Abstract
Cell signaling mediated by the G protein-coupled parathyroid hormone receptor type 1 (PTHR) is fundamental to bone and kidney physiology. It has been unclear how the two ligand systems—PTH, endocrine and homeostatic, and PTH-related peptide (PTHrP), paracrine—can effectively operate with only one receptor and trigger different durations of the cAMP responses. Here we analyze the ligand response by measuring the kinetics of activation and deactivation for each individual reaction step along the PTHR signaling cascade. We found that during the time frame of G protein coupling and cAMP production, PTHrP1–36 action was restricted to the cell surface, whereas PTH1–34 had moved to internalized compartments where it remained associated with the PTHR and Gαs, potentially as a persistent and active ternary complex. Such marked differences suggest a mechanism by which PTH and PTHrP induce differential responses, and these results indicate that the central tenet that cAMP production originates exclusively at the cell membrane must be revised.
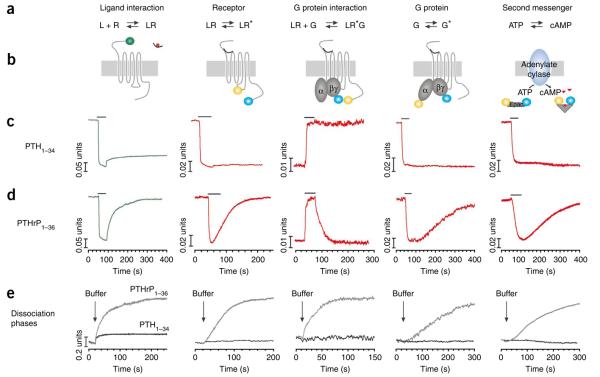
Seminal studies during the past decades established that signaling cascades mediated by a G protein–coupled receptor (GPCR) initially proceed through a succession of biochemical events that take place at the cell membrane and result in the induction and propagation of second messenger molecules1-4 (Fig. 1a). These events begin with the binding of an ‘agonist’ ligand (L) to an inactive-state receptor (R), which causes the receptor to switch to an active-state conformation (R*). The activated receptor then interacts with heterotrimeric G proteins (G, or Gαβγ) to form a transient L–R*–G complex, which exhibits higher affinity for the agonist ligand than does the initial L–R state. The interaction process further involves a conformational change–induced exchange of GDP for GTP on Gα with concomitant release of the activated, GTP-bound Gα (along with Gβγ) from the L–R complex, and the subsequent activation by Gα-GTP of cell membrane–bound effectors such as adenylyl cyclase, which catalyzes the synthesis of the second messenger cyclic AMP (cAMP, 1). Signaling responses are rapidly attenuated by receptor desensitization, typically involving receptor phosphorylation and recruitment of β-arrestin (−1 and/or −2), which drive receptor endocytosis, with ligands either being released at the cell surface or internalized separately from the receptor5. Thus, the removal of receptor from the cell surface by endocytosis is thought to terminate the production of second messengers. This model, however, does not provide a satisfactory explanation for recent studies showing that certain PTH ligands exhibit prolonged cAMP responses in cell culture, and prolonged calcemic responses in animals6,7. These prolonged responses contrast with those observed for PTHrP and related ligands, which promote short-lived signaling responses that are more consistent with the above classical model of L–R–G coupling6,7. The above model also does not provide a rational explanation as to why in clinical testing PTH1–34 stimulates more prolonged increases in serum levels of 1,25-dihydroxy-vitamin D (2), calcium and bone resorption markers than does PTHrP1–36, when the ligands are administered by continuous infusion so as to mimic conditions of primary hyperparathyroidism and humoral hypercalcemia of malignancy8,9.
Figure 1.
Time courses of early reactions in the signaling cascade of PTHR. (a) Biochemical reactions under study. (b) Experimental approaches for FRET measurements of the different kinetic events. (c,d) Time courses of distinct reactions mediated by PTH1–34 (c) and PTHrP1–36 (d) at a saturating concentration. Measurements were performed in single HEK293 cells continuously perfused with buffer or briefly perfused with ligand for the time indicated by the horizontal bar. For binding, the trace represents changes in emission of GFP fluorescence normalized to the initial value. For the other events, traces represent the normalized FRET ratio FYFP/FCFP calculated according to equation (1). Traces are representative of n > 10 independent experiments. (e) Comparison of time courses of ligand dissociation, PTHR deactivation, PTHR and Gs dissociation, Gs deactivation and cAMP degradation upon removal of ligands by washout. Data from the time course of experiments are like those in b and c.
The molecular basis for the different durations of the signaling responses induced by such long/short-acting signaling ligands at the PTHR is unknown. A reasonable possibility, however, is that one or more of the individual biochemical steps that comprise the signal transduction cascade of the PTHR may differ, in terms of reaction mechanism, for the structurally and functionally distinct ligands. We tested this hypothesis by comparing the reaction mechanisms of PTHrP1–36 and PTH1–34. We approached the problem experimentally using fluorescence resonance energy transfer (FRET) methodologies to record the kinetics of key biochemical reactions involved in PTHR signaling from the initial ligand binding step to the generation of cAMP. We also used live cell confocal microscopy to directly visualize the protein-protein colocalization and protein trafficking patterns within the cells. The studies thus permit a correlation analysis, in live cells, between kinetically discrete reaction steps in the signal transduction process and the subcellular trafficking events involved in signal propagation and termination (Fig. 1a,b). By monitoring the effects of brief stimuli of PTH1–34 or PTHrP1–36 on cAMP response and PTHR internalization in cultured cells, we obtained a series of new findings. First, the complex PTH1–34 and PTHR internalizes rapidly into Rab5-positive endosomes in association with adenylyl cyclases. Second, the internalization of PTHR is not associated with desensitization of the Gs or cAMP response. Third, blocking PTHR internalization prevents the sustained cAMP response mediated by PTH. In contrast, PTHrP1–36 actions are completely reversible and limited to the plasma membrane. Our results suggest that PTHR can generate cAMP from intracellular membranes, and that early endosomes serve as a platform for PTHR-mediated sustained cAMP production.
RESULTS
Rate-limiting step for PTHR activation
We previously showed that FRET between the PTHR N-terminally tagged with green fluorescent protein (GFP-N-PTHR) and PTH ligands tagged at Lys13 with tetramethylrhodamine (TMR, 3) (PTH1−34-TMR) reports the initial formation of ligand-receptor complexes in real time in live cells10 (Fig. 1b, left). Here we used this FRET approach to directly compare modes of ligand-receptor association, as well as dissociation, for PTH1–34 and PTHrP1–36. The TMR-labeled peptides and GFP-N-PTHR (stably expressed in HEK293 cells) exhibited binding isotherms and signaling properties similar to those of the respective unmodified peptides and wild-type PTHR (Supplementary Fig. 1). The ligands were applied to the cells using a superfusion system. This system permits a rapid, focal application of the ligand to a single cell under study and the rapid removal of any unbound ligand within a few seconds of the initial application.
