Summary
Erythropoietin (EPO) is the principal cytokine regulating erythropoiesis through its receptor, EPOR. Interestingly, EPORs are also found on immune cells with incompletely understood functions. Here, we show that EPO inhibits the induction of proinflammatory genes including tumor necrosis factor (TNF)-α and inducible nitric oxide (NO) synthase in activated macrophages, which is mechanistically attributable to blockage of nuclear factor (NF)-κB p65 activation by EPO. Accordingly, in systemic Salmonella infection, treatment of mice with EPO results in reduced survival and impaired pathogen clearance because of diminished formation of anti-microbial effector molecules such as TNF-α and NO. However, neutralization of endogenous EPO or genetic ablation of Epor promotes Salmonella elimination. In contrast, in chemically induced colitis, EPO-EPOR interaction decreases the production of NF-κB-inducible immune mediators, thus limiting tissue damage and ameliorating disease severity. These immune-modulatory effects of EPO may be of therapeutic relevance in infectious and inflammatory diseases.
Highlights
► Erythropoietin inhibits NF-κB activation ► EPO impairs Salmonella clearance ► Neutralization of endogenous EPO promotes Salmonella elimination ► In chemically induced colitis, EPO ameliorates diseases severity
Introduction
The renal cytokine hormone erythropoietin (EPO) regulates bone marrow erythrocyte production by stimulating the differentiation and inhibiting the apoptosis of erythroid progenitor cells (De Maria et al., 1999; Liu et al., 2006). However, EPO also bears extrahematopoietic properties that are transduced by EPO receptors (EPORs) expressed on various nonerythroid tissues including immune cells (Brines and Cerami, 2005; Jelkmann, 2007). The erythropoietic response is initiated upon binding of EPO to EPOR homodimers. In nonerythroid tissues by contrast, EPO targets a heteroreceptor complex composed of EPOR subunits assembled with beta common receptors (ßcRs), which are also utilized by other cytokine-specific and growth factor-specific receptors (Brines et al., 2004). Accordingly, EPO has been found to exert protective and antiapoptotic effects in animal models of ischemic, traumatic, and toxic tissue damage involving the nervous system, retina, myocardium, kidney, and liver (Chen et al., 2008; Digicaylioglu and Lipton, 2001; Imamura et al., 2007; Junk et al., 2002; Parsa et al., 2003; Sepodes et al., 2006).
Engagement of EPOR by EPO in erythroid cells results in the induction of Janus kinase-2 (JAK2)- and signal transducer and activator of transcription-5 (STAT5)-dependent signaling cascades (Neubauer et al., 1998; Parganas et al., 1998; Zhu et al., 2008). However, alternative signaling pathways are predicted to exert EPO-mediated effects in nonerythroid tissues (Zhang et al., 2009). In neurons, activation of mitogen-activated protein (MAP) kinase and phosphatidylinositol-3 kinase (PI3K)-Akt pathways have been linked to the antiapoptotic effects of EPO (Sirén et al., 2001). In addition, EPO protects cultured neurons from nitrosative stress-induced apoptosis through activation of JAK2 and nuclear factor (NF)-κB (Digicaylioglu and Lipton, 2001). In contrast, the interaction of EPO with EPORs on cancer cells promotes chemotherapy-induced apoptosis via inhibition of NF-κB (Carvalho et al., 2005). Although contrasting, these results are of interest given that NF-κB and Rel proteins encompass a family of pivotal transcriptional regulators centrally involved in the ligand-induced activation of proinflammatory immune effector pathways in various cell types including macrophages (Akira et al., 2006; Karin and Delhase, 2000).
Taking into consideration the pleiotropic effects of EPO in extraerythroid tissues, the expression of EPORs on immune cells, and the partial similarities between EPO- and cytokine-mediated signal transduction, we questioned whether EPO may exert putative immune-modulatory effects, which could be of clinical relevance in certain inflammatory diseases. We found that EPO induces EPOR-JAK2 signal transduction in myeloid cells thus impairing the classical NF-κB p65 activation pathway. The consequent interference with innate immune response mechanisms resulted in the deterioration of Salmonella infection and ameliorated chemically induced experimental colitis.
Results
Effects of EPO on Macrophage Immune Effector Mechanisms In Vitro
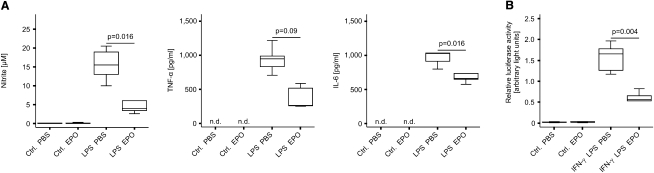
To verify the presence of EPOR complexes, we isolated primary macrophages from different anatomical locations. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) revealed that macrophages expressed considerable quantities of EPOR, β common receptor (ßcR) and JAK2 mRNA (Figures S1A, S1B, and S1C available online). In comparison, CD4+ T cells and hepatocytes displayed low expression, whereas in bone marrow erythroid cells, characterized by the presence of the erythroid-specific cell surface marker Ter119, mainly EPOR and JAK2 mRNA were detected. When subsequently evaluating the impact of EPO on activated macrophages, we found that addition of EPO to primary macrophages in culture significantly reduced the accumulation of nitrite, the stable end-product of the nitric oxide (NO) pathway, as well as of tumor necrosis factor (TNF)-α and interleukin (IL)-6 in culture supernatants derived from lipopolysaccharide (LPS)-stimulated primary peritoneal macrophages as compared to cells treated with solvent (Figure 1A). Moreover, we measured a reduction in interleukin-12p70 (IL-12p70) and IL-23 concentrations under these conditions, although these effects did not reach statistical significance (Figure S1D). These observations were paralleled by reduced mRNA amounts of inducible NO synthase (Nos2), TNF-α, and IL-6 in response to EPO treatment (Figure S1E), whereas mRNA amounts of IL-1β, IL-10, and IL-18 were not affected by EPO treatment (data not shown). Similar results were obtained when stimulating primary peritoneal macrophages or RAW264.7 macrophage-like cells with a combination of interferon-γ (IFN-γ) and LPS, both of which are pivotal inducers of Nos2 expression in these cells (data not shown). These anti-inflammatory effects of EPO on LPS- or IFN-γ and LPS-stimulated macrophages were not affected by pretreatment of EPO-exposed cells with actinomycin D or cycloheximide, suggesting that they were not mediated via alterations of mRNA half-lives or de novo protein synthesis, respectively (data not shown).
Figure 1.
Recombinant EPO Inhibits Proinflammatory Immune Responses in Macrophages In Vitro
(A) Thioglycolate-elicited primary peritoneal macrophages were pretreated with PBS or EPO 30 min before the addition of LPS or solvent. Supernatants were analyzed for concentrations of nitrite and of cytokines and data from at least 3 independent experiments were compared by means of Kruskal-Wallis test. Values are depicted as lower quartile, median and upper quartile (boxes) with minimum and maximum ranges, and statistical significances between PBS- and EPO-treatment are indicated. TNF-α and IL-6 concentrations in supernatants of solvent-treated control macrophages remained below the reported detection limits of the corresponding ELISA kits. n.d. denotes not detectable.
(B) RAW264.7 macrophage-like cells were transiently transfected with a plasmid containing the luciferase gene under the control of the full-length murine Nos2 promoter. Relative luciferase activity is shown. Data were compared and are depicted as in Figure 1A.
However, using a full-length Nos2 promoter firefly luciferase construct in a dual reporter gene assay, we found that EPO treatment resulted in a diminished transcription of the Nos2 gene in transiently transfected macrophages (Figure 1B). These results thus suggested that EPO reduces the production of proinflammatory mediators such as NO by activated macrophages by directly inhibiting Nos2 transcription.
Effects of EPO on the Activity of Proinflammatory Transcription Factors
Because EPO affects iron uptake via the transferrin receptor-1 (TFR1) in erythroid cells (Weiss et al., 1997) and iron inhibits proinflammatory immune effector pathways in macrophages (Weiss et al., 1994), we studied the putative effects of EPO on macrophage iron homeostasis. We found that the effects of EPO on macrophage functions were independent of a putative modulatory effect on macrophage iron homeostasis given that EPO did not modify mRNA or protein expression of TFR1 (Figures S2A, S2B and S2C), and the addition of a blocking TFR1 antibody did not modify the effects of EPO toward Nos2 mRNA expression or NO generation in RAW264.7 cells (data not shown).
