Abstract
For currently available antipsychotic drugs, blockade of dopamine D2 receptors is a critical component for achieving antipsychotic efficacy, but it is also a driving factor in the development of extrapyramidal symptoms (EPS). To inform the clinical development of asenapine, generic mathematical models have been developed for predicting antipsychotic efficacy and EPS tolerability based on D2 receptor occupancy. Clinical data on pharmacokinetics, D2 receptor occupancy, efficacy, and EPS for several antipsychotics were collected from the public domain. Asenapine data were obtained from in-house trials. D2 receptor occupancy data were restricted to published positron emission tomography studies that included blood sampling for pharmacokinetics. Clinical efficacy data were restricted to group mean endpoint data from short-term placebo-controlled trials, whereas EPS evaluation also included some non-placebo-controlled trials. A generally applicable model connecting antipsychotic dose, pharmacokinetics, D2 receptor occupancy, Positive and Negative Syndrome Scale (PANSS) response, and effect on Simpson–Angus Scale (SAS) was then developed. The empirical models describing the D2–PANSS and D2–SAS relationships were used successfully to aid dose selection for asenapine phase II and III trials. A broader use can be envisaged as a dose selection tool for new antipsychotics with D2 antagonist properties in the treatment of schizophrenia.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-010-9247-4) contains supplementary material, which is available to authorized users.
KEY WORDS: asenapine, dopamine D2 receptors, mathematical models
INTRODUCTION
Schizophrenia is a chronic, relapsing illness that is characterized by positive symptoms (e.g., hallucinations and delusions), negative symptoms (e.g., flattened affect and anhedonia), and varying degrees of cognitive impairment. The first- and second-generation antipsychotics approved for the treatment of schizophrenia are primarily effective in managing the positive symptoms of schizophrenia (1).
The efficacy and tolerability of antipsychotics for the positive symptoms of schizophrenia are strongly related to antagonism of dopamine D2 receptors. The D2 occupancy theory suggests that efficacy is optimal when D2 receptor occupancy falls within a certain range (2,3). Data from positron emission tomography (PET) studies in clinical populations indicate that D2 occupancy levels ranging from 65% to 80% elicit optimal antipsychotic effects (4–6). However, risk for D2-related adverse events, particularly extrapyramidal symptoms (EPS), increases substantially when D2 receptor occupancy exceeds 80% (7). Despite these observations, D2 occupancy levels that maximize efficacy and minimize EPS have not been thoroughly quantified across a range of antipsychotics.
Quantitative models that link D2 receptor occupancy of antipsychotic agents to their clinical efficacy and tolerability could be of great utility to predict clinically effective dose ranges for new antipsychotics that act by antagonizing D2 receptors. One such agent, asenapine, is indicated in the USA in adults for the treatment of schizophrenia and the acute treatment, as monotherapy or adjunctive therapy to lithium or valproate, of manic or mixed episodes of bipolar I disorder (8). Asenapine is approved in the European Union for the treatment of moderate to severe manic episodes in bipolar disorder (9). Asenapine exhibits potent in vitro antagonistic activity across a range of cloned human serotonergic, dopaminergic, α-adrenergic, and histaminergic receptors; the Ki of asenapine is 1.3 nM for the D2L receptor and 1.6 nM for the D2S receptor (10).
In early trials, establishing an effective dose range for asenapine was challenging. In an unpublished phase II study, sublingual asenapine doses up to 0.8 mg twice daily (BID) did not reduce symptoms of schizophrenia compared with placebo. In PET studies of healthy subjects, low D2 occupancy was observed after the administration of sublingual asenapine doses of up to 0.3 mg BID (11,12), but D2 receptor occupancy achieved more appropriate levels of 68–93% in patients with schizophrenia after administration of asenapine 2.4–4.8 mg BID (12). Based on these data, it was decided that a model using D2 receptor occupancy levels be developed to predict efficacy across a broader range of asenapine doses. In a next stage, a model to predict EPS tolerability was also included in the framework. Data from multiple marketed antipsychotics (i.e., haloperidol, olanzapine, risperidone, and ziprasidone) were used in the development of these models because the information from asenapine clinical trials alone was not sufficient to generate sufficiently robust models. The resulting model framework was applied to support dose selection decisions for subsequent phase II and phase III trials.
