Abstract
The predominant driver of bioanalysis in supporting drug development is the intended use of the data. Ligand-binding assays (LBA) are widely used for the analysis of protein biotherapeutics and target ligands (L) to support pharmacokinetics/pharmacodynamics (PK/PD) and safety assessments. For monoclonal antibody drugs (mAb), in particular, which non-covalently bind to L, multiple forms of mAb and L can exist in vivo, including free mAb, free L, and mono- and/or bivalent complexes of mAb and L. Given the complexity of the dynamic binding equilibrium occurring in the body after dosing and multiple sources of perturbation of the equilibrium during bioanalysis, it is clear that ex vivo quantification of the forms of interest (free, bound, or total mAb and L) may differ from the actual ones in vivo. LBA reagents and assay formats can be designed in principle to measure the total or free forms of mAb and L. However, confirmation of the forms being measured under the specified conditions can be technically challenging. The assay forms and issues must be clearly communicated and understood appropriately by all stakeholders as the program proceeds through the development process. This paper focuses on monoclonal antibody biotherapeutics and their circulatory L that are either secreted as soluble forms or shed from membrane receptors. It presents an investigation into the theoretical and practical considerations for total/free analyte assessment to increase awareness in the scientific community and offer bioanalytical approaches to provide appropriate PK/PD information required at specific phases of drug development.
Key words: ligand-binding assays, monoclonal antibody biotherapeutics, PK/PD, target biomarkers, total and free assays
INTRODUCTION
Biotherapeutics based on a targeted mechanism have formed a successful drug development paradigm. A major portion of the biotherapeutics consists of monoclonal antibody (mAb) drugs. More than 20 mAb have been approved by the Food and Drug Administration (FDA) as drugs, and more than 100 are projected to be under development (1). Reliable methodologies for measurements of mAb and its target ligand (L) in circulation are crucial for the assessment of exposure–response relationships in support of efficacy and safety evaluations, and dose selection. Ligand-binding assays (LBA) are commonly used for quantification of levels of mAb and the corresponding L (2,3). Multiple forms of mAb and L exist in the biological milieu including free mAb, free L, and mono- and/or bivalent complexes of mAb and L. Additional complexity can result when L is a dimer or multimer. LBA reagents and assay formats can be designed to measure the selected forms of interest, which include the bound or free forms of mAb and L. However, confirmation of the exact forms that are being measured by the LBA can be bioanalytically challenging. Because the data from LBA enable the assessment of the pharmacokinetics/pharmacodynamics (PK/PD) relationship, it is important to clearly understand the interaction of mAb and L using pertinent assays for appropriate PK/PD assessment.
The AAPS Ligand-Binding Assay Bioanalytical Focus Group initiated discussions of these issues in a hot topic session at the 2008 National Biotechnology Conference (Lee et al. LBABFG Jul 08 Newsletter) and formed a discussion group to share the key information on the AAPS Website. [http://community.aapspharmaceutica.com/ShowForum.aspx?ForumID=34]. This manuscript presents the consensus from this group as a result of thorough discussions on: (1) the utility of mAb and L information at various drug development phases, (2) reagent availability considerations, (3) bioanalytical challenges and method caveats, and (4) impact of bioanalytical data on drug development decision-making. This paper provides a practical approach to method choices for total and free assays of mAb and L. It is our attempt to:
▪Increase awareness in the drug development community as to the importance of understanding what forms (free, bound, or total) of mAb or L are being measured by a given LBA.
▪Present common bioanalytical approaches, limitations, and challenges of the methods at various phases of drug development.
▪Identify and bridge potential communication gaps between the data providers (bioanalytical scientists) and the data end users (pharmacokineticists, toxicologists, and PK/PD modelers) when defining data requirements and understanding the inherent trade-offs and risks.
For clarity of discussion, the scope of this paper is limited to mAb biotherapeutics, defined as mAb in the paper, and their circulatory L that are either secreted as soluble forms or shed from membrane receptors. Binding of mAb to anti-drug antibodies (ADA), which may impact pharmacokinetics (PK) assessment and interpretation, and binding of L to other binding proteins, which impacts diagnostic interpretation, deserve separate discussions in future publications. The terminology of free, partially free (also called partially bound), bound, and total mAb and L in the biological fluid are defined in Table I. For mAb, it is practical to define the “free” fraction as the forms that exert biological activities equivalent to those of the unbound forms. Due to the bivalency of mAbs, the free mAb includes both unbound and partially bound (i.e., monovalently bound) forms; these are typically measured using the unbound reference standards in the assay. The “total” mAb would be the sum of fully bound, partially bound, and unbound forms. In addition to binding to the mAb, L can also bind to other proteins, and L may have multiple binding sites with or without co-operative effects. However, for simplicity of the general concept, multivalent binding and proximal protein binding with L will not be discussed in this paper. We herein simplify the determination of L to include only forms of the “free” (unbound) and total (comprised of bound and unbound to mAb) with the corresponding standards being defined by the specified method.
Table I.
Terminology of Analyte Forms in Biological Fluids and Assay Types
| Term | Definition |
|---|---|
| Free drug, mAbfree (interchangeable with “active mAb”) | The sum of bivalent and monovalent unbound forms of mAb |
| Partially free mAb | mAb with one site (monovalent) bound to L and the other site unbound |
| Active mAb (interchangeable with “free mAb”) | The sum of both unbound forms of mAb (bivalent and monovalent) that are able to bind to L to interfere biological actions of L |
| Bound mAb, mAbbound | mAb with both sites bound to L |
| Total mAb, mAbtotal | Sum of bound and free mAb (mAbbound + mAbfree) |
| Free target ligand, Lfree | Unbound L |
| Bound target ligand, Lbound | L bound to mAb |
| Total target ligand, Ltotal | Sum of bound and free (unbound) L (Lbound + Lfree) |
| Specific assay | Measures a known form(s) of the analyte. Example: the combination of the capture and the detection reagents is directed towards a unique epitope of L or mAb. |
| Generic assay | Measures a class of analytes with shared epitope recognized by the reagents; the exact measurements may include more than one analyte. |
| Non-inhibitory assay | The capture and/or the detection reagents are directed toward an epitope that is non-competitive to L (or mAb) of interest, thus no or minimal inhibition is observed from L (or mAb). |
| Inhibitory assay | The capture and/or the detection reagents are directed toward a competitive epitope of L (or mAb), which will inhibit the assay at an appropriate molar ratio. |
“mAb” is defined as a monoclonal antibody therapeutic in the paper, “L” refers to the target ligand in circulation. For simplicity, L is treated as monovalent
While case studies and examples from the literature are presented here, it is not the intent of this paper to be comprehensive. Examples in the tables are given as references but not discussed in detail to avoid a lengthy paper. The reader should refer to the cited examples for detailed descriptions. Future programs and publications will certainly be developed to add to this continually evolving knowledge base.