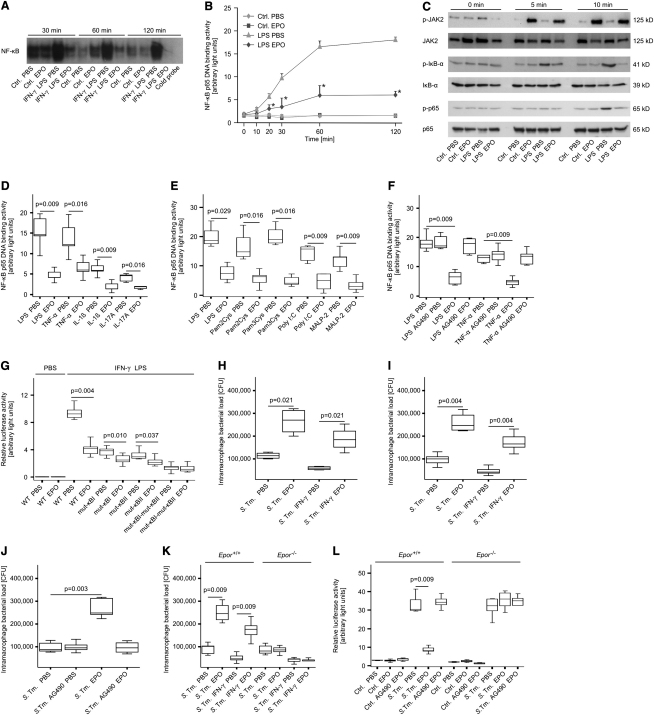
As a next step, we studied the activation of several transcription factors involved in the induction of Nos2 transcription or implicated in signaling events downstream of EPOR. Electromobility shift assays demonstrated that EPO inhibited the DNA binding activity of NF-κB in RAW264.7 cells stimulated with IFN-γ and LPS (Figure 2A), yet did not influence binding activities of STAT1, STAT3, and STAT5, interferon regulatory factor-1 (IRF1), or NF-IL6 (details not shown).
Figure 2.
Recombinant EPO Impairs NF-κB Activation and Salmonella Elimination In Vitro
(A) RAW264.7 macrophage-like cells were treated with EPO, IFN-γ, and LPS for the indicated time periods. NF-κB DNA binding activity in nuclear extracts was evaluated by means of electrophoretic mobility shift assay (EMSA). The specificity of binding was confirmed by cold competition with a 30-fold excess of the same unlabeled oligonucleotide (far right lane) with the nuclear extract of PBS-treated cells stimulated with IFN-γ and LPS for 120 min. One of three representative experiments is shown.
(B) Nuclear proteins were used for specific quantification of NF-κB p65 binding activity by a commercially available chemi-luminescent transcription factor assay at the indicated time points. Data of three independent experiments are expressed as arbitrary light units and shown as means ± SEM and were compared by means of Kruskal-Wallis test. Asterisks indicate statistically significant differences between LPS-stimulated cells pretreated with either PBS or EPO: p < 0.05.
(C) Cytoplasmic proteins from parallel experiments described in the legend for Figure 2B were used for evaluating phosphorylation of JAK2, IκB-α, and of NF-κB p65 by means of western blot as described in Experimental Procedures. Total JAK2, IκB-α, and NF-κB p65 protein, respectively, served as loading controls. One of three representative experiments is shown.
(D–F) RAW 264.7 cells were pretreated with PBS or EPO as above and subsequently stimulated with LPS or recombinant murine cytokines (D). Alternatively, RAW cells were treated with EPO and the Toll-like receptor (TLR) ligands LPS, PAM2Cys, PAM3Cys, Poly(I:C), or MALP-2 (E). RAW cells were treated with LPS or TNF-α after preincubation with the specific JAK2 inhibitor AG490 (F). Nuclear proteins were used for specific quantification of NF-κB p65 binding activity by a commercially available chemi-luminescent transcription factor assay after 120 min. Data of five independent experiments are depicted as arbitrary light units and shown as means ± SEM.
(G) RAW264.7 cells were transiently transfected with murine Nos2 promoter constructs carrying site-specific mutations in one or both NF-κB binding sites (mut-κBI-Nos2-luc and mut-κBII-Nos2-luc or mut-κBI-mut-κBII-Nos2-luc, respectively). Thereafter, macrophages were stimulated with IFN-γ and LPS after preincubation with EPO or solvent. Luciferase activity was measured in a chemi-luminometer and is shown as arbitrary light units.
(H–K) RAW264.7 macrophage-like cells (H) or primary peritoneal macrophages (I-K) were infected with S. typhimurium (S. Tm.) at a MOI of 10. After 1 hr, cells were treated with EPO, IFN-γ, AG490 or solvent and incubated for a total of 24 hr. Thereafter, macrophages were lysed and intramacrophage bacteria were enumerated by plating serial dilutions of cell lysates.
(L) Primary peritoneal macrophages from Epor+/+ and Epor−/− mice were treated as above after transient transfection with a NF-κB reporter construct. Data of five independent experiments are depicted.
Quantification of the specific DNA binding activity of the NF-κB subunit p65 revealed that pretreatment of cells with EPO significantly reduced LPS-induced p65 binding (Figure 2B). Similarly, the effects of IFN-γ and LPS on p65 binding activity were reduced by 48%–64% in macrophages pretreated with EPO (data not shown). Western blot experiments showed that EPO treatment resulted in JAK2 activation, thus inhibiting the LPS-inducible phosphorylation of inhibitor of NF-κB (IκB)-α and the subsequent phosphorylation of the cytoplasmic p65 subunit (Figure 2C).
In contrast, EPO did not influence the IFN-γ- and LPS-inducible phosphorylation of STAT1 and STAT3 (Figure S2D and S2E), whereas STAT5 phosphorylation was substantially increased in response to EPO in both control and IFN-γ- and LPS-activated macrophages (Figure S2F).
Diminished EPO-mediated p65 binding activity was observed after stimulation of RAW264.7 macrophages with TNF-α, IL-1β, and IL-17A (Figure 2D) or with several Toll-like receptor (TLR) ligands (Figure 2E). Notably, pretreatment with the JAK2 inhibitor AG490 abolished the inhibitory effects of EPO on macrophage activation in response to LPS or TNF-α (Figure 2F). In contrast, inhibition of STAT5 resulted in reduced expression of vascular endothelial growth factor (VEGF) after EPO treatment, whereas Nos2 and TNF-α mRNA expression was unresponsive to STAT5 inhibition (Figures S2G, S2H, and S2I).
In RAW264.7 cells transfected with Nos2 promoter constructs that carry mutations in one or both NF-κB binding sites (mut-κBI-Nos2-luc and mut-κBII-Nos2-luc, or mut-κBI-mut-κBII-Nos2-luc, respectively), the inhibitory effect of EPO on IFN-γ- and LPS-induced Nos2-promoter controlled luciferase activity was substantially reduced in mut-κBI-Nos2-luc and mut-κBII-Nos2-luc expressing macrophages and completely abolished in cells transfected with the mut-κBI-mut-κBII-Nos2-luc construct (Figure 2G). Collectively, these data suggested that EPO reduces NF-κB p65 activition in response to various stimuli by inhibiting the phosphorylation of IκB-α in a JAK2-dependent fashion.
Effects of EPO on Salmonella Infection In Vitro
To investigate whether EPO may affect the capacity of macrophages to clear engulfed microbes, we infected cells with Salmonella enterica serovar Typhimurium (S. typhimurium). Quantification of bacterial counts after 24 hr revealed that incubation of infected cells with EPO resulted in increased bacterial loads in both RAW264.7 cells (Figure 2H) and primary peritoneal macrophages (Figure 2I) regardless of IFN-γ supplementation. Furthermore, these increased bacterial loads were accompanied by decreased amounts of Nos2, TNF-α, and IL-6 mRNA (data not shown). This effect was abolished in macrophages upon pharmacological inhibition of JAK2 with AG490 (Figure 2J) and in peritoneal macrophages lacking EPOR (Figure 2K). Correspondingly, transient transfections with NF-κB reporter constructs showed that the negative effects of EPO on NF-κB activation were dependent on the presence of EPOR and the functionality of JAK2 (Figure 2L).