Addition of either PTH1–34-TMR or PTHrP1–36-TMR at a saturating concentration (15 μM) to a cell expressing GFP-N-PTHR led to a rapid (t1/2 ~ 140 ms or 200 ms, respectively) and robust decrease (~20%) in GFP emission, indicating hormone binding to the receptor (Fig. 1c,d). For both ligands, the receptor association process involved fast and slow components, which is consistent with a two-step mechanism, as found previously for PTH1–34 (ref. 10) (Supplementary Fig. 2). Analysis of the fast phase showed that the observed rate constant (kobsfast) linearly depends on hormone concentrations (Supplementary Fig. 3). This strict linearity of the rate constant implies that the fast step reflects a simple bimolecular interaction between the hormone (H) and the PTHR10. The time course of this interaction is defined by kobsfast = koff + (kon × [H]) under pseudo-first-order conditions, where kon and koff represent the binding and dissociation rate constants, respectively. The slope of kobs versus the concentration of hormone gave an association rate constant kon = (0.3 ± 0.1) × 106 M−1 s−1 for PTHrP1–36 and (0.4 ± 0.1) × 106 M−1 s−1 for PTH1–34. The intercept with the ordinate axis gave a similar dissociation rate constant koff = 0.46 ± 0.20 s−1 for PTH1–34 and 0.46 ± 0.23 s−1 for PTHrP1–36, which are in the same range k ≈ 0.70 s−1 for those determined experimentally (Table 1). The calculated ratio (koff/kon) yielded a dissociation constant (Kd) of ≈1 μM for PTH1–34, and 1.50 μM for PTHrP1–36, which is consistent with a low binding affinity with the N-terminal domain of the receptor11.
Table 1.
Kinetic steps of PTHR signaling
| Switch on (s) | Turn off (s) | |||
|---|---|---|---|---|
| PTH1–34 | PTHrP1–36 | PTH1–34 | PTHrP1–36 | |
| (1) L + R ↔ LR | τfast = 0.14 ± 0.01 | τfast = 0.17 ± 0.05 | τfast = 1.50 ± 0.27 | τfast = 1.38 ± 0.23 |
| τslow = 1.15 ± 0.10 | τslow = 1.54 ± 0.15 | τslow NA | τslow = 28.12 ± 0.60 | |
| (2) LR ↔ LR* | τ= 0.95 ± 0.15 | τ= 1.59 ± 0.11 | NA | τ= 58.54 ± 6.42 |
| (3) LR* + G ↔ LR*G | τ= 0.96 ± 0.13 | τ= 1.58 ± 0.19 | NA | τ = 48.14 ± 5.29 |
| (4) G ↔ G* | τ= 1.58 ± 0.13 | τ= 2.04 ± 0.14 | NA | τ= 121.50 ± 6.35 |
| (5) cAMP | τ= 10.89 ± 2.26 | τ= 12.66 ± 1.06 | NA | τ= 296.70 ± 17.47 |
Kinetics of ligand association and dissociation (reaction 1), PTHR activation and deactivation (reaction 2), PTHR and Gs interaction (reaction 3), Gs activation and deactivation (reaction 4), cAMP accumulation and degradation (reaction 5). Reactions were recorded from single HEK293 cells at a saturating concentration of ligand. Values were obtained from the same data as those in Figure 1 and represent the mean ± s.e.m. of the rate constant (τ) of at least six experiments.
In contrast, the rate constants for the slower phase for each ligand followed a hyperbolic dependence on ligand concentration, suggesting that the slow phase involves a receptor conformational change. The maximal slow-phase rate constant observed for PTHrP1–36 (kobs = 0.65 s−1) was moderately lower than that seen for PTH1–34 (kobs = 0.90 s−1, P < 0.001). The kinetic rate for the slow phase of ligand association was similar to the kinetic rate for ligand-induced receptor activation, as assessed by the intramolecular FRET response induced in PTHR-CFP/YFP, a FRET-based PTHR reporter tagged with CFP in the third intracellular loop and YFP in the C-terminal tail (Fig. 1b, second panel). The binding association data are thus consistent with the view10 that the association of both PTH1–34 and PTHrP1–36 with the PTHR proceeds via a two-step mechanism, by which the slower binding step is rate limiting for activation of the PTHR (Supplementary Fig. 2).
Distinct release properties of PTH and PTHrP
After rapid removal of PTHrP1–36-TMR from the perfusate chamber, GFP fluorescence was restored rapidly to its initial level, thus reflecting a complete dissociation of the ligand from the receptor (Fig. 1d,e, left panels). In contrast, most of the decreased GFP emission persisted with PTH1–34-TMR, which is consistent with most of the ligand remaining bound to the receptor after washout (Fig. 1c,e, left panels). The dissociation process for PTHrP1–36 was well represented by the sum of two exponential components, suggesting a two-step mechanism of ligand release (Supplementary Fig. 2). The minor component (~15%) corresponded to a rapid dissociation process (τfast = 1.38 ± 0.23 s), and the major component corresponded to a slower dissociation process (τslow = 28.12 ± 0.60 s). A rapid dissociation process (τfast = 1.50 ± 0.27 s) was also observed for PTH1–34 for a small fraction of LR complexes (~10%), but most of the ligand formed a long-lasting LR complex that remained stable over the course of the experiment (Supplementary Fig. 2). Together, the kinetic data indicate that PTH1–34 and PTHrP1–36 associate with the PTHR by similar two-step processes, but dissociate by distinct mechanisms: PTHrP1–36 dissociates slowly yet fully from the receptor, whereas PTH1–34 forms a more stable and persistent complex with the receptor.
We next analyzed the relative contribution of the receptor's N-terminal extracellular (N) domain and juxtamembrane (J) domain comprising the transmembrane helices and connecting loops to the association and release processes used by PTH1–34 and PTHrP1–36. We used in these studies GFP-ΔN-PTHR, a PTHR variant that lacks the N domain and is tagged extracellularly with GFP. As a control, we used M-PTH1–14, a modified PTH fragment analog that binds only to the receptor's J domain. M-PTH1–14-TMR exhibited similar binding and dissociation kinetics on GFP-ΔN-PTHR and GFP-N-PTHR. Dissociation from GFP-ΔN-PTHR was fully reversible and characterized by a slow, single-step rate constant (τ = 15.84 ± 1.01 s, Supplementary Fig. 4), confirming the exclusive binding of this ligand to the PTHR J domain (Supplementary Fig. 4). The absence of a fast component in the dissociation of M-PTH1–14 from GFP-ΔN-PTHR suggests that the fast component seen in the dissociation of PTHrP1–36 and PTH1–34 from the intact PTHR involves ligand interactions with the receptor's N domain.