UTILITY OF DRUG AND TARGET BIOANALYTICAL DATA
The primary driver of a bioanalytical strategy is the intended use of the data to facilitate decision-making in drug development. Depending on the information required for decision-making, the data required and hence the selection of assay (free, total or both) may differ at each phase of drug development. During the early phase of development, specific reagents may not be available for the development of a free assay. Therefore, the bioanalytical scientist and the data-user(s) should understand and communicate on: (1) the intended use of the bioanalytical assay data, (2) the dynamic equilibrium relationship between mAb and soluble L in circulation, and (3) the technical challenges and practical limitations of the assays.
In the preclinical development stage, mAb concentrations are used to understand PK behavior in non-clinical species and to project the exposure of the first in human (FIH) starting dose (Table II). L concentrations are used to understand the dynamic relationship of mAb to L to assist the identification of the biologically active mAb concentration and to allow the model-based determination of dose and regimen (4). Data in the clinical stage are used to characterize human PK profiles, define PK/PD relationships regarding safety and efficacy, and establish PK/PD models in the target population to support approved use and drug label. Detailed PK/PD data applications are listed in Tables II and III.
Table II.
Utilities of Drug and Target Concentration Data at Preclinical Development Stage
| Concentration dataa | Intended use |
|---|---|
| mAbfree or mAbtotal | To characterize in vivo PK behavior and to project human PK |
| To assess PK and PD response relationships in animal models | |
| To calculate safety margin and determine the safe starting dose and/or efficacious dose in FIH study | |
| Lfree | To assess the inhibition of Lfree following drug treatment and provide proof of concept information; to understand the dynamics of Lfree |
| Ltotal | To assess the re-distribution/modulation of Ltotal following drug treatment and provide proof of concept information; understand the dynamics of Ltotal |
| Lfree/Ltotal ratio | To compare in vivo and in vitro binding affinities |
| mAb and L dynamic relationship | To understand the underlying dynamic relationship between mAb and L to facilitate dose and dosing schedule selection |
mAb monoclonal antibody drugs, L target ligand, PK pharmacokinetics, PD pharmacodynamics, FIH first in human
aThe concentration data required for a specific analyte depend on many factors as discussed in the text
Table III.
Utilities of Drug and Target Ligand Concentration Data at Clinical Development Stage
| Concentration dataa | Intended use |
|---|---|
| mAbfree or mAbtotal | To provide key PK parameters (e.g., area under curve, clearance, volume of distribution, etc.) in human and to allow building the PK structure model |
| To understand PK behavior in different patient populations or in a special population (e.g., pediatric patient) | |
| To correlate exposures to PD or efficacy end points | |
| Model PK/PD profiles to: (1) assess exposure variability and covariate effects, (2) simulate PK/PD outcome for a desired regimen at later stages | |
| To assess potential drug–drug interactions | |
| To correlate exposure to safety end point to assess the maximum tolerated dose | |
| To characterize the mAb–L dynamics to help select dose regimen for later clinical trials | |
| Lfree or Ltotal | To understand kinetics of Lfree inhibition and its correlation to other PD responses or clinical endpoints |
| To correlate the change from baseline L with clinical outcomes and assess its potential as a biomarker for later clinical trials or as a significant covariate for PK parameters |
mAb monoclonal antibody drugs, L target ligand, PK pharmacokinetics, PD pharmacodynamics
aThe concentration data required for a specific analyte depends on many factors as discussed in the text
In many instances, the pharmacologic effect of a biotherapeutic is determined by mAbfree. This is especially true if mAb binds to a soluble ligand to prevent its binding to (or signaling via) its cognate receptor. If a correlation can be established between the binding of Lfree and clinical response, the binding and capture of L could serve as a straightforward biomarker for PK/PD modeling and regimen selection. While mAbfree reflects the availability of mAb for Lfree and binding capacity of mAb to L in vivo, an assessment of mAbtotal can further help characterize the dynamic interaction between mAb and L. mAb/L ratios and the dynamic equilibrium highly influence the specificity required for the bioanalytical assay. Thus, it is important to understand the information needed at different stages of drug development.
Pharmacokineticists are often interested in mAbfree because these levels reflect the availability of the active drug. Toxicologists, however, may have an additional interest in mAbtotal because either on- or off-target effects of mAb may pose safety concerns. It is therefore essential for pharmacokineticists and toxicologists to understand what exactly a particular assay measures before these data are used, and to clarify whether an additional assay is needed. In this regard, the PK or pharmacodynamics (PD) model generated using the results from a particular assay format may need to be re-evaluated if the assay format is changed during later stages of drug development. The LBAs of 21 FDA-approved mAb were recently reviewed in the perspectives of free and/or bound assays (5). Most of these reports did not specify what forms were being measured, which may lead to discordance of PK parameters resulting from different methods. Therefore, when developing the bioanalytical assay strategy for a mAb, it is important to work with key stakeholders in different functional groups to carefully decide on when and how to assess mAb and L for each drug development program, taking into consideration many factors including the stage of drug development.
BINDING EQUILIBRIUM AND CHALLENGES OF TOTAL/FREE ASSAYS
mAb and L binding is governed by the law of mass action first presented by Guldberg and Waage in 1864. It has been widely used to describe reversible binding interactions ranging from steroid hormones with binding proteins to mAb with L (6). Therapeutic mAbs are selected for drug development based on a high binding affinity for L, with equilibrium dissociation constants (Kd) typically in the low nanomolar to picomolar range. The binding kinetics are typically non-linear, capacity-limited, and consistent with second-order association.
 |
 |
Where Kd and Ka are the equilibrium dissociation and association constants, respectively.
