Abstract
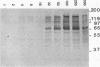
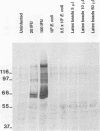
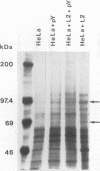
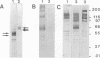
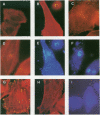
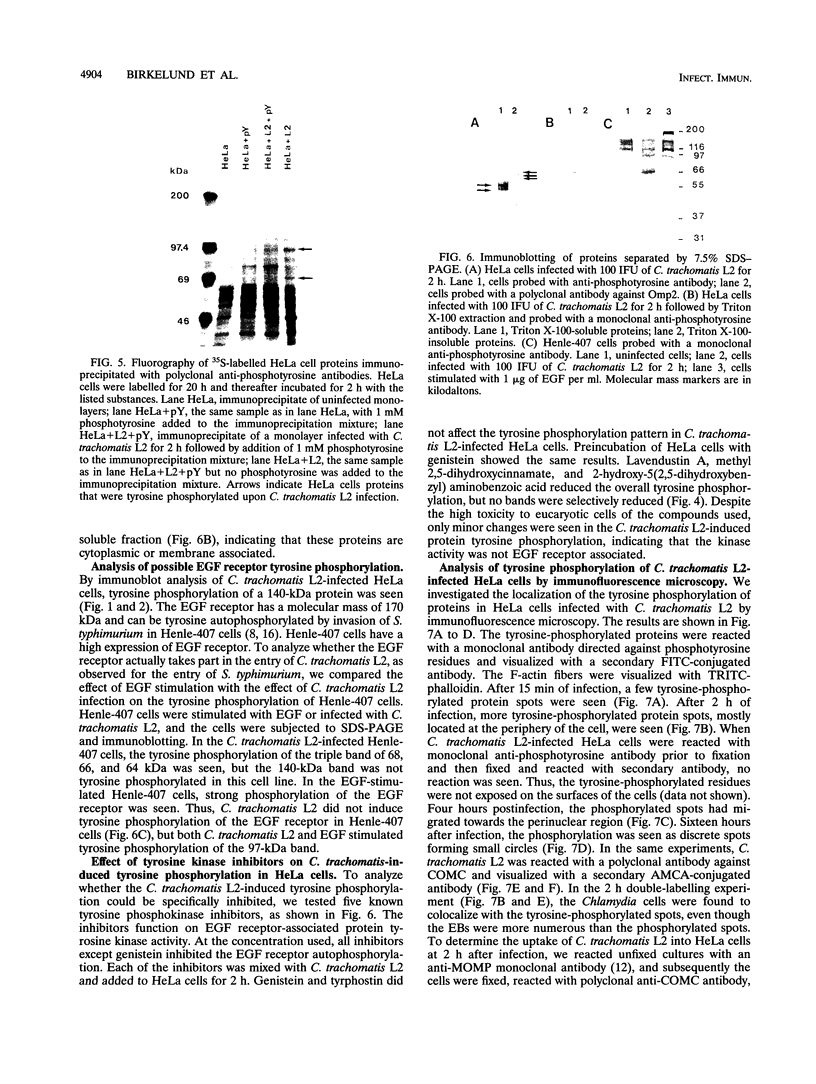
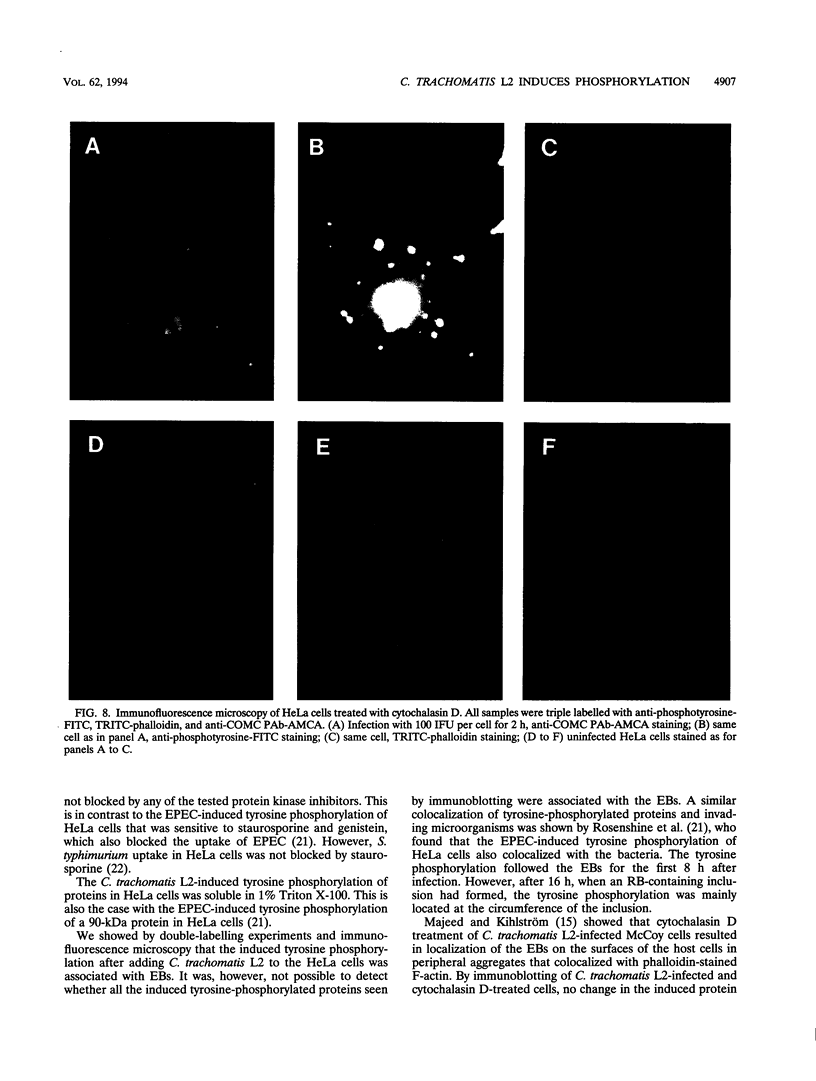
Chlamydia trachomatis L2 is an obligate intracellular microorganism with a unique biphasic life cycle. The extracellular form, the elementary body (EB), is infectious but metabolically inactive. Attachment of EBs to host cells is medicated by a heparan sulfate-like glycosaminoglycan. Following attachment, the EB is internalized within a membrane-bound vesicle, and during the first 8 h of infection the vesicles are transported to a perinuclear location where they aggregate and fuse. By use of a monoclonal antibody against phosphotyrosine, we showed that three classes of proteins are tyrosine phosphorylated: a triple band of 68, 66, and 64 kDa, a 97-kDa band, and a 140-kDa band. The phosphorylation could be detected by immunoblotting from 15 min after infection of HeLa cells. We followed the movement of the EBs and the tyrosine phosphorylation of proteins by double-labelling immunofluorescence microscopy with the same monoclonal anti-phosphotyrosine antibody and a polyclonal antibody against the C. trachomatis L2 outer membrane complex. During the first 8 h of infection, the phosphorylation colocalized with EBs. Sixteen hours after infection, EBs have reorganized to the replicating reticulate bodies, forming an inclusion. At this time, phosphorylation was seen as dotted spots in the periphery of the inclusion.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. E., Stephens R. S. Identification by sequence analysis of two-site posttranslational processing of the cysteine-rich outer membrane protein 2 of Chlamydia trachomatis serovar L2. J Bacteriol. 1989 Jan;171(1):285–291. doi: 10.1128/jb.171.1.285-291.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J. B., Galán J. E., Falkow S. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell. 1993 Jun 4;73(5):903–920. doi: 10.1016/0092-8674(93)90270-z. [DOI] [PubMed] [Google Scholar]
