermarini et al., 2009). The laboratories of Petzel and Gill have both independently cloned two alternatively-spliced cDNAs from Aedes aegypti (AeNHE3) that encode proteins with differing lengths of their C-terminal domains (Hart et al., 2002, Pullikuth et al., 2006). Immunoreactivity for the ‘long’ isoform of AeNHE3 localizes primarily to the basolateral membranes of principal cells in Aedes Malpighian tubules, but immunolabeling of the ‘short’ isoform was not possible with their anti-AeNHE3 antibody (Pullikuth et al., 2006). In contrast, both the ‘long’ and ‘short’ isoforms of the Drosophila ortholog of AeNHE3 (nhe2) localize to the stellate cells of Drosophila Malpighian tubules (Day et al., 2008).
When expressed heterologously in PS120 cells, AeNHE3 can mediate either Na/H or K/H exchange that is not sensitive to amiloride or EIPA (Pullikuth et al., 2006). Thus, AeNHE3 is an unlikely candidate for the amiloride/EIPA-sensitive transporter involved with fluid secretion and regulation of pHi in principal cells. Unfortunately, without a known pharmacological blocker of AeNHE3 activity, the contributions it makes to Malpighian tubule function are difficult to evaluate.
Exit across the apical membrane
In the apical membrane of principal cells, it is widely assumed that there is at least one cation/H+ exchanger that links the H+-transport activity of the apical H+ V-ATPase to the secretion of K+ and/or Na+ (Fig. 3). The assumption is a direct extension of the model proposed by Wieczorek and colleagues for the apical secretion of K+ by the goblet cells in the midgut of the tobacco hornworm Manduca sexta (Wieczorek et al., 1991). However, direct physiological evidence for such an exchanger in principal cells of mosquito Malpighian tubules is lacking. The only supporting evidence is the potent inhibition of tubule fluid secretion by EIPA, which suggests the involvement of a SLC9-like transporter (Petzel, 2000). However, it is unknown whether EIPA inhibits a transporter at the apical membrane, basolateral membrane, or both membranes.
The molecular identity of the presumed apical cation/H+ exchanger in the insect midgut and Malpighian tubules has long eluded insect physiologists, but it is assumed to be a member of the SLC9 CPA superfamily that includes the NHEs and NHAs (Piermarini et al., 2009, Harvey, 2009). Day and colleagues have outlined criteria that the putative apical exchanger should meet, including 1) an enrichment of its transcripts in epithelia with known H+ V-ATPase -driven transport, 2) an apical membrane localization, and 3) a possible preference for mediating K+/H+ exchange over Na+/H+ exchange (Day et al., 2008). Recently, three members of the SLC9 superfamily have been evaluated as the putative apical cation/H+ exchanger in mosquitoes and Drosophila: NHE8, NHA1, and NHA2. Thus, the answer to solving this long-standing mystery of insect physiology appears to be within grasp (vide infra).
NHE8. The first CPA hypothesized to be the apical cation/H+ exchanger in mosquito Malpighian tubules was Aedes NHE8 (AeNHE8), which is an ortholog of mammalian NHE8. Our laboratory and the laboratory of Gill have independently cloned and characterized this transporter from Aedes aegypti (Piermarini et al., 2009, Kang'ethe et al., 2007). The Gill laboratory first reported that AeNHE8-immunoreacitvity localizes to the brush border of principal cells in Aedes Malpighian tubules, making AeNHE8 a viable candidate for the long sought after apical exchanger (Kang'ethe et al., 2007). However, as shown in Fig. 5b, we find that AeNHE8 is an intracellular isoform that occurs in sub-apical vesicles of principal cells, not in the brush border (Piermarini et al., 2009). Our localization is consistent with that of the NHE8-ortholog in Drosophila (nhe1), which also occurs in intracellular compartments of principal cells (Day et al., 2008). Moreover, we have demonstrated that 1) transcripts encoding AeNHE8 are not enriched in Aedes Malpighian tubules; 2) the sub-apical localization of AeNHE8 in principal cells is unaffected after mosquitoes are fed a blood meal or after isolated Malpighian tubules are treated with db-cAMP; and 3) the transport activity of AeNHE8 (when expressed heterologously in Xenopus oocytes) is EIPA-sensitive, but shows a strong preference for Na/H exchange over K/H exchange (Piermarini et al., 2009). Thus, AeNHE8 does not meet any of the criteria established by Day and colleagues for the elusive cation/H+ exchanger (Day et al., 2008).
NHA1 and NHA2. The best remaining candidates for mediating apical cation/H+ exchange in principal cells of mosquito Malpighian tubules are the orthologs of the bacterial NHAs (Harvey, 2009, Rheault et al., 2007). Although the first insect NHA was cloned from the midgut of larval Anopheles mosquitoes, the most convincing evidence for a role of NHAs in Malpighian tubule function is from Drosophila (Day et al., 2008, Rheault et al., 2007). The laboratory of Dow reports that the expression of both nha1 and nha2 transcripts are highly enriched in Drosophila Malpighian tubules and the respective encoded proteins localize exclusively to the brush border of principal cells (Day et al., 2008). The overexpression of nha1 in principal cells appears to increase the spontaneous rates of fluid secretion in Drosophila Malpighian tubules, whereas the overexpression of nha2 increases the concentrations of K+ and/or Na+ in the secreted fluid (Day et al., 2008).
In mosquitoes, the laboratory of Harvey has found prominent intracellular immunoreactivity for NHA1 in principal cells of the proximal segments of larval Anopheles Malpighian tubules (Okech et al., 2008, Rheault et al., 2007). The expression patterns of NHA1 and NHA2 in the Malpighian tubules of adult mosquitoes remain to be determined and are eagerly anticipated. Furthermore, functional characterizations of the NHAs are required to demonstrate the presumed cation/H+ exchange activity of these transporters and to evaluate their pharmacology, cation selectively and stoichiometry.
STELLATE CELLS
Transcellular Cl− transport by stellate cells has been a polarizing issue of Malpighian tubule function among insect physiologists. In brief, the laboratory of O’Donnell has evidence for stellate cells serving the transepithelial secretion of Cl− in Drosophila Malpighian tubules, and our laboratory has evidence for the transepithelial secretion of Cl− through the paracellular pathway in Aedes Malpighian tubules (Beyenbach, 2003a, O'Donnell et al., 1998, Pannabecker et al., 1993). The two views are not incompatible. Both tubules may use stellate cells to secrete Cl− (Fig. 3), but the opening of a paracellular route for Cl− secretion may be particularly well developed in blood-feeding insects because of their need to deal with the huge solute and water loads of the blood meal.
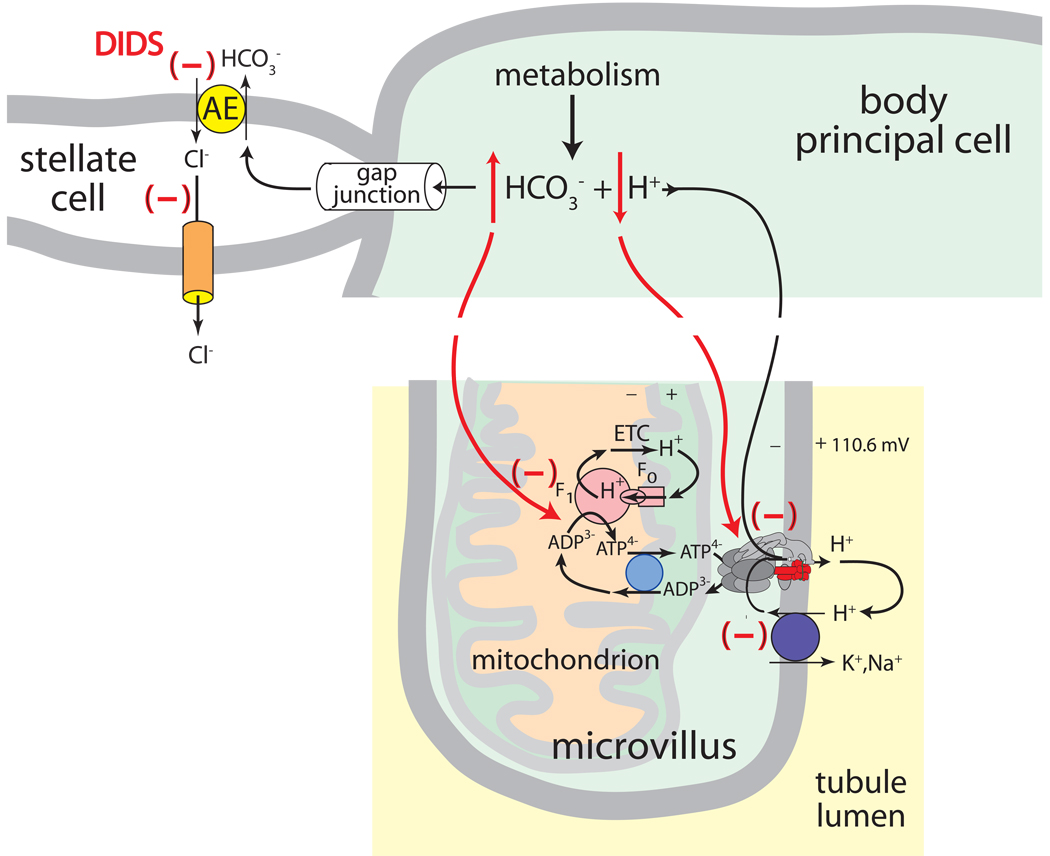
Cl−/HCO3− exchange across the basal membrane. Epithelial cells possessing apical H+ V-ATPase are often opposed by basolateral Cl−/HCO3− anion exchangers (AEs) that export HCO3− generated during oxidative metabolism. As illustrated in Figs. 3 and 6, the extrusion of HCO3− by AE serves to sustain the activity of the proton pump by 1) facilitating the ionization of H2CO3 that provides H+ ions for transport by the H+ V-ATPase, and 2) controlling the intracellular [HCO3−] which is known to inhibit the mitochondrial synthesis of ATP (Lodeyro et al., 2001). Thus, in mosquito Malpighian tubules, one may expect to find an AE in the basolateral membrane of principal cells to support the activity of the apical H+ V-ATPase. Instead, we have found an AE in the basal membrane of stellate cells (Figs. 3, 5C,D).