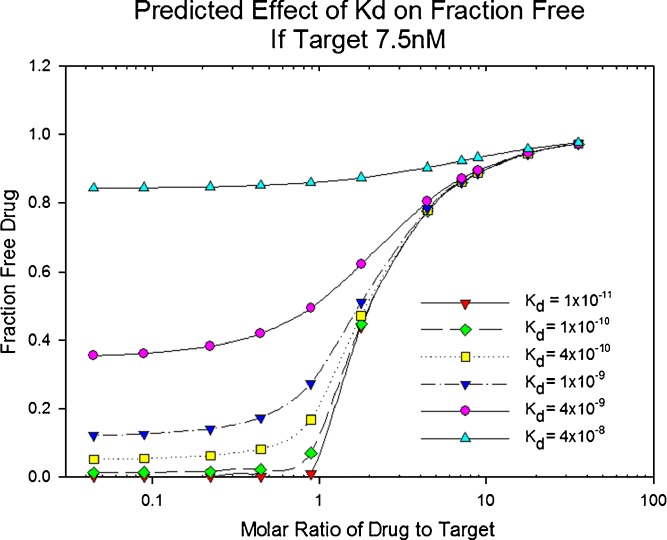
The law of mass action has been used to calculate or predict concentrations of the components in the equilibrium. The tendency of the equilibrium to shift as concentrations change is also described by Le Chatelier’s principle. Figure 1 illustrates that the fraction of mAb bound to L is dependent on the ratio of mAb to L in addition to the Kd.
Fig. 1.
Effect of mAb–L affinity on mAbfree over a range of molar ratios of mAb to L. The concentration of L was fixed at a constant for simulated calculation of mAbfree. The molar ratio in this example assumes one binding site per mole of drug for ease of interpretation; for monoclonal antibodies, there would be two sites per mole of drug
After dosing, binding of mAb to the soluble L is assumed to follow the law of mass action. Thus, concentrations of mAb and L in plasma or serum samples are analyzed to understand the dynamic equilibrium for the PK/PD relationship as discussed in UTILITY OF DRUG AND TARGET BIOANALYTICAL DATA. As predicted by the law of mass action, the in vivo equilibrium of mAb or L will shift to the free or bound form dependent on PK kinetics and the dynamics of L response as categorized in Table IV. The first scenario of high mAb/L represents mAb at high doses with a high binding affinity to L, a frequently encountered case in drug development. However, in some instances, L may accumulate and can result in lower mAb/L at certain time points. Increases in Lfree and/or Ltotal can occur with L accumulation after dosing and may counteract the intended effect of L suppression, or may bring about other safety concerns. Levels of L are dependent on the specific biology as well as disease status.
Table IV.
Dosing Scenarios and Effects on mAb and L In Vivo Equilibrium
| Conditions | Equilibrium favors mAb–L to | Resulting analytes | Comment | |
|---|---|---|---|---|
| Associate | Dissociate | |||
| High mAb/L; high affinity | X | mAbfree, mAbbound, and Lbound | Assumes independent and equivalent binding of L to each of the bivalent binding sites on mAb | |
| mAb/L ∼1; or low affinity | X | Mixed forms | Dosing may increase L to mAb/L ∼1 (16) | |
| High L; slow clearance of L, or increase L production | X | mAbbound, Lbound, and Lfree | L can accumulate if the off rate from mAb is slower than the degradation, resulting in longer T1/2 (17) | |
| Disease status/matrix | Case dependent | Individual matrix variability requires tests on samples from multiple patient donors (18) | ||
mAb monoclonal antibody drugs, L target ligand
In addition to the considerations of the in vivo dynamics of mAb-L binding upon dosing, ex vivo conditions such as sample collection, storage, shipping, and sample analysis may shift equilibrium to conditions very different from those states in vivo. Table V lists some of the conditions that may affect equilibrium dynamics of association or dissociation. The bioanalytical scientist needs to recognize these effects and either try to preserve conditions to be as close to those in vivo as possible or provide the realistic determination for proper interpretation by the data users.
Table V.
Ex Vivo Conditions Contributing to mAb and L Equilibrium Shifts
| Conditions | Equilibrium favors mAb–L to | Comment | |
|---|---|---|---|
| Associate | Dissociate | ||
| Sample dilution | X | Most mAb assays require high dilutions for sample analysis (19). | |
| Dilution’s effect on the equilibrium shift is less relevant for low abundant L assays in general. | |||
| Freeze/thaw cycles | X | (20) | |
| Assay incubation times | Case dependent | Experimental equilibrium may not be achieved during assay and the data generated may not match the model. Time to reach the equilibrium is dependent on the equilibrium rate constants and concentrations of mAb and L (19) | |
| Assay reagent | Case dependent | High binding affinity to reagent may compete with mAb–L binding (21) | |
| High concentration of capture reagent | X | (22) | |
mAb monoclonal antibody drugs, L target ligand
The ex vivo quantification of the different mAb and/or L forms of interest (free, bound, and total L and mAb) may deviate from the actual values in vivo due to possible sources of perturbation of that equilibrium during sample collection and analysis. In addition, the extent of the deviation depends on the time of sample collection during a study, which makes it harder to predict how well an experimentally determined PK/PD profile truly resembles that of the in vivo.
In the cases when it is difficult to control the equilibrium dynamics of the specific forms of mAb and/or L in a sample that contains mixtures of those forms, it may be useful to drive the equilibrium primarily to the free or bound (total) form for determination. Alternatively, a separation step can be used to separate the free from bound forms and measure the specified forms to derive the PK or PD information required at each stage of drug development. Many LBA assay platforms and conditions are flexible enough to accomplish this. Thus, the different methods and approaches of quantification of the specific forms of mAb and L are discussed in BIOANALYTICAL APPROACHES TO QUANTIFY THERAPEUTIC ANTIBODY and BIOANALYTICAL APPROACHES FOR TARGET LIGAND, respectively.
