Abstract
The evolutionarily conserved heat shock transcription factor Hsf1 plays a central role in thermal adaptation in the major fungal pathogen of humans, Candida albicans. Hsf1 becomes hyperphosphorylated in response to heat shock and activates the transcription of genes with heat shock elements (HSEs) in their promoters, these genes contributing to thermal adaptation. However, the relevance of Hsf1 activation to C. albicans virulence is not clear as this pathogen is thought to be obligately associated with warm blooded animals, and this issue has not been tested because HSF1 is essential for viability in C. albicans. In this study, we demonstrate that the HSE regulon is active in C. albicans cells infecting the kidney. We also show the CE2 region of Hsf1 is required for activation and that the phosphorylation of specific residues in this domain contributes to Hsf1 activation. C. albicans HSF1 mutants that lack this CE2 region are viable. However, they are unable to activate HSE-containing genes in response to heat shock, and they are thermosensitive. Using this HSF1 CE2 deletion mutant we demonstrate that Hsf1 activation, and hence thermal adaptation, contributes significantly to the virulence of C. albicans.
Keywords: Candida albicans, Heat shock, Hsf1, Transcriptional activation, Phosphorylation, Pathogenesis
1. Introduction
The major fungal pathogen of humans, Candida albicans, exists in many healthy individuals as a relatively harmless commensal organism in the microflora of the oral cavity and the gastrointestinal and urogenital tracts (Odds, 1988; Calderone, 2002). However, C. albicans is also a frequent cause of mucosal infections such as oral thrush and vaginitis (Ruhnke, 2002). These infections generally arise when host immune defenses are weakened or the local microflora is disturbed. In patients with severely compromised immune defenses, e.g. in chemotherapy or transplant patients, C. albicans can establish potentially fatal systemic infections of the bloodstream and of major organs such as the kidney, liver and brain (Filler and Kullberg, 2002; Kullberg and Filler, 2002).
A combination of virulence factors and fitness attributes contribute to the pathogenicity of C. albicans. Virulence attributes are thought to impact directly upon interactions between the fungus and its host (Odds et al., 2003). For example, yeast-hypha morphogenesis balances the context-dependent requirements of the fungus for rapid growth, nutrient foraging, dissemination and invasion (Gow et al., 2002, 2003; Sundstrom, 2006). The secretion of aspartyl proteinases and (phospho)lipases enhance invasion and nutrient provision (Hube and Naglik, 2002). Also, a battery of adhesins promotes attachment to host tissues (Staab et al., 1999; Hoyer et al., 2008), whilst invasins enhance uptake by host cells (Phan et al., 2007).
In contrast, fitness attributes enhance the physiological robustness of C. albicans cells in the diverse niches they can occupy within the host. For example, effective metabolic adaptation enhances nutrient assimilation and growth (Brown, 2005), whereas robust stress responses are thought to help protect the fungus against environmental insults and host immune defences (Quinn and Brown, 2007). Oxidative, nitrosative, osmotic and thermal stress responses in particular have attracted significant interest in the field (San Jose et al., 1996; Alonso-Monge et al., 1999; Smith et al., 2004; Ullmann et al., 2004; Hromatka et al., 2005; Chauhan et al., 2006; Enjalbert et al., 2006; Brown et al., 2009).
The generally held view is that fungal virulence factors and fitness attributes have evolved relatively rapidly, allowing species to adapt to the diverse niches they occupy. With regard to stress regulation in the fungi, sensors and upstream signalling components display a high degree of evolutionary divergence, as do the transcription factors that drive the corresponding adaptive responses (Butler et al., 2009; Nikolaou et al., 2009; Rispail et al., 2009). Also, while core stress regulatory modules are highly conserved, in some cases their cellular roles have diverged. For example, while AP1-like transcription factors such as Yap1, Cap1 and Pap1 drive transcriptional responses to oxidative stress (Cohen et al., 2002; Chen et al., 2008; Znaidi et al., 2009), the stress activated protein kinases Hog1 and Sty1 mediate responses to different types of stress in evolutionarily divergent yeasts (Chen et al., 2003; O’Rourke and Herskowitz, 2004; Enjalbert et al., 2006). Nevertheless, the downstream adaptive responses driven by these pathways are well conserved. For example, glycerol is generally accumulated as an osmolyte during adaptation to osmotic stress, and thioredoxin and glutaredoxin systems are induced to mediate redox adaptation in response to reactive oxygen species.
Historically, the heat shock response in C. albicans has been of interest because, although additional factors probably influence morphogenesis in the host, temperature up-shifts are associated with the yeast-to-hyphal transition (Swoboda et al., 1995; Shapiro et al., 2009) and because Hsp90 fragments are immuno-protective in systemic candidiasis (Matthews et al., 1991). We became interested in the heat shock response and its regulation in C. albicans because this pathogen is thought to be obligately associated with warm blooded animals (Odds, 1988). We confirmed that C. albicans does activate a bona fide transcriptional response to an acute heat shock (Enjalbert et al., 2003; Nicholls et al., 2009), and that an evolutionarily conserved heat shock transcription factor, Hsf1, plays a central role in this response (Nicholls et al., 2009). We also demonstrated that Hsf1 becomes hyperphosphorylated in response to heat shock, that Hsf1 activates transcription via heat shock elements (HSEs) in the promoters of its target genes, and that transcriptional activation by Hsf1 is specific for heat shock and is not triggered by other medically relevant stresses (Nicholls et al., 2009).
Why then has a heat shock response been retained in this pathogen during evolution if it is obligately associated with warm blooded animals? We reasoned that the primary role of Hsf1 in C. albicans in vivo might be to mediate thermal homeostasis rather than adaptation to acute heat shocks. In other words, Hsf1 might play an essential role in vivo by tuning the levels of essential C. albicans chaperones such as Hsp70, Hsp90 and Hsp104 to the growth temperature of the host niche. This is not possible to test using hsf1/hsf1 null mutants because HSF1 is essential for viability in C. albicans (Nicholls et al., 2009). Therefore, in this study we have tested this hypothesis by generating C. albicans HSF1 mutants in which basal Hsf1 activity is retained, but heat shock activation has been blocked. We show that a specific domain of C. albicans Hsf1 is required for heat shock activation and that phosphorylation at specific sites within this domain contribute to Hsf1 activation. We confirm that HSE-containing genes are activated in C. albicans during systemic infection of the kidney and that Hsf1 activation is required for the full virulence of C. albicans. Hence, thermal homeostasis is integral to the adaptation of this fungus during pathogenesis.
2. Materials and methods
2.1. Strains and growth conditions
C. albicans strains used in this study are listed in Table 1. Strains were grown in yeast–peptone–dextrose (YPD) medium (Sherman, 1991). The expression of tetracycline-regulated HSF1 alleles was down-regulated by the addition of doxycycline to a final concentration of 20 μg/ml for at least 6 h (Nicholls et al., 2009). To heat stress C. albicans, cells were grown in YPD at 30 °C for at least 6 h to mid-exponential phase, and then rapidly transferred to pre-warmed flasks at 45 °C for 30 min.
Table 1.