Fig. 6.
The metabolic support hypothesis of stellate cells in Aedes Malpighian tubules. The Cl−/HCO3− exchanger AeAE is present in stellate cells and not principal cells (see Figs. 3 and 5C,D). The disulfonic stilbene DIDS reverses the additional fluid secretion stimulated by two different diuretic hormones by 1) raising intracellular HCO3− which inhibits mitochondrial ATP synthesis, and 2) reducing intracellular [H+] thereby limiting substrate from the motor of transepithelial electrolyte and fluid secretion, the H+ V-ATPase located in the brush border apical membrane of principal cells.
Our laboratory has cloned and characterized a SLC4-like AE (AeAE) from Malpighian tubules of Aedes aegypti (Piermarini et al., 2010). AeAE, expressed heterologously in Xenopus oocytes, mediates Cl−/HCO3− exchange that is inhibited by the stilbene derivative DIDS (Piermarini et al., 2010). As shown in Fig. 5c,d AeAE immunoreactivity localizes exclusively to the basal regions of stellate cells—not principal cells—in Aedes Malpighian tubules (Piermarini et al., 2010). Whether AeAE mediates transepithelial Cl− secretion in Aedes Malpighian tubules is questionable. On the one hand, the presence of AeAE in the basal membrane and the presence of Cl− channels in the apical membrane set the stage for transcellular Cl− secretion through stellate cells. On the other hand, the experimental evidence favors far more a paracellular route for Cl− secretion (vide infra).
Role of Na/K ATPase? The role of the Na/K ATPase in Malpighian tubules of Aedes is unclear. In previous studies we have observed that ouabain, an inhibitor of the Na/K ATPase, significantly inhibits the spontaneous rates of fluid secretion in distal segments of the tubule, and Patrick and colleagues have immunochemical evidence for the presence of the α-subunit of the Na/K ATPase in stellate cells of this tubule segment (Hegarty et al., 1991, Patrick et al., 2006). However, we find no significant ATPase activity of the Na/K pump in distal segments (Weng et al., 2003). Most, if not all ATPase activity can be attributed to the H+ V-ATPase activity. One explanation for these contrary observations proposes a non-pumping role of the α-subunit of the Na,K-ATPase. Increasingly, transport-independent functions of the α and β subunits of the Na/K ATPase are being discovered (Krupinski and Beitel, 2009, Rajasekaran et al., 2008). The two subunits are part of adhesion complexes, particularly in vertebrate tight junctions as well as invertebrate septate junctions.
Apical Cl− channels. Using sharp jeweler broaches it is possible to open Malpighian tubules in order to expose the luminal surfaces of epithelial cells for patch clamp experiments. We did not succeed in obtaining membrane patches of the tall brush border of principal cells, but apical membrane patches of stellate cells were readily produced (O'Connor and Beyenbach, 2001). We have observed two types of anion conductive channels in unstimulated Malpighian tubules of Aedes aegypti: 1) ‘type I’ channels with intermediate conductance and slow kinetics and 2) ‘type II’ channels with low conductance and fast kinetics. Type I channels are sensitive to known chloride-channel blockers such as diphenylamine-2-carboxylate (DPC) and niflumic acid, but they are not blocked by SITS or DIDS. The type I channels exhibits a type I Eisenman halide selectivity sequence (i.e., I- > Br- > Cl > F > isethionate), which matches that of the tubule epithelium during unstimulated conditions, but not of that during kinin stimulation. Moreover, type I channels are not directly influenced by the Ca2+ concentration which is known to increase during kinin stimulation (O'Connor and Beyenbach, 2001). Thus, in unstimulated tubules, the type I channels may contribute to transcellular Cl− secretion cooperating with the Cl−/HCO3− exchange across the basal membrane (Fig. 3), but they are not likely to play a role during kinin stimulation, because kinins do not require stellate cells in Aedes Malpighian tubules to mediate their effects on transepithelial Cl− secretion (Yu and Beyenbach, 2004). The molecular identities of type I and type II chloride channels are unknown.
In contrast to mosquito Malpighian tubules, O’Donnell and colleagues have identified ‘maxi’ channels in apical patches of stellate cells from the Malpighian tubules of Drosophila that 1) exhibit a large conductance and fast kinetics and 2) are blocked by DPC, niflumic acid, and DIDS (O'Donnell et al., 1998). Furthermore, using a vibrating probe approach, they found current sinks in the vicinity of stellate cells that are consistent with transcellular Cl− transport in both unstimulated and kinin-stimulated Malpighian tubules (O'Donnell et al., 1998). However, vibrating electrodes do not have the geometric resolution to distinguish between transcellular and paracellular currents especially when transepithelial current sinks are identified near small cells such as stellate cells. RT-PCR and transcriptomic data indicate that Malpighian tubules of Drosophila express three genes that encode ClC-like chloride channels, of which two are enriched in the tubules (Dow and Davies, 2003, Wang et al., 2004). The localization of each gene’s expression and the characterization of their encoded proteins remain to be determined.
GAP JUNCTIONS
Two-electrode voltage clamping experiments of principal cells in our laboratory have provided evidence for the electrical coupling between principal cells in Aedes Malpighian tubules. First, the input resistances of principal cells are relatively low compared to that of the transepithelial resistance, suggesting the coupling of approximately 5–6 principal cells (Masia et al., 2000). Second, the spontaneous oscillations of the resting basolateral membrane potential (Vbl) in different principal cells of the same isolated Malpighian tubule are almost perfectly synchronized (Weng et al., 2008). We estimate 6000 as the number of gap junctions that connect a single principal cell to neighboring principal cells. The junctions appear to close their connections when the mitochondrial synthesis of ATP is inhibited with dinitrophenol (Weng et al., 2008). Thus, when ATP levels drop, principal cells not only close K+ channels in basolateral membranes (vide supra), but they also block communication to other principal cells. Again, the shutdown of K+ entry and traffic between principal cells may allow principal cells to protect intracellular milieus during periods of metabolic stress.
The presence of gap junctions between stellate cells and principal cells cannot be examined with electrophysiological methods because stellate cells are too thin for the impalement with microelectrodes (Figs. 1,5) (Weng et al., 2008). Accordingly, gap junctions between stellate and principal cells must be demonstrated by other methods such as molecular and immunochemical approaches. RT-PCR experiments in our laboratory indicate that four genes encoding putative gap junction proteins (i.e., innexins) are expressed in Aedes Malpighian tubules (Weng et al., 2008). In situ hybridization and/or immunohistochemical localization experiments are now required to determine if stellate cells express any of these innexins. Proof of the existence of gap junctions between principal cells and stellate cells would fortify the ‘metabolic support’ hypothesis of stellate cells described below.
THE METABOLIC SUPPORT HYPOTHESIS OF STELLATE CELLS
The maintenance of Malpighian tubule function during periods of enhanced function (e.g., diuretic fluid secretion) requires the elevated metabolism of mitochondria to generate additional ATP. Intracellular ATP not only fuels the activity of the apical H+ V-ATPase, but it also maintains the membrane and gap junction conductances (Weng et al., 2008, Wu and Beyenbach, 2003). One metabolic consequence of increased transport activity is the generation of additional intracellular CO2 and consequently H+ and HCO3−. Accordingly, the prompt export of HCO3− supports the increased mitochondrial synthesis of ATP by preventing an accumulation of HCO3−, and it explains the acidification of principal pHi by ~0.2 units upon stimulating fluid secretion (and metabolism) with db-cAMP (Lodeyro et al., 2001, Petzel et al., 1999). In turn, the intracellular acidification is expected to stimulate the proton pump by providing it with additional substrate and to rev up the transport of Na+ and K+ into the tubule lumen (Fig. 3) (Petzel et al., 1999). This hypothetical series of events requires AeAE and gap junctions between principal and stellate cells (Fig. 6). We have already identified AeAE in Malpighian tubules as the first and so far only transporter of HCO3− in mosquito Malpighian tubules (Piermarini et al., 2010). As shown in Fig. 5c,d, AeAE is expressed exclusively in stellate cells. The presence of gap junctions between principal and stellate cells remains to be documented.