Agonist-specific PTHR conformations
Coupling of a receptor to a G protein is classically associated with the stabilization of an active state of the receptor that has high affinity for agonist ligands. We therefore sought to analyze how G protein coupling modulates the receptor dissociation processes used by PTH1–34 and PTHrP1–36. We thus co-expressed the PTHR with a dominant-negative form of Gαs (DN-Gs), which forms a stable yet signaling-inactive complex with Gαs-coupled receptors6,12. Co-expression of DN-Gs had no effect on PTH1–34-TMR release, but eliminated most (~80%) of the slow phase of the PTHrP1–36 dissociation process, and produced instead mostly stable, irreversible binding (Supplementary Fig. 2). The effect of DN-Gs on the dissociation of M-PTH1–14 was markedly similar to that of PTHrP1–36: the slow phase was eliminated and replaced by mostly irreversible binding (Supplementary Fig. 4). These results suggest that the slow dissociation component seen with these two ligands depends on the release of G proteins from the receptor, a process inhibited by DN-Gs. The data are also concordant with those of our previous radioligand binding studies performed in membranes, which showed that 125I-PTH1–34 binding remains mostly stable in the presence of GTPγS (guanosine 5′-O-(γ-thio) triphosphate, a nonhydrolyzable GTP analog), whereas 125I-PTHrP1–36 and 125I-M-PTH1–15 binding is mostly destabilized by GTPγS (ref. 6). 125I-M-PTH1–15 was also shown to bind poorly to membranes prepared from cells lacking Gαs, whereas PTH1–34 bound strongly11. The combined radioligand and FRET data thus support the hypothesis that PTH1–34 forms an unusually persistent high-affinity complex with the PTHR that is insensitive to GTPγS, and thus not dependent on classical G protein coupling, but that can nevertheless be associated with G proteins (see below), whereas the other ligands, PTHrP1–36 and M-PTH1–14, form more conventional, high-affinity complexes that are transient and depend on cross-allosteric effects of coupling to G proteins.
The differing kinetics by which PTH1–34 and PTHrP1–36 dissociated from the PTHR support our hypothesis that these two ligands preferentially stabilize distinct high-affinity conformational states of the receptor. We previously showed by recording FRET changes in HEK293 cells expressing PTHR-CFP/YFP that the rate constant of PTHR activation by PTH1–34 follows a hyperbolic dependence on ligand concentration13. This suggests a two-step process by which a fast ligand binding step is followed by a slower conformational change13. We reasoned that if PTHrP1–36 induces the same active-state receptor conformation as PTH1–34, then the slow-phase activation rate constant should follow the same hyperbolic dependence on ligand concentration. FRET measurements of receptor activation induced by the two ligands at various concentrations revealed that this was not the case. Indeed, PTH1–34 mediated PTHR-CFP/YFP activation with a time constant of 0.95 ± 0.15 s, whereas PTHrP1–36 yielded a significantly higher time constant of 1.59 ± 0.11 s (n = 10, P < 0.001). The kinetics of PTHR activation were not only different for the two ligands, but PTH1–34, as well as PTH1–84 distinguished themselves from PTHrP1–36 and M-PTH1–14 by their capacity to induce a stable active state that persisted well after removal of ligand from the perfusion chamber (Fig. 1c,d, second panel; Supplementary Fig. 5).
Active conformations of PTHR locked by PTH but not PTHrP
Next, we assessed whether PTH1–34 or PTHrP1–36 binding to the PTHR differentially affects receptor coupling to heterotrimeric G proteins, as assessed by intermolecular FRET14,15 (Fig. 1b, third and fourth panels). First, we examined coupling to heterotrimeric G protein using PTHR-YFP and Gs-CFP (Gαs/Gβ1/Gγ2-CFP) as acceptor-donor pair. Application of either PTH1–34 or PTHrP1–36 at a saturating concentration induced a fast interaction between PTHR and G proteins (Fig. 1c,d, third panel). The kinetics of the PTHR-Gs interactions were dependent on the relative expression levels of the PTHR-YFP and Gs-CFP, in accord with a diffusion-controlled process (Fig. 2a). The maximum rate constant obtained for each ligand at the highest level of Gs-CFP (τ = 0.96 ± 0.13 s and τ = 1.58 ± 0.19 s for PTH1–34 and PTHrP1–36, respectively) was as high as that obtained for the corresponding PTHR activation response (Fig. 2a and Table 1). These findings imply, therefore, that in the context of a low level of G protein, the kinetics of the PTHR-Gs interaction are not determined by the time course of receptor activation, but rather by a diffusion-controlled collision process.
Figure 2.
Coupling, activation and trafficking of Gs proteins. (a) Kinetics of PTHR-Gs interaction. HEK293 cells were transfected with an increasing amount of CFP-labeled Gγ2 in combination with Gβ1 and Gαs, and with a fixed amount of PTHR-YFP cDNAs. To ensure that relative levels of receptor and G proteins varied in examined cells, we performed experiments in cells displaying different ratios of YFP and CFP fluorescence emission as an indicator of the relative PTHR-YFP and Gαs-YFP expression levels. Western blots of lysates from transfected cells verified the varying level of Gα and Gγ expressions. Loading was controlled by detection of β-actin (n = 4). (b) Relationship between the observed time constant τ and agonist concentration. Values were obtained from fitting the time course of PTHR-GS interaction as shown in Figure 1. Data represent the mean ± s.e.m. of n > 5 experiments. (c,d) HEK293 cells stably expressing PTHR and transiently transfected with cDNAs encoding Gαs-GFP, Gβ1 and Gγ2 were perfused with PTH1–34-TMR (c) or PTHrP1–36-TMR (d) for 10–20 s and then rapidly washed out by buffer. Upon PTH1–34-TMR wash, co-localization between PTH1–34-TMR and GαS-GFP persisted over a period of 30 min in endosomes but not at the cell surface (c). In contrast, after cell wash, only ligand-containing vesicles are observed in the case of PTHrP1–36-TMR-activated Gαs (d). Pearson's correlation coefficients r ± s.d. (n = 30) at 30 min are 0.800 ± 0.019 for PTH1–34 and 0.034 ± 0.057 for PTHrP1–36. Individual channel data are shown in Supplementary Figure 6.