C. albicans strains.
| Strain | Genotype | Source |
|---|---|---|
| SC5314 | Clinical isolate | Gillum et al. (1984) |
| CAI4 | ura3::λ imm434/ura3::λ imm434 | Fonzi and Irwin (1993) |
| CLM60-1 | ade2::hisG/ade2::hisG, ura3::λ imm434/ura3::λ imm434, ENO1/eno1::ENO1-tetR-ScHAP4AD-3XHA-ADE2, hsf1::hisG-URA3-hisG/HSF1 | Nicholls et al. (2009) |
| CLM61-1 | ade2::hisG/ade2::hisG, ura3::λ imm434/ura3::λ imm434, ENO1/eno1::ENO1-tetR-ScHAP4AD-3XHA-ADE2, hsf1::hisG/HSF1 | Nicholls et al. (2009) |
| CLM62-1 | ade2::hisG/ade2::hisG, ura3::λ imm434/ura3::λ imm434, ENO1/eno1::ENO1-tetR-ScHAP4AD-3XHA-ADE2, hsf1::hisG/URA3-tetp-HSF1 | Nicholls et al. (2009) |
| SN1 | ura3::λ imm434/ura3::λ imm434, pBasal-lacZ(URA3) | Nicholls et al. (2009) |
| SN2 | ura3::λ imm434/ura3::λ imm434, pHSE-lacZ(URA3) | Nicholls et al. (2009) |
| SN65 | ade2::hisG/ade2::hisG, ura3::λ imm434/ura3::λ imm434, ENO1/eno1::ENO1-tetR-ScHAP4AD-3XHA-ADE2, hsf1::hisG/HSF1 pBasal-lacZ(URA3) | Nicholls et al. (2009) |
| SN66 | ade2::hisG/ade2::hisG, ura3::λ imm434/ura3::λ imm434, ENO1/eno1::ENO1-tetR-ScHAP4AD-3XHA-ADE2, hsf1::hisG/HSF1, pHSE-lacZ(URA3) | Nicholls et al. (2009) |
| SN127 | ade2::hisG/ade2::hisG, ura3::λ imm434/ura3::λ imm434, ENO1/eno1::ENO1-tetR-ScHAP4AD-3XHA-ADE2, hsf1::hisG/HSF1-CE2t-hisG-URA3-hisG | This study |
| SN128 | ade2::hisG/ade2::hisG, ura3::λ imm434/ura3::λ imm434, ENO1/eno1::ENO1-tetR-ScHAP4AD-3XHA-ADE2, hsf1::hisG/HSF1-CTMt-hisG-URA3-hisG | This study |
| SN138 | ade2::hisG/ade2::hisG, ura3::λ imm434/ura3::λ imm434, ENO1/eno1::ENO1-tetR-ScHAP4AD-3XHA-ADE2, hsf1::hisG/HSF1-CE2t-hisG pBasal-lacZ(URA3) | This study |
| SN141 | ade2::hisG/ade2::hisG, ura3::λ imm434/ura3::λ imm434, ENO1/eno1::ENO1-tetR-ScHAP4AD-3XHA-ADE2, hsf1::hisG/HSF1-CE2t-hisG pHSE-lacZ(URA3) | This study |
| SN148 | ade2::hisG/ade2::hisG, ura3::λ imm434/ura3::λ imm434, ENO1/eno1::ENO1-tetR-ScHAP4AD-3XHA-ADE2, hsf1::hisG/HSF1-CTMt-hisG pBASAL-lacZ(URA3) | This study |
| SN151 | ade2::hisG/ade2::hisG, ura3::λ imm434/ura3::λ imm434, ENO1/eno1::ENO1-tetR-ScHAP4AD-3XHA-ADE2, hsf1::hisG/HSF1-CTMt-hisG pHSE-lacZ(URA3) | This study |
| SN250 | ura3::λ imm434/ura3::λ imm434, pSDM-WT(URA3) | This study |
| SN251 | ura3::λ imm434/ura3::λ imm434, pSDM-A(URA3) | This study |
| SN252 | ura3::λ imm434/ura3::λ imm434, pSDM-E(URA3) | This study |
| SN253 | ura3::λ imm434/ura3::λ imm434, pΔCE2(URA3) | This study |
| SN254 | ade2::hisG/ade2::hisG, ura3::λ imm434/ura3::λ imm434, ENO1/eno1::ENO1-tetR-ScHAP4AD-3XHA-ADE2, hsf1::hisG/URA3-tetp-HSF1, pSDM-WT(NAT1) | This study |
| SN255 | ade2::hisG/ade2::hisG, ura3::λ imm434/ura3::λ imm434, ENO1/eno1::ENO1-tetR-ScHAP4AD-3XHA-ADE2, hsf1::hisG/URA3-tetp-HSF1, pSDM-A(NAT1) | This study |
| SN256 | ade2::hisG/ade2::hisG, ura3::λ imm434/ura3::λ imm434, ENO1/eno1::ENO1-tetR-ScHAP4AD-3XHA-ADE2, hsf1::hisG/URA3-tetp-HSF1, pSDM-E(NAT1) | This study |
| SN257 | ade2::hisG/ade2::hisG, ura3::λ imm434/ura3::λ imm434, ENO1/eno1::ENO1-tetR-ScHAP4AD-3XHA-ADE2, hsf1::hisG/URA3-tetp-HSF1, pSDM-ΔCE2 (NAT1) | This study |
2.2. Strain construction
Doxycycline-conditional C. albicans HSF1 mutants were generated as previously described for CLM62-1 (Table 1) (Nicholls et al., 2009). HSF1 truncation mutants were created using the mini Ura-blaster cassette (Wilson et al., 2000) in an hsf1/HSF1 background (CLM61-1: Nicholls et al., 2009). Briefly, HSF1::hisG-URA3-hisG truncation cassettes were created by PCR amplification using the primers HSF1-CTMt-F, HSF1-CTMt-R, HSF1-CE2t-F and HSF1-CE2t-R (Table 2). These cassettes were then transformed into C. albicans CLM61-1 to delete codons 566–762 of the HSF1 open reading frame to create the allele CE2t (strain SN127), and to delete codons 732–762 of HSF1 to generate the allele CTMt (strain SN128: Table 1). Ura3-minus segregant of SN127 and SN128 were then selected by growth on media containing 5-fluoroorotic acid (Wilson et al., 2000), and the genotypes of these strains confirmed by PCR diagnosis. The HSE-lacZ and Basal-lacZ reporters (Nicholls et al., 2009) were then transformed into these C. albicans strains selecting for the URA3 marker (Murad et al., 2000). Correct integration was confirmed by PCR diagnosis with oligonucleotides RPS1-GEN and LacZ-F (Table 2).
Table 2.
Oligonucleotides used in this study.
| Primer | Sequence (5′–3′) | Application |
|---|---|---|
| HSF-F | GTTTGTGGCACTGACAGA | Diagnosis of HSF1 allele |
| HSF-Diag | GACTGTTATTAGCTGGGC | Diagnosis of HSF1 allele |
| HSF1-CE2t | TCAGACTGCACAACCTACTTATGAATCACCATTATCAACCAGCGATACCAATAATAATAACAACAACAGTACCTTTGAATATCAACAAGCTGTCAATtaaGTTTTCCCAGTCACGACGTT | Disruption cassette for creating truncation |
| HSF1-CTMt | GCTACTACAACTCCTGGTTCTAATGTGCCAAATGGTGGCCATTATAATAATGGGAATATAAATTTTGTTGATTCACCAATTGCAATGACTCCAGGCTaAGTTTTCCCAGTCACGACGTT | Disruption cassette for creating truncation |
| HSF-C-Term-R | CGAGGTGAAAGAAAATGCTAGGCATTAGGTAGACTACAACAGATTGTATTTCGTAAACTTATTGTATTAAACTTAAAATTTATCTATATACCTAAACAACGTGTGGAATTGTGAGCGGATA | Disruption cassette for creating truncation |
| D1 | GCACGGATTTGCTGATTTCAG | Diagnosis of Hsf1 truncation |
| D2 | GTGAAATAGTCCGAACTACCC | Diagnosis of Hsf1 truncation |
| WT-F | GATCCGCACATTCAAGACGTCCAAGTATGTCTAGAACCAAATCTACAG | oligo to recreate CE2 domain |
| WT-R | GATCCTGTAGATTTGGTTCTAGACATACTTGGACGTCTTGAATGTGCG | oligo to recreate CE2 domain |
| ALL A-F | GATCCGCACATTCAAGACGTCCAGCTATGTCTAGAGCTAAAGCTGCTG | oligo to recreate CE2 domain with phosphosites changed to A |
| ALL A-R | GATCCAGCAGCTTTAGCTCTAGACATAGCTGGACGTCTTGAATGTGCG | oligo to recreate CE2 domain with phosphosites changed to A |
| ALL E-F | GATCCGCACATTCAAGACGTCCAGAAATGTCTAGAGAAAAAGAAGAAG | oligo to recreate CE2 domain with phosphosites changed to E |
| ALL E-R | GATCCTTCTTCTTTTTCTCTAGACATTTCTGGACGTCTTGAATGTGCG | oligo to recreate CE2 domain with phosphosites changed to E |
| CE2 DEL F | TATGGATCCCCAGAAGGGTCCATAGAAGAT | Inverse PCR oligo to delete CE2 domain |
| CE2 DEL R | TATGGATCCTCGGTTAGTCAACATGAGACG | Inverse PCR oligo to delete CE2 domain |
| RPS1-GEN | GTGTGGGATTAAGTGAATACG | Diagnosis of insertion of CIp10-based plasmids at RPS1 |
| LACZ-F | GCTTCAAGGTTTTGGTTCTCC | Diagnosis of insertion of CIp10-based plasmids at RPS1 |
| ACT1-F | GATGAAGCCCAATCCAAAAG | PCR-amplification of ACT1 probe |
| ACT1-R | GGAGTTGAAAGTGGTTTGGT | PCR-amplification of ACT1 probe |
| HSP90-F | TAGTCGACTATGGCTGACGCAAAAGTTG | PCR-amplification of HSP90 probe |
| HSP90-R | ACATGGTACCACGACCCAAT | PCR-amplification of HSP90 probe |
| HSP104-F | TTGCTGCATTTATCCCATCA | PCR-amplification of HSP104 probe |
| HSP104-R | CAGCATCACCAATCAACACC | PCR-amplification of HSP104 probe |
| HSP70-F | TGATGCTGCCAAGAATCAAG | PCR-amplification of HSP70 probe |
| HSP70-R | TCACCAGCAGTGGCTTTAACT | PCR-amplification of HSP70 probe |
| qENO1-F | AAAACCCAGAATCCGACCC | qRT-PCR oligo |
| qENO1-R | AAGCATCCCAGTCATCTTCAG | qRT-PCR oligo |
| qACT1-F | GCTGAACGTATGCAAAAG | qRT-PCR oligo |