Effects of EPO Administration on Salmonella Infection In Vivo
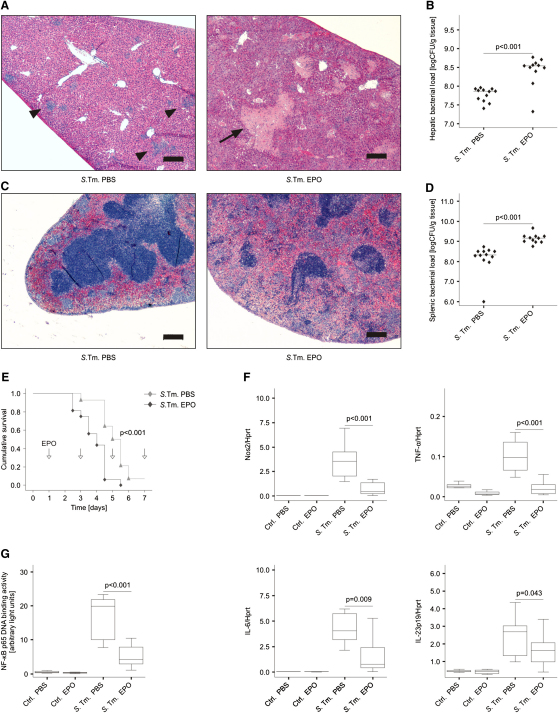
For assessing the effects of EPO toward a systemic infection in vivo, C57BL/6 mice were inoculated intraperitoneally (i.p.) with 500 colony forming units (CFU) of S. typhimurium and then with phosphate buffered saline (PBS) or EPO injection at a dose of 5 U/g body weight on days 3 and 4 after infection. Mice were sacrificed on day 5 for further analyses. Histopathological examination revealed that Salmonella-infected mice treated with PBS had defined microabscesses in livers and spleens (Figures 3A and 3C). In contrast, EPO-treated Salmonella-infected mice showed hepatic macroabscesses and scattered inflammatory foci in spleens. These observations were likewise secondary to enhanced pathogen proliferation given that EPO-treated mice exhibited significantly higher numbers of bacterial colonies in both organs (Figures 3B and 3D).
Figure 3.
EPO Administration Impairs Pathogen Clearance in Salmonella Infection In Vivo
(A and C) C57BL/6 mice were infected i.p. with 500 CFU of S. typhimurium and treated with PBS or EPO on days 3 and 4 after infection. Livers (A) and spleens (C) were removed and formalin-fixed samples were further processed for HE staining. Whereas PBS-treated Salmonella-infected (S. Tm.) animals had microabscesses in livers (indicated by arrowheads) and preserved splenic organ architecture, EPO-treated Salmonella-infected mice presented with macroabscesses in livers (indicated by an arrow) and scattered inflammatory foci in the spleens due to multiple microabscesses. No signs of thrombo-embolic events were observed in either group. Scale bars represent 200 μm.
(B and D) Bacterial loads were determined in livers (B) and spleens (D) on day 5 after infection. Data were combined from three independent experiments with similar results. Values from 11 or 12 mice per group were log-transformed and compared by means of Student's t test. Individual values and means are depicted and statistical significances between PBS- and EPO-treatment are indicated.
(E) C57BL/6 mice were infected i.p. with 500 CFU of S. typhimurium and treated with PBS or EPO every second day starting on day 1 (24 hr) after infection (n = 14–16 per group). Time points of EPO (or PBS) applications are indicated by arrows. Survival was monitored during an observation period of 7 days. The cumulative survival was analyzed by the log-rank test: p < 0.001 for the comparison of the two groups.
(F) Spleen samples were subjected to RNA preparation and quantitative determination of immune gene expression by RT-PCR. Data from 8–12 samples per group are shown as relative abundance of target gene expression in relation to the house-keeping gene hypoxanthin phospho-ribosyl transferase (Hprt).
(G) Spleen samples (n = 8–12 per group) were used for the preparation of nuclear extracts and NF-κB p65 binding activity was measured.
When investigating whether EPO treatment affects host survival in Salmonella infection, an independent series of experiments revealed that the median survival time of EPO-treated mice was markedly reduced as compared to solvent-treated animals (108 versus 132 hr, p < 0.001 when compared by log-rank test; Figure 3E). The mRNA expression of critical inflammatory genes was comparable in spleens and livers of infected mice. Importantly, EPO treatment significantly reduced mRNA expression of Nos2, TNF-α, IL-6, IL-12p35, and IL-23p19 and to a lesser extent of IL-1β in spleens of Salmonella-infected mice on day 5 after infection (Figure 3F and data not shown). The reduced expression of proinflammatory cytokines in Salmonella-infected spleens of EPO-treated animals was accompanied by the reduction of serum concentrations of theses mediators (Figure S3A) as well as by diminished splenic NF-κB p65 binding activity in comparison to PBS-treated controls (Figure 3G). Notably, the treatment of infected mice with EPO did not influence the mRNA expression of other key T helper (Th) cell cytokines such as IFN-γ, IL-4, IL-13, IL-17A, and IL-17F or of the master switch transcription factors t-bet, GATA-3, RORγt, and Foxp3 (Figure S3B and data not shown). Altogether, these results demonstrated that EPO impairs the clearance of engulfed Salmonella by macrophages by inhibiting the activation of NF-κB and thus the induction of proinflammatory immune response genes.
Role of the Endogenously Produced EPO in Salmonella typhimurium Infection
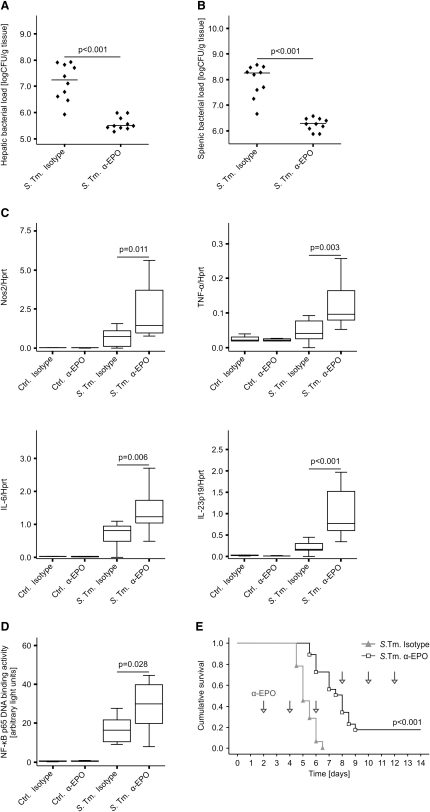
For determining whether endogenously produced EPO modifies the course of Salmonella septicemia, groups of 4-8 C57BL/6 mice were inoculated intraperitoneally (i.p.) with 500 CFU of S. typhimurium and treated with solvent, an isotype control antibody or a neutralizing EPO antibody on days 1 and 2 after infection. Treatment with the EPO antibody resulted in a significant reduction of bacterial counts in livers (Figure 4A) and spleens (Figure 4B) on day 4 of infection, whereas no difference in bacterial numbers between animals receiving PBS or the isotype control antibody was observed (details not shown). Accordingly, the enhanced resistance of animals treated with the EPO antibody was associated with increased splenic Nos2, TNF-α, IL-6, and IL-23p19 mRNA expression (Figure 4C) and higher NF-κB p65 binding activity (Figure 4D) as compared to isotype-treated controls. Of note, antibody-mediated neutralization of EPO resulted in improved survival of Salmonella-infected mice (120 versus 192 hr, p < 0.001 when compared by log-rank test; Figure 4E).
Figure 4.
Neutralization of Endogenous EPO Reduces Bacterial Loads and Stimulates Antibacterial Immune Effector Pathways
(A and B) C57BL/6 mice were infected i.p. with 500 CFU of S. typhimurium and treated with a neutralizing EPO antibody (α-EPO) or isotype control on days 1 and 2 after infection. Bacterial loads were determined in livers (A) and spleens (B) on day 4 after infection.