MATERIALS AND METHODS
Overall Approach
An overview of the model framework is presented in Fig. 1. In brief, for each drug and dose regimen for which suitable efficacy and EPS data were available, the time course of D2 occupancy was predicted from pharmacokinetic–pharmacodynamic models. Clinical efficacy and EPS data at these dose regimens were linked to summary measures of these predicted D2 occupancy time courses.
Fig. 1.
Schematic overview of the relationship among the models used to develop the overall PANSS–D2 and EPS–D2 models. PANSS Positive and Negative Syndrome Scale, PET positron emission tomography, SAS Simpson–Angus Scale
Data
Data were obtained from multiple sources. Asenapine data were obtained from an in-house database of clinical studies. Data sources for haloperidol, olanzapine, risperidone, and ziprasidone included public domain regulatory documents, advisory committee documents, and the published literature. Although haloperidol is considered a first-generation antipsychotic, it was included in the model because it is frequently used as a positive control and was anticipated to provide important EPS data at high D2 occupancy levels.
Pharmacokinetics
The pharmacokinetics of each drug was described by compartmental models. A one-compartment model was used for haloperidol, olanzapine, and ziprasidone (13,14). A two-compartment model was used for risperidone (15) and asenapine (based on internal reports). Parameter uncertainty and inter-individual variability were derived from reported parameter standard errors (SE) and variability if available. If not directly available for the modeled parameters, they were derived from the variability in reported area under the curve and maximum concentration (Cmax) values. The pharmacokinetic models were entered into Pharsight trial simulator v2.1 (Pharsight Corporation, London, UK) where they were combined with the concentration–D2 models.
Dopamine D2 Receptor Occupancy
Dopamine D2 receptor occupancy data were obtained from PET studies in which plasma concentrations were available following the administration of single or multiple doses of different antipsychotics (see “Data” above) to healthy volunteers or schizophrenia patients. Although data from both PET and single-photon emission computed tomography (SPECT) studies could be used to ascertain receptor occupancy levels, in the present models, only PET data were used because there were only limited SPECT data that included plasma concentration observations. All of the PET studies used 11C-raclopride as the radioligand, except for one in-house PET study with asenapine that used NCQ-115 (12).
The relationship between D2 occupancy and plasma concentrations was described by an Emax model. Since D2 occupancy corresponds to a direct 1:1 interaction between the ligand and the receptor, a Hill coefficient of 1 was assumed. It was also assumed that D2 occupancy is 0 in the absence of drug. Based on these assumptions, the simplified Hill equation was used:
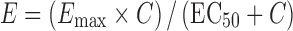 |
where E is the D2 occupancy, Emax is the maximal D2 occupancy, EC50 is the drug concentration producing a half-maximal D2 occupancy, and C is the drug plasma concentration. Models were developed using the NLME function in S-Plus (Insightful Corporation, Palo Alto, California).
Clinical Parameters
Data
Clinical data for the five antipsychotics were linked to predictions from the D2 occupancy model to characterize the relationships between clinical efficacy or EPS liability and D2 occupancy levels. The most common primary efficacy measure to assess treatment effects in schizophrenia is change in total score on the Positive and Negative Syndrome Scale (PANSS) (16). The mean change in PANSS total score from baseline at endpoint compared with placebo, using the last observation carried forward (LOCF) method to account for missing values, was the dependent variable in the efficacy model. Because of this choice of efficacy parameter, only placebo-controlled trials were included. In the case of flexible-dose studies, the reported mean daily dose of study medication was used. Before the use of the PANSS, the Brief Psychiatric Rating Scale (BPRS) was the main efficacy measure used in antipsychotic trials. To assess whether BPRS data should be disregarded or used, in-house data from individual patients where both PANSS and BPRS were recorded were analyzed using simple linear regression. This analysis (not presented) suggested that the two endpoints were indeed correlated; hence, BPRS data were included in the present analysis.