| qACT1-R | GAACAATGGATGGACCAG | qRT-PCR oligo |
| qHSP90-F | CTGGTGCTGACGTTTCTA | qRT-PCR oligo |
| qHSP90-R | ACCAGCGTTAGATTCCCA | qRT-PCR oligo |
| qLACZ-F | CACCTCAAGTTCCTCAAGAA | qRT-PCR oligo |
| qLACZ-R | CCTACGAAGTTACCATTGAC | qRT-PCR oligo |
| qHSP104-F | GAAGGCTCAACACAGTATTT | qRT-PCR oligo |
| qHSP104-R | GGTCGTATTTCATCTGGAGG | qRT-PCR oligo |
2.3. Plasmid construction
Hsf1 phosphorylation mutants were created by mutagenesis of plasmid pACT1pHSF1 (Nicholls et al., 2009). First, the CE2 domain was deleted (codons 564–578) by inverse PCR using oligos CE2 del F/R (Table 2) thereby introducing an internal BamHI site. The resultant plasmid was called pΔCE2. Second, oligonucleotides All WT-F/R (Table 2) were annealed and cloned into this new BamHI site to recreate the wild type sequence (pSDM-WT). Third, oligonucleotides All E-F/R and All A-F/R (Table 2) were annealed and cloned into the BamHI site to generate HSF1 alleles with either S571E, T575E, S577E, T578E mutations (pSDM-E) or S571A, T575A, S577A, T578A mutations (pSDM-A). These plasmids were integrated into C. albicans CAI4 selecting for the URA3 marker, and into C. albicans CLM62-1 using the NAT1 marker. Correct genomic integration was confirmed by PCR using oligos D1, D2, HSF-F and RPS1-GEN (Table 2).
2.4. mRNA analyses
Published methods were used for RNA preparation, and northern analyses of the ACT1, HSP90 and HSP104 mRNAs (Wicksteed et al., 1994; Brown et al., 2001). A non-radioactive kit was used for detection of the probe (ECL Direct™ Nucleic Acid Labelling and Detection Systems, Amersham, UK) (Nicholls et al., 2009).
qRT-PCR was used to quantify the levels of the lacZ, HSP90, HSP104, ENO1 transcripts relative to the internal ACT1 mRNA control using the primers listed in Table 2. RNA samples (2 μg) were incubated in 20 μl reactions with 1.5 μl of DNase I, 1.5 μl of RNase OUT, 2 μl of DNase I buffer (Invitrogen; Paisley, UK) at room temperature for 15 min. cDNA was then prepared using Superscript II (Invitrogen) as per the manufacturer’s protocols. Real-time (RT)-PCR SYBR green (Roche; Welwyn Garden City, UK) assays were carried out as per the manufacturer’s instructions on a Roche LightCycler® 480.
2.5. Reporter assays
LacZ expression levels were assayed in quadruplicate on independent transformants as described previously (Rupp, 2002; Nicholls et al., 2009). Briefly, C. albicans cells were grown for at least 6 h to exponential phase. Half of each culture was subjected to a stress for 30 min and the other half acted as the untreated control. Cells were harvested and resuspended in 1 ml of Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4 10 mM KCl, 1 mM MgSO4, 50 mM β-mercaptoethanol), and then 50 μl of chloroform and 20 μl of 1% SDS were added. Samples were equilibrated at 37°C for 10 min, and then reactions started by addition of 200 μl of pre-warmed OPNG (4 mg/ml). Samples were incubated until a yellow colour developed whereupon the reaction was stopped by addition of 0.4 ml of 1 M Na2CO3. β-galactosidase activities were measured in Miller Units.
2.6. Protein extraction and western blots
Protein extracts were prepared and subjected to Western blotting using published protocols (Smith et al., 2004). Briefly, cells were resuspended in 250 μl lysis buffer (0.1 M Tris–HCl, pH 8, 10% glycerol, 1 mM DTT, pepstatin A, Protease Inhibitor Cocktail) and sheared with glass beads in a Mini-bead beater (6 × 30 s with 1 min intervals on ice). Lysates were centrifuged at 13,000 rpm for 10 min at 4 °C. Protein extracts were dephosphorylated using lambda phosphorylase (NEB) as per the manufacturer’s protocols. Protein extracts (15 g) were subjected to SDS–PAGE electrophoresis, blotted for 2 h at 30 V, and membranes blocked for at least 1 h at room temperature using 5% milk. Membranes were probed overnight at 4 °C with a rabbit anti-FLAG-HRP conjugated antibody (diluted 1/200,000) (Sigma). Membranes were then washed and signals detected with an HRP Western blotting kit (Amersham, UK).
2.7. Virulence assays and in vivo samples for qRT-PCR
All animal experimentation conformed to the requirements of United Kingdom Home Office legislation and of the Ethical Review Committee of the University of Aberdeen.
The virulence of C. albicans strains SC5314, CE2t, CTMt and Basal-lacZ (Table 1) was assessed in the mouse intravenous challenge model of systemic candidiasis (MacCallum et al., 2010). Female BALB/c mice (6–8 weeks old; Harlan, UK) were infected intravenously with a saline suspension of C. albicans cells (5.6–5.8 × 104 CFU/g mouse body weight) from cultures grown overnight in NGY medium (MacCallum and Odds, 2005). After 72 h of infection, animals were weighed, humanely terminated, and their kidneys removed aseptically. One kidney from each animal was used to assay fungal burdens. Infection Outcome Scores were calculated on the basis of fungal burdens and animal weights, as described previously (MacCallum et al., 2010). The second kidney from each animal was snap frozen for RNA extraction and qRT-PCR analysis (Walker et al., 2009).
To analyse gene expression in vivo, C. albicans strains SC5314, Basal-lacZ and HSE-lacZ (Table 1) were used to initiate infections in mice. For each strain, a saline suspension (2.4–4.0 × 104 CFU/g mouse body weight), prepared from overnight cultures in NGY medium, was used to intravenously infect six female BALB/c mice (6–8 weeks old; Harlan, UK). Mice were monitored daily and culled when they showed signs of illness and/or when they had lost 20% body weight. Mice were humanely terminated and the kidneys aseptically removed. One kidney was used for fungal burden determination and the other was snap frozen in liquid nitrogen for subsequent RNA extraction and qRT-PCR analyses (Walker et al., 2009).
For virulence assays, kidney fungal burdens and infection outcome scores were compared by Kruskal–Wallis and Mann–Whitney U tests. All statistic analyses were carried out using SPSS Statistics package 17.0.
3. Results
3.1. The CE2 domain is required for Hsf1-mediated transcriptional activation
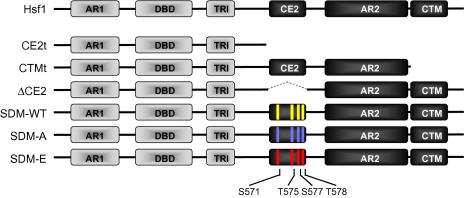
Eukaryotic heat shock transcription factors are well conserved and have similar structures. Saccharomyces cerevisiae Hsf1 is an 833 amino acid protein that contains a winged-helix-turn-helix DNA-binding domain (DBD: residues 168–284), a hydrophobic repeat region necessary for coiled-coil formation during Hsf1p trimerization (residues 326–424), amino-terminal and carboxy-proximal transcriptional activation domains (AR1 and AR2: residues 1–424 and 583–782, respectively), a negative regulatory domain (CE2: residues 535–550), and a carboxy-terminal modulator domain that alleviates the repression by CE2 in response to heat shock (CTM: residues 783–833) (Santoro et al., 1998; Sakurai and Fukasawa, 2001; Hashikawa and Sakurai, 2004). C. albicans Hsf1 displays homology to these domains (Fig. 1).
