Abstract
Acamprosate is clinically used to treat alcoholism. However, the precise molecular functionality of acamprosate in the central nervous system remains unclear, although it is known to antagonize glutamate action in the brain. Since elevated glutamate signaling, especially in the nucleus accumbens (NAc), is implicated in several aspects of alcoholism, we utilized mice lacking type 1 equilibrative nucleoside transporter (ENT1), which exhibit increased glutamate levels in the NAc as well as increased ethanol drinking behaviors. We found that acamprosate significantly reduced ethanol drinking of mice lacking ENT1 (ENT1–/–) while having no such effect in wild-type littermates. We then analyzed the basal and acamprosate-treated accumbal metabolite profiles of ENT1–/– and wild-type mice using in vivo 16.4T proton magnetic resonance spectroscopy (MRS). Our data show that basal glutamate+glutamine (Glx), glutamate, glutamine and N-acetylaspartatic acid (NAA) levels are increased in the nucleus accumbens (NAc) of ENT1–/– compared to wild-type mice. We then found that acamprosate treatment significantly reduced Glx and glutamine levels while increasing taurine levels in the NAc of only ENT1–/– compared to their saline-treated group while normalizing other metabolite compared to wild-type mice. This study will be useful in the understanding of the molecular basis of acamprosate in the brain.
Keywords: alcoholism, ENT1, glutamate, taurine, in vivo MRS, acamprosate
INTRODUCTION
Acamprosate (Campral®) is a taurine homologue, which has been FDA-approved and is clinically used to treat alcoholism [34]. However, the effect of acamprosate is not uniform in all patients, with some patients responding better to this drug than others [15]. Therefore, understanding the molecular function of acamprosate and how brain metabolites are affected by its administration will help in the prediction of a successful reduction in alcohol drinking in humans [12, 18].
Adenosine is one of the main inhibitory neuromodulators that regulates glutamate levels in response to alcohol [1]. An ethanol-sensitive nucleoside transporter, type 1 equilibrative nucleoside transporter (ENT1), regulates adenosine levels in response to acute ethanol treatment [16]. Human genetic association studies indicate that allelic variants of SLC29A1 (human ENT1) are associated with an alcohol abuse phenotype in women [10]. A number of recent studies also suggest that ENT1 gene expression is inversely correlated with ethanol drinking and sensitivity to the acute intoxicating effects of ethanol in several rodent models [1, 5, 19, 24, 25]. Specifically, mice lacking ENT1 (ENT1–/–) exhibit reduced ataxic and hypnotic effects of acute ethanol intoxication as well as increased ethanol drinking compared to wild-type littermates [4, 5, 17]. Based on our previous studies, these behaviors appear to be mediated by constitutively increased glutamate levels in the NAc of ENT1–/– mice [4, 5, 17]. Since chronic ethanol treatment and withdrawal are known to increase extracellular glutamate levels in several brain regions [22, 23], ENT1–/– mice may be used as a model representing adenosine-mediated, constitutively-increased glutamate signaling in alcoholism [4, 5, 17].
Here we report that acamprosate significantly reduces ethanol drinking in ENT1–/– mice. Using in vivo 16.4T [1H] MRS, we investigated the basal and acamprosate treated metabolite profile in the NAc of ENT1–/– mice. Our results indicate that acamprosate may reduce ethanol drinking by normalizing Glx levels and increasing taurine levels in the NAc of ENT1–/– mice.
MATERIALS AND METHODS
ENT1–/– mice were generated as described previously [5]. F1 generation mice were generated by crossing 129X1/SvJ background heterozygous ENT1 mice with C57BL/6J wild-type mice. The C57BL/6J and 129X1/SvJ mice were purchased from the Jackson laboratory (Bar Harbor, ME). The heterozygous F1 mice were then crossed to generate F2 generation mice. Only 8-16 week old male F2 generation homozygote wild-type and ENT1–/– mice were used in all experiments. Animal care and handling procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committees in accordance with NIH guidelines.
The effect of acamprosate on oral alcohol self-administration and preference were examined using a modified two-bottle choice drinking experiment [13]. To examine the effect of acamprosate on ethanol consumption and preference, mice were given 200 mg/kg (i.p.) acamprosate (Estechpharma, Korea) every 12 h for four additional days after mice steadily consumed 10% ethanol in a two-bottle choice drinking experiment.