Spleen samples of PBS-treated controls (n = 4-6 per group) and of Salmonella-infected mice (n = 10 per group) were used to study the expression of immune response genes (C) and NF-κB p65 binding activity (D).
(E) For analysis of survival, C57BL/6 mice were infected i.p. with 500 CFU of S. typhimurium and treated with a neutralizing EPO antibody (or isotype control) every other day starting 2 days after infection as indicated by arrows.
Role of EPOR Signaling in Systemic Salmonella Infection
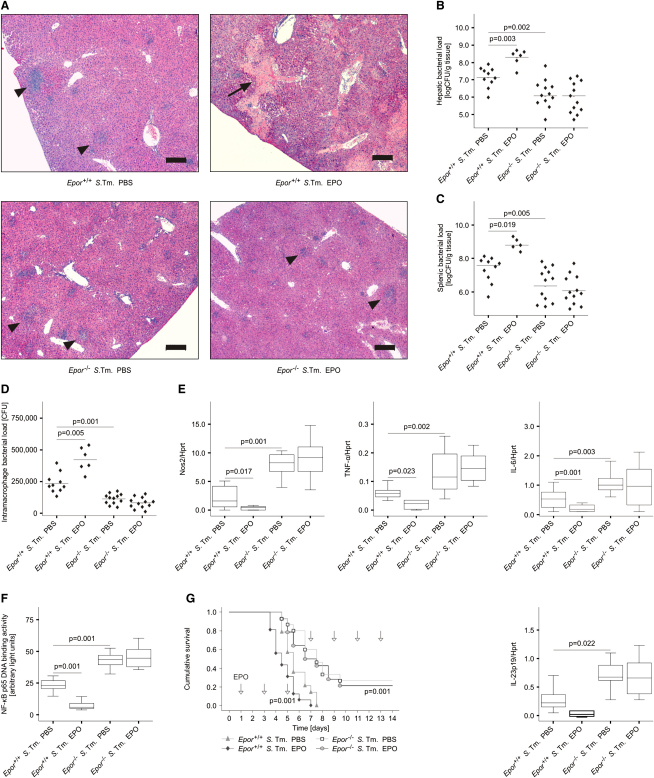
To study whether these effects can be linked to the direct interaction of EPO with its receptor, we infected Epor−/− mice and Epor+/+ littermates with S. typhimurium and treated them with EPO or PBS on days 3 and 4 after infection. Of relevance, EPO-treated Epor+/+ mice displayed hepatic macroabscesses, whereas EPO-treated Epor−/− did not (Figure 5A). Furthermore, both solvent- and EPO-treated Epor−/− mice had reduced bacterial colonization in livers and spleens as compared to their Epor+/+ littermates (Figures 5B and 5C). Similarly, Epor+/+ peritoneal macrophages isolated from EPO-treated mice were impaired in controlling intracellular Salmonella replication (Figure 5D). In contrast, Epor−/− macrophages displayed reduced bacterial burdens independent of preceding treatment of mice with PBS or EPO. In parallel, we measured increased mRNA expression of Nos2, TNF-α, IL-6, IL-12p35, and IL-23p19 in the spleens of Epor−/− mice as compared to Epor+/+ mice (Figure 5E and data not shown). Of note, NF-κB p65 binding activity was reduced in peritoneal macrophages isolated from Salmonella-infected Epor+/+ mice after EPO treatment as compared to PBS treatment, whereas this effect was not seen in Epor−/− macrophages (Figure 5F). As revealed by two independent experiments, in which Salmonella-infected mice were treated with EPO or solvent every other day, the enhanced immune activation present in Salmonella-infected Epor−/− mice translated into improved survival of these animals (113 versus 149 hr, p < 0.001 for comparison of Epor+/+ mice treated with either EPO or PBS by log-rank test; 149 versus 206 hr, p < 0.001 for comparison of PBS-treated Epor+/+ and Epor−/− mice; Figure 5G).
Figure 5.
EPOR Functionality on Nonerythroid Cells Regulates Immune Response and Outcome in Salmonella Infection
(A) Epor+/+ and Epor−/− C57BL/6 mice were infected i.p. with 500 CFU of S. typhimurium and treated with PBS or EPO (5 U/g body weight) on days 3 and 4 after infection. Liver histology showed macroabscesses (indicated by an arrow) in EPO-treated Epor+/+ mice and micro-abscesses (indicated by arrowheads) in animals assigned to the other three treatment groups (A). Scale bars represent 200 μm.
(B and C) Bacterial loads were determined in livers (B) and spleens (C) on day 5 after infection.
(D) Peritoneal macrophages of these mice were seeded in gentamicin-containing RPMI and intracellular bacterial loads were evaluated after 1 hr. Data were compared by Kruskal-Wallis test and statistical significances are indicated.
(E and F) Spleen samples of these mice (n = 6–12 per group) were used to measure the expression of immune response genes (E) and NF-κB p65 binding activity (F). Statistical significant differences between Epor+/+ treated with either PBS or EPO and between Epor+/+ and Epor−/− treated with PBS are indicated.
(G) For comparison of survival, Epor+/+ and Epor−/− C57BL/6 mice were infected i.p. with 500 CFU of S. typhimurium and treated with EPO at a dose of 5 U/g body weight (or PBS) every other day starting 1 day after infection as indicated by arrows. The cumulative survival was analyzed by the log-rank test: p < 0.001 for the comparison of Epor+/+ treated mice with either PBS or EPO; p < 0.001 for the comparison of Epor+/+ and Epor−/− mice treated with PBS.
Effects of EPO on the Course of Experimental Colitis
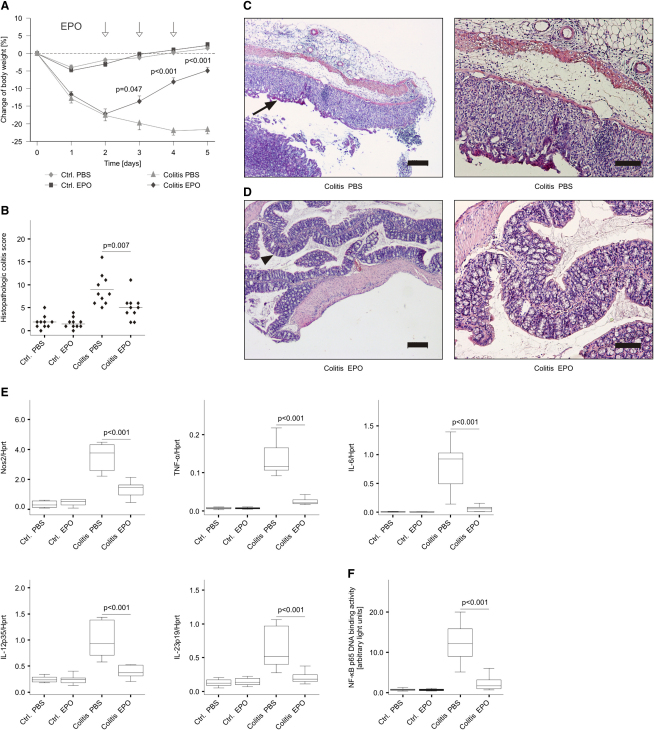
To evaluate the effects of EPO treatment in noninfectious inflammatory disease models, we first studied mice suffering from trinitrobenzene sulfonic acid (TNBS)-induced colitis. SJL/J mice were treated with solvent or EPO on 3 consecutive days starting on day 2 after induction of TNBS colitis. When studying the clinical course of the disease, EPO-treated TNBS-treated mice presented with an improved weight gain when compared to solvent-treated animals (Figure 6A).
Figure 6.
EPO Treatment Downregulates Proinflammatory Immune Pathways and Improves Disease Activity in TNBS-Induced Colitis
(A) SJL/J mice were subjected to cutaneous immunization of TNBS diluted in EtOH and then to intrarectal administration of TNBS diluted in EtOH; control mice were treated with PBS diluted in EtOH. Subsequently, mice were injected with EPO (or PBS) on days 2, 3, and 4 after induction of colitis as indicated by arrows. The change in weight is expressed as percentage of body weight from day 0, and data are shown as means ± SEM for 10 mice per group.