BIOANALYTICAL APPROACHES TO QUANTIFY THERAPEUTIC ANTIBODY
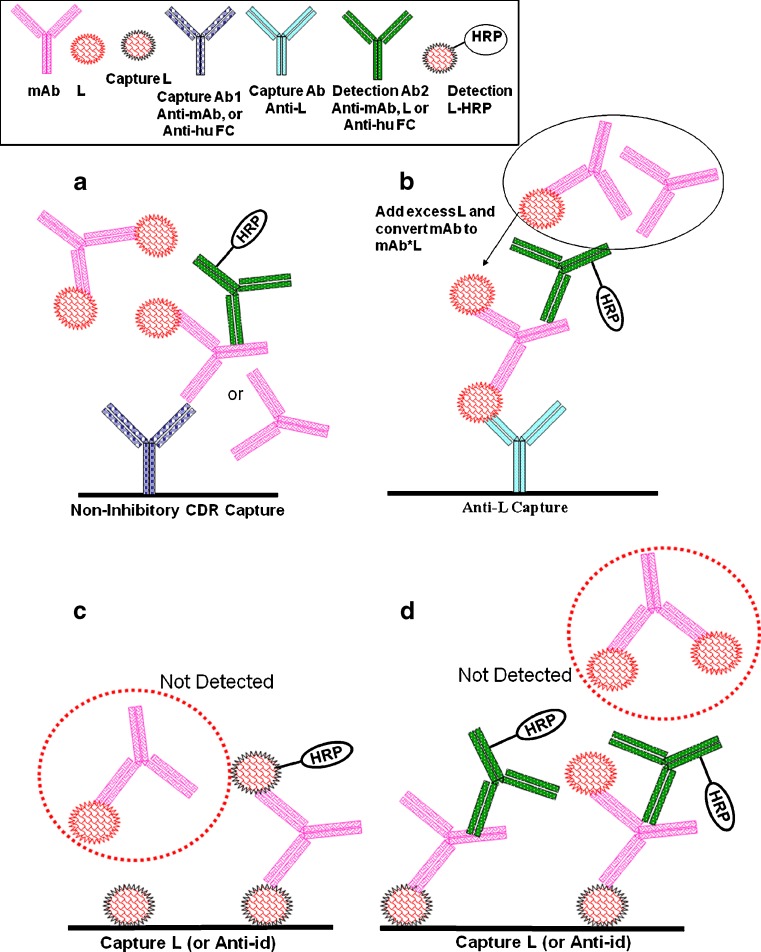
Although LBA can be designed to measure either mAbfree or mAbtotal, reagent limitations, sample dilution, etc., may result in uncertainty as to an assay’s ability to measure only mAbfree (5). This may lead to the alternative strategy of measuring mAbtotal instead. Assay formats for mAbtotal and mAbfree are described in Table VI, and typical ELISA formats are depicted in Fig. 2.
Table VI.
Assay Formats Designed for the Measurement of Putative Total and Free mAb
| Measurea | Type | Capture reagent | Detection reagent | Comment |
|---|---|---|---|---|
| mAbtotal. | Generic bridging format | Anti-human IgG | Anti-human IgG | Large size L may interfere due to steric hindrance. Not applicable to clinical sample due to interference from endogenous human IgG. |
| Complementary paired format | Non-inhibitory anti-CDR | Anti-human IgG | Applicable to clinical samples | |
| Preincubate with L, followed by mAb*L assay | Non-inhibitory anti-L | Anti-human IgG or non-inhibitory anti-CDR | Affinity of mAb binding to L must be sufficiently high. Same caveat as the generic format. | |
| Dissociation, followed by mAbfree assay | Pre-treatment may introduce assay errors | |||
| mAbfree | Bridging assay | Inhibitory anti-ids, or L | Inhibitory anti-ids or L | Measures bivalent mAbfree |
| Antigen capture | L | Non-inhibitory anti-ids or anti-human IgG | Usually, a recombinant L is used. Bivalent and monovalent mAbfree | |
| Complementary paired format | Inhibitory anti-ids | L or anti-human IgG | Measures bivalent and monovalent mAbfree | |
| Competitive assay (labeled mAb compete with unlabeled mAb in sample) | L or anti-id | (Labeled mAb) | Measures bivalent and monovalent mAbfree |
mAb monoclonal antibody drugs, L target ligand, IgG Immunoglobulin G, CDR complementarity-determining regions
aAlthough mAbtotal.and mAbfree can theoretically be measured based on the assay types above, putative values of each analyte are frequently obtained due to many factors that can alter the mAb–L equilibrium as discussed in the text
Fig. 2.
Schematics of typical ELISA for total and free mAb. Symbols for mAb and L, capture and detection reagents are shown in the inset of (a) and (b). Total drug assays: a mAbtotal: non-inhibitory anti-CDR capture, anti-hu IgG detection. b mAbtotal: preincubate with L to convert to mAb*L, followed by non-inhibitory anti-L capture and anti-human IgG or non-inhibitory anti-CDR detection. Free drug assays: c For bivalent mAbfree: bridging assay with L capture and detection. d For bivalent and monovalent mAbfree–L capture and antibody detection
Generic Formats: Used for Measuring mAbtotal
Because specific reagents are often not available, “generic” assays for measuring mAbtotal are commonly adopted during the preclinical phase. In order to minimize the cross-reactivity with the test species’ IgGs, anti-light-chain and/or subclass-specific reagents can be used (e.g., Fig. 2a, b using anti-Fc reagents). The same format may be used for multiple animal species, and for variants of drug candidates, nonetheless, the method validation for each mAb is still required for each species. It can be used as an “off-the-shelf” assay with minimal optimization needed in early development before reagents for specific assays are generated. However, this type of assay format is not applicable to clinical samples due to interference from milligrams per milliliter levels of endogenous human IgG in these samples. Results from a spike recovery test should be used to evaluate and confirm that there is no interference from the endogenous components. In addition, the method is not specific for the active drug and may react with denatured, chemically, or proteolytically modified forms of mAb.
Complementary Paired Formats: Used for Measuring mAbtotal or mAbfree
A complementary paired approach using a non-inhibitory anti-CDR antibody reagent (where the antibody reagent recognizes an epitope on the hyper-variable region of mAb that does not participate in the L binding site) and a generic reagent would be a format that can be applied to clinical samples (e.g., Fig. 2a using anti-mAb reagents). This complementarity-determining regions (CDR) epitope would not be present on the endogenous human IgG in clinical samples. However, these non-inhibitory anti-CDR mAb reagents can be difficult to obtain. Alternatively, if a polyclonal antibody reagent is used, there is the added challenge of maintaining the lot-to-lot consistency over the lifetime of a program.
For formats that are specific for mAbfree, at least one component of the pair of reagents must be binding to the same site of the analyte. These reagents might be anti-idiotypic antibodies which compete with L for binding (i.e., inhibitory anti-ids), or L itself (Fig. 2c, d). A variation of this format would be the combinatorial use of the reagents (e.g., “bridging” assays in which the same reagent is used for both the capture and detection, L as capture paired with an anti-id detection, or vice versa). One advantage of the bridging assay is that, in order to be detected, mAb must have two functionally free binding sites. The L capture format requires mAb to have only one free binding site to be detected, and it is specific for both free and partially free drug. However, the results do not reveal the relative proportions of these two forms.
In a competitive assay for mAbfree, labeled mAb (e.g., with biotin or horseradish peroxidase) is allowed to compete with unlabeled mAb in the sample for binding to specific capture reagent. The amount of labeled mAb bound will be inversely related to the amount of mAb in the sample. However, competitive assays can be less robust than non-competitive formats.