In vivo magnetic resonance spectroscopy (MRS) was performed on a 16.4 T (700 MHz) Bruker Avance III 700WB spectrometer with an 89 mm vertical bore (Bruker BioSpin, Billerica, MA). Mice were anesthetized in an isolated chamber using 3.5% isoflurane. For the basal metabolite level measurements, mice were injected with saline (i.p.) and to analyze the effect of acamprosate, mice were given a 200 mg/kg i.p. injection of acamprosate. Mice were immediately placed in the anesthesia chamber for 5-10 min following injections and then inserted into the probe for scanning. For both experiments, the mouse typically spent about 95 minutes inside the magnet (5 minutes preparation time and 90 minutes scan time). Accumbal metabolites were quantified using LCModel Version 6.2-2 software [20]. Metabolites were quantified as percent ratio to total creatine (creatine + phosphocreatine) levels as an internal control to standardize for animal and instrumental variations as well as for the total number of cells analyzed within the volume of interest (VOI). Pilot fast low-angle shot (FLASH) images were recorded for placement of an 8 μl (2 × 2 × 2 mm3) accumbal VOI [28]. Then, method specific local magnet field homogenization was performed. A point-resolved spectroscopy (PRESS) sequence was used with a repetition time (TR) of 1768 ms, an echo-time (TE) of 10 ms, and a number of averages (NA) of 3072. Consistent spectral resolution was ensured by considering LCModel calculated Cramér-Rao lower bounds. LCModel quantified glutamate + glutamine (Glx) with a Cramér-Rao lower bounds of less than 10%; N-acetylaspartatic acid (NAA), glutamate, glutamine, glycerophosphocholine + phosphocholine (GPC + PCh), guanosine, taurine, γ-aminobutyric acid (GABA), myo-inositol, and phosphocreatine with a Cramér-Rao lower bounds of less than 20%; and N-acetylaspartylglutamic acid (NAAG), alanine and lactate with a Cramér-Rao lower bounds of less than 35% in all spectra.
All data are presented as the mean ± SEM (standard error of the mean). Data were analyzed by two-tailed t-test, one-way or two-way repeated measures ANOVA. Results were considered significantly different when p < 0.05.
RESULTS
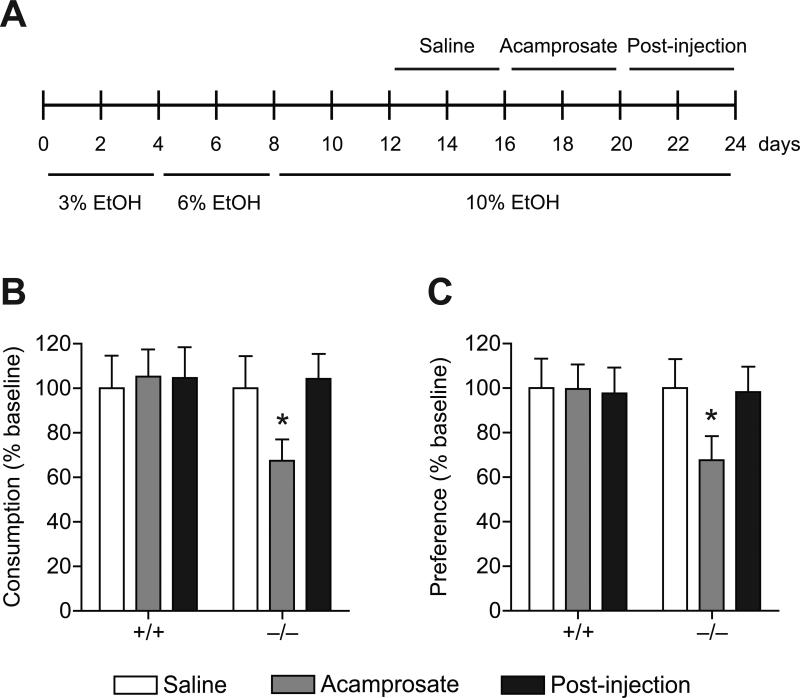
We examined whether acamprosate could reduce ethanol drinking behaviors in ENT1–/– mice in a two-bottle drinking experiment. When analyzing the drinking behaviors of ENT1–/– and wild-type mice we found that acamprosate significantly reduced ethanol consumption (Figure 1B; n = 16 per genotype) and preference (Figure 1C; n = 15~16 per genotype) of ENT1–/– mice compared to respective saline injection periods in a two-bottle choice drinking experiment. As reported, ENT1–/– mice consume more ethanol than wild-type mice under basal conditions [5]. Thus, to examine the antidipsotrophic effect of acamprosate, we analyzed the data after normalization by baseline. For ethanol consumption, one-way repeated measures ANOVA on ENT1–/– mice indicated a significant effect of acamprosate treatment in ENT1–/– mice (F2,24 = 8.395, p = 0.002) but not in wild-type littermates. For ethanol preference, one-way repeated measures ANOVA on ENT1–/– mice also indicated a significant effect of acamprosate treatment in ENT1–/– mice (F2,22 = 3.712, p = 0.041) but not in wild-type littermates. These data indicate that acamprosate treatment (i.p.) is effective in reducing ethanol consumption and preference in ENT1–/– mice but not in wild-type littermates. Additionally, during the post-injection phase (the four days following the acamprosate administration period), ethanol drinking returned to levels similar to that of baseline in ENT1–/– mice.