Fig. 1.
Construction of C. albicans HSF1 mutants. The top line illustrates the domain structure of C. albicans Hsf1, roughly to scale, by analogy to S. cerevisiae Hsf1. Hsf1 domains include two transcriptional activation regions (AR1 and AR2), a DNA-binding domain (DBD), a trimerisation region (TRI), a control element (CE2) and a carboxy-terminal region (CTM). Schematic representations of C. albicans mutations created in this study lie below the wild type Hsf1 structure (Hsf1). Truncations of the CTM region (CTMt) and all of the carboxy-terminus up to and including the CE2 region (CE2t) were created at the HSF1 locus in an HSF1/hsf1 heterozygote, these constructs being expressed from the endogenous HSF1 promoter. Hsf1 phosphorylation sites in the CE2 region are highlighted in yellow. BamHI sites were introduced on each site of the CE2 region to create the wild type control (SDM-WT) for the subsequent site directed mutagenesis of these sites to alanine, in blue (SDM-A) or glutamate, in red (SDM-E). The CE2 region was also deleted from SDM-WT to create the mutation ΔCE2. These constructs were expressed from the ACT1 promoter (Section 2).
Our first aim was to test the functional significance of the putative regulatory domains located in the carboxy-terminal half of C. albicans Hsf1. Therefore we set out to create HSF1 truncation mutants in which the CTM domain alone, the AR2 and CTM domains together, and then all three carboxy-terminal domains (CE2-AR2-CTM) were selectively deleted. The approach was to create the truncations by integrating a URA3 cassette at the 3′ end of the remaining wild type allele in the hsf1/HSF1 heterozygote, CLM61-1 (Table 1). Strains carrying the CTM (CTMt) and CE2-AR2-CTM (CE2t) truncations were successfully generated (Fig. 1), but parallel attempts to generate an AR2-CTM truncation were not.
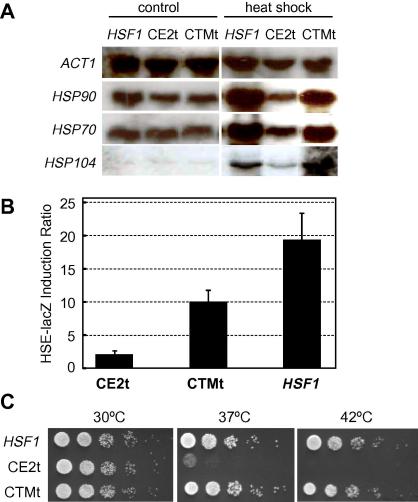
We then tested the impact of the CTMt and CE2t truncations upon Hsf1 function. Hsf1 is known to mediate the activation of HSE-containing genes in response to heat shock (Nicholls et al., 2009). Therefore, we compared HSP70, HSP90 and HSP104 mRNA levels in strains expressing wild type and truncated forms of Hsf1 by northern analysis (Fig. 2A). As expected, untreated cells expressing wild type Hsf1 expressed HSP70 and HSP90 at high basal levels, and the levels of these mRNAs increased following heat shock (30 °C to 45 °C for 30 min). HSP104 mRNA levels were below detectable levels in untreated cells and were significantly induced in heat shocked cells. These data are entirely consistent with our previous observations (Nicholls et al., 2009). Interestingly, truncation of the CTM domain did not affect the basal levels of the HSP70, HSP90 or HSP104 mRNAs, nor did it impair the ability of Hsf1 to increase their expression in response to heat shock (Fig. 2A). This suggests that the CTM domain is not required to alleviate repression of Hsf1 activity in C. albicans, in contrast to the situation in S. cerevisiae (Sakurai and Fukasawa, 2001). In contrast the CE2t truncation attenuated the heat shock induction of the HSP70, HSP90 and HSP104 mRNAs.
Fig. 2.
Removal of the CE2 and AR2 regions attenuates the ability of Hsf1 to activate the HSE regulon in response to heat shock and confers thermosensitivity. (A) Northern analysis of the HSP70, HSP90 and HSP140 mRNAs using ACT1 as an internal loading control. RNA was isolated from C. albicans strains hsf1/HSF1 (CLM61-1), CE2t (SN127) and CTMt (SN128) (Table 1) grown at 30 °C in YPD to exponential phase and then subjected to a 30 to 45 °C heat shock for 30 min, or maintained at 30 °C (control). (B) Activation of the HSE-lacZ reporter was measured by assaying β-galactosidase activities in heat shocked C. albicans hsf1/HSF1, CE2t and CTMt cells expressing either the basal-lacZ or HSE-lacZ reporter (SN65, SN66, SN138, SN141, SN148, and SN151; Table 1). Fold-induction in HSE-lacZ expressing cells is shown relative to equivalent cells expressing the basal-lacZ reporter. (C) Impact of Hsf1 truncations upon thermotolerance. C. albicans cells were serially diluted, spotted onto YPD, and incubated overnight at 30 °C, 37 °C or 42 °C: HSF1, hsf1/HSF1 (CLM61-1); CE2t (SN127); CTMt (SN128).
This finding was assessed further by assaying the activation of the HSE-lacZ reporter in HSF1, CE2t and CTMt cells (Fig. 2B). The heat shock activation of this HSE-lacZ reporter in C. albicans has been shown to be dependent upon Hsf1 (Nicholls et al., 2009). Not surprisingly, therefore, this reporter was strongly activated in response to heat shock in cells expressing wild type Hsf1. Some attenuation of this activation was observed following truncation of the CTM domain. However, activation was almost completely inhibited in cells expressing the CE2 truncation, thereby confirming that Hsf1 is unable to activate HSE-containing promoters following deletion of the CE2, AR2 and CTM domains.
Taken together our data suggested that sequences in the CE2-AR2 region are required for Hsf1-mediated transcriptional activation in response to heat shock. To test whether deletion of the CE2-AR2 region also alters the thermotolerance of C. albicans, strains expressing wild type or truncated versions of Hsf1 were grown at different temperatures (Fig. 2C). Cells expressing wild type or CTMt versions of Hsf1 were able to grow up to 42 °C (the maximum temperature tested at which wild type cells grew). In contrast cells expressing the CE2 truncation were thermo-sensitive. Therefore, there was a strong correlation between the ability of Hsf1 molecules to activate transcription in C. albicans and the thermotolerance of the corresponding cells. This result is consistent with the behaviour of Hsf1 truncations in S. cerevisiae, where removal of sequences after Hsf1 residue 583 blocks Hsf1 activation and renders cells temperature sensitive for growth at 37 °C (Morano et al., 1999).
3.2. Phosphorylation within the Hsf1 CE2 domain affects thermotolerance
C. albicans Hsf1 is known to be activated by hyperphosphorylation in response to heat shock (Nicholls et al., 2009), but the residues phosphorylated in response to heat shock have not yet been identified. Recently, two independent studies of the C. albicans phosphoproteome have identified four residues within the CE2 domain of Hsf1 that can be phosphorylated: S571, T575, S577 and T578 (Beltrao et al., 2009; Kaffarnik and Peck, unpublished). The impact of heat shock on Hsf1 phosphorylation was not investigated in these studies. Nevertheless, phosphorylation at these residues might contribute to the heat shock activation of Hsf1.
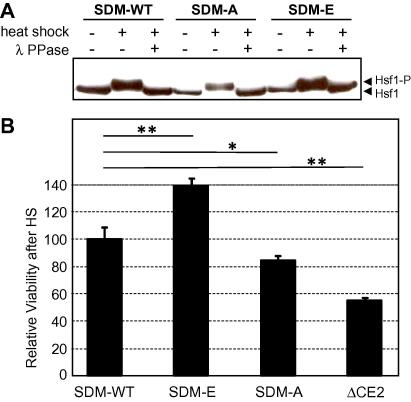
To test this possibility, the corresponding HSF1 codons were all mutated either to alanine (SDM-A) or glutamate codons (SDM-E) to generate non-phosphorylatable and phosphomimetic versions of Hsf1, respectively. Cells expressing these alleles were compared with cells expressing an equivalent wild type construct (SDM-WT: Fig. 1). These constructs were expressed with amino-terminal FLAG tags to permit detection by immunoblot analysis (Section 2). Immunoblotting revealed that, as expected the FLAG-tagged wild type Hsf1 was phosphorylated in response to heat shock (Fig. 3A). The SDM-A and SDM-E also showed gel shifts following heat shock that were resolved by treatment with λ phosphatase. Therefore, further residues in C. albicans Hsf1, in addition to S571, T575, S577 and T578, are phosphorylated in response to heat shock.