Challenges of Obtaining True Values of mAbfree
Although mAbfree represents the forms that exert biological activities and is preferred by the pharmacokineticists, in practice, in vivo mAbfree concentrations are challenging to obtain even using well-designed assay formats. As discussed in BINDING EQUILIBRIUM AND CHALLENGES OF TOTAL/FREE ASSAYS, conditions of sample collection, manipulation, or assay may perturb the equilibrium and change the proportion of mAbfree. As an alternative approach, the concentrations of mAbfree, Lfree, and mAb*L in the sample can be calculated from mAbtotal and Ltotal. However, the calculation is based on the equilibrium equation, which requires a good estimate of the equilibrium dissociation constant, Kdin vivo (7).
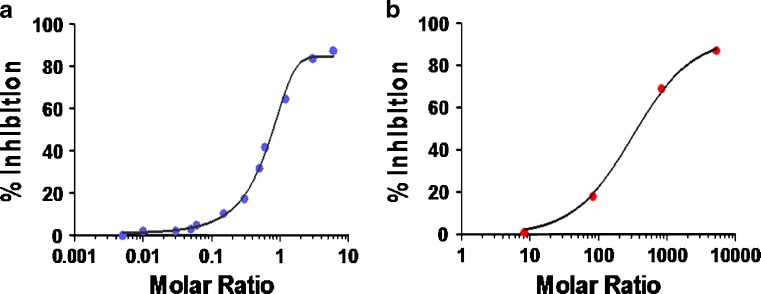
Because the dynamic equilibrium varies with different mAb and the corresponding L concentrations, it is important to test the mAb assay in the presence of excess L to determine empirically if it is indeed a total or free assay. Figure 3 shows the examples of using interference testing of mAb by L. L and mAb were preincubated at various molar ratios to reach equilibrium, and then mAb concentration was determined by the specified ELISA. The data were plotted with molar ratio of L/mAb at the X axis and percent inhibition at the Y axis. For a mAbfree assay, the IC50 would approach 1. However, it should be noted that the recombinant L used for the test may not bind to mAb exactly as the endogenous form, which may cause the IC50 to deviate from the true value.
Fig. 3.
Interference test of a mAb by L. The recombinant L and the mAb were co-incubated at 37°C at various molar ratios and followed by ELISA determination of mAb. The X axis represents the molar ratio of L/mAb and the Y axis represents the percent inhibition for binding assay of mAb. a Example of a mAbfree assay that showed 50% inhibition at molar ratio of approximately 0.7. b Example of a essentially mAbtotal assay that showed 50% inhibition at a L/mAb molar ratio of approximately 300
Additionally, it is important to select a consistent and robust method to support the product through clinical development. If a method change is necessary, method comparison should be carried out using both spiked samples and study samples to compare the impact of method change on PK interpretation.
BIOANALYTICAL APPROACHES FOR TARGET LIGAND
Assays for Measuring Total Target Ligand (Ltotal)
Ltotal provides information on the effect of mAb on L accumulation. Because mAb generally has a longer half life than the circulating L, mAb*L formed after dosing may not be cleared as fast as Lfree. Furthermore, up-regulation of the membrane receptor form of L or synthesis of soluble L as a response to dosing in some cases may increase the circulatory L concentration (8).
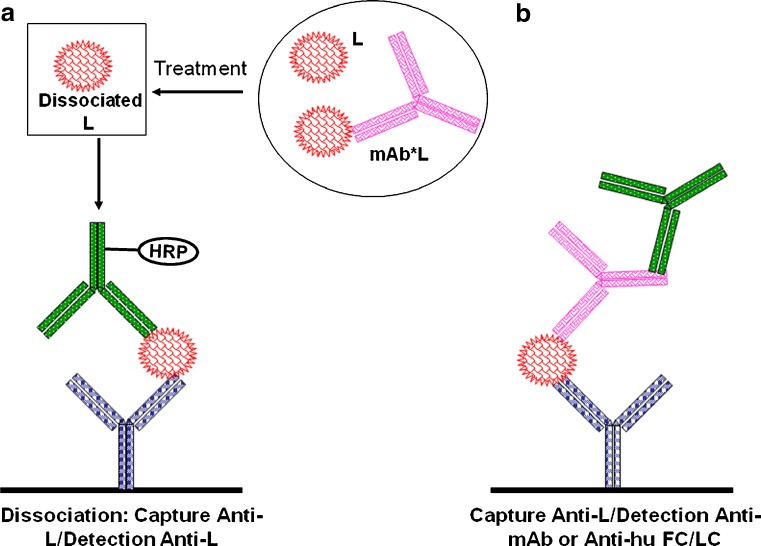
Several methods can be adopted to measure Ltotal. These methods are summarized in Table VII with comments on method application and limitations. Typical assay formats are depicted in Fig. 4. To measure Ltotal, specific Ab against L at epitopes that are different from that of mAb (non-inhibitory) can be used (Fig. 4b). For this assay format, if the anti-L reagent has overlapping regions of recognition with mAb, the presence of mAb could interfere with the assay, resulting in under-estimation. Conversely, mAb may form complexes with L, which may enhance the assay signal, resulting in over-estimation. Dilution of the sample may increase dissociation of the mAb*L complexes for an Ltotal assay. Pretreatments may also be used to drive the bound L forms into the dissociated form (Fig. 4a). The dissociation methods are dependent on the relative denaturation of mAb as opposed to L (9).
Table VII.
Assay Approaches for the Measurement of Putative Total Target Ligand for Preclinical and Clinical Development Stages
| Pre-treatment | Capture | Detection | Comment |
|---|---|---|---|
| None | First specific anti-L Ab | Second specific anti-L Ab | Specific non-inhibitory Abs |
| Add excess mAb to convert all to monovalent mAb*L | Specific anti-L Ab | Specific anti-mAb or anti-human FC or anti-human LC | Measures mAb*L |
| Dissociation by acid, urea, or alcohol (23) | First specific anti-L Ab | Second specific anti-L Ab | Dissociation may be incomplete; L may be labile to pre-treatment |
| Alkaline (pH > 13), or acid/guanidine (9) | First specific anti-L Ab | Second specific anti-L Ab | L may be labile to pre-treatment |
| Acid dissociation followed by solid phase extraction and ELISA | First specific anti-L Ab | Second specific anti-L Ab | For low molecular weight L; L may be labile to pre-treatment |
| Acid extraction followed by solid phase extraction and LC-MS (24) | NA | LC-MS | For low molecular weight L; L may be labile to pre-treatment |
| Immuno-affinity capture of L followed by LC-MS (25) | Specific non-inhibitory anti-L Ab | LC-MS | LC-MS less sensitive than ELISA |
| Competitive ELISA | Specific non-inhibitory anti-L Ab | L-conjugate | Free and bound may not compete equally |
| Dilution and incubation of sample prior to LBA analysis | First specific anti-L Ab | Second specific anti-L Ab | Dilution, temperature, and time may drive equilibrium toward Lfree |
mAb monoclonal antibody drugs, L target ligand, FC fragment cystallizable region, LC liquid chromatography LC-MS liquid chromatography-mass spectrometry, NA not applied, LBA ligand-binding assays, ELISA enzyme-linked immunosorbent assay
Fig. 4.