Figure 1.
Reduced ethanol consumption and preference by acamprosate administration in ENT1–/– mice. (A) Mice were given 12 days to acclimate to a 10% (v/v) ethanol concentration. Then mice were injected with saline and acamprosate (4 days each) during the two-bottle drinking experiment. The post-injection period refers to the 4 days following the acamprosate injection period. (B) Reduced ethanol consumption and (C) preference in ENT1–/– mice during the acamprosate administration period compared to the saline injection period. n = 15~16 mice per genotype and per treatment group. All data are expressed as mean ± SEM. *p < 0.05 compared to respective saline treatment groups by one-way repeated measures ANOVA.
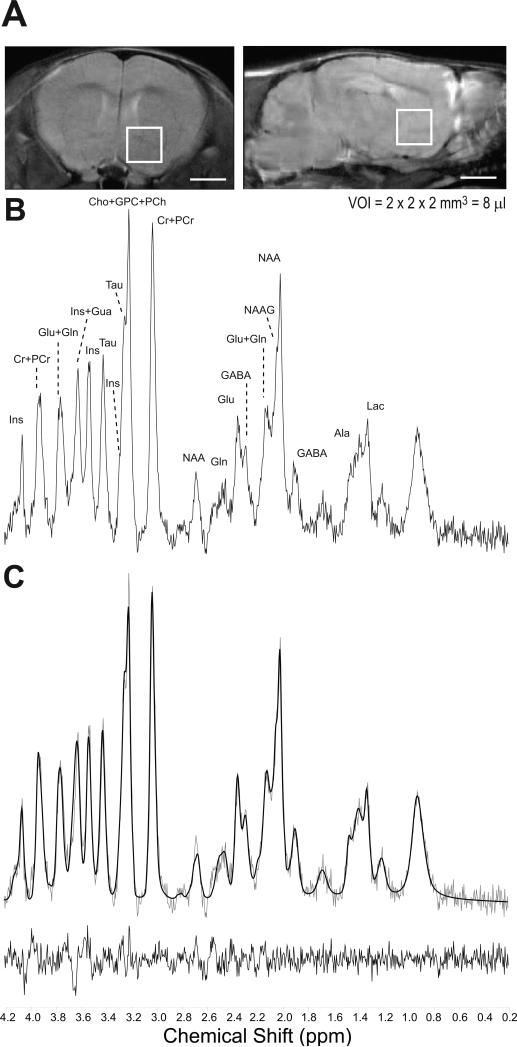
Since acamprosate is capable of altering the drinking behaviors of ENT1–/– mice, we investigated whether acamprosate treatment could alter the brain metabolite profile of mice lacking ENT1 using in vivo proton MRS. Figure 2A is a representation of the position of the 8 μl VOI on 1.0 mm thick coronal and sagittal sections of the mouse brain using a rapid acquisition relaxation enhanced (RARE; RARE factor = 4, TE = 9.6 ms, TR = 4000 ms, NA = 4) sequence for increased resolution. The data from three consecutive 30 min acquisitions were added together to maximize spectral sensitivity and resolution making the total scan time 90 min (Figure 2B). Spectra were fit using LCModel. Figure 2C is the LCModel best fit metabolite profile of the example spectrum and the residual difference.