We next investigated rates of G protein activation using Gαs-YFP and Gγ2-CFP (Gs-YFP/CFP)15, which reports the association status of the heterotrimeric complex (Fig. 1b, fourth panel). Addition of PTH1–34 or PTHrP1–36 to cells co-expressing the PTHR and Gs-CFP/YFP yielded a decrease in FRET, consistent with ligand-induced conformational rearrangement and/or disassembly of the αβγ heterotrimer (Fig. 1c,d, fourth panel). The time constants for induction of these Gs activation responses reached maximal values of τ = 1.58 ± 0.13 s and 2.05 ± 0.14 s for PTH1–34 and PTHrP1–36, respectively (at saturating ligand concentration) (Fig. 2b), which are close to the time constants for receptor-Gs coupling. After washout of PTHrP1–36, the PTHR-Gs complex dissociated with a time constant (τ = 48.14 ± 5.29 s) comparable to that of receptor inactivation (τ = 58.54 ± 6.42 s, P = 0.08, n = 7; Fig. 1d, second and third panels), and Gs deactivated with a time constant (τ = 121.50 ± 6.35 s) about two times slower than that for receptor deactivation (Fig. 1d, fourth panel). In contrast, after washout of PTH1–34, neither uncoupling of the PTHR-Gs complex nor deactivation of Gs was observed (Fig. 1c, third and fourth panels). These finding are consistent with a persistently activated PTHR state induced by PTH1–34 but not by PTHrP1–36.
The data so far thus indicate that the receptor and G protein activation processes induced by PTH1–34 and PTHrP1–36 follow similar kinetic mechanisms (switch-on kinetics for PTHR signaling were slightly but significantly faster for PTH1–34; P < 0.001; Table 1), whereas the corresponding deactivation processes follow markedly divergent kinetic mechanisms. Thus, for both ligands, receptor binding, as opposed to receptor conformational change, is the rate-limiting step for receptor activation. Also, the conformational or dissociational event between the Ga and Gbg subunits, as opposed to receptor and G protein interaction, is the rate-limiting step for G protein activation. In contrast, the limiting step for termination of PTHR signaling diverges markedly for PTH and PTHrP: PTH1–34 induces a ‘locked on’ active-state PTHR conformation that can mediate persistent Gs protein activation, whereas PTHrP1–36 dissociates rapidly from the PTHR, prompting rapid signal termination.
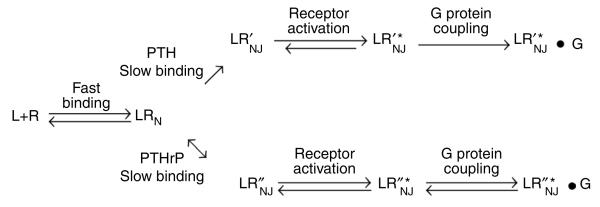
Our new data extend our previous model, in which we suggested differential stabilization of transitional intermediary states of the receptor6,11, to better account for the difference between various ligands, such as PTH and PTHrP. The new kinetic data (Supplementary Fig. 2) suggest that both PTH and PTHrP initially interact with the N domain of the same or similar receptor conformation. Then, in a second, slower step, each ligand interacts differently with the receptor's J domain so as to induce or stabilize a distinct intermediary conformation that can couple to G proteins; the bimolecular state induced by PTH (LR¢) is more stable than that induced by PTHrP (LR′). This refined hypothesis is illustrated in Scheme 1.
Scheme 1.
A hypothesis for PTH and PTHrP signaling. L is the ligand; R is the PTHR; LRN is a low-affinity complex between the ligand and the PTHR N domain; LR′NJ and LR″NJ are high-affinity complexes formed by PTH and PTHrP, respectively, and involving interactions to both the N and J domains of the PTHR; LR′*NJ and LR″*NJ are the active receptor states stabilized by PTH and PTHrP, respectively; and LR′*NJ•G and LR″*NJ•G represent distinct high-affinity complexes coupled to a G protein.
PTHR endocytosis and prolonged cAMP signaling by PTH
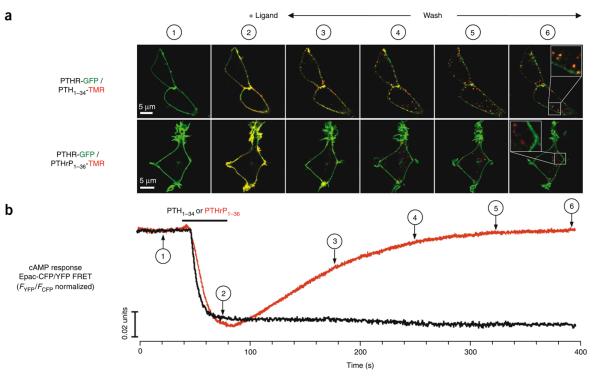
To probe further the mechanisms underlying the divergent signaling responses induced by PTH1–34 and PTHrP1–36, we used live cell imaging to examine the subcellular localization of the ligands, receptor and Gαs at times after ligand binding and washout. Within seconds of a rapid perfusion application of either PTH1–34-TMR or PTHrP1–36-TMR, both ligands co-localized with GFP-N-PTHR as well as with Gαs-GFP at the cell membrane (Figs. 2 and 3, and Supplementary Fig. 6). At later time points after ligand washout, PTHrP1–36-TMR was largely absent from the cell surface and could be detected as small puncta at internalized sites. These sites did not co-localize with either GFP-N-PTHR or Gαs-GFP, but the latter protein nevertheless showed a diffuse pattern of ligand-induced internalization. In contrast, PTH1–34-TMR appeared as large puncta at internalized sites, and these sites showed strong co-localization with both GFP-N-PTHR and Gas-GFP. A quantitative analysis of co-localization using Pearsons's coefficient showed a strong overlap distribution of fluorescences representing PTH-TMR and GFP-N-PTHR or Gs-GFP (Figs. 2 and 3). A three-dimensional view of this experiment reveals a distinct spatial resolution pattern for PTH1–34-TMR and PTHrP1–36-TMR in cells expressing GFP-N-PTHR (Supplementary Videos 1 and 2). We further assessed whether the PTHR and Gs would also co-localize internally upon PTH1–34 stimulation by using Gαs-GFP and a PTHR N-terminally tagged with the monomeric RFP, mRFP-N-PTHR. As shown in Supplementary Figure 7, the punctate distribution of PTHR matched that of Gs. The marked differences seen in the subcellular trafficking patterns for PTH1–34 and PTHrP1–36 paralleled the differences seen for these ligands in the kinetic FRET studies. Indeed, because the kinetic FRET recordings report bulk cellular fluorescence, and the imaging studies show that the bulk cellular fluorescence is internalized, the combined data strongly suggest that the L–R–G complexes formed by PTH1–34 are maintained even as they move to internalized subcellular domains.
Figure 3.