Following exploratory analyses of EPS data collected using a combination of rating scales [Simpson–Angus Scale (SAS), Extrapyramidal Symptom Rating Scale (ESRS), Barnes Akathisia Rating Scale, Abnormal Involuntary Movement Scale] as well as reported frequencies of specific EPS-related adverse events, the SAS (17) was selected as a general assessment of EPS-like symptoms. The risperidone clinical trial program used the ESRS as a comprehensive measure of EPS. As with the PANSS–BPRS relationship, a decision as to whether to exclude the ESRS data or to attempt to “map” the ESRS scale to the SAS scale was considered. The approach used mapped ESRS scores to SAS scores by anchoring the extreme ends of each scale; linearity was assumed between the extremes. The bottom end of the scale was determined from mean placebo EPS scores and the top end from mean haloperidol EPS scores. With these parameters in place, the following equation was used to calculate predicted SAS scores from observed ESRS scores:
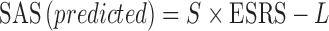 |
where S = (SAS haloperidol mean − SAS placebo mean)/(ESRS haloperidol mean − ESRS placebo mean) and L = (S × ESRS placebo mean) − SAS placebo mean. This process is depicted schematically in Fig. 2. In most cases, placebo-controlled trials were used to determine the difference in EPS effect from placebo. However, in three clinical trials that did not include placebo arms, low-dose antipsychotic treatments were considered to represent placebo. The olanzapine (1 mg once daily, QD), risperidone (0.5 mg BID), and ziprasidone (2 mg BID) doses used were much lower than the optimally effective clinical doses (olanzapine 10 mg QD; risperidone 4–8 mg/day; ziprasidone 20–80 mg BID) (13,18,19). As such, it would be unlikely that these doses would induce EPS, given that the optimal clinical doses of these agents are associated with minimal liability for EPS.
Fig. 2.
Schematic depiction of the conversion of ESRS scores to SAS scores for the extrapyramidal symptom modeling. ESRS Extrapyramidal Symptom Rating Scale, SAS Simpson–Angus Scale
Models
Two separate generic models were developed to describe the relationships of PANSS versus D2 occupancy (PANSS–D2) and SAS versus D2 occupancy (SAS–D2) across the five antipsychotics. Initially, the efficacy model was developed to support dose selection in subsequent phase II trials. Following completion of phase II, the model for EPS was added to the overall framework.
In developing these models, several basic assumptions were made. Assumption 1: D2 occupancy is a primary driver of efficacy and EPS. Assumption 2: Deriving PANSS and SAS scores from the BPRS and ESRS, respectively, does not introduce bias, and the additional information provided by these conversions outweighs the uncertainty surrounding these transforms. The validity of this assumption was assessed by investigating the effect of including or excluding BPRS and ESRS data on the fitted relationships. Assumption 3: Individual covariates (smoking, age, sex, etc.) are of relatively minor importance compared with study-to-study variability as a result of differences in, e.g., dropout rate and placebo response. A recent pooled analysis of the individual clinical trial data from the asenapine program in schizophrenia supports this assumption (20). Assumption 4: The use of both daily peak D2 occupancy and average D2 occupancy will mitigate the impact of pharmacokinetic differences across agents. Overall, the approach was to develop mathematical models that described the data well and generated predictions with sufficient precision to establish a basis for decision making.
The range of D2 occupancy values that elicit antipsychotic efficacy with minimal risk for inducing EPS (60%–85%) is narrow. Most of the clinical data were found to be associated with occupancy values within this range. Therefore, a log transformation of the D2 occupancy level [i.e., log(100% − D2 occupancy)] was performed to make the scale more suitable for parametric modeling. This transformation stretches the area of interest on the x-axis (D2 occupancy, 60–95%) and contracts areas of less interest (D2 occupancy, <60%). Models were developed in SAS version 8 (SAS Institute Inc., Cary, North Carolina).
Simulations
The complete model framework was applied to predict the dose response of asenapine on PANSS and SAS. Simulations were performed aiming to characterize the ”true” dose response for asenapine in a typical patient. For different asenapine dose regimens, predictions were made of pharmacokinetics, D2 occupancy time profiles, and resulting PANSS or SAS scores. Model uncertainty in the parameter estimates of each model component was included in the simulations through univariate sampling on the basis of standard errors (pharmacokinetic model) or multivariate sampling from the variance–covariance matrix (D2 occupancy, D2–PANSS, and D2–SAS models). An external validation of the efficacy model was obtained through comparison to results from subsequent, independent phase II studies.