Figure 2.
Metabolite profile from the mouse nucleus accumbens using in vivo 16.4T MRS and LCModel. (A) Representative RARE images of 1.0 mm thick coronal and sagittal brain sections where the 8 μl volume of interest (VOI) is centered on the nucleus accumbens. Scale bar = 2 mm. (B) Representative brain metabolite spectrum acquired using in vivo 16.4T. See Table 1 for abbreviation of labeled brain metabolites. (C) LCModel best fit of the brain metabolite spectrum in Fig. 2B and residual difference.
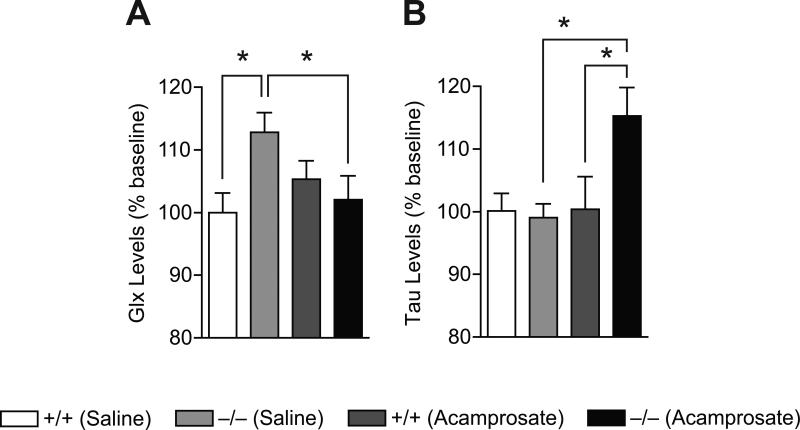
In the present study using in vivo 16.4T MRS, we measured elevated basal total glutamate levels in the NAc of ENT1–/– mice compared to wild-type littermates (Figure 3A and Table 1). When comparing wild-type and ENT1–/– mice metabolite levels (n = 10~12 per genotype and metabolite), two-tailed t-test illustrated that ENT1–/– mice display elevated glutamate+glutamine (Glx; p = 0.01), glutamate (p = 0.04), glutamine (p = 0.009) and NAA (p = 0.008) levels in the NAc while other metabolites were not altered (Figure 3B and Table 1). According to our previous work, elevated basal glutamate levels in the NAc might contribute to some of the observed behavioral phenotypes of mice lacking ENT1 including reduced ataxic and hypnotic effects of acute ethanol intoxication as well as increased voluntary ethanol consumption compared to wild-type littermates [5].
Figure 3.
The effect of acamprosate on basal accumbal metabolite levels of wild-type and ENT1–/– mice presented as percent baseline. (A) Basal glutamate+glutamine (Glx) is significantly increased in ENT1–/– mice compared wild-type mice and acamprosate treatment normalizes this increase. (B) Acamprosate administration significantly increases taurine (Tau) levels in ENT1–/– mice compared to ENT1–/– mice basal levels and compared to wild-type acamprosate treatment levels. n = 10~11 mice per genotype, treatment and metabolite. All data are expressed as mean ± SEM. *p < 0.05 by two-tailed t-test.
Table 1.