PTHR endocytosis and cAMP responses mediated by PTH1–34 and PTHrP1–36. (a) Trafficking of TMR-labeled ligands and GFP-N-PTHR were monitored in live HEK293 cells by spinning disc confocal microscopy. PTH1–34-TMR or PTHrP1–36-TMR co-localized with GFP-N-PTHR at the time of injection. Fluorescence micrographs in a showed that upon ligand wash, PTH1–34-TMR (red) and GFP-N-PTHR (green) co-localized (yellow) and remained closely associated during receptor internalization (bottom panels). In contrast, PTHrP1–36-TMR alone is detected as small puntae at internalized sites. Pearson's correlation coefficients r ± s.d. (n = 30) are 0.767 ± 0.030 for PTH1−34 and 0.060 ± 0.090 for PTHrP1−36. Individual channel data are shown in Supplementary Figure 6. (b) TMR-labeled or unlabeled ligands (100 nM) were added by perfusion for about 30 s (horizontal bars); numbers 1–6 correlate live cells spinning disc microscopy and FRET time points for cAMP induction as measured by FRET changes in the Epac-CFP/YFP. Cells were HEK293 stably expressing GFP-N-PTHR alone (a) or coexpressing PTHR and Epac-CFP/YFP (b). The recording is representative of n > 10 experiments.
We examined further the nature of these intracellular domains using the GFP-tagged Rab GTPases Rab5-GFP and Rab7-GFP, which are associated with early and late endosomes, respectively. In Rab5-GFP- transfected cells, all of the internalized PTH1–34-TMR co-localized with Rab5-labeled endosomes (Supplementary Fig. 8a). Consistent with this co-localization with Rab5, PTH1–34-TMR also co-localized with the early endosomal antigen 1 (EEA-1) tagged with GFP (data not shown). No co-localization was seen for PTH1–34-TMR and Rab7-GFP, even after 30 min of incubation (Supplementary Fig. 8b). These findings suggest that within the 30-min timeframe of our experiments, the PTH1–34/PTHR/Gs complex that initially forms at the cell surface internally redistributes to, and is persistently maintained in, Rab5-containing compartments, with little or no advancement through later-stage endosomal processing steps.
As a means to assess the potential for signal transduction in the internalized compartments, we used BODIPY-labeled forskolin (BODIPY-Fsk, 4) to fluorescently label cellular adenylyl cyclases. In these experiments, BODIPY-Fsk labels all cells, whereas PTH1–34-TMR labels only those cells successfully transfected (transiently) with PTHR. After PTH1–34-TMR binding to the PTHR-expressing cells, the BODIPY-Fsk in those cells strongly co-localized with the ligand as internalized punctae (Supplementary Fig. 9). This punctate pattern of BODIPY-Fsk staining was not observed in neighboring untransfected cells that did not bind PTH. In cells that bound PTHrP1–36-TMR, the labeled forskolin remained diffuse. These data suggest that adenylyl cyclase molecules are also associated with the internalized PTH1–34/PTHR/Gs complexes.
To test whether the internalized PTH1–34-induced complexes produce cAMP, we used Epac-CFP/YFP, a FRET-based reporter of cAMP production in live cells16 (Fig. 1b, fifth panel, cAMP binding produces a reduction in FRET). Addition of PTH1–34 or PTHrP1–36 (100 nM) to these cells caused a rapid change in the FRET signal (~10% reduction within ~10 s; Fig. 3b), to nearly identical extents for the two ligands, consistent with the equivalent capacity of the two ligands to stimulate a maximal cAMP response in cells expressing recombinant or native PTHR, as measured in dose-response experiments using a cAMP radioimmunoassay (Supplementary Fig. 1c,d). After ligand washout, the Epac-FRET signal induced by PTHrP1–36 returned back to its initial level (Fig. 3b), whereas that induced by PTH1−34 (1−10 nM), as well as that of PTH1−84, remained fully stable after washout (Fig. 3b and Supplementary Fig. 5d). These Epac-CFP/YFP FRET data reveal that the cAMP-generating response induced by PTH1–34 is maintained at times when PTH1–34, the PTHR and Gs molecules have together redistributed to an internalized compartment.
Collectively, the data suggest a model by which the prolonged cAMP response induced by PTH1–34 arises from an active holo-enyzme complex of ligand-receptor and Gs that is located within an endocytic domain containing the early endosome marker Rab5. We further addressed the hypothesis that the prolonged cAMP response originates from an endosomal domain (as opposed to a plasma membrane surface domain) using a dominant-negative form of dynamin, Dyn K44A, which prevents internalization of ligand-PTHR complexes17,18. As expected, in HEK293 cells co-expressing GFP-N-PTHR and Dyn K44A, both GFP-N-PTHR and PTH1–34-TMR remained exclusively co-localized at the cell surface (Fig. 4a). In control cells, the cAMP levels induced by PTH1–34 stayed at the maximal level for at least 20 min after ligand washout, as observed in studies above. In contrast, the cAMP levels induced by PTH1–34 in the Dyn K44A-expressing cells diminished within ~5 min after ligand washout (Fig. 4b,c). Thus, blocking PTHR endocytosis shortened the duration of the cAMP response induced by PTH1–34. These data thus support a correlation between endosomal L–R–G complexes, as opposed to cell surface complexes, and the prolonged cAMP response induced by PTH1–34.
Figure 4.
PTHR endocytosis and prolonged cAMP responses. (a) HEK293 cells stably expressing GFP-N-PTHR (upper panels) and transfected with Dyn K44A (lower panels) were perfused with s100 nM PTH1–34-TMR for about 20 s and then with buffer alone for the remainder of the experiment. 20 min after ligand exposure and washout, PTH1–34-TMR and GFP-N-PTHR complexes redistributed in endosomes in control cells, but remained exclusively localized at the plasma membrane of Dyn K44A-expressing cells. Scale bars, 5 μm. (b) Averaged cAMP response over a 50-min time course measured by FRET changes from HEK293 cells stably expressing PTHR and transiently expressing Epac-CFP/YFP with or without Dyn K44A. Cells were continuously perfused with control buffer or 100 nM PTH1–34 (arrow). Data represent the mean ± s.e.m. of n = 9. (c) Bars represent the average cAMP response. In these experiments, the ligand was washed out 20 s after application by perfusion to remove the unbound ligand.
DISCUSSION
For the ligand-induced receptor activation process, the slower of the two ligand binding components likely reflects interaction of ligand with the receptor's J domain. This step is rate limiting for receptor activation. In our earlier work, we established that ligand analogs modeled on PTH1–34 cause prolonged signaling, not only in vitro but also in vivo on serum calcemic, phosphoturic and bone turnover responses7. Here we concentrate on the actions of the physiological ligands PTH and PTHrP. PTH is represented by PTH1–84, or, more frequently, PTH1–34, which has been shown to be equivalent; the physiologically relevant forms of PTHrP in blood and tissue are unknown, but PTHrP1–36 is sufficient to fully activate the PTHR. To investigate the actions of PTH and PTHrP, we used FRET and live cell imaging to analyze the kinetics of activation induced by each ligand from the initial step of receptor binding to the step of generation of cAMP. In parallel to these kinetic processes, we evaluated the subcellular movements of the signaling machinery over the same time courses.