Schematic of typical sandwich ELISA for Ltotal. a Pre-treatment to dissociate mAb*L complex before assay. b No pre-treatment to dissociate. Symbols are the same as in Fig. 2a, b
Assays for Measuring Free Target Ligand (Lfree)
Monitoring Lfree during dosing is informative for determining the efficacious dose. Reagent choice affects Lfree measurement in a way similar to that of mAbfree, however, with more complexity. In many cases, the soluble L is present at low abundance compared with the membrane-bound L in the tissue and may require a highly sensitive assay to measure the normal levels. The high molar ratio of mAb/L in most cases prohibits accurate measurements of the Lfree after dosing. However, the relative trend of Lfree can be obtained to provide valuable information about the effect of mAb and levels required for maintenance.
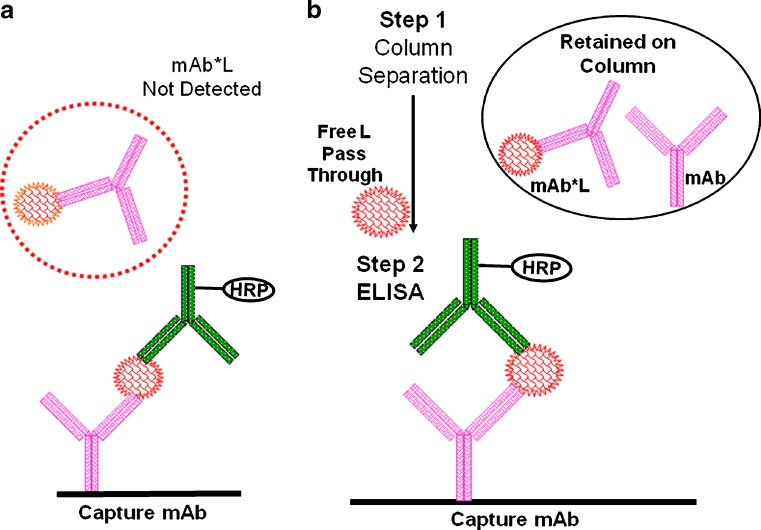
Methods that have been used to measure Lfree in preclinical and clinical stages are summarized in Table VIII with typical assay formats depicted in Fig. 5. A specific inhibitory anti-L antibody or mAb (or mAb analog) can be used as the capture reagent and another specific non-inhibitory anti-L antibody for detection (Fig. 5a). However, sample process steps and assay conditions that dissociate the bound form (see BINDING EQUILIBRIUM AND CHALLENGES OF TOTAL/FREE ASSAYS) may result in over-estimation (9). Another approach is to remove the bound forms prior to LBA by molecular sieve, solid phase extraction, or affinity separations (Fig. 5b, e.g., removal by protein G, protein A, or anti-human FC column). However, the additional processes may introduce error due to adsorption to the column or filter surfaces. Dissociation may also occur during the process, and the processes are labor-intensive. Therefore, Lfree data may only be able to show a relative trend rather than providing absolute quantification.
Table VIII.
Assay Approaches for the Measurement of Putative Free Target Ligand Measurement
| Pre-treatment | Capture | Detection | Comment |
|---|---|---|---|
| NA | First specific anti-L Ab or mAb | Second specific anti-L Ab | Specific inhibitory reagents |
| Solid phase extraction followed by ELISA or LC-MS | First specific anti-L Ab | Second specific anti-L Ab | Applicable to low molecular weight L |
| Filtration using spin column (e.g. 5,000 MW cut off) | First specific anti-L Ab | Second specific anti-L Ab | Applicable to low molecular weight L |
| Affinity separation of bound and free ligand | First specific anti-L Ab | Second specific anti-L Ab | Applicable to large molecule L |
mAb monoclonal antibody drugs, L target ligand LC-MS liquid chromatography-mass spectrometry, ELISA enzyme-linked immunosorbent assay, NA not applied
Fig. 5.
Schematic of typical sandwich ELISA for Lfree. a No pre-treatment for separation; b Use μ-affinity column to separate free from bound before ELISA. Symbols are the same as in Fig. 2a, b
When using sample preparation methods to develop total and free assays, it is important to evaluate the L recovery across the expected concentration range and in the intended matrix with and without mAb and to test for interference from matrix and other related binding proteins. It is important to confirm whether the assay is measuring total or free L using the final method. The interference test with various molar ratios of mAb/L similar to the experiment described in Fig. 3 may be used.
Relative Assay of Target Ligand
The measurement of L (Ltotal or Lfree) can provide important information that includes proof of in vivo binding of mAb to L, target occupancy, efficacious mAb levels, and PK/PD relationship. However, the accuracy of L measurement depends on the quality of the reference standard and the reagents. When native endogenous L standards cannot be obtained, recombinant and/or synthetic standards of L are used. The accuracy of measurement depends on relative binding activity of the standard compared with that of the endogenous analyte. Parallelism should be evaluated for Ltotal during analytical validation to determine if the assay recognizes the endogenous L in the same way as the reference standard. A lack of parallelism indicates that the assay is only quasi-quantitative and the data must be interpreted in this context. When samples with sufficiently high endogenous levels are not available to evaluate parallelism, caution should be used in interpreting the data in absolute terms. In this case, the relative change in L will be more reliable (3).
CONCLUSIONS AND PERSPECTIVES
In order to use and interpret bioanalytical data appropriately, it is important to understand the reliability and limitations of the data. The LBA Bioanalytical Focus Group of AAPS first organized a forum for discussion on the utility of total and free binding assays during a hot topic session at the 2008 National Biotech Conference. The interactions that ensued brought to light the complexities that must be considered when developing a total or free assay. Experts in bioanalysis, PK/PD modeling, and toxicology have continued this discussion to share their experiences in best practices and associated challenges. This paper summarizes the major points of those discussions.