- Brundage R. A., Smith G. A., Camilli A., Theriot J. A., Portnoy D. A. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11890–11894. doi: 10.1073/pnas.90.24.11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Moulder J. W. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun. 1978 Feb;19(2):598–606. doi: 10.1128/iai.19.2.598-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen G., Pedersen L. B., Koehler J. E., Lundemose A. G., Birkelund S. Interaction between the Chlamydia trachomatis histone H1-like protein (Hc1) and DNA. J Bacteriol. 1993 Mar;175(6):1785–1795. doi: 10.1128/jb.175.6.1785-1795.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis C. L., Starnbach M. N., Falkow S. Morphological and cytoskeletal changes in epithelial cells occur immediately upon interaction with Salmonella typhimurium grown under low-oxygen conditions. Mol Microbiol. 1992 Nov;6(21):3077–3087. doi: 10.1111/j.1365-2958.1992.tb01765.x. [DOI] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Pace J., Hayman M. J. Involvement of the epidermal growth factor receptor in the invasion of cultured mammalian cells by Salmonella typhimurium. Nature. 1992 Jun 18;357(6379):588–589. doi: 10.1038/357588a0. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Taga T., Akira S. Cytokine signal transduction. Cell. 1994 Jan 28;76(2):253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundemose A. G., Birkelund S., Larsen P. M., Fey S. J., Christiansen G. Characterization and identification of early proteins in Chlamydia trachomatis serovar L2 by two-dimensional gel electrophoresis. Infect Immun. 1990 Aug;58(8):2478–2486. doi: 10.1128/iai.58.8.2478-2486.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed M., Ernst J. D., Magnusson K. E., Kihlström E., Stendahl O. Selective translocation of annexins during intracellular redistribution of Chlamydia trachomatis in HeLa and McCoy cells. Infect Immun. 1994 Jan;62(1):126–134. doi: 10.1128/iai.62.1.126-134.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed M., Gustafsson M., Kihlström E., Stendahl O. Roles of Ca2+ and F-actin in intracellular aggregation of Chlamydia trachomatis in eucaryotic cells. Infect Immun. 1993 Apr;61(4):1406–1414. doi: 10.1128/iai.61.4.1406-1414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed M., Kihlström E. Mobilization of F-actin and clathrin during redistribution of Chlamydia trachomatis to an intracellular site in eucaryotic cells. Infect Immun. 1991 Dec;59(12):4465–4472. doi: 10.1128/iai.59.12.4465-4472.