Fig. 3.
Influence of Hsf1 phosphorylation sites upon Hsf1 phosphorylation and thermotolerance in C. albicans. (A) Mutagenesis of putative phosphorylation sites at S571, T575, S577 and S578 does not prevent Hsf1 phosphorylation. C. albicans cells expressing different FLAG-tagged Hsf1 mutants were subjected to a 30 to 45 °C heat shock or maintained at 30 °C, and protein extracts analysed by Western blotting with an anti-FLAG antibody: SDM-WT, SN250; SDM-A, SN251; SDM-E, SN252 (Table 1). Some extracts were also treated with λ phosphatase to confirm that the observed band shifts represented Hsf1 phosphorylation. Bands corresponding to phosphorylated (Hsf1-P) and unphosphorylated Hsf1 are highlighted by arrows on the right. (B) Mutations in the Hsf1 CE2 domain affect thermotolerance. C. albicans hsf1/tet-HSF1 cells (CLM62-1) transformed with various plasmids were grown for 6 h in the presence of 20 μg/ml doxycycline to down-regulate Hsf1, subjected to a 30 to 45 °C heat shock for 30 min and then viability assayed by plating on YPD and determining colony forming units (CFU): SDM-WT (SN254); SDM-A (SN255); SDM-E (SN256); ΔCE2 (SN257) (Table 1). Viability was measured relative to heat stressed control cells (SDM-WT, SN254). Means and standard deviations from triplicate experiments are shown: *p < 0.05; **p < 0.01.
We tested whether mutagenesis of the Hsf1 phosphosites at S571, T575, S577 and T578 affects the thermotolerance of C. albicans by examining cell viability after a 30–45 °C heat shock (Fig. 3B). The SDM-WT, SDM-A and SDM-E HSF1 alleles were transformed into the doxycycline-conditional hsf1/tet-HSF1 mutant (CLM62-1) to generate strains SN254-257 (Table 1). Cells were grown in YPD containing doxycycline at 30 °C for 6 h to down-regulate the tet-HSF1 conditional allele (Nicholls et al., 2009). Cells were then either subjected to heat shock from 30 °C to 45 °C for 30 min, or maintained at 30 °C as a control, and then cell viabilities measured by plating (Fig. 3B). Cells expressing the phosphomimetic SDM-E allele displayed a significant increase in heat resistance compared to the SDM-WT control, whereas cells expressing the non-phosphorylatable SDM-A allele showed a small but statistically significant decrease in thermotolerance. These results suggest that, although additional Hsf1 residues appear to be phosphorylated in response to heat shock, the observed phosphorylation at S571, T575, S577 and T578 contributes to thermotolerance in C. albicans.
3.3. Deletion of the Hsf1 CE2 domain blocks Hsf1 phosphorylation and confers thermosensitivity upon C. albicans
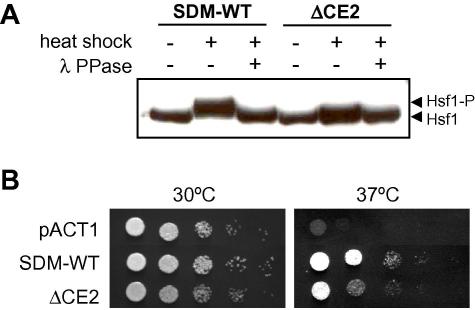
To test whether the additional phosphorylation sites in Hsf1 are located within the CE2 domain, we generated a new HSF1 allele that lacks this domain (ΔCE2: Fig. 1). This HSF1 allele was expressed with an analogous amino-terminal FLAG tag to the SDM alleles described above (Section 2). Immunoblot analysis of the wild type SDM-WT control once again confirmed that Hsf1 is phosphorylated in response to heat shock (Fig. 4A). In contrast, no detectable phosphorylation of Hsf1 lacking the CE2 domain, was observed, suggesting that most, if not all, of the phosphorylated residues lie within this domain.
Fig. 4.
Deletion of the CE2 domain blocks detectable Hsf1 phosphorylation and renders C. albicans thermosensitive. (A) CE2 deletion blocks Hsf1 phosphorylation. C. albicans cells expressing FLAG-tagged versions of Hsf1 were heat shocked or maintained at 30 °C, and protein extracts examined by western blotting: SDM-WT, SN250; ΔCE2, SN253 (Table 1). (B) The CE2 domain is required for thermotolerance. Serially diluted C. albicans hsf1/tet-HSF1 cells (CLM62-1) expressing different HSF1 alleles, or the empty pACT1 vector as a control, were spotted onto YPD containing doxycycline and incubated overnight at 30 °C or 37 °C.
We tested whether deletion of the Hsf1 CE2 domain has a concomitant effect upon thermotolerance. This was the case when growth of the ΔCE2 mutant was compared with the wild type control on plates at 37 °C (Fig. 4B) and when their viabilities were compared after a 30–42 °C heat shock (Fig. 3B). Significantly, the viability of the ΔCE2 mutant (in which Hsf1 phosphorylation was blocked) was lower than the SDM-A mutant (in which some Hsf1 phosphorylation was retained) (Fig. 3B). Therefore, all of the mutants we examined exhibited a strong correlation between the extent of Hsf1 phosphorylation and the degree of thermotolerance. These results reinforce the idea that thermal adaptation in C. albicans is dependent upon Hsf1 activation by phosphorylation in the CE2 domain.
3.4. The C. albicans Hsf1-HSE regulon is activated during systemic infection of the kidney
Is the Hsf1-HSE regulon required to drive thermal homeostasis during C. albicans infections? Our first step towards addressing this major question was to test whether the Hsf1-HSE regulon is expressed during systemic kidney infections by qRT-PCR.
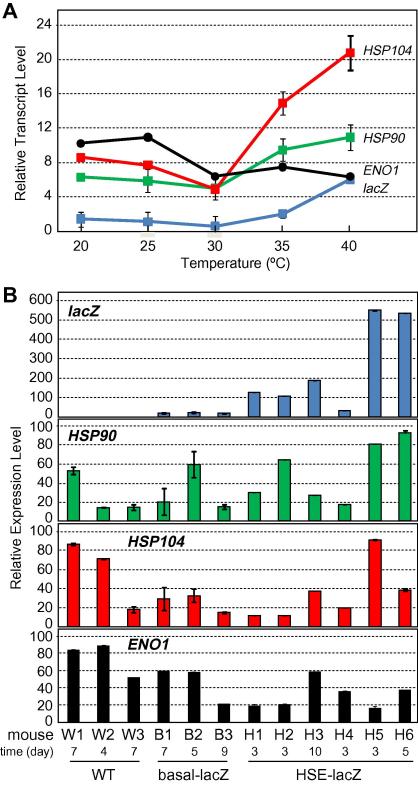
Our starting point involved the calibration of our qRT-PCR quantification methods using C. albicans cells grown at defined temperatures in vitro. C. albicans cells expressing HSE-lacZ in a wildtype HSF1/HSF1 background (SN2: Table 1) were grown at 20 °C, 25 °C, 30 °C, 35 °C or 40 °C. RNA was isolated from these cultures and the expression of the lacZ, HSP90 and HSP104 mRNAs measured relative to the internal ACT1 mRNA control. In addition, we examined ENO1 mRNA levels (relative to ACT1) as a non-heat shock inducible control. lacZ mRNA levels increased when the growth temperature increased above 30 °C (Fig. 5A), which was entirely consistent with the growth temperature-related increase in β-galactosidase expression levels that we observed previously (Nicholls et al., 2009). The qRT-PCR data confirmed that the expression of this Hsf1-dependent reporter is modulated at the transcript level in response to growth temperature. Furthermore, analogous increases in the levels of the Hsf1-regulated transcripts HSP90 and HSP104 were observed at growth temperatures above 30 °C (Fig. 5A). Indeed the correlation between the lacZ, HSP90 and HSP104 transcript levels was very strong (Table 3). In contrast, ENO1 mRNA levels did not increase with growth temperature (Fig. 5A), and the correlation between ENO1 and HSP104 transcript levels, for example, was weak (−0.43 in vitro, and −0.13 in vivo). Data from independent experiments confirmed the reproducibility of these observations, thereby reinforcing the idea that the Hsf1-HSE regulon tunes the expression of essential chaperones to the growth temperature of C. albicans.