Diuretic hormones such as aedeskinin and Anoga-DH31 are thought to activate cell metabolism to enhance the activity of the apical H+ V-ATPase and to increase rates of transepithelial electrolyte and fluid secretion (Coast, 2007). In a recent study we have observed that the diuresis mediated by two different diuretic hormones, aedeskinin and Anoga-DH31, is reversed by blocking the activity of AeAE with DIDS (Piermarini et al., 2010). It raises the question as to how the inhibition of Cl−/HCO3− exchange in stellate cells reverses the transcellular cation secretion in principal cells induced by two different diuretic peptides using two different signaling pathways.
If the HCO3− that is generated during periods of elevated metabolic activity in principal cells is transported to stellate cells by gap junctions for extrusion by AeAE, as illustrated in Fig. 6, then DIDS would 1) bring about the accumulation of metabolic HCO3− in principal cells and hamper ATP synthesis, and 2) increase pHi thereby depriving the H+ V-ATPase with additional H+ for transport. The metabolic support hypothesis of stellate cells would also explain why the inhibitory effects of peritubular disulfonic stilbenes (DIDS, SITS) are small on Aedes Malpighian tubules that secrete fluid at relatively low spontaneous rates, but much greater on tubules that secreted fluid at relatively high spontaneous rates (Hegarty et al., 1991, Piermarini et al., 2010). Thus, AeAE in stellate cells appears to serve the metabolic support of transepithelial transport rather than the transepithelial secretion of Cl−.
DYNAMIC REGULATION OF THE PARACELLULAR PATHWAY
As shown in Fig. 3, Cl− may pass from the hemolymph into the tubule lumen taking a transcellular route through stellate cells or a paracellular route between the epithelial cells of the tubule. We have observed no evidence for the transepithelial passage of Cl− through principal cells. Neither the apical nor the basolateral membrane of principal cells exhibit Cl− diffusion potentials that would be expected from the presence of Cl− channels (Pannabecker et al., 1993). We have observed rapid electroneutral Cl− fluxes across the basolateral membrane of principal cells (unpublished observations), but we have no evidence for tying these fluxes to transepithelial Cl− secretion. A transcellular Cl− route through stellate cells is also unlikely in Aedes Malpighian tubules because DIDS has no effect on fluid secretion under control, non-diuretic conditions, and SITS inhibits spontaneous rates of fluid secretion by only 24% (Hegarty et al., 1991, Piermarini et al., 2010). More profound reductions of fluid secretion would be expected if AeAE in the basal membrane and Cl− channels in the apical membrane of stellate cells mediated the transepithelial secretion of Cl−. Although a hemolymph to lumen flux of Cl− through stellate cells cannot be entirely excluded, the evidence for the paracellular Cl− flux is unequivocal, especially under conditions of the diuresis triggered by one family of diuretic peptides, the kinins. What is more, the paracellular pathway for Cl− can be opened and closed with switch-like speed (Beyenbach, 2003a, Pannabecker et al., 1993).
The evidence for regulating the paracellular Cl− permeability
Since the idea of a paracellular pathway with channel-like properties of selectivity and gating remains foreign in the minds of many, we summarize here the evidence for the rapid, post-translational regulation of the septate junction in Malpighian tubules of the yellow fever mosquito:
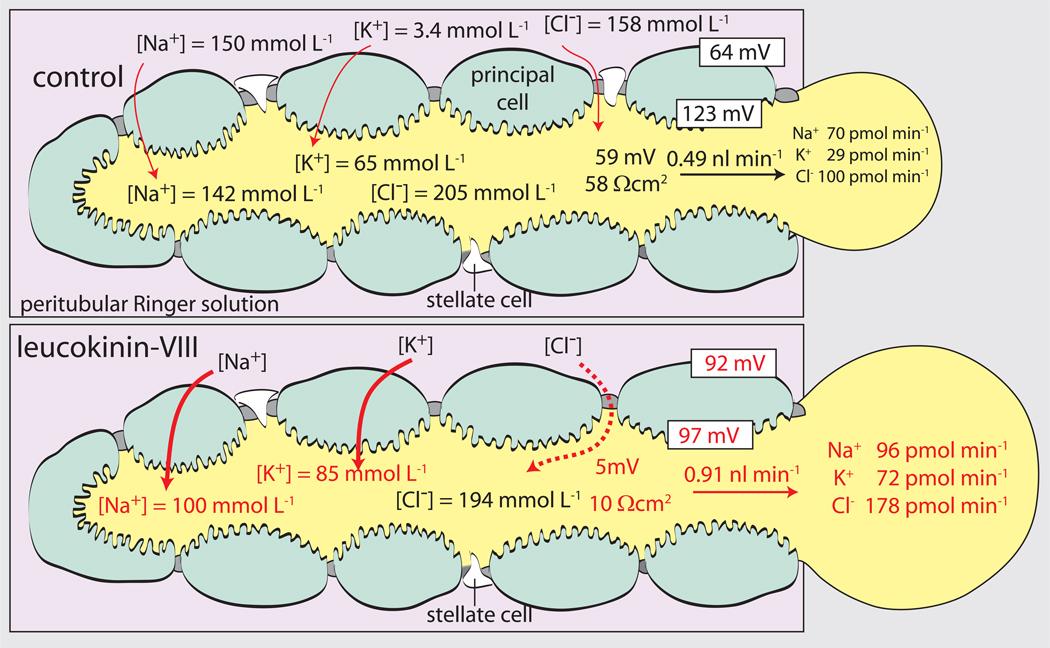
As shown in Fig. 7, the diuretic peptide leucokinin significantly increases the rate of both transepithelial NaCl and KCl secretion consistent with the activation of a transport pathway for Cl− rather than Na+ or K+ (Pannabecker et al., 1993, Schepel et al., 2010).
Leucokinin produces a nearly complete transepithelial short-circuit as the transepithelial voltage suddenly drops from 59 mV to 5 mV together with the drop in the transepithelial resistance from 58 Ωcm2 to 10 Ωcm2 (Fig. 7). Thus, the Aedes Malpighian tubule is an epithelium capable of switching between the functional properties of a moderately tight epithelium to a leaky epithelium.
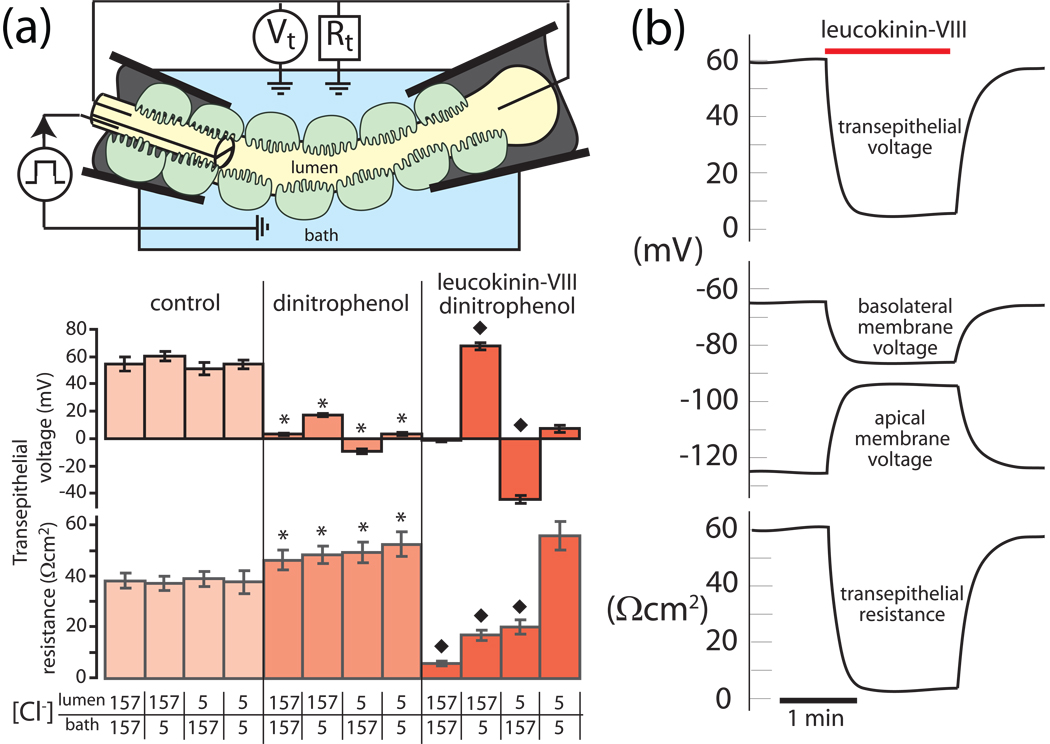
The functional properties of the paracellular pathway can be studied directly in Malpighian tubules of Aedes aegypti by inhibiting transcellular active transport of Na+ and K+ with mitochondrial poisons such as dinitrophenol (Pannabecker et al., 1992, Pannabecker et al., 1993, Wu and Beyenbach, 2003). Mitochondrial poisons reduce intracellular ATP concentrations nearly 10-fold with the effect of inhibiting the H+ V-ATPase and the transepithelial secretion of in Na+ and K+ (Wu and Beyenbach, 2003). As a result, the transepithelial voltage drops from 54 mV to 3 mV as shown in Fig. 8a. In parallel, the input resistance of principal cells increases nearly 10-fold from 334 kΩ to 3151 kΩ (Wu and Beyenbach, 2003). In view of this large increase, measures of the transepithelial resistance in the presence of dinitrophenol (DNP) approach the resistance of the paracellular pathway (Pannabecker et al., 1992, Pannabecker et al., 1993). As shown in Fig. 8a, the resistance of the paracellular pathway is approximately 46 Ωcm2 in the presence of DNP and 157 mmol L−1 Cl− on both sides of the epithelium.