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis B. L., Lax I., Kris R., Dombalagian M., Honegger A. M., Howk R., Givol D., Ullrich A., Schlessinger J. All autophosphorylation sites of epidermal growth factor (EGF) receptor and HER2/neu are located in their carboxyl-terminal tails. Identification of a novel site in EGF receptor. J Biol Chem. 1989 Jun 25;264(18):10667–10671. [PubMed] [Google Scholar]
- McClain D. A., Maness P. F., Edelman G. M. Assay for early cytoplasmic effects of the src gene product of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2750–2754. doi: 10.1073/pnas.75.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran M. F., Polakis P., McCormick F., Pawson T., Ellis C. Protein-tyrosine kinases regulate the phosphorylation, protein interactions, subcellular distribution, and activity of p21ras GTPase-activating protein. Mol Cell Biol. 1991 Apr;11(4):1804–1812. doi: 10.1128/mcb.11.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlner J., Krämer J., Meyer T. F. A plasmid system for high-level expression and in vitro processing of recombinant proteins. Gene. 1993 Aug 16;130(1):121–126. doi: 10.1016/0378-1119(93)90354-6. [DOI] [PubMed] [Google Scholar]
- Reynolds D. J., Pearce J. H. Endocytic mechanisms utilized by chlamydiae and their influence on induction of productive infection. Infect Immun. 1991 Sep;59(9):3033–3039. doi: 10.1128/iai.59.9.3033-3039.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenshine I., Donnenberg M. S., Kaper J. B., Finlay B. B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992 Oct;11(10):3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenshine I., Duronio V., Finlay B. B. Tyrosine protein kinase inhibitors block invasin-promoted bacterial uptake by epithelial cells. Infect Immun. 1992 Jun;60(6):2211–2217. doi: 10.1128/iai.60.6.2211-2217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wyrick P. B., Choong J., Davis C. H., Knight S. T., Royal M. O., Maslow A. S., Bagnell C. R. Entry of genital Chlamydia trachomatis into polarized human epithelial cells. Infect Immun. 1989 Aug;57(8):2378–2389. doi: 10.1128/iai.57.8.2378-2389.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. P., Stephens R. S. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell. 1992 May 29;69(5):861–869. doi: 10.1016/0092-8674(92)90296-o. [DOI] [PubMed] [Google Scholar]