(B) Histopathological colitis scores for EtOH-instilled and TNBS-treated mice described in the legend to Figure 5A (n = 10 per group). Each point represents an individual mouse. Values are depicted as lower quartile, median, and upper quartile (boxes) with minimum and maximum ranges, and statistically significant differences between means of TNBS mice treated with either PBS or EPO are indicated.
(C and D) Photomicrographs of HE-stained colonic sections showed mucosal thickening, epithelial hyperplasia and inflammation (arrow) in PBS-treated TNBS-mice (C) and nearly unaffected mucosa (arrow head) observed in EPO-treated TNBS-mice (D). Colon histology is shown at low (left) and high (right) magnification. Scale bars: 200 μm (left) or 100 μm (right).
(E and F) qRT-PCR analysis of immune response genes (E) and NF-κB p65 binding activity (F) in colons of these mice (n = 10 per group).
Histological sections revealed no substantial disease activity in solvent-treated control mice (sections not shown), whereas in TNBS-treated animals, severe inflammation could be detected (Figures 6B, 6C, and 6D). Of note, the histopathological disease score was significantly reduced in TNBS-treated mice receiving EPO treatment as compared to PBS (Figure 6B).
Correspondingly, we found that in inflamed colons of TNBS-treated mice, EPO treatment significantly reduced the mRNA expression of Nos2, TNF-α, IL-6, IL-12p35, and IL-23p19 (Figure 6E) without affecting the mRNA expression of several other anti-inflammatory and cytoprotective cytokines such as IL-10 and IL-22 (data not shown). Accordingly, supernatants of primary colonic organ cultures obtained from EPO treated TNBS-exposed mice contained significantly reduced concentrations of nitrite, TNF-α, IL-6, IL-12p70, and IL-23 (data not shown) as compared to colonic supernatants of solvent-treated TNBS-exposed mice. Relevantly, the binding of NF-κB p65 to its consensus sequence was significantly lower in colonic extracts from EPO-treated than from solvent-treated TNBS-exposed mice (Figure 6F). Isolation of lamina propria cells revealed that EPO predominately influenced effector functions of CD11b+ myeloid cells resulting in reduced expression of Nos2, TNF-α, IL-6, IL-12p35, and IL-23p19 along with diminished p65 binding activity (Figure S4A). In contrast, no significant effects of EPO on cytokine expression in CD4+ T cells were observed (Figure S4B). Moreover, treatment of purified CD4+ T cells with EPO had no pronounced effect on cytokine production after in vitro stimulation of cells with either phorbol myristate acetate (PMA) and ionomycin or antibodies against CD3 and CD28 (Figure S4C and data not shown).
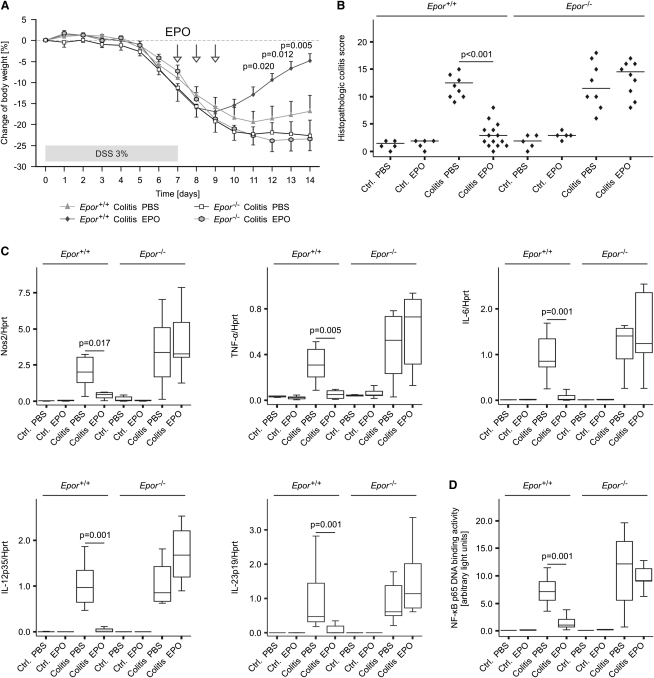
To confirm the specificity and relevance of EPO-EPOR signaling in experimental colitis, we next subjected Epor−/− mice and Epor+/+ littermates to oral dextran sulfate sodium (DSS) treatment for 7 consecutive days and followed them up for another 7 days. Body weight determinations and histological scoring on day 14 revealed that EPO was able to reduce the severity of DSS-induced colitis selectively in Epor+/+ mice, whereas Epor−/− mice did not benefit from EPO administration (Figures 7A and 7B). Furthermore, DSS-treated Epor+/+ displayed reduced colonic mRNA amounts of Nos2, TNF-α, and IL-6 and IL-12 and IL-23 subunits in response to EPO treatment (Figure 7C). In contrast, all DSS-fed Epor−/− mice as well as PBS-treated Epor+/+mice presented with high expression of these proinflammatory genes along with high p65 binding activities (Figure 7D). Taken together, these data convincingly show that EPO inhibits NF-κB activation and proinflammatory gene expression in lamina propria myeloid cells thus reducing the severity of chemically induced experimental colitis.
Figure 7.
EPO Treatment Downregulates Proinflammatory Immune Pathways and Improves Disease Activity in DSS-Induced Colitis
Epor−/− mice and Epor+/+ littermates on a C57BL/6 background were administered 3% DSS dissolved in water or water alone (controls) for 7 consecutive days. Thereafter, DSS was replaced by drinking water and all animals were followed up for another 7 days. Subsequently, mice were injected with EPO (or PBS) on days 7, 8, and 9 after induction of colitis as indicated by arrows.
(A) Changes in body weight as combined from two independent experiments and 5–14 DSS-treated mice per group are presented and were compared as detailed in the legend to Figure 6. Data of mice receiving drinking water are not depicted. Statistical significant differences between DSS-treated Epor+/+ mice receiving either PBS or EPO are indicated.
(B) Histopathological colitis scores for mice administered either water or DSS (n = 5–14 per group) with each point representing an individual mouse.
(C and D) qRT-PCR analysis of immune response genes (C) and NF-κB p65 binding activity (D) in colons of these mice (n = 5–14 per group).
Discussion
Herein, we have provided evidence that EPO acts as a potent anti-inflammatory immune modulator by specifically targeting NF-κB p65-driven inflammatory effector pathways. This evidence is based on in vitro data using both primary and cell line macrophages as well as on in vivo observations obtained from mouse models of Salmonella septicemia and experimental colitis, respectively. Our data concerning the effects of antibody-mediated neutralization of circulating EPO in systemic Salmonella infection and the results obtained from Epor−/− mice further support the idea that EPO-EPOR-JAK2 signaling crucially regulates macrophage effector functions. We observed that EPO subverted cell-mediated immune responses in Salmonella-infected macrophages by impairing the production of several macrophage-derived inflammatory mediators such as NO, TNF-α, and IL-6 both in vitro and in vivo. Accordingly, EPO decreased disease activity in chemically induced colitis. In contrast, the elimination of S. typhimurium, a facultatively intracellular bacterium controlled by macrophage immune effector mechanisms (Mastroeni et al., 2000; Valdez et al., 2009), was impaired upon EPO treatment. The observed effects were specific for the interaction of EPO with the EPOR as demonstrated by enhanced NF-κB p65 binding activity and improved control of bacterial replication in Epor−/− macrophages, in which exogenous EPO administration had no regulatory effects. Moreover, experiments with pharmacological inhibitors revealed that the anti-inflammatory effects of EPO observed were strictly dependent on JAK2 phosphorylation, whereas interactions of JAK2 with STAT5 were dispensable for EPO to exert its inhibitory functions on macrophages. In contrast, induction of VEGF in response to EPO treatment required STAT5 activity, implying that at least two signaling pathways downstream of EPOR and JAK2 are functional in macrophages. Inhibition of PI3K or MEK1 and MEK2 did not affect Nos2 mRNA expression after EPO treatment, suggesting that these kinases were not involved in the inhibitory EPO-EPOR signaling cascade (data not shown). Furthermore, the fact that a TRAF-6 inhibitory peptide did not alter the effects of EPO on stimulated macrophages suggested that the link between EPOR signaling and p65 activation may not directly involve TRAF-6 (data not shown).