RESULTS
Pharmacokinetics
Figure 3 shows pharmacokinetic profiles across agents after dose, clearance, and bioavailability normalization for illustrative purposes. Considerable differences exist in the peak/trough ratios for the different antipsychotics, with asenapine having the highest peak/trough ratio, even at BID dosing. The mean population pharmacokinetic parameters and relative standard errors for the agents that were used in the simulations are reported in Table I.
Fig. 3.
Plasma concentrations of antipsychotics over time. Concentrations are normalized for dose, clearance, and bioavailability. BID twice daily, QD once daily
Table I.
Parameter Estimates Used in the Population Pharmacokinetic Models
| Model | Asenapine | Haloperidol | Olanzapine | Risperidone | Ziprasidone |
|---|---|---|---|---|---|
| 2-Compartment | 1-Compartment | 1-Compartment | 2-Compartment | 1-Compartment | |
| Parameter, mean (relative SE) | |||||
| K a (h−1) | 2.33 (89%) | 0.36 (26%) | 0.54 (30%) | 2.19 (6%) | 0.147 (5%) |
| CL (L/h) | 138 (32%) | 26 (10%) | 20.6 (4.5%) | 5.64 (4%) | 31.5 (5%) |
| V c (L) | 1070 (63%) | 672 (8%) | 1121 (12%) | 75 (3%) | 105 (10%) |
| V p (L) | 1430 (NE) | 73 (3%) | |||
| Q (L/h) | 65.3 (56%) | ||||
| F (%) | –a | 60 (13%) | –a | –a | 60 (15%) |
CL clearance, F absolute bioavailability, K a absorption rate constant, NE not estimated, Q intercompartmental clearance, SE standard error, V c central volume of distribution, V p peripheral volume of distribution
aParameters are corrected for F (i.e., CL/F, V/F, etc.)
Dopamine D2 Receptor Occupancy
Positron emission tomography data for which pharmacokinetic information was available were obtained for asenapine (28 data points), haloperidol (36 points), olanzapine (19 points), risperidone (43 points), and ziprasidone (8 points). An overview of the data sources for the PET data is presented in Table II.
Table II.
Overview of the PET Data Sources Used in the Development of the D2 Occupancy–Pharmacokinetic Model
| Agent | Dosesa | Population | No. of PET observationsb | D2 occupancy range (%) | Data source |
|---|---|---|---|---|---|
| Asenapine | 0.1 mg | Healthy volunteers | 3 | 12–23 | (11) |
| Asenapine | 0.6 mg/day | Healthy volunteers | 24 | 0–45 | (12) |
| Asenapine | 4–9.6 mg/day | Schizophrenia patients | 18 | 9–93 | (12) |
| Haloperidol | 4–12 mg/day | Schizophrenia patients | 5 | 75–89 | (3) |
| Haloperidol | 2 mg/day | Schizophrenia patients | 6 | 53–74 | (5) |
| Haloperidol | 1–5 mg/day | Schizophrenia patients | 21 | 38–87 | (21) |
| Haloperidol | 7.5 mg | Schizophrenia patients | 4 | 83–92 | (33) |
| Olanzapine | 5–60 mg/day | Schizophrenia patients | 17 | 43–89 | (2) |
| Olanzapine | 10 mg | Healthy volunteers | 3 | 59–63 | (34) |
| Risperidone | 2–6 mg/day | Schizophrenia patients | 9 | 59–83 | (35) |
| Risperidone | 2–12 mg/day | Schizophrenia patients | 16 | 63–89 | (2) |
| Risperidone | 1 mg | Healthy volunteers | 3 | 40–55 | (36) |
| Risperidone | 3–6 mg/day | Schizophrenia patients | 15 | 53–85 | (37) |
| Ziprasidone | 40 mg | Healthy volunteers | 1 | 77 | (38) |
| Ziprasidone | 0–40 mg | Healthy volunteers | 7 | 0–79 | (39) |
PET positron emission tomography
aDose regimens labeled as “mg” refer to single-dose administrations; “mg/day” refers to multiple-dose administration prior to PET scan
bOnly PET observations with pharmacokinetic information were included in the analysis
The direct Emax model provided an adequate description of the data (Fig. 4a). No notable delay between plasma concentrations and D2 occupancy observations was detected. Limitations in the data (e.g., generally only one occupancy observation per individual) may have contributed to the inability of the model to establish such time effects, especially when of relatively small (several hours) magnitude.