Effect of acamprosate treatment in the NAc
| Metabolites | Wild-type |
ENT1–/– |
||
|---|---|---|---|---|
| Saline | Acamprosate | Saline | Acamprosate | |
| N-acetylaspartatic acid (NAA) | 0.76 ± 0.03 (12) | 0.77 ± 0.03 (10) | 0.87 ± 0.02* (12) | 0.77 ± 0.04 (12) |
| Glutamate (Glu) | 0.89 ± 0.03 (10) | 0.94 ± 0.03 (10) | 0.98 ± 0.03* (10) | 0.92 ± 0.03 (10) |
| Glutamine (Gln) | 0.40 ± 0.01 (10) | 0.39 ± 0.02 (10) | 0.45 ± 0.01* (10) | 0.37 ± 0.02† (10) |
| Glutamate+Glutamine (Glx) | 1.26 ± 0.04 (10) | 1.33 ± 0.04 (10) | 1.43 ± 0.04* (10) | 1.29 ± 0.05† (10) |
| Glycerophosphocholine+Phosphocholine (GPC+PCh) | 0.34 ± 0.01 (12) | 0.33 ± 0.01 (10) | 0.35 ± 0.01 (12) | 0.31 ± 0.01† (12) |
| Taurine (Tau) | 1.22 ± 0.03 (10) | 1.23 ± 0.06 (10) | 1.21 ± 0.03 (12) | 1.41 ± 0.06*,† (11) |
| N-acetylaspartylglutamic acid (NAAG) | 0.17 ± 0.02 (10) | 0.12 ± 0.01 (4) | 0.15 ± 0.01 (6) | 0.16 ± 0.01 (7) |
| Alanine (Ala) | 0.26 ± 0.04 (8) | 0.19 ± 0.01 (7) | 0.21 ± 0.02 (9) | 0.21 ± 0.02 (9) |
| γ-aminobutyric acid (GABA) | 0.62 ± 0.04 (12) | 0.61 ± 0.04 (10) | 0.68 ± 0.03 (12) | 0.65 ± 0.04 (12) |
| Guanosine (Gua) | 0.59 ± 0.04 (11) | 0.57 ± 0.03 (10) | 0.53 ± 0.02 (12) | 0.56 ± 0.03 (11) |
| Lactate (Lac) | 0.40 ± 0.03 (10) | 0.48 ± 0.11 (7) | 0.49 ± 0.06 (10) | 0.46 ± 0.04 (6) |
| myo-Inositol (Ins) | 1.08 ± 0.05 (12) | 0.97 ± 0.10 (10) | 1.12 ± 0.04 (12) | 1.02 ± 0.03 (12) |
| Phosphocreatine (PCr) | 0.84 ± 0.04 (12) | 0.87 ± 0.05 (10) | 0.77 ± 0.03 (11) | 0.84 ± 0.04 (12) |
p < 0.05 compared to wild-type mice within each treatment group by two-tailed t-test.
p < 0.05 compared to saline treated group within each genotype by two-tailed t-test.
Metabolites are measured relative to total creatine. All data are expressed as mean ± SEM. Number in parentheses is number of mice used in statistical analyses based on Cramér-Rao lower bounds. See “Materials and Methods” for detailed inclusion criteria for statistical analyses.
Since acamprosate is thought to regulate glutamate levels or neurotransmission [8, 14, 21, 26], we analyzed the effect of acamprosate treatment on the metabolite profile of ENT1–/– mice using in vivo 16.4T MRS. We found that acamprosate treatment significantly reduced Glx and glutamine levels and increased taurine and glycerophosphocholine+phosphocholine (GPC+PCh) levels compared to saline treated ENT1–/– mice, while having no such effect in wild-type mice (Figure 3 and Table). When comparing acamprosate treatment and an equivalent volume of saline in wild-type mice, no significant alterations in any metabolite levels were observed (Figure 3 and Table 1). However, when analyzing acamprosate treatment compared to an equivalent volume of saline in ENT1–/– mice, two-tailed t-test indicated a significant difference between acamprosate treatment and saline treatment for Glx (p = 0.003), glutamine (p = 0.011), taurine (p = 0.003) and GPC+PCh (p = 0.024; Figure 3 and Table 1). Taurine was also significantly increased during acamprosate treatment in ENT1–/– mice compared to acamprosate treatment in wild-type mice (p = 0.042). Additionally, two-way ANOVA indicated an effect of genotype (F1,42 = 3.849, p = 0.05) and treatment (F1,42 = 5.215, p = 0.028) without a significant interaction between genotype and treatment. Taken together, these results indicate that reduced Glx and glutamine levels and/or increased taurine levels in the NAc might be important in the regulation of ethanol consumption.
Discussion
Our results indicate that basal accumbal glutamate+glutamine (Glx), glutamate and glutamine are significantly increased in ENT1–/– mice compared to their wild-type littermates. Deletion or inhibition of ENT1 increases extracellular glutamate levels by inhibiting presynaptic adenosine A1 receptors (A1R) [5] and/or astrocytic excitatory amino acid transporter type 2 (EAAT2) [33]. Therefore, it is likely that the increased basal accumbal glutamate levels of ENT1–/– mice compared to wild-type mice measured by MRS are at least in part, owing to increased extracellular glutamate levels. Basal glutamine, as well as Glx, levels were also significantly increased compared to wild-type littermates which may be a result of the astrocytic glutamate-glutamine cycle [1]. We have also found that N-acetylaspartatic acid (NAA) is significantly increased in the NAc of ENT1–/– mice compared to wild-type littermates. Some studies suggest that NAA is important as a measure of neuronal function and structural integrity, while reduced levels are implicated in neurodegenerative disorders [29]. Decreased cortical NAA is also correlated with neuronal loss, relative decreased viability, and/or impaired function [9]. In contrast, elevated striatal NAA levels may reflect increased neuronal activity [11] which is consistent with the observation of increased glutamate excitatory postsynaptic currents in the NAc of ENT1–/– mice [5].