From a molecular perspective, the immune-modulatory effects of EPO could be traced back to inhibition of NF-κB p65-mediated transcription of inflammatory target genes in macrophages. The activation of NF-κB proteins involves the phosphorylation and proteasomal degradation of IκBs via the IκB kinase (IKK) complex, which results in the release and nuclear translocation of active NF-κB homo- and heterodimers consisting of various combinations of p65, c-Rel, RelB, p50, and p52 subunits (Karin and Ben-Neriah, 2000). Specifically, EPO inhibited the phosphorylation of IκB-α and subsequently the phosphorylation and activation of p65. Genes known to be trans-activated by phosphorylated p65 dimers include proinflammatory cytokines, chemokines, and inflammatory enzymes including Nos2 (Pasparakis, 2009). The linkage of EPO-EPOR-JAK2 signaling to NF-κB activation appears to be of clinical relevance given that NF-κB functionality is essential for adequate host defense in a variety of infectious diseases, whereas in immune-mediated conditions including inflammatory colitis, the activation of NF-κB transcription factors within macrophages is a major component of immune-driven tissue damage (Asquith et al., 2010; Bouma and Strober, 2003; Karin and Ben-Neriah, 2000; Kaser et al., 2010; Pasparakis, 2009). This assumption is substantiated by the data provided herein that demonstrated deterioration of Salmonella septicemia and improvement of experimental colitis after EPO treatment. Although epithelial NF-κB is essential for mucosal tissue homeostasis and regeneration, genetic ablation or pharmacological inhibition of IKK-dependent p65 activation in myeloid cells limits disease progression in experimental colitis (Greten et al., 2004; Lawrance et al., 2003; Neurath et al., 1996).
Recombinant EPO is widely used for the treatment of various types of anemia including chemotherapy-induced anemia, anemia of end-stage renal disease and anemia of inflammation (AI) (Weiss and Goodnough, 2005). Although recombinant EPO substitutes the lack of endogenously produced EPO in renal anemia, its mode of action in AI is less clear given that patients suffering from this condition are able to generate even higher amounts of EPO than nonanemic controls (Theurl et al., 2006). According to the data presented here, administration of EPO may reduce this proinflammatory immune status thus promoting erythroid progenitor cell proliferation.
The immune-modulatory effects of EPO may raise the question of whether or not the therapeutic administration of EPO for the treatment of anemia could impact on the course of the diseases underlying AI such as cancer, infection, or autoimmune disorders. These concerns go along with EPOR expression on cancer cells (Jelkmann, 2007) and reduced survival rates in certain cancer patients receiving recombinant EPO (Bohlius et al., 2009); however, no such life-shortening effect has been observed in a meta-analysis of chemotherapy-induced anemia treated with recombinant EPO (Ludwig et al., 2009). An increased incidence of thrombo-embolic events or the induction of tumor growth by EPO treatment as well as the addition of iron rather than EPO administration are hypothesized to cause adverse outcomes in some patients (Bohlius et al., 2009; Pfeffer et al., 2009; Weiss and Goodnough, 2005). Our data on the inhibitory effects of EPO on NF-κB-driven macrophage functions disclose the additional possibility that EPO may directly impair immune responses directed against neoplastic cells.
Currently, little information is available on putative effects of EPO therapy on the clinical course of infections. In intensive care unit patients including individuals with pneumonia or sepsis receiving appropriate antimicrobial therapy, the administration of EPO appears to be safe (Corwin et al., 2007). Nevertheless, our data suggested that EPO impairs clearance of living pathogens, which are controlled by proinflammatory macrophage effector pathways including intracellular pathogens such as Salmonella spp. or Mycobacterium spp. (Schaible and Kaufmann, 2004). This may be of relevance in patients not receiving appropriate antibiotic therapy or suffering from chronic or latent infections such as tuberculosis. On the basis of our results, the inhibitory effect of EPO toward the formation of NO and of proinflammatory cytokines may thus be problematic in the presence of viable bacteria. In contrast, by inhibiting an overwhelming proinflammatory immune response induced by circulating LPS or bacterial superantigens, EPO may beneficially affect outcome after the bacteria have been killed by appropriate antibiotic treatment (Aoshiba et al., 2009). Thus, the net clinical effect of EPO administration in bacterial septicemia and in the setting of chronic or latent infections has to be carefully evaluated prospectively.
In patients with rheumatoid arthritis, treatment with recombinant EPO not only resulted in amelioration of anemia but also improved disease activity (Kaltwasser et al., 2001). This clinical observation goes along with our results that demonstrated improved disease control in murine experimental colitis, which could be attributed to inhibitory effects of EPO toward NF-κB-driven immune effector pathways. The potential therapeutic benefits of high-dose EPO therapy in humans, however, may be outweighed by its primary effect of expanding the erythrocyte mass with a subsequent increase in the risk for thrombo-embolic complications (Ehrenreich et al., 2002). Of interest, EPO derivatives without erythropoietic effects have been developed (Adembri et al., 2008; Bunn, 2007; Erbayraktar et al., 2006; Imamura et al., 2007; Leist et al., 2004), which could serve as valuable therapeutic tools in the treatment of pathologic inflammation.
Experimental Procedures
Cell Isolation and Culture
Primary peritoneal macrophages were harvested and cultured as described in detail in Supplemental Experimental Procedures. Cells were incubated with 5 U/mL EPO diluted in PBS or PBS alone. After another 30 min, macrophages were stimulated with 200 ng/mL LPS (Escherichia coli 055:B5; obtained from Sigma) and/or 50 U/mL recombinant murine IFN-γ (rmuIFN-γ; purchased from R&D) for 6 or 24 hr. Control samples were treated with PBS. Thereafter, supernatants were harvested and macrophages were subjected to RNA preparation.
RAW264.7 murine macrophage-like cells were maintained in complete DMEM containing 10% heat-inactivated fetal calf serum (FCS; purchased from PAA), 100 U/mL penicillin, and 0.1 mg/mL streptomycin (Biochrom AG) at 37°C in humidified air containing 5% CO2. Cells were pretreated with 5 U/mL EPO or PBS for 30 min and subsequently stimulated with 50 U/mL rmuIFN-γ and/or 100 ng/mL LPS.
Salmonella Infection In Vitro
Primary peritoneal macrophages and RAW264.7 cells were used for in vitro infection assays generating comparable results. Prior to in vitro infection, macrophages were incubated in complete medium without antibiotics. Wild-type Salmonella enterica serovar Typhimurium (S. typhimurium) strain ATCC14028 was used for all experiments and grown under sterile conditions in LB broth (Sigma) to late-logarithmic phase. Macrophages were infected with S. typhimurium at a multiplicity of infection (MOI) of 10 and harvested as described (Nairz et al., 2009a).
Salmonella Infection In Vivo
All animal experiments were performed according to the guidelines of the Medical University of Innsbruck and the Austrian Ministry for Science and Education based on the Austrian Animal Testing Act of 1988 (BMWF-66.011/0008-II/10b/2008, BMWF-66.011/0084-II/10b/2008, and BMWF-66.011/0157-II/10b/2009). C57BL/6 mice were housed under specific pathogen-free conditions at the central animal facilities of the Medical University of Innsbruck. Male mice were used at 8–10 weeks of age and infected i.p. with 500 CFU of S. typhimurium diluted in 200 μl of PBS. Mice were monitored twice daily for signs of illness.
In one series of experiments, mice received recombinant human EPO (rhuEPO) i.p. (5 U/g body weight) diluted in 200 μl PBS on days 3 and 4 after the experimental infection. Infected control mice received 200 μl PBS. Animals were sacrificed on day 5 of infection. For survival studies, infection was performed as above and EPO or PBS was administered on days 1, 3, and 5 of infection. In independent experiments, Epor−/−rescued mice (Suzuki et al., 2002) (herein termed Epor−/− mice), generated as described in Supplemental Experimental Procedures were used for in vivo infections.