The results suggest two major conclusions. First, PTH and PTHrP induce distinctive receptor conformations that can be distinguished kinetically at several steps of binding and activation. Second, the prolonged signaling so far noted only for PTH is closely correlated with the formation of Rab5-positive endosomal compartments, which, surprisingly, contain ligand, receptor, Gs and adenylyl cyclase.
The differences noted in the ‘switch on’ rate constants for PTH versus PTHrP, while small (Table 1), support a distinctive receptor conformation formed by the two ligands. More importantly, the turn-off rates are completely different, with markedly prolonged activation seen with PTH (Table 1). Under conditions of optimal PTHR activity, the time constants for subsequent G protein coupling and activation are rate limited by the slower process of receptor activation. This principle also applies for other receptors such as the adenosine A2A receptor15, where the speed of receptor activation determines the kinetics of G protein coupling, as measured in the context of high levels of receptors and G protein expression. The next step, involving the production of cAMP by adenylyl cyclase, takes place at a kinetic rate about six times slower than that of G protein activation (Table 1). This indicates that processes other than G protein activation limit the induction of cAMP formation.
It is currently thought that cyclic AMP production mediated by activated GPCRs is limited to the plasma membrane by mechanisms involving receptor and arrestin interactions that prevent further receptor-G protein coupling, recruit phosphodiesterases to the cell surface and promote receptor internalization19. Our data suggest that other mechanisms are operative for the PTHR, in that sustained cAMP production can occur and is prolonged even when most of the PTH-PTHR complexes have moved from the plasma membrane to the endocytic domains, and few, if any, complexes are detected at the cell surface. Our FRET and live cell microscopy experiments reveal a correlation between the long-lived cAMP production and the selective capacity of PTH1–34 to form a stable ligand-receptor-Gs ternary complex that remains intact even upon redistribution from the cell surface to endosomal sites.
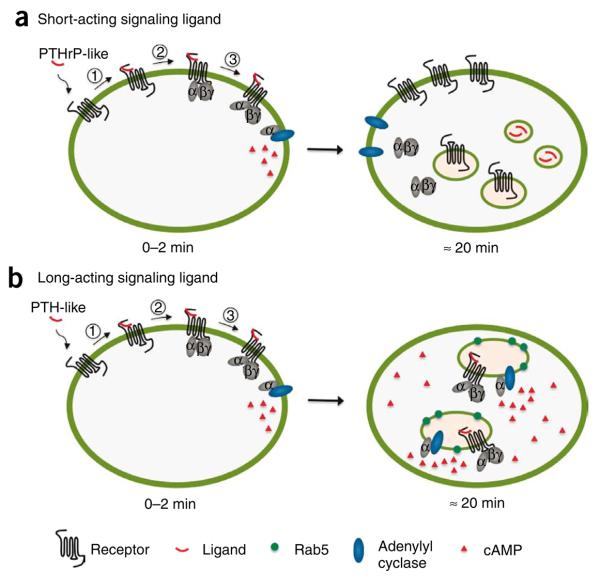
The precise catalytic and subcellular mechanisms that mediate the observed prolonged cAMP-generating activity remain unclear, but the strong co-localization of PTH1–34 with the PTHR, Gs and adenylyl cyclase within the endocytic domain raises the novel possibility that the internalized complexes are enzymatically active (Fig. 5). However, it is important to consider alternative possibilities: for example, earlier studies showed that the PTHR recycled back to the cell membrane within 1 h after removal of the PTH1–34 agonist20,21. Although the possibility therefore exists that an active PTH-PTHR complex continuously internalizes and returns to the cell surface to repeatedly trigger a cAMP response, our experiments indicate that this is not the case. Importantly, the lifetime of the cAMP response was shortened by more than half when the receptor internalization was blocked, suggesting that desensitization mechanisms that operate on the plasma membrane and on endosomes are different. After internalization, little or no TMR fluorescence from PTH1–34-TMR was detected at the cell surface (Figs. 3a and 4a, and Supplementary Video 1). It seems unlikely, therefore, that the persistent cAMP responses observed are due to the presence of the PTH-PTHR complex in the cell membrane
Figure 5.
Mode of activation of the PTHR by long-acting and short-acting signaling ligands. (a) The action of a short-acting signaling ligand is well represented by the classical model for G protein signaling. The ligand interacts first with the receptor (1). The receptor is then switched on to lead to G protein recruitment (2) and activation (3), which in turn initiates adenylyl cyclase activation. In the classical model—that is, the PTHrP-like hormone model—the ligand rapidly dissociates from the receptor, which deactivates and ultimately terminates the signaling. Receptor and ligand traffic through distinct compartments and pathways. (b) In our model of long-acting signaling ligand, the hormone interacts tightly with the receptor in a conformationally dependent manner. The receptor is then locked into a prolonged active state inducing sustained receptor–G protein coupling and sustained G protein activation. The ternary ligand–receptor–G protein heterocomplex is preserved during its internalization in Rab5-containing endosomes and persists over time to appear as a key structure in the prolonged downstream signaling of PTH-like hormone.
The differential effects of PTH1–34 and PTHrP1–36 on the duration of complex assembly and signaling, as well as on subcellular trafficking, may be viewed as an example of biased agonism at the PTHR, insofar as the altered selectivity with which the two ligands bind to distinct receptor conformations drives the resulting complexes along altered paths of signaling and trafficking.
Our data may provide mechanistic clues for understanding how PTH and PTHrP could induce the differing effects on bone remodeling responses suggested from clinical studies8,9. Given that the duration of PTHR agonist exposure and/or action strongly determines the net physiological effect on the opposing processes of bone formation and bone resorption, with prolonged action favoring a net catabolic response, it would stand that under otherwise equivalent conditions, PTHrP1–36 would be more bone-anabolic than PTH1–34, as the latter induces a more stable and productive complex with the PTHR, and thus would have a higher propensity to activate the bone-resorption responses than would PTHrP. Any mechanistic basis for a differential effect of the ligands in humans remains to be determined. This could in part be related to the differential capacity of the ligands to induce altered types of ternary complexes with receptor and Gas, and to induce differential modes of internalization. Further studies are needed to explore this hypothesis, the relationship between signal duration and complex internalization in cells, and the effects of the ligands on bone and mineral ion regulation in vivo.
Many questions remain to be explored. Among them are the following: What events at the cell surface trigger the formation of distinct endosomal trafficking routes for PTH and PTHrP? What cellular elements other than those identified are involved in the divergent vesicle formation and trafficking? What intracellular mechanisms permit extremely long signaling, given that signal durations much longer than that seen with PTH have been observed with modified PTH analogs6? Further understanding of the processes described here will require studies with multiple tools, including electron microscopy, vesicle isolation and proteomic analysis.