The paracellular pathway displays transepithelial Cl− diffusion potentials. No significant transepithelial Cl− diffusion potentials (which reflect the transepithelial Cl− permeability) were observed under control conditions when the [Cl−] was reduced in either the peritubular bath or tubule lumen (Fig. 8a). In contrast, transepithelial Cl− diffusion potentials reach significant levels when transcellular active transport is inhibited with dinitrophenol (DNP). The lumen-positive and lumen-negative transepithelial voltages measured respectively in the case of lumen-to-bath and bath-to-lumen diffusion of Cl− reflect the Cl− permeability of the paracellular septate junctional pathway (Fig. 8a). Transepithelial Cl− diffusion potentials (~13 mV) which are symmetrical for the diffusion of Cl− from lumen-to-bath and vice versa, are expected from a single diffusion barrier such as the septate junction.
Transepithelial Cl− diffusion potentials increase substantially in the presence of leucokinin. Remarkably, kinin diuretic peptides still exert their effects on the transepithelial Cl− permeability in tubules poisoned with DNP (Fig. 8a). In particular, the addition of leucokinin-VIII to the peritubular bath reduces the resistance of the paracellular septate junctional pathway from 52 Ωcm2 to 6 Ωcm2, documenting a substantial increase in paracellular conductance (permeability). Moreover, the permeability increase is for Cl− in view of the large, symmetrical transepithelial Cl− diffusion potentials up to 77% of Nernst potentials (Pannabecker et al., 1993).
That indeed an extracellular Cl− pathway has opened up in the presence of leucokinin-VIII is proven by the fact that the transepithelial resistance (i.e. paracellular septate junctional resistance) returns to 56 Ωcm2, the pre-leucokinin magnitude (52 Ωcm2) when the [Cl−] on both sides of the epithelium is lowered to 5 mmol L−1 (Fig. 8a). Here, the paracellular septate junctional pathway displays channel-like properties where the resistance of an open Cl− channel increases with decreasing Cl− concentration (Goldman rectification).
Fig. 7.
The diuretic peptide leucokinin-VIII increases the transepithelial secretion rates of NaCl, KCl and water in Malpighian tubules of Aedes aegypti. The kinin also switches the tubule from a moderately tight epithelium (transepithelial voltage of 59 mV and resistance of 58 Ωcm2) to a leaky epithelium (transepithelial voltage of 5 mV and resistance of 10 Ωcm2). Numbers in red indicate statistical significance difference from control. Data are from (Pannabecker et al., 1993) and modified from(Beyenbach, 2003a).
Fig. 8.
Kinin diuretic peptide targets the Cl− permeability of the paracellular pathway. A, dinitrophenol was used to inhibit transcellular active transport mechanisms such that measures of the transepithelial resistance approach the resistance of the paracellular septate junctional pathway. B, time course and the reversibility of the effect of leucokinin-VIII on tubule electrophysiology. Data are taken from (Pannabecker et al., 1993) and modified from (Beyenbach, 2003a)
The electrophysiological on/off effects of leucokinin take place with switch-like speed (Fig. 8b), indicating the post-translational regulation of the paracellular septate junctional Cl− permeability.
The kinin signaling pathway
Malpighian tubules of Aedes aegypti are thought to have only one gene for the receptor of kinins (Pietrantonio et al., 2005). It is a G protein-coupled receptor (Fig. 9). In a recent study we have learned that all three natural aedeskinins increase the rate of fluid secretion in isolated Malpighian tubules together with the electrophysiological changes shown in Figs. 7 and 8 for leucokinin-VIII, a kinin of the cockroach Leucophaea (Schepel et al., 2010). However, some synthetic kinin analogs increase the rate of fluid secretion without effects on tubule electrophysiology and vice versa. These observations suggest that more than one signaling pathway is activated by kinins. One signal pathway triggers the electrophysiological response, and the other triggers fluid secretion. Since there is only one kinin receptor gene, separate and independent signaling pathways emanating from the receptor may indicate agonist-directed signaling, where partial ligand binding may trigger one signaling pathway and not the other. Alternatively, there may be two receptor isoforms which - depending on their glycosylation - may selectively couple to different signaling pathways.
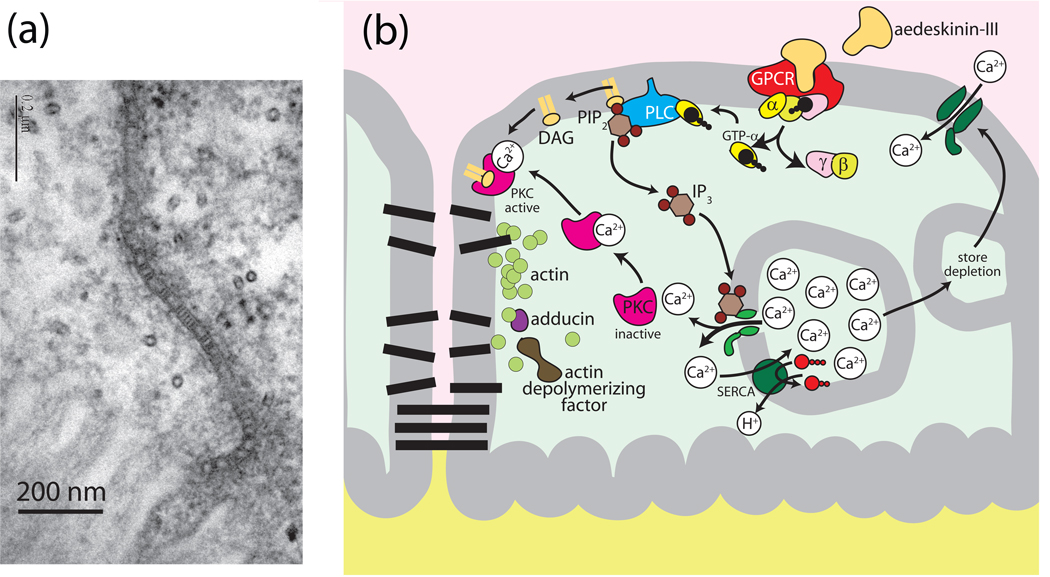
Fig. 9.
Hypothetical model for kinin signaling to the septate junction via protein kinase C (PKC) in Aedes Malpighian tubules. A, electron micrograph of the septate junction in Aedes Malpighian tubules (modified from (Rajasekaran et al., 2008) Note the septa near the apical brush border. The proteins forming the septa are unknown. B, model of reversible opening and closing of the paracellular pathway. A conformational change in the septate junctional proteins or a break in the septa (protein strands ?) are expected to lower the resistance of the paracellular pathway.
Agonist-directed signaling and glycosylation-dependent signaling is not uncommon in the case of G protein-coupled receptors (Berg and Clarke, 2006, Rasmussen et al., 2007, Simmons, 2005). Moreover, although Drosophila Malpighian tubules express a single transcript encoding a kinin GPCR (consistent with one receptor), two immunochemical forms of the drosokinin GPCR are detected: one is N-glycosylated and the other is not (Radford et al., 2002). Malpighian tubules of the Anopheles mosquito also express two analogous immunochemical forms of the kinin GPCR (Radford et al., 2004).
From previous studies we know that binding of leucokinin-VIII to the Aedes GPCR activates in part a Ca2+ signaling pathway via phospholipase C and IP3 (Yu and Beyenbach, 2001, Isnard-Bagnis et al., 2003, Yu and Beyenbach, 2004). The latter causes the release of Ca2+ from intracellular stores (Fig. 9). Subsequently, store depletion triggers the crucial signaling step: the opening of Ca2+-channels in the basolateral membrane of principal cells. Indeed, the entry of Ca2+ into principal cells from the peritubular bath is essential for the sustained and full effect of leucokinin-VIII. How elevated intracellular Ca2+ concentrations go on to increase the Cl− permeability of the paracellular septate junction is unknown. The hypothesis offered in Fig. 9 proposes a role of protein kinase C.