In an additional series of experiments, mice were infected as above and injected i.p. with 200 μg of a monoclonal EPO antibody or the identical amount of an isotype control antibody (from R&D) as indicated. Mice were then sacrificed on day 4 or 14 of infection. We determined the bacterial load of organs by plating serial dilutions of organ homogenates on LB agar (Sigma) under sterile conditions and calculated the number of bacteria per gram of tissue.
Establishment of TNBS-Colitis
Male SJL/J mice were used for experiments at the age of 6–8 weeks. Colitis was induced by rectal administration of 1 mg of 2,4,6-trinitrobenzene sulfonic-acid (TNBS; purchased from Sigma) in 50% ethanol after cutaneous presensitization 7 days before rectal challenge as described in Supplemental Experimental Procedures. Mice were monitored daily for body weight and signs of illness. On days 2, 3, and 4 after the rectal administration of TNBS, mice were administered rhuEPO (5 U/g body weight) or PBS as a control. Animals were killed on day 5 after administration of TNBS.
Establishment of DSS-Colitis
DSS-colitis was induced in male C57BL/6 Epor+/+ and Epor−/− age-matched littermates (10–14 weeks) with 3% dextran sulfate sodium (DSS; from MP Biomedicals) in accordance with an established protocol with modifications as described in Supplemental Experimental Procedures.
RNA Extraction and Quantitative Reverse Transcription Polymerase Chain Reaction
Preparation of total RNA and quantification of mRNA expression by quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed exactly as described (see Supplemental Experimental Procedures) (Nairz et al., 2009a).
Western Blot Analysis
Protein extracts were prepared with cytoplasmic lysis buffer (25 mM Tris-HCl [pH 7.4], 40 mM KCl, and 1% Triton X-100) supplemented with 1 μg/mL aprotinin and 1 μg/mL leupeptin (all from Sigma). Ten to twenty micrograms of total protein were run on 10%–15% SDS-polyacrylamide gels, and western blotting was performed exactly as described (Theurl et al., 2006) with the antibodies listed in Supplemental Experimental Procedures.
Transcription Factor Assays
Nuclear protein extracts were prepared with the Nuclear and Cytoplasmic Extraction Reagent (Pierce). Oligonucleotide sequences and EMSA conditions are detailed in Supplemental Experimental Procedures. NF-κB p65 binding activity of nuclear extracts was assessed with a commercially available chemi-luminescent transcription factor assay kit in exact accordance with the manufacturer's instructions (Pierce).
Transient Transfections
Transient transfections of RAW264.7 macrophages or primary peritoneal macrophages were performed by electroporation following protocols optimized by the manufacturer (Amaxa). Nos2 promoter or NF-κB activities were determined by the Dual Luciferase system (Promega) in accordance with the manufacturer's instructions. Firefly luciferase activity was corrected by cotransfection of cells with the constitutively expressed Renilla luciferase vector pRL-SV40. Reporter constructs are described in further detail in Supplemental Experimental Procedures.
Detection of Cytokines and Reactive Species
Determination of cytokines in culture supernatants and sera, respectively, was performed with ELISA kits for TNF-α, IL-1β, IL-6, IL-10, IL-12p70, and IFN-γ (BD PharMingen), for IL-23 (eBioscience), and for IL-17A (from R&D). Determination of nitrite, the stable oxidation product of nitric oxide (NO), was carried out with the Griess-Ilosvay's nitrite reagent (Merck) as described (Nairz et al., 2009b).
Statistical Analysis
Statistical analysis was carried out with a SPSS statistical package. We determined significance by unpaired two-tailed Student's t tests or by Mann-Whitney U test to assess data where only two groups existed. Analysis of variance combined with Bonferroni correction or Kruskall-Wallis test, as appropriate, was used for all other experiments. Unless otherwise specified, data are depicted as lower quartile, median and upper quartile (boxes) with minimum and maximum ranges. Survival was compared by log-rank test. For the comparison of organ bacterial loads, data were log-transformed prior to Student's t test or analysis of variance. Individual values and means of log-transformed values are depicted. Generally, P values less than 0.05 were considered significant in any test.
Additional Experimental Procedures
Detailed methodology is described in Supplemental Experimental Procedures.
Acknowledgments
The authors are grateful to K. Auer, S. Berger, I. Brosch, S. Engl, B. Enrich, D. Hilber, M. Seifert, and U. Stanzl for excellent technical support. The authors are indebted to K.R. Morris and E.D. Chan (Department of Medicine, University of Texas-Houston Health Science Center, Houston, Texas, USA) as well as T. Bittorf (Institute of Medical Biochemistry, University of Rostock, Germany) for generously providing luciferase constructs. The authors would like to thank T. Sakai (Experimental Animal Division, RIKEN BioResource Center, Ibaraki, Japan), N. Suzuki and M. Yamamato (Center for TARA, University of Tsukuba, Tsukuba, Japan) for providing Epor−/− rescued mice. This work was supported by grants from the European Union (EUROIRON 1, G.W.) and the Austrian Research Fund, FWF (P-23551, G.W.) and a IFTZ grant by the Medical University of Innsbruck (L.A.H. and N.T.). G.W. received research support for a study on cellular iron transport from Amgen Inc., Thousand Oaks, California, U.S.A. which is unrelated to the topic of this manuscript.
Published online: January 20, 2011
Footnotes
Supplemental Information includes four figures and Supplemental Experimental Procedures can be found with this article online at doi:10.1016/j.immuni.2011.01.002.
Supplemental Information
References
- Adembri C., Massagrande A., Tani A., Miranda M., Margheri M., De Gaudio R., Pellegrini-Giampietro D.E. Carbamylated erythropoietin is neuroprotective in an experimental model of traumatic brain injury. Crit. Care Med. 2008;36:975–978. doi: 10.1097/CCM.0B013E3181644343. [DOI] [PubMed] [Google Scholar]
- Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Aoshiba K., Onizawa S., Tsuji T., Nagai A. Therapeutic effects of erythropoietin in murine models of endotoxin shock. Crit. Care Med. 2009;37:889–898. doi: 10.1097/CCM.0b013e31819b8371. [DOI] [PubMed] [Google Scholar]
- Asquith M.J., Boulard O., Powrie F., Maloy K.J. Pathogenic and protective roles of MyD88 in leukocytes and epithelial cells in mouse models of inflammatory bowel disease. Gastroenterology. 2010;139:519–529. doi: 10.1053/j.gastro.2010.04.045. 529, e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlius J., Schmidlin K., Brillant C., Schwarzer G., Trelle S., Seidenfeld J., Zwahlen M., Clarke M., Weingart O., Kluge S. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373:1532–1542. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- Bouma G., Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- Brines M., Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat. Rev. Neurosci. 2005;6:484–494. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- Brines M., Grasso G., Fiordaliso F., Sfacteria A., Ghezzi P., Fratelli M., Latini R., Xie Q.W., Smart J., Su-Rick C.J. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc. Natl. Acad. Sci. USA. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H.F. New agents that stimulate erythropoiesis. Blood. 2007;109:868–873. doi: 10.1182/blood-2006-08-019083. [DOI] [PubMed] [Google Scholar]
- Carvalho G., Lefaucheur C., Cherbonnier C., Métivier D., Chapel A., Pallardy M., Bourgeade M.F., Charpentier B., Hirsch F., Kroemer G. Chemosensitization by erythropoietin through inhibition of the NF-kappaB rescue pathway. Oncogene. 2005;24:737–745. doi: 10.1038/sj.onc.1208205. [DOI] [PubMed] [Google Scholar]
- Chen J., Connor K.M., Aderman C.M., Smith L.E. Erythropoietin deficiency decreases vascular stability in mice. J. Clin. Invest. 2008;118:526–533. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin H.L., Gettinger A., Fabian T.C., May A., Pearl R.G., Heard S., An R., Bowers P.J., Burton P., Klausner M.A., Corwin M.J., EPO Critical Care Trials Group Efficacy and safety of epoetin alfa in critically ill patients. N. Engl. J. Med. 2007;357:965–976. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]