Acamprosate treatment successfully antagonized glutamate/glutamine signaling in the NAc of only ENT1–/– mice. Since ENT1–/– mice appear to mimic either a chronic ethanol treated or withdrawn status, it is possible that an addictive phenotype such as elevated glutamate levels is required for acamprosate to be effective [14, 27]. Consistently, in clinical studies, acamprosate treatment has been shown to reduce glutamate levels in the anterior cingulate of detoxified alcohol-dependent patients as well as in the cortex of healthy individuals, both measured by MRS [2, 30]. While other recent studies show that acamprosate may interact with glycine receptors in the NAc and attenuate dopamine release, which may have an effect on alcohol consumption in rats [3]. Moreover, as ethanol enhances taurine-activated glycine receptor function [32], the blockade of glycine transporter-1 has been shown to reduce relapse-like alcohol drinking in rats [31]. However, the effect of acamprosate on specific receptors still awaits further investigation.
The present study is also consistent with a microdialysis study, which showed that acamprosate increased taurine levels in the NAc of rats [6]. Because a taurine injection (45 mg/kg, i.p.) blocks accumbal glutamate levels in rats [7], our findings suggest that increased taurine levels may reduce glutamate levels in ENT1–/– mice. Thus, acamprosate-induced elevated taurine levels might contribute to the normalization of glutamate or glutamine which could be associated with decreased voluntary ethanol consumption of ENT1–/– mice.
Since the voxel used is a cube, it is likely other brain regions, especially some portions of the dorsal striatum, might be included within the selected VOI. Nonetheless, the majority of the VOI consisted of the NAc. In future studies, it would be interesting to analyze the metabolite profiles and drug effects in other brain regions including the anterior cingulate or dorsal striatum. The long-term effect of acamprosate on the accumbal metabolite profile as measured by MRS also awaits further investigation.
In summary, our findings demonstrate that acamprosate treatment reduced ethanol drinking of ENT1–/– mice. Then, using in vivo 16.4T [1H] MRS, we quantified several brain metabolites related to ethanol intoxication and consumption. Our findings indicate that Glx and glutamine levels were normalized while taurine levels were significantly increased by acamprosate administration. This study suggests that manipulating taurine levels or the receptors associated with taurine metabolism could be a potential therapeutic target for alcohol use disorders and in vivo MRS could be useful to diagnose or monitor the therapeutic process in humans.
ACKNOWLEDGMENTS
We thank E. Knight and D. Frederixon for preparing the manuscript and S. Provencher for providing LCModel basis sets. This research was supported by the Samuel Johnson Foundation for Genomics of Addiction Program at Mayo Clinic and by grants from the National Institutes of Health (NIH) to D.-S. C. (Ro1 AA015164, P20 AA017830-Project 1) and to S. I. M. (P20 AA017830-Project 3).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Acamprosate reduced ethanol drinking in mice lacking ethanol-sensitive ENT1.
- Using 16.4T MRS, we detected increased basal glutamate levels in ENT1 null mice.
- Acamprosate reduced glutamine and Glx levels in the NAc of ENT1 null mice.
- Acamprosate increased taurine levels as well in the NAc of ENT1 null mice.