Taking a proteomic approach to elucidate the signaling pathway of kinin diuretic peptides, we have observed that the stimulation of Malpighian tubules with aedeskinin for only 1 minute decreases the presence of subunits A and B of the H+ V-ATPase in the cytosol, consistent with their move to the membrane where they join the V0 complex to activate the proton pump and transepithelial secretion (Beyenbach et al., 2009). In contrast, adducin, actin, regucalcin, and actin-depolymerizing factor all increase in the cytosol which we hypothetically associate with the disassembly of the septate junctional complex (Fig. 9). We hypothesize further that the dissociation of the junctional complex on the intracellular side breaks the septate junctional barrier, perhaps the septa themselves, with the effect of reducing the paracellular resistance (Fig. 9a). The proteins forming the septa of septate junctions are unknown as are the proteins that give rise to Cl− permselectivity of septate junctions.
Tight junctions and septate junctions
Compared to all that is known about vertebrate tight junctions, our understanding of invertebrate septate junctions is rather meager. Functionally, tight and septate junctions may be analogous. However, structurally, the two are quite different. In vertebrate epithelia, tight junctions surround epithelial cells at the most apical region and extend into the paracellular pathway for a very short distance, typically less than 1 µm (Fig. 10a). Tight junctions are formed by the membrane protein families of claudins and occludins. Interactions between the extracellular loops of claudins pull the outer membrane leaflets of adjoining cells so close to each other as to nearly fuse them, thereby forming what Jared Diamond first described as epithelial “kisses” (Diamond, 1974).
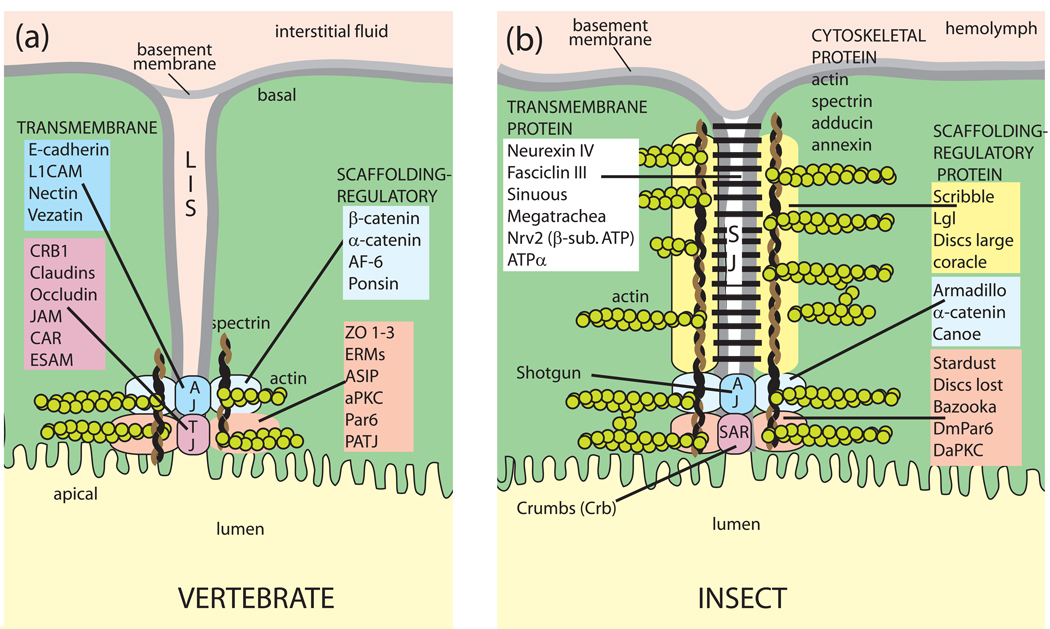
Fig. 10.
The paracellular pathway in vertebrates and insects. Vertebrate tight junctions and invertebrate septate junctions share in common a structural organization that includes 1) transmembrane spanning proteins that reach into the extracellular space of the junction, and 2) intracellular scaffolding and regulatory proteins that anchor the transmembrane proteins to the cytoskeleton. A, the paracellular pathway in vertebrate epithelia: LIS, lateral interstitial space; AJ, adherens junction; TJ, tight junction; B, the paracellular pathway in invertebrate epithelia SJ, septate junction; SAR, subapical region. See also Fig. 9a for electron micrographs of the septate junction in Aedes Malpighian tubules. Gap junctions and desmosomes are omitted altogether. Adopted from (Furuse and Tsukita, 2006, Knust and Bossinger, 2002).
In contrast, septate junctions occupy the basal portion of the paracellular pathway for a considerable length (Figs. 9B, 10B) (Lane and Skaer, 1980). Importantly, they are not formed by membrane fusions. Rungs that form a ‘paracellular ladder’ assure that the cell membranes of neighboring epithelial cells are kept apart by 15–20 nm (Fig. 9a). The rungs (septa) are thought to spiral around epithelial cells, from apical to basal poles, thereby giving rise to a ladder-like appearance in apico-basal sections. Towards the apical side of the paracellular pathway, adherens junctions and the subapical region may contain membrane fusions (Fig. 10b).
Among the more than 40 proteins of tight junctions, the claudins, occludins and junctional adhesion molecules (JAMs) are the major transmembrane proteins (Fig. 10a) (Anderson and Van Itallie, 2008). Tricellulin occupies the apical spots where three epithelial cells make contact (Ikenouchi et al., 2005). The transmembrane proteins are attached to a cytoplasmic network of multi-PDZ proteins (ZO-1, ZO-2, ZO-3, MAGI-1, PatJ, PALS1 and MUPP1) that are known as anchoring or scaffolding proteins that recruit claudins, occludins, tricellulin, and JAMs to the tight junction and anchor them to the cytoskeleton (Fig.10a). Actin dynamics appear to be key to 1) the assembly of junctions during development, and 2) the physiological and pathological control of the paracellular pathway (Anderson and Van Itallie, 2008).
Far less is known about the molecular structure of the paracellular pathway in invertebrate epithelia. Although we know a large number of proteins associated with this pathway, we are far less certain about the exact location of these proteins (Fig. 10b). Most authors associate the identified proteins with the septate junction, as the septate junction is usually taken to be synonymous with the paracellular pathway. However, the septate junction is one of three junctions (Fig. 10b). The claudin-like proteins sinuous and megatrachea are more likely to form membrane fusions in the subapical region (SAR) or adherens junctions (AJ) than they are in the region of the septate junction. Similar uncertainties exist about the location of scaffolding and regulatory proteins along the paracellular pathway. Nevertheless, the resemblance of vertebrate tight junctions and invertebrate septate junctions is striking at the molecular level. Knowing where these proteins are located and how they interact in Malpighian tubules is critical to understanding 1) the dynamic regulation of the paracellular pathway in insect Malpighian tubules, and 2) the mechanism of action of diuretic peptides and hormones.
Acknowledgements
The authors thank the National Science Foundation and the National Institutes of Health for funding our research over the years. Whatever ‘conflict of interest’ could be seen in placing scientific knowledge in the public domain eludes our imagination.