- De Maria R., Testa U., Luchetti L., Zeuner A., Stassi G., Pelosi E., Riccioni R., Felli N., Samoggia P., Peschle C. Apoptotic role of Fas/Fas ligand system in the regulation of erythropoiesis. Blood. 1999;93:796–803. [PubMed] [Google Scholar]
- Digicaylioglu M., Lipton S.A. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H., Hasselblatt M., Dembowski C., Cepek L., Lewczuk P., Stiefel M., Rustenbeck H.H., Breiter N., Jacob S., Knerlich F. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol. Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- Erbayraktar S., de Lanerolle N., de Lotbinière A., Knisely J.P., Erbayraktar Z., Yilmaz O., Cerami A., Coleman T.R., Brines M. Carbamylated erythropoietin reduces radiosurgically-induced brain injury. Mol. Med. 2006;12:74–80. doi: 10.2119/2006-00042.Erbayraktar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten F.R., Eckmann L., Greten T.F., Park J.M., Li Z.W., Egan L.J., Kagnoff M.F., Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Imamura R., Isaka Y., Ichimaru N., Takahara S., Okuyama A. Carbamylated erythropoietin protects the kidneys from ischemia-reperfusion injury without stimulating erythropoiesis. Biochem. Biophys. Res. Commun. 2007;353:786–792. doi: 10.1016/j.bbrc.2006.12.099. [DOI] [PubMed] [Google Scholar]
- Jelkmann W. Erythropoietin after a century of research: Younger than ever. Eur. J. Haematol. 2007;78:183–205. doi: 10.1111/j.1600-0609.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- Junk A.K., Mammis A., Savitz S.I., Singh M., Roth S., Malhotra S., Rosenbaum P.S., Cerami A., Brines M., Rosenbaum D.M. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA. 2002;99:10659–10664. doi: 10.1073/pnas.152321399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltwasser J.P., Kessler U., Gottschalk R., Stucki G., Möller B. Effect of recombinant human erythropoietin and intravenous iron on anemia and disease activity in rheumatoid arthritis. J. Rheumatol. 2001;28:2430–2436. [PubMed] [Google Scholar]
- Karin M., Ben-Neriah Y. Phosphorylation meets ubiquitination: The control of NF-[kappa]B activity. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Karin M., Delhase M. The I kappa B kinase (IKK) and NF-kappa B: Key elements of proinflammatory signalling. Semin. Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- Kaser A., Zeissig S., Blumberg R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrance I.C., Wu F., Leite A.Z., Willis J., West G.A., Fiocchi C., Chakravarti S. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-kappa B. Gastroenterology. 2003;125:1750–1761. doi: 10.1053/j.gastro.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Leist M., Ghezzi P., Grasso G., Bianchi R., Villa P., Fratelli M., Savino C., Bianchi M., Nielsen J., Gerwien J. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- Liu Y., Pop R., Sadegh C., Brugnara C., Haase V.H., Socolovsky M. Suppression of Fas-FasL coexpression by erythropoietin mediates erythroblast expansion during the erythropoietic stress response in vivo. Blood. 2006;108:123–133. doi: 10.1182/blood-2005-11-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig H., Crawford J., Osterborg A., Vansteenkiste J., Henry D.H., Fleishman A., Bridges K., Glaspy J.A. Pooled analysis of individual patient-level data from all randomized, double-blind, placebo-controlled trials of darbepoetin alfa in the treatment of patients with chemotherapy-induced anemia. J. Clin. Oncol. 2009;27:2838–2847. doi: 10.1200/JCO.2008.19.1130. [DOI] [PubMed] [Google Scholar]
- Mastroeni P., Vazquez-Torres A., Fang F.C., Xu Y., Khan S., Hormaeche C.E., Dougan G. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J. Exp. Med. 2000;192:237–248. doi: 10.1084/jem.192.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M., Fritsche G., Crouch M.L., Barton H.C., Fang F.C., Weiss G. Slc11a1 limits intracellular growth of Salmonella enterica sv. Typhimurium by promoting macrophage immune effector functions and impairing bacterial iron acquisition. Cell. Microbiol. 2009;11:1365–1381. doi: 10.1111/j.1462-5822.2009.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M., Theurl I., Schroll A., Theurl M., Fritsche G., Lindner E., Seifert M., Crouch M.L., Hantke K., Akira S. Absence of functional Hfe protects mice from invasive Salmonella enterica serovar Typhimurium infection via induction of lipocalin-2. Blood. 2009;114:3642–3651. doi: 10.1182/blood-2009-05-223354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer H., Cumano A., Müller M., Wu H., Huffstadt U., Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- Neurath M.F., Pettersson S., Meyer zum Büschenfelde K.H., Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat. Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- Parganas E., Wang D., Stravopodis D., Topham D.J., Marine J.C., Teglund S., Vanin E.F., Bodner S., Colamonici O.R., van Deursen J.M. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- Parsa C.J., Matsumoto A., Kim J., Riel R.U., Pascal L.S., Walton G.B., Thompson R.B., Petrofski J.A., Annex B.H., Stamler J.S., Koch W.J. A novel protective effect of erythropoietin in the infarcted heart. J. Clin. Invest. 2003;112:999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat. Rev. Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- Pfeffer M.A., Burdmann E.A., Chen C.Y., Cooper M.E., de Zeeuw D., Eckardt K.U., Feyzi J.M., Ivanovich P., Kewalramani R., Levey A.S., TREAT Investigators A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N. Engl. J. Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- Schaible U.E., Kaufmann S.H. Iron and microbial infection. Nat. Rev. Microbiol. 2004;2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- Sepodes B., Maio R., Pinto R., Sharples E., Oliveira P., McDonald M., Yaqoob M., Thiemermann C., Mota-Filipe H. Recombinant human erythropoietin protects the liver from hepatic ischemia-reperfusion injury in the rat. Transpl. Int. 2006;19:919–926. doi: 10.1111/j.1432-2277.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- Sirén A.L., Fratelli M., Brines M., Goemans C., Casagrande S., Lewczuk P., Keenan S., Gleiter C., Pasquali C., Capobianco A. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc. Natl. Acad. Sci. USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Ohneda O., Takahashi S., Higuchi M., Mukai H.Y., Nakahata T., Imagawa S., Yamamoto M. Erythroid-specific expression of the erythropoietin receptor rescued its null mutant mice from lethality. Blood. 2002;100:2279–2288. doi: 10.1182/blood-2002-01-0124. [DOI] [PubMed] [Google Scholar]
- Theurl I., Mattle V., Seifert M., Mariani M., Marth C., Weiss G. Dysregulated monocyte iron homeostasis and erythropoietin formation in patients with anemia of chronic disease. Blood. 2006;107:4142–4148. doi: 10.1182/blood-2005-08-3364. [DOI] [PubMed] [Google Scholar]
- Valdez Y., Ferreira R.B., Finlay B.B. Molecular mechanisms of Salmonella virulence and host resistance. Curr. Top. Microbiol. Immunol. 2009;337:93–127. doi: 10.1007/978-3-642-01846-6_4. [DOI] [PubMed] [Google Scholar]
- Weiss G., Goodnough L.T. Anemia of chronic disease. N. Engl. J. Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- Weiss G., Werner-Felmayer G., Werner E.R., Grünewald K., Wachter H., Hentze M.W. Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J. Exp. Med. 1994;180:969–976. doi: 10.1084/jem.180.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G., Houston T., Kastner S., Jöhrer K., Grünewald K., Brock J.H. Regulation of cellular iron metabolism by erythropoietin: Activation of iron-regulatory protein and upregulation of transferrin receptor expression in erythroid cells. Blood. 1997;89:680–687. [PubMed] [Google Scholar]
- Zhang Y.L., Radhakrishnan M.L., Lu X., Gross A.W., Tidor B., Lodish H.F. Symmetric signaling by an asymmetric 1 erythropoietin: 2 erythropoietin receptor complex. Mol. Cell. 2009;33:266–274. doi: 10.1016/j.molcel.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B.M., McLaughlin S.K., Na R., Liu J., Cui Y., Martin C., Kimura A., Robinson G.W., Andrews N.C., Hennighausen L. Hematopoietic-specific Stat5-null mice display microcytic hypochromic anemia associated with reduced transferrin receptor gene expression. Blood. 2008;112:2071–2080. doi: 10.1182/blood-2007-12-127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.