Fig. 5.
Expression of HSE-containing genes in C. albicans cells from infected mouse kidneys. HSP90, HSP104, lacZ and ENO1 mRNA levels were measured relative to the internal ACT1 mRNA control by qRT-PCR. (A) Control experiments assaying transcript levels in C. albicans SN1 cells (expressing HSE-lacZ) grown in vitro in YPD at different temperatures. Error bars are from triplicate assays from two independent qRT-PCR analyses on each sample. Similar results were obtained for two independent experiments. (B) C. albicans mRNA levels in cells from infected kidneys of mice with systemic candidiasis: W1–W3, three different mice infected with SC5314; B1–B3, three mice infected with SN1 (expressing basal-lacZ); H1–H6, six mice infected with SN2 (expressing HSE-lacZ). The time post-infection (in days) that each mouse was sacrificed is indicated.
Table 3.
Correlation coefficients for comparisons of lacZ mRNA levels with other transcripts.
| Transcript | Expression in vitro | Expression in vivo |
|---|---|---|
| lacZ | 1 | 1 |
| HSP90 | 0.88 | 0.85 |
| HSP104 | 0.93 | 0.80 |
| ENO1 | −0.46 | −0.13 |
Correlation coefficients were calculated by comparing the relative expression level of HSP or ENO1 transcripts with the corresponding level of the lacZ transcript. Correlation coefficients were calculated using in vitro data for each experimental condition examined (Fig. 5A), and in vivo data from each animal infected with C. albicans cells expressing HSE-lacZ (H1–H6 in Fig. 5B).
Next we examined lacZ, HSP90, HSP104 and ENO1 transcript levels in vivo, in C. albicans cells from infected kidneys. Mice were sacrificed between days 3–10 after being injected intravenously with C. albicans SC5314 (HSF1/HSF1 control), SN1 (HSF1/HSF1 cells containing Basal-lacZ) or SN2 (HSF1/HSF1 cells containing HSE-lacZ) (Table 1), once infection had progressed to a point when high kidney fungal burdens were obtained (kidney fungal burdens = 6.1 ± 0.7 log10 CFU/g). We used the clinical isolate, SC5314, as the infection control in this experiment because the virulence of this strain has been shown to be equivalent to C. albicans CAI4 cells carrying CIp10 (Walker et al., 2009), the vector used to generate pBasal-lacZ and pHSE-lacZ (Nicholls et al., 2009). To maintain the in vivo transcriptome, kidneys were rapidly dissected, snap frozen and the tissues fixed before RNA preparation (Walker et al., 2009: Materials and methods). Transcript levels were then measured by qRT-PCR relative to the internal ACT1 mRNA control (Fig. 5B).
As expected, no detectable lacZ mRNA was seen in C. albicans SC5134 cells, and minimal lacZ mRNA levels were observed in SN1 cells (Basal-lacZ) from kidney infections in independent animals (Fig. 5B). In contrast significant lacZ mRNA levels were observed in SN2 cells (HSE-lacZ) infecting the kidney, indicating that the Hsf1-HSE regulon is activated in C. albicans during systemic infections. Also HSP90 and HSP104 were expressed during experimental infections with C. albicans SC5134 and SN1 (Fig. 5B). These C. albicans strains do not contain the HSE-lacZ reporter, but they do contain an intact Hsf1-HSE regulon. Therefore, these data strengthen the idea that the Hsf1-HSE regulon is activated during systemic infections of the kidney.
HSE-lacZ expression levels did vary significantly between individual infected animals. However, HSE-lacZ mRNA levels correlated well with the HSP90 and HSP104 transcripts in these infections (Fig. 5B; Table 3). In contrast the levels of these transcripts did not correlate with ENO1 mRNA levels, indicating that this experimental variation was not due to technical noise but to bona fide regulation of the Hsf1-HSE regulon in vivo. This Hsf1-HSE regulon appears to be induced specifically in response to temperatures above about 30 °C (Nicholls et al., 2009). Taken together our data suggest variation in the temperatures of individual animals with systemic candidiasis. Such variation has been observed previously in animals with severe C. albicans infection (Spellberg et al., 2005).
3.5. Hsf1 activation contributes significantly to the virulence of C. albicans
The above observations suggested that thermal homeostasis might contribute significantly to the physiological fitness of C. albicans cells in vivo, and hence that Hsf1 might contribute to the virulence of this pathogen. However, HSF1 is an essential gene in C. albicans (Nicholls et al., 2009) and therefore it was not possible to test this hypothesis using hsf1/hsf1 null mutants. Nevertheless, having constructed a C. albicans mutant in which Hsf1 is not activated (CE2t) it became possible to test whether activation of the Hsf1-HSE regulon is required for virulence in C. albicans.
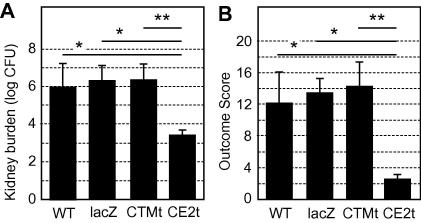
Mice were infected with C. albicans SN127 (CE2t) and with the control strains SC5314 and SN128 (Table 1). The clinical isolate SC5314 represented a wild type control. SN128 expressed the heat shock-inducible Hsf1 CTMt truncation mutant, thereby controlling for the molecular manipulations used to generate the Hsf1 CE2t mutant. The challenge dose for the virulence assays was chosen to produce infection outcome scores of approximately 14 at day 3 post-infection for the wild type control strain (challenge dose = 5.6–5.8 × 104 CFU/g body weight). Virulence was assayed by determining infection outcome scores, based upon weight change and kidney burdens at 3 days post-infection (Materials and methods; MacCallum et al., 2010) (Fig. 6). The CTMt cells displayed similar fungal burdens and outcome scores to the wild type control, indicating that our molecular approaches per se had not impaired the virulence of C. albicans. Furthermore, inclusion of strain SN2 in these virulence experiments confirmed that the HSE-lacZ construct used in the above experiments (Section 3.4) did not affect the virulence of C. albicans (Fig. 6).
Fig. 6.
C. albicans mutants that are unable to activate Hsf1 display attenuated virulence in the mouse model of systemic candidiasis. (A) Kidney burdens measured at day 3: WT, SC5314; HSE-lacZ (SN2); CTMt (SN128); CE2t (SN127) (Table 1). (B) Infection Outcome Scores calculated after three days (means and standard deviation for data from six animals): ∗p < 0.05; ∗∗p < 0.01. Higher outcome scores reflect more severe infection.
The CE2t mutant displayed significantly reduced fungal burdens and outcome scores compared with the control strains (Fig. 6). Furthermore, a 10-fold increase in challenge inoculum did not increase infection outcome score (not shown), demonstrating the severe virulence attenuation of this strain. This indicated that Hsf1 induction is required for the full virulence of C. albicans.
4. Discussion
In this study, we report two important conclusions relating to heat shock regulation in the fungal pathogen C. albicans. First, we conclude that the CE2 domain of the essential heat shock transcription factor, Hsf1, is required for its activation in response to heat shock. This CE2 domain lies between the trimerisation region and the activation domain AR2 of Hsf1 (Fig. 1). Mutations that remove this CE2 domain block heat shock activation of the HSP70, HSP90 and HSP104 mRNAs and of the Hsf1-dependent HSE-lacZ reporter (Fig. 2A and B), confer thermosensitivity upon C. albicans (Figs. 2C, 3B and 4B), and block the heat shock inducible hyperphosphorylation of Hsf1 (Fig. 4A). These data highlight the importance of the CE2 domain in the regulation of Hsf1 activity, and also demonstrate the importance of Hsf1 activation for thermal adaptation in C. albicans.