References
- Ahearn GA, Mandal PK, Mandal A. Biology of the 2Na/1H antiporter in invertebrates. J. exp. Biol. 2001;289:232–244. doi: 10.1002/1097-010x(20010401/30)289:4<232::aid-jez4>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Van Itallie CM. Tight junctions. Curr Biol. 2008;18:R941–R943. doi: 10.1016/j.cub.2008.07.083. [DOI] [PubMed] [Google Scholar]
- Bennett AF. Experimental evolution and the Krogh Principle: generating biological novelty for functional and genetic analyses. Physiol. Biochem. Zool. 2003;76:1–11. doi: 10.1086/374275. [DOI] [PubMed] [Google Scholar]
- Benoit JB, Denlinger DL. Meeting the challenges of on-host and off-host water balance in blood-feeding arthropods. J Insect Physiol. 2010 doi: 10.1016/j.jinsphys.2010.02.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg KA, Clarke WP. Development of functionally selective agonists as novel therapeutic agents. Drug Discov Today Ther Strateg. 2006;3:421–428. [Google Scholar]
- Beyenbach KW. Energizing epithelial transport with the vacuolar H+-ATPase. News Physiol Sci. 2001;16:145–151. doi: 10.1152/physiologyonline.2001.16.4.145. [DOI] [PubMed] [Google Scholar]
- Beyenbach KW. Regulation of tight junction permeability with switch-like speed. Curr Opin Nephrol Hypertens. 2003a;12:543–550. doi: 10.1097/00041552-200309000-00010. [DOI] [PubMed] [Google Scholar]
- Beyenbach KW. Transport mechanisms of diuresis in Malpighian tubules of insects. J Exp Biol. 2003b;206:3845–3856. doi: 10.1242/jeb.00639. [DOI] [PubMed] [Google Scholar]
- Beyenbach KW, Baumgart S, Lau K, Piermarini PM, Zhang S. Signaling to the apical membrane and to the paracellular pathway: changes in the cytosolic proteome of Aedes Malpighian tubules. J Exp Biol. 2009;212:329–340. doi: 10.1242/jeb.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyenbach KW, Masia R. Membrane conductances of principal cells in Malpighian tubules of Aedes aegypti. J. Insect Physiol. 2002;48:375–386. doi: 10.1016/s0022-1910(02)00057-4. [DOI] [PubMed] [Google Scholar]
- Beyenbach KW, Oviedo A, Aneshansley DJ. Malpighian tubules of Aedes-aegypti - Five tubules, one function. J. Insect Physiol. 1993;39:639–648. [Google Scholar]
- Beyenbach KW, Pannabecker TL, Nagel W. Central role of the apical membrane H+-ATPase in electrogenesis and epithelial transport in Malpighian tubules. J Exp Biol. 2000;203:1459–1468. doi: 10.1242/jeb.203.9.1459. [DOI] [PubMed] [Google Scholar]
- Beyenbach KW, Piermarini PM. Osmotic and Ionic Regulation in Insects. In: Evans DH, editor. Osmotic and Ionic Regulation: Cells and Animals. Boca Raton, FL: CRC Press; 2009. [Google Scholar]
- Beyenbach KW, Skaer H, Dow JA. The developmental, molecular, and transport biology of Malpighian tubules. Annu Rev Entomol. 2010;55:351–374. doi: 10.1146/annurev-ento-112408-085512. [DOI] [PubMed] [Google Scholar]
- Beyenbach KW, Wieczorek H. The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J. Exp. Biol. 2006;209:577–589. doi: 10.1242/jeb.02014. [DOI] [PubMed] [Google Scholar]
- Bradley TJ, Stuart AM, Satir P. The ultrastructure of the larval malpighian tubules of a saline-water mosquito. Tissue Cell. 1982;14:759–773. doi: 10.1016/0040-8166(82)90064-7. [DOI] [PubMed] [Google Scholar]
- Brett CL, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol. 2005;288:C223–C239. doi: 10.1152/ajpcell.00360.2004. [DOI] [PubMed] [Google Scholar]
- Cabrero P, Pollock VP, Davies SA, Dow JA. A conserved domain of alkaline phosphatase expression in the Malpighian tubules of dipteran insects. J Exp Biol. 2004;207:3299–3305. doi: 10.1242/jeb.01156. [DOI] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- Coast G. The endocrine control of salt balance in insects. Gen Comp Endocrinol. 2007;152:332–338. doi: 10.1016/j.ygcen.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Coast GM, Garside CS, Webster SG, Schegg KM, Schooley DA. Mosquito natriuretic peptide identified as a calcitonin-like diuretic hormone in Anopheles gambiae (Giles) J Exp Biol. 2005;208:3281–3291. doi: 10.1242/jeb.01760. [DOI] [PubMed] [Google Scholar]
- Dames P, Zimmermann B, Schmidt R, Rein J, Voss M, Schewe B, et al. cAMP regulates plasma membrane vacuolar-type H+-ATPase assembly and activity in blowfly salivary glands. Proc Natl Acad Sci U S A. 2006;103:3926–3931. doi: 10.1073/pnas.0600011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JP, Wan S, Allan AK, Kean L, Davies SA, Gray JV, et al. Identification of two partners from the bacterial Kef exchanger family for the apical plasma membrane V-ATPase of Metazoa. J. Cell Sci. 2008;121:2612–2619. doi: 10.1242/jcs.033084. [DOI] [PubMed] [Google Scholar]
- Diamond JM. Tight and leaky junctions of epithelia: a perspective on kisses in the dark. Fed. Proc. 1974;33:2220–2224. [PubMed] [Google Scholar]
- Dow JA, Davies SA. The Malpighian tubule: rapid insights from post-genomic biology. J Insect Physiol. 2006;52:365–378. doi: 10.1016/j.jinsphys.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Dow JT, Davies SA. Integrative physiology and functional genomics of epithelial function in a genetic model organism. Physiol Rev. 2003;83:687–729. doi: 10.1152/physrev.00035.2002. [DOI] [PubMed] [Google Scholar]
- Evans JM, Allan AK, Davies SA, Dow JA. Sulphonylurea sensitivity and enriched expression implicate inward rectifier K+ channels in Drosophila melanogaster renal function. J Exp Biol. 2005;208:3771–3783. doi: 10.1242/jeb.01829. [DOI] [PubMed] [Google Scholar]
- Filippov V, Aimanova K, Gill SS. Expression of an Aedes aegypti cation-chloride cotransporter and its Drosophila homologues. Insect Mol Biol. 2003;12:319–331. doi: 10.1046/j.1365-2583.2003.00415.x. [DOI] [PubMed] [Google Scholar]
- Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Dow JA. Characterization of the Drosophila melanogaster alkali-metal/proton exchanger (NHE) gene family. J Exp Biol. 2001;204:3703–3716. doi: 10.1242/jeb.204.21.3703. [DOI] [PubMed] [Google Scholar]
- Hart SJ, Knezetic JA, Petzel DH. Cloning and tissue distribution of two Na+/H+ exchangers from the Malpighian tubules of Aedes aegypti. Arch Insect Biochem Physiol. 2002;51:121–135. doi: 10.1002/arch.10057. [DOI] [PubMed] [Google Scholar]
- Harvey WR. Voltage coupling of primary H+ V-ATPases to secondary Na+- or K+-dependent transporters. J Exp Biol. 2009;212:1620–1629. doi: 10.1242/jeb.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert SC, Mount DB, Gamba G. Molecular Physiology of cation-coupled Cl− cotransport: the SLC12 family. Pfluegers Arch. 2004:580–593. doi: 10.1007/s00424-003-1066-3. [DOI] [PubMed] [Google Scholar]
- Hegarty JL, Zhang B, Carroll MC, Cragoe EJJ, Beyenbach KW. Effects of amiloride on isolated Malpighian tubules of the yellow fever mosquito (Aedes aegypti) J. Insect Physiol. 1992;38:329–337. [Google Scholar]
- Hegarty JL, Zhang B, Pannabecker TL, Petzel DH, Baustian MD, Beyenbach KW. Dibutyryl cAMP activates bumetanide-sensitive electrolyte transport in Malpighian tubules. Am. J. Physiol. Cell Physiol. 1991;261:C521–C529. doi: 10.1152/ajpcell.1991.261.3.C521. [DOI] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function and physiological roles. Physiol. Rev. 2010;90:291–306. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Wang Y, Jefferies K, Qi J, Hinton A, Forgac M. Structure and regulation of the V-ATPases. J Bioenerg Biomembr. 2005;37:393–398. doi: 10.1007/s10863-005-9478-8. [DOI] [PubMed] [Google Scholar]
- Isnard-Bagnis C, Da Silva N, Beaulieu V, Yu AS, Brown D, Breton S. Detection of ClC-3 and ClC-5 in epididymal epithelium: immunofluorescence and RT-PCR after LCM. Am J Physiol Cell Physiol. 2003;284:C220–C232. doi: 10.1152/ajpcell.00374.2001. [DOI] [PubMed] [Google Scholar]
- Kane PM. Disassembly and reassembly of the yeast vacuolar H+-ATPase in vivo. J Biol Chem. 1995;270:17025–17032. [PubMed] [Google Scholar]
- Kane PM, Parra KJ. Assembly and regulation of the yeast vacuolar H+-ATPase. J. Exp. Biol. 2000;203:81–87. doi: 10.1242/jeb.203.1.81. [DOI] [PubMed] [Google Scholar]
- Kang'ethe W, Aimanova KG, Pullikuth AK, Gill SS. NHE8 mediates amiloride-sensitive Na+/H+ exchange across mosquito Malpighian tubules and catalyzes Na+ and K+ transport in reconstituted proteoliposomes. Am. J. Physiol. Renal Physiol. 2007;292:F1501–F1512. doi: 10.1152/ajprenal.00487.2005. [DOI] [PubMed] [Google Scholar]