One conclusion can be drawn. The quite different patterns of receptor activation by PTH versus PTHrP with this single receptor, PTHR, help to explain how the two physiological systems, the endocrine and the paracrine, function in vivo without mutual interference. The findings also provide an understanding of the observed differences in pharmacological response to infusion or injection of the two natural ligands of the PTHR7,8.
METHODS
FRET
Early reactions in the signaling cascade of PTHR were measured by FRET. First, ligand-receptor interactions were measured by FRET between GFP-tagged PTH receptor and TMR-labeled peptides. Second, PTHR activation was monitored by a decrease in FRET between CFP and YFP inserted in the third intracellular loop and the C terminus of the receptor, respectively. Third, the interaction between PTHR and G protein was measured as an increase in FRET between YFP-labeled PTHR and CFP-labeled Gγ2 in combination with Gαs and Gβ1 proteins. Fourth, G protein activation in cells expressing PTHR was monitored by recording FRET between YFP-labeled Gα and CFP-labeled Gγ2 subunits. Finally, cAMP production was measured as a decrease of FRET in the cAMP sensor Epac-YFP/CFP. Cells plated on poly-d-lysine-coated glass coverslips and maintained in HEPES/BSA buffer (HEPES buffer containing 0.1% (w/v) bovine serum albumin (BSA)) at room temperature (~22 °C) were placed on a Zeiss inverted microscope (Axiovert 200) equipped with an oil immersion 100× Plan-Neofluar objective and a dual emission photometric system (TILL Photonics). Cells were excited with light from a polychrome V (TILL Photonics).
For binding events, the illumination time was typically set to 5 ms applied with a frequency of 40 Hz. FRET was monitored as the decrease emission of GFP (FGFP) from GFP-N-PTHR in the presence of TMR-labeled peptides, recorded at 520 ± 20 nm (beam splitter 530 DCLP) upon excitation at 480 ± 20 nm (beam splitter 498 DCLP). Note that during the recording of binding events, TMR-labeled peptides were continuously superfused, and the presence of TMR fluorescence in the FRET channel (emission at 580 nm) due to the direct excitation of TMR (that is, bound and unbound PTH1–34-TMR) at 480 nm does not permit differentiation of the TMR emission due to FRET or due to direct excitation. Because the diminution of GFP fluorescence was due to FRET between GFP and TMR10, we used the decline of GFP fluorescence as readout for the binding event. The spillover of TMR into the 520 nm channel (1.5%) was negligible at concentrations lower than 3 μM of TMR-labeled peptides, and we subtracted the spillover from the signal of the 520 nm channel at concentrations of 3 μM or higher to give a corrected FGFP. FRET between GFP-N-PTHR and TMR-labeled peptides was also determined by donor (GFP) recovery after acceptor (TMR) photobleaching after 3 consecutive cycles of 6 min of continuous illumination at 550 nm (orange glass 530 nm, beam splitter 555 DCLP, emission filter HQ605/75).
For inter- or intramolecular FRET between CFP and YFP, FRET was monitored as a YFP/CFP emission intensity ratio upon excitation at 436 nm (filter 436 ± 10 nm and a beam splitter dichroic long-pass (DCLP) 460 nm). The emission fluorescence intensities were determined at 535 ± 15 nm (YFP) and 480 ± 20 nm (CFP) with a beam splitter DCLP of 505 nm. The FRET ratio for single experiments was corrected according to equation (1):
| (1) |
where FYFPex436/em535 and FCFPex436/em480 represent respectively the emission intensities of YFP (recorded at 535 nm) and CFP (recorded at 480 nm) upon excitation at 436 nm; a and b represent correction factors for the bleed-through of CFP into the 535 nm channel (a = 0.35) and the cross-talk due to the direct YFP excitation by light at 436 nm (b = 0.06). FYFPex500/em535 represents the emission intensity of YFP (recorded at 535 nm) upon direct excitation at 500 nm, and was recorded at the beginning of each experiment. Note that the bleed-through of YFP into the 480 nm channel was negligible. For each measurement, changes in fluorescence emissions due to photobleaching were subtracted. To ensure that CFP- and YFP-labeled molecule expression were similar in examined cells, we performed experiments in cells displaying comparable fluorescence levels.
The means of FRET experiments were calculated according to equation (2)22, which normalizes for different expression levels of CFP and YFP molecules:
| (2) |
Recording of ligand-induced changes in FRET
A single cell expressing GFPN-PTHR was continuously superfused with HEPES/BSA buffer and PTH1–34-TMR at different concentrations using a computer-assisted solenoid valve rapid superfusion device (ALA-VM8, ALA Scientific Instruments, solution exchange 5 to 10 ms). Signals detected by avalanche photodiodes were digitalized using an analog-digital converter (Digidata1422A, Axon Instruments) and stored on a personal computer using Clampex 10.0 software (Axon Instruments). The change in the fluorescence emission of GFP as a function of time due to FRET was analyzed using nonlinear regression to one- and two-exponential models:
| (3) |
where A1 and A2 are the amplitudes of the two phases, A0 is the baseline at t = ∞, k1 and k2 are the rate constants (s−1) for the two phases and t is the time. The best fits were judged by the analysis of residuals (that is, differences between the experimental data and the calculated curve fits). Changes in emission due to photobleaching were systematically subtracted. Data were analyzed using Origin 7.1 software (Microcal).
Live cell imaging by spinning disc confocal microscopy
Experiments were performed at room temperature on HEK293 cells grown onto a FluoroDish (World Precision Instruments, Inc.) and 48 h post-transfection. Cells were washed with HEPES/BSA buffer before visualization and recording with an oil immersion 63× Plan-Neofluar objective using an UltraView spinning disc confocal microscope (Perkin Elmer). Ligands were added and removed by perfusion. Images were acquired at 0.3-μm intervals along the z axis and processed using Volocity software (Improvision Inc.).
Analysis of data
Kinetic data were fitted by nonlinear least-square equations using OriginLab (version 7.00). Binding and signaling data were analyzed using GraphPad Prism (version 4.00, GraphPad Software). Numerical data were expressed as means ± s.e.m. Statistical significance, using the Student's t-test, was taken as P < 0.05. Colocalization studies were analyzed by Image J (US National Institutes of Health), which provided Pearson's correlation coefficient (r) as a means to measure the degree of colocalization of objects in dual-color images.
Additional methods
Information on the ligands used and details of the molecular biology and cell culture experiments can be found in the Supplementary Methods and Supplementary Figure 10.