The CE2 domain harbours four amino acid residues at which phosphorylation has been detected in C. albicans cells: S571, T575, S577 and T578 (Beltrao et al., 2009; Kaffarnik and Peck, unpublished). However, these high throughput studies did not look at the proteome under heat shock conditions. Therefore, we tested whether phosphorylation at these residues contributes Hsf1 activation. We showed that blocking phosphorylation at S571, T575, S577 and T578 (SDM-A) attenuated the thermal resistance of C. albicans slightly, whereas phosphomimetic mutations at these sites (SDM-E) slightly increased thermotolerance (Fig. 3B). However, some Hsf1 phosphorylation was retained in these mutants (Fig. 3A), and deletion of the whole CE2 domain conferred a greater effect upon C. albicans thermotolerance than mutagenesis of S571, T575, S577 and T578 alone (Fig. 3B). We conclude that while phosphorylation at these four residues contributes to Hsf1 activation, additional phosphorylation sites exist within Hsf1, probably in the CE2 domain (e.g. at S567 and/or S573). However, we do not exclude the possibility that mutagenesis of CE2 inhibits phosphorylation elsewhere in Hsf1.
Our second important conclusion is that activation of the Hsf1-HSE regulon contributes significantly to the virulence of C. albicans. Two key observations underpin this conclusion. Firstly, preventing the activation of Hsf1 severely attenuates the virulence of C. albicans (Fig. 6). Deleting the Hsf1 CTM domain did not attenuate virulence (CTMt), whereas further truncation of CE2 and AR2 domains (CE2t) significantly attenuated virulence, although the slow growth of this latter mutant at 37 °C probably contributed to this phenotype (Fig. 2). Secondly, the Hsf1-HSE regulon is activated during systemic kidney infections (Fig. 5). Interestingly, there was variation in the degree of activation of the Hsf1-HSE regulon between experimental infections. Some of this variability may be intrinsic to the mice and in particular to the differing rates of progression in individual animals. This affected the times at which samples were taken for analysis post-infection, which might have contributed to this variation (Fig. 5). Nevertheless, this observation is consistent with variation in the temperatures of individual animals with systemic candidiasis, some animals being febrile, others not (Spellberg et al., 2005). Taken together, these data are entirely consistent with the hypothesis that Hsf1 is essential for thermal homeostasis in C. albicans, tuning the levels of essential chaperones to the temperature of the local microenvironment. This proposed function does not preclude a role for Hsf1 in protection against acute heat shocks in C. albicans. However, it is not clear when this pathogen might be exposed to the sudden and dramatic temperature up-shifts associated with the experimental heat shocks induced in vitro, given that C. albicans is thought to be obligately associated with warm blooded animals (Odds, 1988).
Acknowledgments
This work was supported by a Grant from the UK Biotechnology and Biological Research Council (BB/D009308/1). AB is also supported by the BBSRC (BB/F00513X/1), the Wellcome Trust (080088) and the European Commission (PITN-GA-2008-214004; ERC-2009-AdG-249793). DM is supported by the Wellcome Trust (089930) and the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs).
References
- Alonso-Monge R., Navarro-Garcia F., Molero G., Diez-Orejas R., Gustin M., Pla J., Sanchez M., Nombela C. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J. Bacteriol. 1999;181:3058–3068. doi: 10.1128/jb.181.10.3058-3068.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrao P., Trinidad J.C., Fiedler D., Roguev A., Lim W.A., Shokat K.M., Burlingame A.L., Krogan N.J. Evolution of phosphoregulation: comparison of phosphorylation patterns across yeast species. PLoS Biol. 2009;7:e1000134. doi: 10.1371/journal.pbio.1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.J.P. Integration of metabolism with virulence in Candida albicans. In: Brown A.J.P., editor. Fungal Genomics Mycota XIII. Springer-Verlag; Heidelberg: 2005. pp. 185–203. [Google Scholar]
- Brown A.J.P., Haynes K., Quinn J. Nitrosative and oxidative stress responses in fungal pathogenicity. Curr. Opin. Microbiol. 2009;12:384–391. doi: 10.1016/j.mib.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.J.P., Planta R.J., Restuhadi F., Bailey D.A., Butler P.R., Cadahia J.L., Cerdan M.E., De Jonge M., Gardner D.C.J., Gent M.E., Hayes A., Kolen C.P.A.M., Lombardia L.J., Murad A.M.A., Oliver R.A., Sefton M., Thevelein J., Tournu H., van Delft Y.J., Verbart D.J., Winderickx J., Oliver S.G. Transcript analysis of 1003 novel yeast genes using high-throughput northern hybridisations. EMBO J. 2001;20:3177–3186. doi: 10.1093/emboj/20.12.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler G., Rasmussen M.D., Lin M.F., Santos M.A.S., Sakthikumar S., Munro C.A., Rheinbay E., Grabherr M., Forche A., Reedy J.L., Agrafioti I., Arnaud M.B., Bates S., Brown A.J.P., Brunke S., Costanzo M.C., Fitzpatrick D.A., de Groot P.W.J., Harris D., Hoyer L.L., Hube B., Klis F.M., Kodira C., Lennard N., Logue M.E., Martin R., Neiman A.M., Nikolaou E., Quail M.A., Quinn J., Santos M.C., Schmitzberger F.F., Sherlock G., Shah P., Silverstein K., Skrzypek M.S., Soll D., Staggs R., Stansfield I., Stumpf M.P.H., Sudbery P.E., Thyagarajan S., Zeng Q., Berman J., Berriman M., Heitman J., Gow N.A.R., Lorenz M.C., Birren B.W., Kellis M., Cuomo C.A. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. 2009;459:657–662. doi: 10.1038/nature08064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone R.A. ASM Press; Washington, DC: 2002. Candida and Candidiasis. [Google Scholar]
- Chauhan N., Latge J.P., Calderone R.A. Signaling and oxidant adaptation in Candida albicans and Aspergillus fumigatus. Nat. Microbiol. Rev. 2006;4:435–444. doi: 10.1038/nrmicro1426. [DOI] [PubMed] [Google Scholar]
- Chen D., Toone W.M., Mata J., Lyne R., Burns G., Kivinen K., Brazma A., Jones N., Bahler J. Global transcriptional responses of fission yeast to environmental stress. Mol. Biol. Cell. 2003;14:214–229. doi: 10.1091/mbc.E02-08-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Wilkinson C.R.M., Watt S., Penkett C.J., Toone W.M., Jones N., Bahler J. Multiple pathways differentially regulate global oxidative stress responses in fission yeast. Mol. Biol. Cell. 2008;19:308–317. doi: 10.1091/mbc.E07-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B.A., Pilpel Y., Mitra R.D., Church G.M. Discrimination between paralogs using microarray analysis: application to the Yap1p and Yap2p transcriptional networks. Mol. Biol. Cell. 2002;13:1608–1614. doi: 10.1091/mbc.01-10-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert B., Nantel A., Whiteway M. Stress-induced gene expression in Candida albicans: absence of a general stress response. Mol. Biol. Cell. 2003;14:1460–1467. doi: 10.1091/mbc.E02-08-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert B., Smith D.A., Cornell M.J., Alam I., Nicholls S., Brown A.J.P., Quinn J. Role of the Hog1 stress-activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans. Mol. Biol. Cell. 2006;17:1018–1032. doi: 10.1091/mbc.E05-06-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filler S.G., Kullberg B.J. Deep-seated candidal infections. In: Calderone R.A., editor. Candida and Candidiasis. ASM Press; Washington, DC: 2002. pp. 341–348. [Google Scholar]
- Gow N.A.R., Brown A.J.P., Odds F.C. Fungal morphogenesis and host invasion. Curr. Opin. Microbiol. 2002;5:366–371. doi: 10.1016/s1369-5274(02)00338-7. [DOI] [PubMed] [Google Scholar]
- Gow N.A.R., Knox Y., Munro C.A., Thompson W.D. Infection of chick chorioallantoic membrane (CAM) as a model for invasive hyphal growth and pathogenesis of Candida albicans. Med. Mycol. 2003;41:331–338. doi: 10.1080/13693780310001600859. [DOI] [PubMed] [Google Scholar]