- Karas K, Brauer P, Petzel D. Actin redistribution in mosquito Malpighian tubules after a blood meal and cyclic AMP stimulation. J Insect Physiol. 2005;51:1041–1054. doi: 10.1016/j.jinsphys.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Klowden MJ. Blood, sex and the mosquito. Bioscience. 1995:326–331. [Google Scholar]
- Knust E, Bossinger O. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298:1955–1959. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- Krebs HA. The August Krogh Principle: "For many problems there is an animal on which it can be most conveniently studied". J. Exp. Zool. 1975;194:221–226. doi: 10.1002/jez.1401940115. [DOI] [PubMed] [Google Scholar]
- Krogh A. On the hydrostatic mechanism of the Corethra larva with an account of methods of microscopical gas analysis. Skand. Archiv Physiologie. 1911;25:181–203. [Google Scholar]
- Krogh A. Osmotic Regulation in Aquatic Animals. Cambridge, MA: Cambridge University Press; 1939. [Google Scholar]
- Krogh A. Croonian Lecture: The active and passive exchanges of inorganic ions through the surfaces of living cells and through living membranes generally. Proc. Roy. Soc. London B. 1946;133:140–200. doi: 10.1098/rspb.1946.0008. [DOI] [PubMed] [Google Scholar]
- Krupinski T, Beitel GJ. Unexpected roles of the Na-K-ATPase and other ion transporters in cell junctions and tubulogenesis. Physiology. 2009;24:192–201. doi: 10.1152/physiol.00008.2009. [DOI] [PubMed] [Google Scholar]
- Lane NJ, Skaer Hl. Intercellular junctions in insect tissues. In: Berridge MJ, Treherne JE, Wigglesworth VB, editors. Advances in Insect Physiology. London: Academic Press; 1980. [Google Scholar]
- Lodeyro AF, Calcaterra NB, Roveri OA. Inhibition of steady-state mitochondrial ATP synthesis by bicarbonate, an activating anion in ATP hydrolysis. Biochim Biophys Acta. 2001;1506:236–243. doi: 10.1016/s0005-2728(01)00221-3. [DOI] [PubMed] [Google Scholar]
- Maddrell SHP. Fluid secretion by the Malpighian tubules of insects. Philos Trans R Soc Lond B Biol Sci. 1971;262:197–207. [Google Scholar]
- Masia R, Aneshansley D, Nagel W, Nachman RJ, Beyenbach KW. Voltage clamping single cells in intact Malpighian tubules of mosquitoes. Am. J. Physiol. 2000;279:F747–F754. doi: 10.1152/ajprenal.2000.279.4.F747. [DOI] [PubMed] [Google Scholar]
- Melton IC, Gilligan DM, Wood MA, Ellenbogen KA. Optimal cardiac pacing after heart transplantation. Pacing Clin Electrophysiol. 1999;22:1510–1527. doi: 10.1111/j.1540-8159.1999.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Morillo CA, Camacho ME, Wood MA, Gilligan DM, Ellenbogen KA. Diagnostic utility of mechanical, pharmacological and orthostatic stimulation of the carotid sinus in patients with unexplained syncope. J Am Coll Cardiol. 1999;34:1587–1594. doi: 10.1016/s0735-1097(99)00365-4. [DOI] [PubMed] [Google Scholar]
- Murata T, Yamato I, Kakinuma Y, Leslie AGW, Walker JE. Structure of the rotor of the V-type Na+-ATPase from Enterococcus hirae. Science. 2005;308:654–659. doi: 10.1126/science.1110064. [DOI] [PubMed] [Google Scholar]
- O'Connor KR, Beyenbach KW. Chloride channels in apical membrane patches of stellate cells of Malpighian tubules of Aedes aegypti. J. Exp. Biol. 2001;204:367–378. doi: 10.1242/jeb.204.2.367. [DOI] [PubMed] [Google Scholar]
- O'Donnel MJ. Too much of a good thing: How insects cope with excess ions or toxins in the diet. J. Exp. Biol. 2009;212:363–372. doi: 10.1242/jeb.023739. [DOI] [PubMed] [Google Scholar]
- O'Donnell MJ, Rheault MR, Davies SA, Rosay P, Harvey BJ, Maddrell SH, et al. Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am. J. Physiol. 1998;274:R1039–R1049. doi: 10.1152/ajpregu.1998.274.4.R1039. [DOI] [PubMed] [Google Scholar]
- Okech BA, Boudko DY, Linser PJ, Harvey WR. Cationic pathway of pH regulation in larvae of Anopheles gambiae. J. Exp. Biol. 2008;211:957–968. doi: 10.1242/jeb.012021. [DOI] [PubMed] [Google Scholar]
- Palatroni P, Gabrielli MG, Scattolini B. Histochemical localization of carbonic anhydrase in Malpighian tubules of Culex pipiens. Experientia. 1981;37:409–411. doi: 10.1007/BF01970135. [DOI] [PubMed] [Google Scholar]
- Pannabecker TL, Aneshansley DJ, Beyenbach KW. Unique electrophysiological effects of dinitrophenol in Malpighian tubules. Am. J. Physiol. 1992;263:R609–R614. doi: 10.1152/ajpregu.1992.263.3.R609. [DOI] [PubMed] [Google Scholar]
- Pannabecker TL, Hayes TK, Beyenbach KW. Regulation of epithelial shunt conductance by the peptide leucokinin. J. Membr. Biol. 1993;132:63–76. doi: 10.1007/BF00233052. [DOI] [PubMed] [Google Scholar]
- Patrick ML, Aimanova K, Sanders HR, Gill SS. P-type Na+/K+-ATPase and V-type H+-ATPase expression patterns in the osmoregulatory organs of larval and adult mosquito Aedes aegypti. J Exp Biol. 2006;209:4638–4651. doi: 10.1242/jeb.02551. [DOI] [PubMed] [Google Scholar]
- Petzel DH. Na/H exchange in mosquito Malpighian tubules. Am. J. Physiol. Reg. Physiol. 2000;279:R1996–R2003. doi: 10.1152/ajpregu.2000.279.6.R1996. [DOI] [PubMed] [Google Scholar]
- Petzel DH, Berg MM, Beyenbach KW. Hormone-controlled cAMP-mediated fluid secretion in yellow-fever mosquito. Am J Physiol Regulatory Integrative Comp Physiol. 1987;253:R701–R711. doi: 10.1152/ajpregu.1987.253.5.R701. [DOI] [PubMed] [Google Scholar]
- Petzel DH, Hagedorn HH, Beyenbach KW. Preliminary isolation of mosquito natriuretic factor. Am. J. Physiol. Reg. Physiol. 1985;249:R379–R386. doi: 10.1152/ajpregu.1985.249.4.R379. [DOI] [PubMed] [Google Scholar]
- Petzel DH, Hagedorn HH, Beyenbach KW. Peptide nature of two mosquito natriuretic factors. Am J Physiol. 1986;250:R328–R332. doi: 10.1152/ajpregu.1986.250.3.R328. [DOI] [PubMed] [Google Scholar]
- Petzel DH, Pirotte PT, Van Kerkhove E. Intracellular and luminal pH measurements of Malpighian tubules of the mosquito Aedes aegypti: The effects of cAMP. J. Insect Physiol. 1999;45:973–982. doi: 10.1016/s0022-1910(99)00076-1. [DOI] [PubMed] [Google Scholar]
- Piermarini PM, Grogan L, Lau K, Wang L, Beyenbach KW. A SCL4-like anion exchanger from renal tubules of the mosquito (Aedes aegypti): Evidence for a novel role of stellate cells in diuretic fluid secretion. Am J Physiol Regul Integr Comp Physiol. 2010;298:642–660. doi: 10.1152/ajpregu.00729.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piermarini PM, Weihrauch D, Meyer H, Huss M, Beyenbach KW. NHE8 is an intracellular cation/H+ exchanger in renal tubules of the yellow fever mosquito Aedes aegypti. Am J Physiol Renal Physiol. 2009;296:F730–F750. doi: 10.1152/ajprenal.90564.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrantonio PV, Jagge C, Taneja-Bageshwar S, Nachman RJ, Barhoumi R. The mosquito Aedes aegypti (L.) leucokinin receptor is a multiligand receptor for the three Aedes kinins. Insect Mol Biol. 2005;14:55–67. doi: 10.1111/j.1365-2583.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- Plawner L, Pannabecker TL, Laufer S, Baustian MD, Beyenbach KW. Control of diuresis in the yellow fever moquito Aedes aegypti: Evidence for similar mechanisms in the male and female. J. Insect Physiol. 1991;37:119–128. [Google Scholar]
- Pullikuth AK, Aimanova K, Kang'ethe W, Sanders HR, Gill SS. Molecular characterization of sodium/proton exchanger 3 (NHE3) from the yellow fever vector, Aedes aegypti. J Exp Biol. 2006;209:3529–3544. doi: 10.1242/jeb.02419. [DOI] [PubMed] [Google Scholar]
- Radford JC, Davies SA, Dow JA. Systematic G-protein-coupled receptor analysis in Drosophila melanogaster identifies a leucokinin receptor with novel roles. J Biol Chem. 2002;277:38810–38817. doi: 10.1074/jbc.M203694200. [DOI] [PubMed] [Google Scholar]
- Radford JC, Terhzaz S, Cabrero P, Davies SA, Dow JA. Functional characterisation of the Anopheles leucokinins and their cognate G-protein coupled receptor. J Exp Biol. 2004;207:4573–4586. doi: 10.1242/jeb.01317. [DOI] [PubMed] [Google Scholar]
- Rajasekaran SA, Beyenbach KW, Rajasekaran AK. Interactions of tight junctions with membrane channels and transporters. Biochim. Biophys. Acta. 2008;1778:757–769. doi: 10.1016/j.bbamem.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Ramsay JA. Osmotic regulation in mosquito larvae; the role of the Malpighian tubules. J Exp Biol. 1951;28:62–73. doi: 10.1242/jeb.28.1.62. [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, et al. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- Rehberg PB. August Krogh, November 15, 1874-September 13, 1949. Yale J Biol Med. 1951;24:83–102. [PMC free article] [PubMed] [Google Scholar]