References
- 1.Asatryan L, Nam HW, Lee MR, Thakkar MM, Dar MS, Davies DL, Choi DS. Implication of the Purinergic System in Alcohol Use Disorders. Alcohol. Clin. Exp. Res. doi: 10.1111/j.1530-0277.2010.01379.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolo N, Nedelec JF, Muzet M, De Witte P, Dahchour A, Durbin P, Macher JP. Central effects of acamprosate: part 2. Acamprosate modifies the brain in-vivo proton magnetic resonance spectrum in healthy young male volunteers. Psychiatry Res. 1998;82:115–127. doi: 10.1016/s0925-4927(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 3.Chau P, Stomberg R, Fagerberg A, Soderpalm B, Ericson M. Glycine receptors involved in acamprosate's modulation of accumbal dopamine levels: an in vivo microdialysis study. Alcohol. Clin. Exp. Res. 2010;34:32–38. doi: 10.1111/j.1530-0277.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Nam HW, Lee MR, Hinton DJ, Choi S, Kim T, Kawamura T, Janak PH, Choi DS. Altered glutamatergic neurotransmission in the striatum regulates ethanol sensitivity and intake in mice lacking ENT1. Behav. Brain Res. 2010;208:636–642. doi: 10.1016/j.bbr.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, Diamond I, Bonci A, Messing RO. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat. Neurosci. 2004;7:855–861. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- 6.Dahchour A, De Witte P. Ethanol and amino acids in the central nervous system: assessment of the pharmacological actions of acamprosate. Prog. Neurobiol. 2000;60:343–362. doi: 10.1016/s0301-0082(99)00031-3. [DOI] [PubMed] [Google Scholar]
- 7.Dahchour A, De Witte P. Taurine blocks the glutamate increase in the nucleus accumbens microdialysate of ethanol-dependent rats. Pharmacol. Biochem. Behav. 2000;65:345–350. doi: 10.1016/s0091-3057(99)00197-5. [DOI] [PubMed] [Google Scholar]
- 8.De Witte P, Littleton J, Parot P, Koob G. Neuroprotective and abstinence-promoting effects of acamprosate: elucidating the mechanism of action. CNS Drugs. 2005;19:517–537. doi: 10.2165/00023210-200519060-00004. [DOI] [PubMed] [Google Scholar]
- 9.Frye MA, Thomas MA, Yue K, Binesh N, Davanzo P, Ventura J, O'Neill J, Guze B, Curran JG, Mintz J. Reduced concentrations of N-acetylaspartate (NAA) and the NAA-creatine ratio in the basal ganglia in bipolar disorder: a study using 3-Tesla proton magnetic resonance spectroscopy. Psychiatry Res. 2007;154:259–265. doi: 10.1016/j.pscychresns.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Gass N, Ollila HM, Utge S, Partonen T, Kronholm E, Pirkola S, Suhonen J, Silander K, Porkka-Heiskanen T, Paunio T. Contribution of adenosine related genes to the risk of depression with disturbed sleep. J. Affect. Disord. 2010;126:134–139. doi: 10.1016/j.jad.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Harte MK, Bachus SB, Reynolds GP. Increased N-acetylaspartate in rat striatum following long-term administration of haloperidol. Schizophr. Res. 2005;75:303–308. doi: 10.1016/j.schres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Kiefer F, Mann K. Acamprosate: how, where, and for whom does it work? Mechanism of action, treatment targets, and individualized therapy. Curr. Pharm. Des. 2010;16:2098–2102. doi: 10.2174/138161210791516341. [DOI] [PubMed] [Google Scholar]
- 13.Lee MR, Hinton DJ, Song JY, Lee KW, Choo C, Johng H, Unal SS, Richelson E, Choi DS. Neurotensin receptor type 1 regulates ethanol intoxication and consumption in mice. Pharmacol. Biochem. Behav. 2010;95:235–241. doi: 10.1016/j.pbb.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mann K, Kiefer F, Spanagel R, Littleton J. Acamprosate: recent findings and future research directions. Alcohol. Clin. Exp. Res. 2008;32:1105–1110. doi: 10.1111/j.1530-0277.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- 15.Morley KC, Teesson M, Reid SC, Sannibale C, Thomson C, Phung N, Weltman M, Bell JR, Richardson K, Haber PS. Naltrexone versus acamprosate in the treatment of alcohol dependence: A multi-centre, randomized, double-blind, placebo-controlled trial. Addiction. 2006;101:1451–1462. doi: 10.1111/j.1360-0443.2006.01555.x. [DOI] [PubMed] [Google Scholar]