- Hashikawa N., Sakurai H. Phosphorylation of the yeast heat shock transcription factor is implicated in gene-specific activation dependent on the architecture of the heat shock element. Mol. Cell. Biol. 2004;24:3648–3659. doi: 10.1128/MCB.24.9.3648-3659.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer L.L., Green C.B., Oh S.H., Zhao X. Discovering the secrets of the Candida albicans Agglutinin-Like Sequence (ALS) gene family – a sticky pursuit. Med. Mycol. 2008;46:1–15. doi: 10.1080/13693780701435317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hromatka B.S., Noble S.M., Johnson A.D. Transcriptional response of Candida albicans to nitric oxide and the role of the YHB1 gene in nitrosative stress and virulence. Mol. Biol. Cell. 2005;16:4814–4826. doi: 10.1091/mbc.E05-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hube B., Naglik J. Extracellular hydolases. In: Calderone R.A., editor. Candida and Candidiasis. ASM Press; 2002. pp. 107–122. [Google Scholar]
- Kullberg B.J., Filler S.G. Candidemia. In: Calderone R.A., editor. Candida and Candidiasis. ASM Press; Washington, DC: 2002. pp. 327–340. [Google Scholar]
- MacCallum D.M., Odds F.C. Temporal events in the intravenous challenge model for experimental Candida albicans infections in female mice. Mycoses. 2005;48:151–161. doi: 10.1111/j.1439-0507.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- MacCallum D.M., Coste A., Ischer F., Jacobsen M.D., Odds F.C., Sanglard D. Genetic dissection of azole resistance mechanisms in Candida albicans and their validation in a mouse model of disseminated infection. Antimicrob. Agents Chemother. 2010;54:1476–1483. doi: 10.1128/AAC.01645-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R.C., Burnie J.P., Howat D., Rowland T., Walton F. Autoantibody to HSP90 can mediate protection against systemic candidosis. Immunology. 1991;74:20–24. [PMC free article] [PubMed] [Google Scholar]
- Morano K.A., Santoro N., Koch K.A., Thiele D.J. A trans-activation domain in yeast heat shock transcription factor is essential for cell cycle progression during stress. Mol. Cell. Biol. 1999;19:402–411. doi: 10.1128/mcb.19.1.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad A.M.A., Lee P.R., Broadbent I.D., Barelle C.J., Brown A.J.P. CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast. 2000;16:325–327. doi: 10.1002/1097-0061(20000315)16:4<325::AID-YEA538>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Nicholls S., Leach M., Priest C., Brown A.J.P. The role of the heat shock transcription factor, Hsf1, in a fungal pathogen thought to be obligately associated with warm blooded animals. Mol. Microbiol. 2009;74:844–861. doi: 10.1111/j.1365-2958.2009.06883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaou E., Agrafioti I., Stumpf M., Quinn J., Stansfield I., Brown A.J.P. Phylogenetic diversity of stress signalling pathways in fungi. BMC Evol. Biol. 2009;9:44. doi: 10.1186/1471-2148-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F.C. second ed. Bailliere Tindall; London, United Kingdom: 1988. Candida and Candidosis. [Google Scholar]
- Odds F.C., Calderone R.A., Hube B., Nombéla C. Virulence in Candida species: views and suggestions from a peer-group workshop. ASM News. 2003;69:54–55. [Google Scholar]
- O’Rourke S.M., Herskowitz I. Unique and redundant roles for HOG MAPK pathway components as revealed by whole-genome expression analysis. Mol. Biol. Cell. 2004;15:532–542. doi: 10.1091/mbc.E03-07-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan Q.T., Myers C.L., Fu Y., Sheppard D.C., Yeaman M.R., Welch W.H., Ibrahim A.S., Edwards J.E., Filler S.G. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol. 2007;5:e64. doi: 10.1371/journal.pbio.0050064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J., Brown A.J.P. Stress Responses in Candida albicans. In: Hube B., d’Enfert C., editors. Candida: Comparative and Functional Genomics. Horizon Scientific Press; 2007. pp. 217–261. [Google Scholar]
- Rispail N., Soanes D.M., Ant C., Czajkowski R., Grünler A., Huguet R., Perez-Nadales E., Poli A., Sartorel E., Valiante V., Yang M., Beffa R., Brakhage A.A., Gow N.A.R., Kahmann R., Lebrun M.H., Lenasi H., Perez-Martin J., Talbot N.J., Wendland J., Di Pietro A. Comparative genomics of MAP kinase and calcium–calcineurin signalling components in plant and human pathogenic fungi. Fungal Genet. Biol. 2009;46:287–298. doi: 10.1016/j.fgb.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Rupp S. LacZ assays in yeast. Methods Enzymol. 2002;350:112–131. doi: 10.1016/s0076-6879(02)50959-9. [DOI] [PubMed] [Google Scholar]
- Ruhnke M. Skin and mucous membrane infections. In: Calderone R.A., editor. Candida and Candidiasis. ASM Press; Washington, DC: 2002. pp. 307–325. [Google Scholar]
- Sakurai H., Fukasawa T. A novel domain of the yeast heat shock factor that regulates its activation function. Biochem. Biophys. Res. Commun. 2001;285:696–701. doi: 10.1006/bbrc.2001.5234. [DOI] [PubMed] [Google Scholar]
- San Jose C., Monge R.A., Perez-Diaz R., Pla J., Nombela C. The mitogen-activated protein kinase homolog HOG1 gene controls glycerol accumulation in the pathogenic fungus Candida albicans. J. Bacteriol. 1996;178:5850–5952. doi: 10.1128/jb.178.19.5850-5852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro N., Johansson N., Thiele D.J. Heat shock element architecture is an important determinant in the temperature and transactivation domain requirements for heat shock transcription factor. Mol. Cell. Biol. 1998;18:6340–6352. doi: 10.1128/mcb.18.11.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro R., Uppuluri P., Zaas A.K., Collins C., Senn H., Perfect J.R., Heitman J., Cowen L.E. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signalling. Curr. Biol. 2009;19:1–9. doi: 10.1016/j.cub.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Smith D.A., Nicholls S., Morgan B.A., Brown A.J.P., Quinn J. A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans. Mol. Biol. Cell. 2004;15:4179–4190. doi: 10.1091/mbc.E04-03-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B., Ibrahim A.S., Edwards J.E., Jr., Filler S.G. Mice with disseminated candidiasis die of progressive sepsis. JID. 2005;192:336–343. doi: 10.1086/430952. [DOI] [PubMed] [Google Scholar]
- Staab J.F., Bradway S.D., Fidel P.L., Sundstrom P. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science. 1999;283:1535–1538. doi: 10.1126/science.283.5407.1535. [DOI] [PubMed] [Google Scholar]
- Sundstrom P. Candida albicans hypha formation and virulence. In: Heitman J., Filler S.G., Edwards J.E., Mitchell A.P., editors. Molecular Principles of Fungal Pathogenesis. ASM Press; Washington DC: 2006. pp. 45–47. [Google Scholar]
- Swoboda R.K., Bertram G., Budge S., Gooday G.W., Gow N.A.R., Brown A.J.P. Structure and regulation of the HSP90 gene from the pathogenic fungus Candida albicans. Infect. Immun. 1995;63:4506–4514. doi: 10.1128/iai.63.11.4506-4514.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann B.D., Myers H., Chiranand W., Lazzell A.L., Zhao Q., Vega L.A., Lopez-Ribot J.L., Gardner P.R., Gustin M.C. Inducible defense mechanism against nitric oxide in Candida albicans. Eukaryot. Cell. 2004;3:715–723. doi: 10.1128/EC.3.3.715-723.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L.A., MacCallum D.M., Bertram G., Gow N.A.R., Odds F.C., Brown A.J.P. Genome-wide analysis of Candida albicans gene expression patterns during infection of the mammalian kidney. Fungal Genet. Biol. 2009;46:210–219. doi: 10.1016/j.fgb.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicksteed B.L., Collins I., Dershowitz A., Stateva L.I., Green R.P., Oliver S.G., Brown A.J.P., Newlon C.S. A physical comparison of chromosome III in six strains of Saccharomyces cerevisiae. Yeast. 1994;10:39–57. doi: 10.1002/yea.320100105. [DOI] [PubMed] [Google Scholar]
- Wilson R.B., Davis D., Enloe B.M., Mitchell A.P. A recyclable Candida albicans URA3 cassette for PCR product-directed disruptions. Yeast. 2000;16:65–70. doi: 10.1002/(SICI)1097-0061(20000115)16:1<65::AID-YEA508>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Znaidi S., Barker K.S., Weber S., Alarco A.M., Liu T.T., Boucher G., Rogers P.D., Raymond M. Identification of the Candida albicans Cap1p regulon. Eukaryot. Cell. 2009;8:806–820. doi: 10.1128/EC.00002-09. [DOI] [PMC free article] [PubMed] [Google Scholar]