In LBA, capture and detection reagents are critical components in determining assay specificity for free, bound, or total. An understanding of the mAb/L ratio and dynamic equilibrium is paramount to selecting the most appropriate approach for method development. It is equally important to understand the variability in mAb/L ratio found in different species and disease status since the disease model or specific patient population may have a very different L profile than the healthy controls. Even in the presence of highly characterized reagents, it should be understood that the mAb*L exists in vivo in a dynamic equilibrium and thus ex vivo assay conditions (e.g., sample dilution and incubation times) can impact both mAb and L being measured and the equilibration of the two. While the mAb-L equilibrium and assay manipulations can be investigated, the experimental results should be evaluated to understand if they truly reflect the binding relationships in the study samples. That being understood, the sample processing strategy and the assay formats listed in the tables are frequently employed to develop assays aimed at assessing free, total, and complexed mAb and L forms.
mAb and L concentration data are utilized to make specific decisions at different stages of development. In the preclinical setting, reagents may not be available to develop a mAbfree method. mAbfree or mAbtotal (when a mAbfree method is not available) determinations are used to evaluate systemic exposure-time course, its relationship to toxicity findings, and predict the safety margin for dosing in human. Typically, mAb is dosed in excess of L, and mAbtotal would approximate mAbfree, which may serve as an indicator of mAb activity for use in model-based dosing decisions. During clinical evaluations, mAbfree or mAbtotal assayed with specific reagents are used to characterize drug disposition in humans and to correlate exposure with safety and efficacy while a better understanding of the mAb-L dynamics may inform dosing regimen selections for later stage clinical trials.
L data has been increasingly used in drug development to guide decisions. For instance, Lfree data may be useful in dose and schedule selection. The understanding of the L kinetics could help to define the efficacious mAbfree levels required to maintain receptor occupancy. In many cases, because Lfree concentration is so low and may vary with assay conditions, quantification may be unreliable. Another approach is to examine the trend of change after dosing rather than rely on the absolute values. Ltotal may indicate a proof of activity and may raise safety concern if the mAb–L binding alters the target expression profile (e.g., high L accumulation), Ltotal may be used in PK/PD models to deduce Lfree. With the appropriate information of Ltotal, Lfree, as well as mAbfree or mAbtotal, one may estimate the in vivo binding affinity and inhibition of Lfree using PK/PD modeling, which may assist the dose and dosing schedule selection.
Since the analyte forms (free/total/complex) being measured and the bioanalytical methods used to quantify these forms impact the determination of the drug exposure-time course, it is critical to align bioanalytical data interpretation within the context of the overall drug development program. Moreover, it is important to design bioanalytical methods that address the pertinent scientific questions. Because of the complexity and uniqueness of each target and its associated disease biology, bioanalytical assay strategies for quantifying relevant forms should be carefully crafted for each development program in consultation with data end-users, taking into consideration drug-target biology, the development stage, and practical challenges including reagents availability. While a satisfactory bioanalytical method that meets all the requirements is highly desirable and may be developed with additional effort, it is often the case that a less optimal assay has to be used to support a certain stage of development. In the later case, caveats of the method, impact on data interpretation, and risks to the program should therefore be clearly communicated to ensure fully informed decisions.
There were a few publications using tandem high-performance chromatography mass spectrometry (LC-MS/MS) methods to quantify mAb (10–15). The method approach involves enzyme digestion to reduce mAb into small peptides to be within the mass range of the mass spectrometry. However, the mAb has to be denatured before enzyme digestion, resulting dissociation of the mAb from L. Thus, the LC-MS/MS method would quantify total mAb.
While the issues and examples described in this paper have applications to many types of biological therapeutics, in the interest of clarity, the authors limited the focus to mAb and their associated L. The complexity of other biotherapeutics such as proteins, peptides, and oligonucleotides and their interactions will require additional consideration to expand the concepts presented here and will be the subject of future publications.
We acknowledge the challenges of providing clear and decisive recommendations on practical methods development and appropriate use of the resulting data. We have attempted to identify the usefulness of the data at each stage of drug development so that assay development may be directed to serve that specific purpose. More importantly, we have highlighted the assay limitations for appropriate data usage and interpretation. Discussions will continue on to the issues and challenges of both mAbs and non-mAb biotherapeutics. Of special interest is the topic of binding components, especially ADA.
Acknowledgment
We thank other members of the discussion team besides the authors at the AAPS Ligand-Binding Bioanalytical Focus Group for their participation in the discussions and reviewing the manuscript: Huifen Faye Wang, Laura Hong, Thomas McIntosh, Valerie Quarmby, Bing Kuang, Jaya Goyal, Alyssa Morimoto, Murli Krishna, Tracey Clark, John Nowak, Chun-Hua Cai, Eric Blasko, Barbara Duncan, Melissa Jezuit, Mark Peterson, Manoj Rajadhyaksha, Frank Spriggs, Martin Ullmann, Steven Martin, Ellen Wang, Yuling Wu, Jie Guo, and Tatiana Plavina. We also thank Jayanthi Wolf, Danuta Herzyk, Hong Wang, and Scott Chandler for reviewing the manuscript.