Supplementary Material
ACKNOWLEDGMENTS
J.-P.V. thanks M.J. Lohse (University of Wuerzburg) and N.O. Nikolaev (University of Wuerzburg) for generously sharing their plasmid encoding Epac-CFP/YFP, C. Berlot (Weis Center for Research) for the plasmid encoding Gαs-CFP, M. Bünemann (University of Wuerzburg) for the Gγ2-CFP construct and A. Bisello (University of Pittsburgh) for Dyn K44A. The authors thank D. Altschuler for the use of his FRET imaging microscope. This work was supported by start-up funds from the Department of Medicine, Massachusetts General Hospital, and the Department of Pharmacology and Chemical Biology, University of Pittsburgh (to J.-P.V.). M.C. is at the Department of Pharmacology, School of Pharmacy, University of Santiago de Compostella (Spain) and received a fellowship from the Xunta de Galicia (Spain) for a research sabbatical at the J.-P.V. laboratory at the Endocrine Unit of the Massachusetts General Hospital. R.B. was supported by US National Institutes of Health grant DK38451 and received an investigator award from the National Kidney Foundation. J.-P.V. thanks P. Friedman and G. Romero for critically commenting on the manuscript.
Footnotes
AUTHOR CONTRIBUTIONS
S.F. and T.N.F. performed most of the experiments with the support of M.C., B.W. and R.B.; J.-P.V. designed and supervised the experiments and wrote the manuscript with the support of T.J.G., J.T.P., S.F. and T.N.F.
Note: Supplementary information and chemical compound information is available on the Nature Chemical Biology website.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/.
References
- 1.Lamb TD. Gain and kinetics of activation in the G-protein cascade of phototransduction. Proc. Natl. Acad. Sci. USA. 1996;93:566–570. doi: 10.1073/pnas.93.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohse MJ, et al. Optical techniques to analyze real-time activation and signaling of G-protein-coupled receptors. Trends Pharmacol. Sci. 2008;29:159–165. doi: 10.1016/j.tips.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 4.Pugh EN, Jr., Lamb TD. Amplification and kinetics of the activation steps in phototransduction. Biochim. Biophys. Acta. 1993;1141:111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- 5.Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ. Res. 2006;99:570–582. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- 6.Dean T, Vilardaga JP, Potts JT, Jr., Gardella TJ. Altered selectivity of parathyroid hormone (PTH) and PTH-related protein (PTHrP) for distinct conformations of the PTH/PTHrP receptor. Mol. Endocrinol. 2008;22:156–166. doi: 10.1210/me.2007-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okazaki M, et al. Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation. Proc. Natl. Acad. Sci. USA. 2008;105:16525–16530. doi: 10.1073/pnas.0808750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horwitz MJ, et al. Direct comparison of sustained infusion of human parathyroid hormone-related protein-(1-36) [hPTHrP-(1-36)] versus hPTH-(1-34) on serum calcium, plasma 1,25-dihydroxyvitamin D concentrations, and fractional calcium excretion in healthy human volunteers. J. Clin. Endocrinol. Metab. 2003;88:1603–1609. doi: 10.1210/jc.2002-020773. [DOI] [PubMed] [Google Scholar]
- 9.Horwitz MJ, et al. Continuous PTH and PTHrP infusion causes suppression of bone formation and discordant effects on 1,25(OH)2 vitamin D. J. Bone Miner. Res. 2005;20:1792–1803. doi: 10.1359/JBMR.050602. [DOI] [PubMed] [Google Scholar]
- 10.Castro M, Nikolaev VO, Palm D, Lohse MJ, Vilardaga JP. Turn-on switch in parathyroid hormone receptor by a two-step parathyroid hormone binding mechanism. Proc. Natl. Acad. Sci. USA. 2005;102:16084–16089. doi: 10.1073/pnas.0503942102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean T, et al. Mechanisms of ligand binding to the parathyroid hormone (PTH)/PTH-related protein receptor: selectivity of a modified PTH(1-15) radioligand for GalphaS-coupled receptor conformations. Mol. Endocrinol. 2006;20:931–943. doi: 10.1210/me.2005-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berlot CH. A highly effective dominant negative alpha s construct containing mutations that affect distinct functions inhibits multiple Gs-coupled receptor signaling pathways. J. Biol. Chem. 2002;277:21080–21085. doi: 10.1074/jbc.M201330200. [DOI] [PubMed] [Google Scholar]
- 13.Vilardaga JP, Bunemann M, Krasel C, Castro M, Lohse MJ. Measurement of the millisecond activation switch of G protein-coupled receptors in living cells. Nat. Biotechnol. 2003;21:807–812. doi: 10.1038/nbt838. [DOI] [PubMed] [Google Scholar]
- 14.Hein P, Frank M, Hoffmann C, Lohse MJ, Bunemann M. Dynamics of receptor/G protein coupling in living cells. EMBO J. 2005;24:4106–4114. doi: 10.1038/sj.emboj.7600870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hein P, et al. Gs activation is time-limiting in initiating receptor-mediated signaling. J. Biol. Chem. 2006;281:33345–33351. doi: 10.1074/jbc.M606713200. [DOI] [PubMed] [Google Scholar]
- 16.Nikolaev VO, Bunemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J. Biol. Chem. 2004;279:37215–37218. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- 17.Syme CA, Friedman PA, Bisello A. Parathyroid hormone receptor trafficking contributes to the activation of extracellular signal-regulated kinases but is not required for regulation of cAMP signaling. J. Biol. Chem. 2005;280:11281–11288. doi: 10.1074/jbc.M413393200. [DOI] [PubMed] [Google Scholar]
- 18.Sneddon WB, et al. Activation-independent parathyroid hormone receptor internalization is regulated by NHERF1 (EBP50) J. Biol. Chem. 2003;278:43787–43796. doi: 10.1074/jbc.M306019200. [DOI] [PubMed] [Google Scholar]
- 19.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 20.Chauvin S, Bencsik M, Bambino T, Nissenson RA. Parathyroid hormone receptor recycling: role of receptor dephosphorylation and beta-arrestin. Mol. Endocrinol. 2002;16:2720–2732. doi: 10.1210/me.2002-0049. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari SL, Behar V, Chorev M, Rosenblatt M, Bisello A. Endocytosis of ligand-human parathyroid hormone receptor 1 complexes is protein kinase C-dependent and involves b-arrestin2. Real-time monitoring by fluorescence microscopy. J. Biol. Chem. 1999;274:29968–29975. doi: 10.1074/jbc.274.42.29968. [DOI] [PubMed] [Google Scholar]
- 22.Xia Z, Liu Y. Reliable and global measurement of fluorescence resonance energy transfer using fluorescence microscopes. Biophys. J. 2001;81:2395–2402. doi: 10.1016/S0006-3495(01)75886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.