- 16.Nagy LE, Diamond I, Casso DJ, Franklin C, Gordon AS. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J. Biol. Chem. 1990;265:1946–1951. [PubMed] [Google Scholar]
- 17.Nam HW, Lee MR, Hinton DJ, Choi DS. Reduced effect of NMDA glutamate receptor antagonist on ethanol-induced ataxia and striatal glutamate levels in mice lacking ENT1. Neurosci. Lett. 2010;479:277–281. doi: 10.1016/j.neulet.2010.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ooteman W, Naassila M, Koeter MW, Verheul R, Schippers GM, Houchi H, Daoust M, van den Brink W. Predicting the effect of naltrexone and acamprosate in alcohol-dependent patients using genetic indicators. Addict. Biol. 2009;14:328–337. doi: 10.1111/j.1369-1600.2009.00159.x. [DOI] [PubMed] [Google Scholar]
- 19.Parkinson FE, Xiong W, Zamzow CR, Chestley T, Mizuno T, Duckworth ML. Transgenic expression of human equilibrative nucleoside transporter 1 in mouse neurons. J. Neurochem. 2009;109:562–572. doi: 10.1111/j.1471-4159.2009.05991.x. [DOI] [PubMed] [Google Scholar]
- 20.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 21.Putzke J, Spanagel R, Tolle TR, Zieglgansberger W. The anti-craving drug acamprosate reduces c-fos expression in rats undergoing ethanol withdrawal. Eur. J. Pharmacol. 1996;317:39–48. doi: 10.1016/s0014-2999(96)00696-6. [DOI] [PubMed] [Google Scholar]
- 22.Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J. Neurosci. 2004;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur. J. Pharmacol. 1995;283:177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- 24.Sharma R, Engemann S, Sahota P, Thakkar MM. Role of adenosine and wake-promoting basal forebrain in insomnia and associated sleep disruptions caused by ethanol dependence. J. Neurochem. 2010 doi: 10.1111/j.1471-4159.2010.06980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Short JL, Drago J, Lawrence AJ. Comparison of ethanol preference and neurochemical measures of mesolimbic dopamine and adenosine systems across different strains of mice. Alcohol. Clin. Exp. Res. 2006;30:606–620. doi: 10.1111/j.1530-0277.2006.00071.x. [DOI] [PubMed] [Google Scholar]
- 26.Spanagel R, Kiefer F. Drugs for relapse prevention of alcoholism: ten years of progress. Trends Pharmacol. Sci. 2008;29:109–115. doi: 10.1016/j.tips.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat. Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- 28.Tkac I, Dubinsky JM, Keene CD, Gruetter R, Low WC. Neurochemical changes in Huntington R6/2 mouse striatum detected by in vivo 1H NMR spectroscopy. J. Neurochem. 2007;100:1397–1406. doi: 10.1111/j.1471-4159.2006.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai G, Coyle JT. N-acetylaspartate in neuropsychiatric disorders. Prog. Neurobiol. 1995;46:531–540. doi: 10.1016/0301-0082(95)00014-m. [DOI] [PubMed] [Google Scholar]
- 30.Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L, Adams LJ, Vengeliene V, Spanagel R, Zhang Y, Shen J, George DT, Hommer D, Heilig M. Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch. Gen Psychiatry. 2010;67:1069–1077. doi: 10.1001/archgenpsychiatry.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vengeliene V, Leonardi-Essmann F, Sommer WH, Marston HM, Spanagel R. Glycine transporter-1 blockade leads to persistently reduced relapse-like alcohol drinking in rats. Biol. Psychiatry. 2010;68:704–711. doi: 10.1016/j.biopsych.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 32.Welsh BT, Kirson D, Allen HM, Mihic SJ. Ethanol enhances taurine-activated glycine receptor function. Alcohol. Clin. Exp. Res. 2010;34:1634–1639. doi: 10.1111/j.1530-0277.2010.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Lee MR, Choi S, Kim T, Choi DS. ENT1 regulates ethanol-sensitive EAAT2 expression and function in astrocytes. Alcohol. Clin. Exp. Res. 2010;34:1110–1117. doi: 10.1111/j.1530-0277.2010.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zalewska-Kaszubska J, Gorska D, Dyr W, Czarnecka E. Effect of chronic acamprosate treatment on voluntary alcohol intake and beta-endorphin plasma levels in rats selectively bred for high alcohol preference. Neurosci. Lett. 2008;431:221–225. doi: 10.1016/j.neulet.2007.11.041. [DOI] [PubMed] [Google Scholar]