Abbreviations
- ADA
Anti-drug antibodies
- Anti-id
Anti-idiotypic
- CDR
Complementarity-determining regions
- ELISA
Enzyme-linked immunosorbent assay
- FIH
First in human
- Fc
Fragment cystallizable region
- IC50
The concentration of the interfering compound at which the analyte recovery is 50% of that in the absence of the interfering compound (50% inhibition point)
- IgG
Immunoglobulin G
- Kd
Dissociation equilibrium constant
- LBA
Ligand-binding assay
- L
Target ligand
- Lfree
Target ligand not bound to drug
- Ltotal
Sum of bound and unbound target ligand
- mAb
Monoclonal antibody
- mAbfree
The unbound and partially free forms of the monoclonal antibody drug that are able to bind to the target ligand to mediate biological actions
- mAbtotal
Sum of bound partially bound and unbound forms of the monoclonal antibody drug
- mAb*L
Complex of monoclonal antibody drug and target ligand
- pAb
Polyclonal antibodies
- PD
Pharmacodynamics
- PK
Pharmacokinetics
References
- 1.Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84(5):548–58. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- 2.DeSilva B, Smith W, Weiner R, Kelley M, Smolec J, Lee B, et al. Recommendations for the bioanalytical method validation of ligand-binding assays to support pharmacokinetic assessments of macromolecules. Pharm Res. 2003;20(11):1885–900. doi: 10.1023/B:PHAM.0000003390.51761.3d. [DOI] [PubMed] [Google Scholar]
- 3.Lee JW, Devanarayan V, Barrett YC, Weiner R, Allinson J, Fountain S, et al. Fit-for-purpose method development and validation for successful biomarker measurement. Pharm Res. 2006;23(2):312–28. doi: 10.1007/s11095-005-9045-3. [DOI] [PubMed] [Google Scholar]
- 4.Betts AM, Clark TH, Yang J, Treadway JL, Li M, Giovanelli MA, et al. The application of target information and preclinical pharmacokinetic/pharmacodynamic modeling in predicting clinical doses of a Dickkopf-1 antibody for osteoporosis. J Pharmacol Exp Ther. 2010;333(1):2–13. doi: 10.1124/jpet.109.164129. [DOI] [PubMed] [Google Scholar]
- 5.Kuang B, King L, Wang H. Therapeutic monoclonal antibody concentration monitoring: free or total? Bioanalysis. 2010;2(6):1125–40. doi: 10.4155/bio.10.64. [DOI] [PubMed] [Google Scholar]
- 6.Baulieu EE. Some aspects of the mechanism of action of steroid hormones. Mol Cell Biochem. 1975;7(3):157–74. doi: 10.1007/BF01731406. [DOI] [PubMed] [Google Scholar]
- 7.Reverberi R, Reverberi L. Factors affecting the antigen-antibody reaction. Blood Transfus. 2007;5(4):227–40. doi: 10.2450/2007.0047-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93(11):2645–68. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]
- 9.Salimi-Moosavi H, Lee J, DeSilva B, Dollgast G. Novel approaches using alkaline or acid/guanidine treatment to eliminate therapeutic antibody interference in the measurement of total target ligand. J Pharm Biomed Anal. 2010;51:1128–33. doi: 10.1016/j.jpba.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Ezan E, Dubois M, Becher F. Bioanalysis of recombinant proteins and antibodies by mass spectrometry. Analyst. 2009;134(5):825–34. doi: 10.1039/b819706g. [DOI] [PubMed] [Google Scholar]
- 11.Dubois M, Fenaille F, Clement G, Lechmann M, Tabet JC, Ezan E, et al. Immunopurification and mass spectrometric quantification of the active form of a chimeric therapeutic antibody in human serum. Anal Chem. 2008;80(5):1737–45. doi: 10.1021/ac7021234. [DOI] [PubMed] [Google Scholar]
- 12.Hagman C, Ricke D, Ewert S, Bek S, Falchetto R, Bitsch F. Absolute quantification of monoclonal antibodies in biofluids by liquid chromatography-tandem mass spectrometry. Anal Chem. 2008;80(4):1290–6. doi: 10.1021/ac702115b. [DOI] [PubMed] [Google Scholar]
- 13.Heudi O, Barteau S, Zimmer D, Schmidt J, Bill K, Lehmann N, et al. Towards absolute quantification of therapeutic monoclonal antibody in serum by LC-MS/MS using isotope-labeled antibody standard and protein cleavage isotope dilution mass spectrometry. Anal Chem. 2008;80(11):4200–7. doi: 10.1021/ac800205s. [DOI] [PubMed] [Google Scholar]
- 14.Luna LG, Williams TL, Pirkle JL, Barr JR. Ultra performance liquid chromatography isotope dilution tandem mass spectrometry for the absolute quantification of proteins and peptides. Anal Chem. 2008;80(8):2688–93. doi: 10.1021/ac701945h. [DOI] [PubMed] [Google Scholar]
- 15.Wang KY, Chuang SA, Lin PC, Huang LS, Chen SH, Ouarda S, et al. Multiplexed immunoassay: quantitation and profiling of serum biomarkers using magnetic nanoprobes and MALDI-TOF MS. Anal Chem. 2008;80(16):6159–67. doi: 10.1021/ac800354u. [DOI] [PubMed] [Google Scholar]
- 16.Lowe PJ, Tannenbaum S, Gautier A, Jimenez P. Relationship between omalizumab pharmacokinetics, IgE pharmacodynamics and symptoms in patients with severe persistent allergic (IgE-mediated) asthma. Br J Clin Pharmacol. 2009;68(1):61–76. doi: 10.1111/j.1365-2125.2009.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lachmann HJ, Lowe P, Felix SD, Rordorf C, Leslie K, Madhoo S, et al. In vivo regulation of interleukin 1beta in patients with cryopyrin-associated periodic syndromes. J Exp Med. 2009;206(5):1029–36. doi: 10.1084/jem.20082481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hormbrey E, Gillespie P, Turner K, Han C, Roberts A, McGrouther D, et al. A critical review of vascular endothelial growth factor (VEGF) analysis in peripheral blood: is the current literature meaningful? Clin Exp Metastasis. 2002;19(8):651–63. doi: 10.1023/A:1021379811308. [DOI] [PubMed] [Google Scholar]
- 19.Beum PV, Kennedy AD, Taylor RP. Three new assays for rituximab based on its immunological activity or antigenic properties: analyses of sera and plasmas of RTX-treated patients with chronic lymphocytic leukemia and other B cell lymphomas. J Immunol Meth. 2004;289(1–2):97–109. doi: 10.1016/j.jim.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Beer PM, Wong SJ, Hammad AM, Falk NS, O’Malley MR, Khan S. Vitreous levels of unbound bevacizumab and unbound vascular endothelial growth factor in two patients. Retina. 2006;26(8):871–6. doi: 10.1097/01.iae.0000233327.68433.02. [DOI] [PubMed] [Google Scholar]
- 21.Blasco H, Lalmanach G, Godat E, Maurel MC, Canepa S, Belghazi M, et al. Evaluation of a peptide ELISA for the detection of rituximab in serum. J Immunol Meth. 2007;325(1–2):127–39. doi: 10.1016/j.jim.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Ceze N, Ternant D, Piller F, Degenne D, Azzopardi N, Dorval E, et al. An enzyme-linked immunosorbent assay for therapeutic drug monitoring of cetuximab. Ther Drug Monit. 2009;31(5):597–601. doi: 10.1097/FTD.0b013e3181b33da3. [DOI] [PubMed] [Google Scholar]
- 23.Davis RA, Butterfield AM, Konrad RJ, Bourdage JS. A novel method for quantitative measurement of a biomarker in the presence of a therapeutic monoclonal antibody directed against the biomarker. J Pharm Biomed Anal. 2008;48(3):897–901. doi: 10.1016/j.jpba.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Rose MJ, Tran L, Zhang J, Miranda LP, James CA, et al. Development of a method for the sensitive and quantitative determination of hepcidin in human serum using LC-MS/MS. J Pharmacol Toxicol Meth. 2009;59(3):171–80. doi: 10.1016/j.vascn.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Wang R, Sweeney D, Gandy SE, Sisodia SS. The profile of soluble amyloid beta protein in cultured cell media. Detection and quantification of amyloid beta protein and variants by immunoprecipitation-mass spectrometry. J Biol Chem. 1996;271(50):31894–902. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]