- Rheault MR, Okech BA, Keen SB, Miller MM, Meleshkevitch EA, Linser PJ, et al. Molecular cloning, phylogeny and localization of AgNHA1: the first Na+/H+ antiporter (NHA) from a metazoan, Anopheles gambiae. J. Exp. Biol. 2007;210:3848–3861. doi: 10.1242/jeb.007872. [DOI] [PubMed] [Google Scholar]
- Roitberg BD, Mondor EB, Tyerman JGA. Pouncing spider, flying mosquito: blood acquisition increases predation risk in mosqitoes. Behavioral Ecol. 2003;14:736–740. [Google Scholar]
- Satmary WM, Bradley TJ. The distribution of cell types in the Malpighian tubules of Aedes taeniorhynchus Diptera Culicidae. Int J Insect Morphol Embryol. 1984;13:209–214. [Google Scholar]
- Sautin YY, Lu M, Gaugler A, Zhang L, Gluck SL. Phosphatidylinositol 3-kinase-mediated effects of glucose on vacuolar H+-ATPase assembly, translocation, and acidification of intracellular compartments in renal epithelial cells. Mol. Cell Biol. 2005;25:575–589. doi: 10.1128/MCB.25.2.575-589.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer DB, Beyenbach KW. Dibutyryl-cAMP increases basolateral sodium conductance of mosquito Malpighian tubules. Am. J. Physiol. 1985;248:R339–R345. doi: 10.1152/ajpregu.1985.248.3.R339. [DOI] [PubMed] [Google Scholar]
- Schepel SA, Fox AJ, Miyauchi JT, Sou T, Yang JD, Lau K, et al. The single kinin receptor signals to separate and independent physiological pathways in Malpighian tubules of the yellow fever mosquito. Am J Physiol Regul Integr Comp Physiol. 2010 doi: 10.1152/ajpregu.00068.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Nielsen B. August and Marie Krogh. Lives in Science. New York, NY: Oxford University Press; 1995. [Google Scholar]
- Scott BN, Yu M-J, Lee LW, Beyenbach K. Mechanisms of K+ transport across basolateral membranes of principal cells in Malpighian tubules of the yellow fever mosquito, Aedes aegypti. J Exp Biol. 2004;207:1655–1663. doi: 10.1242/jeb.00932. [DOI] [PubMed] [Google Scholar]
- Sieglaff DH, Dunn WA, Xie XS, Megy K, Marinotti O, James AA. Comparative genomics allows the discovery of cis-regulatory elements in mosquitoes. Proc Natl Acad Sci U S A. 2009;106:3053–3058. doi: 10.1073/pnas.0813264106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons MA. Functional selectivity, ligand-directed trafficking, conformation-specific agonism: what's in a name? Mol Interv. 2005;5:154–157. doi: 10.1124/mi.5.3.4. [DOI] [PubMed] [Google Scholar]
- Smith KE, Vanekeris LA, Linser PJ. Cloning and characterization of AgCA9, a novel alpha-carbonic anhydrase from Anopheles gambiae Giles sensu strictu (Diptera: Culicidae) larvae. J. Exp. Biol. 2007;210 doi: 10.1242/jeb.008342. 3919-3030. [DOI] [PubMed] [Google Scholar]
- Sozen MA, Armstrong JD, Yang M, Kaiser K, Dow JA. Functional domains are specified to single-cell resolution in a Drosophila epithelium. Proc. Natl. Acad. Sci. USA. 1997;94:5207–5212. doi: 10.1073/pnas.94.10.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JP, Dow JA, Earley FG, Klein U, Jäger D, Wieczorek H. Regulation of plasma membrane V-ATPase activity by dissociation of peripheral subunits. J. Biol. Chem. 1995;270:5649–5653. doi: 10.1074/jbc.270.10.5649. [DOI] [PubMed] [Google Scholar]
- Sun Q, Tian E, Turner RJ, Ten Hagen KG. Developmental and functional studies of the SLC12 gene family members of Drosophila melanogaster. Am. J. Physiol. Cell Physiol. 2010;298:C26–C37. doi: 10.1152/ajpcell.00376.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhzaz S, Southall TD, Lilley KS, Kean L, Allan AK, Davies SA, et al. Differential gel electrophoresis and transgenic mitochondrial calcium reporters demonstrate spatiotemporal filtering in calcium control of mitochondria. J Biol Chem. 2006;281:18849–18858. doi: 10.1074/jbc.M603002200. [DOI] [PubMed] [Google Scholar]
- Vitavska O, Merzendorfer H, Wieczorek H. The V-ATPase subunit C binds to polymeric F-actin as well as monomeric G-actin and induces cross-linking of actin filaments. J. Biol. Chem. 2005;280:1070–1076. doi: 10.1074/jbc.M406797200. [DOI] [PubMed] [Google Scholar]
- Voss M, Schmidt R, Walz B, Baumann O. Stimulus-induced translocation of the protein kinase A catalytic subunit to the apical membrane in blowfly salivary glands. Cell Tissue Res. 2008;335:657–662. doi: 10.1007/s00441-008-0673-x. [DOI] [PubMed] [Google Scholar]
- Voss M, Vitavska O, Walz B, Wieczorek H, Baumann O. Stimulus-induced phosphorylation of vacuolar H+-ATPase by protein kinase A. J. Biol. Chem. 2007;282:33735–33742. doi: 10.1074/jbc.M703368200. [DOI] [PubMed] [Google Scholar]
- Wang J, Kean L, Yang J, Allan AK, Davies SA, Herzyk P, et al. Function-informed transcriptome analysis of Drosophila renal tubule. Genome Biol. 2004;5:R69. doi: 10.1186/gb-2004-5-9-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng XH, Huss M, Wieczorek H, Beyenbach KW. The V-type H+-ATPase in Malpighian tubules of Aedes aegypti: localization and activity. J. Exp. Biol. 2003;206:2211–2219. doi: 10.1242/jeb.00385. [DOI] [PubMed] [Google Scholar]
- Weng XH, Piermarini PM, Yamahiro A, Yu MJ, Aneshansley DJ, Beyenbach KW. Gap junctions in Malpighian tubules of Aedes aegypti. J. Exp. Biol. 2008;211:409–422. doi: 10.1242/jeb.011213. [DOI] [PubMed] [Google Scholar]
- Wessing A, Zierold K. The formation of type-I concretions in Drosophila Malpighian tubules studied by electron microscopy and X-ray microanalysis. J Insect Physiol. 1999;45:39–44. doi: 10.1016/s0022-1910(98)00097-3. [DOI] [PubMed] [Google Scholar]
- Wessing A, Zierold K, Hevert F. Two types of concretions in Drosophila Malpighian tubules as revealed by X-ray microanalysis: A study of urine formation. J. Insect Physiol. 1992;38:543–554. [Google Scholar]
- Wessing A, Zierold K, Schafer D. Intracellular storage of sodium and magnesium in Drosophila Malpighian tubules. X-ray microanalysis of native cryosections. Eur J Cell Biol. 1988;47:1–6. [PubMed] [Google Scholar]
- Wheelock GD, Petzel DH, Gillett JD, Beyenbach KW, Hagedorn HH. Evidence for hormonal control of diuresis after a blood meal in the mosquito Aedes aegypti. ARCH INSECT BIOCHEM PHYSIOL. 1988;7:75–90. [Google Scholar]
- Wieczorek H, Beyenbach KW, Huss M, Vitavska O. Vacuolar-type proton pumps in insect epithelia. J. Exp. Biol. 2009;212:1611–1619. doi: 10.1242/jeb.030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek H, Putzenlechner M, Zeiske W, Klein U. A vacuolar-type proton pump energizes K+/H+ antiport in an animal plasma membrane. J. Biol. Chem. 1991;266:15340–15347. [PubMed] [Google Scholar]
- Williams JC, Beyenbach KW. Differential effects of secretagogues on Na and K secretion in the Malpighian tubules of Aedes aegypti (L.) J. Comp. Physiol. 1983;149:511–517. [Google Scholar]
- Williams JC, Beyenbach KW. Differential effects of secretagogues on the electrophysiology of the Malpighian tubules of the yellow fever mosquito. J. Comp. Physiol. [B] 1984;154:301–309. [Google Scholar]
- Williams JC, Hagedorn HH, Beyenbach KW. Dynamic changes in flow rate and composition of urine during the post-bloodmeal diuresis in Aedes aegypti (L.) J Comp Physiol [A] 1983;153:257–265. [Google Scholar]
- Wu DS, Beyenbach KW. The dependence of electrical transport pathways in Malpighian tubules on ATP. J. Exp. Biol. 2003;206:233–243. doi: 10.1242/jeb.00066. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang Z, Miyamoto Y, Reinach PS. Cell signaling pathways mediating epidermal growth factor stimulation of Na:K:2Cl cotransport activity in rabbit corneal epithelial cells. J. Membr. Biol. 2001;183:93–101. doi: 10.1007/s00232-001-0057-6. [DOI] [PubMed] [Google Scholar]
- Yu M, Beyenbach KW. Leucokinin and the modulation of the shunt pathway in Malpighian tubules. J Insect Physiol. 2001;47:263–276. doi: 10.1016/s0022-1910(00)00084-6. [DOI] [PubMed] [Google Scholar]
- Yu MJ, Beyenbach KW. Effects of leucokinin-VIII on Aedes Malpighian tubule segments lacking stellate cells. J Exp Biol. 2004;207:519–526. doi: 10.1242/jeb.00772. [DOI] [PubMed] [Google Scholar]
- Zimmermann B, Dames P, Walz B, Baumann O. Distribution and serotonin-induced activation of vacuolar-type H+-ATPase in the salivary glands of the blowfly Calliphora vicina. J Exp Biol. 2003;206:1867–1876. doi: 10.1242/jeb.00376. [DOI] [PubMed] [Google Scholar]