Fig. 4.
a Curve fits for separate E max models. b Predicted mean time course of D2 occupancy across antipsychotics. BID twice daily, QD once daily
The model parameters were estimated to be relatively precise with standard errors ranging from 3% (risperidone, haloperidol) to 11% (ziprasidone) for Emax and from 12% (asenapine) to 33% (ziprasidone) for EC50. Estimates of Emax and of the concentration producing 50% receptor occupancy (EC50) obtained from the concentration–D2 occupancy differed across agents (Table III and Fig. 4a). There was a close similarity between EC50 values for D2 occupancy (when corrected for protein binding and molecular weight) and receptor affinity rankings (Table III). Furthermore, Emax estimates appeared to be related to receptor-binding affinity (Table III), with Emax and EC50 values being highest for asenapine (101.8% and 0.528 ng/mL, respectively).
Table III.
Comparison of Relative EC50 Values Derived from Human In Vivo PET Studies to K d Values Derived from In Vitro Studies, with Haloperidol Used as the Reference Agent
| Drug | E max (%) (estimate, SE) | EC50 (ng/mL) (estimate, SE) | Relative PET EC50 a | K d b (nM) | Relative K d |
|---|---|---|---|---|---|
| Asenapine | 101.8 (4.5%) | 0.528 (0.063) | 0.5 | 1.3–1.4 | 0.9 |
| Haloperidol | 92.0 (3.3%) | 0.532 (0.085) | 1.0 | 1.4–1.7 | 1.0 |
| Risperidone | 91.2 (3.0%) | 4.43 (0.75) | 7.0 | 6.2–8.5 | 5.0 |
| Ziprasidone | 98.0 (10.7%) | 15.4 (5.1) | 2.0 | 8.1–10 | 6.0 |
| Olanzapine | 87.5 (3.9%) | 5.29 (1.22) | 8.0 | 21–26 | 15.0 |
EC 50 dose producing half-maximal D2 occupancy, E max maximum D2 occupancy, PET positron emission tomography
aCorrected for molecular weight and plasma protein binding
b K d = in vitro dissociation constant based on Shahid et al. (10)
To address the observed differences in Emax between agents, three derivations of maximal D2 occupancy (Emax) were considered for both efficacy and EPS models: Fixed Emax, Different Emax, and Same Emax. The primary scenario (Fixed Emax) assumed the different Emax values to be an artifact of the PET studies, and after fitting compound-specific Emax and EC50 values, all Emax values were fixed to the theoretical 100% value. With that, each of the concentration–D2 occupancy relationships was scaled from 0% to 100%. In the Different Emax model, the agent-specific Emax value was used. In the Same Emax model, a single Emax value was estimated across all five compounds; its estimate was 93%.
The predicted mean time course of D2 occupancy for each agent is depicted in Fig. 4b. Most of the agents exhibited significant variation between minimum and maximum D2 occupancy over a 24-h period. For each of the Emax derivations, values of average daily D2 occupancy and maximum D2 occupancy over the dosing interval were obtained as potential drivers for efficacy and EPS.
Clinical Data
An overview of the clinical data sources is presented in Supplementary Table I. Positive and Negative Syndrome Scale data for five agents (asenapine, haloperidol, olanzapine, risperidone, and ziprasidone) from 12 studies (45 different treatment arms, of which 33 were active) were included. The average sample size across the active treatment arms was 68 (range, 41–106). Because of this variation in sample size across studies, weighting by sample size was used to ensure that those trials with larger sample sizes, and consequently more precise estimates of change from placebo, contributed more heavily to the analysis. For those studies reporting change from placebo on the BPRS, a median scaling factor of 1.66 was used to convert these scores to PANSS total scores. This scaling factor was obtained from a regression analysis of individual PANSS and PANSS-derived BPRS data from the in-house trial (041-002), and its value was similar to the theoretical scaling factor of 1.67 based on the number of items in both rating scales.
Data on EPS were collected from 15 studies (61 different treatment arms, of which 49 were active) and across the same antipsychotic agents used for the PANSS model. The average sample size across active treatment arms was 80 (range, 17–230). For risperidone studies included in this model, the conversion of ESRS scores to SAS scores yielded the following equation:
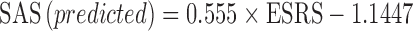 |
D2 Occupancy–PANSS Model
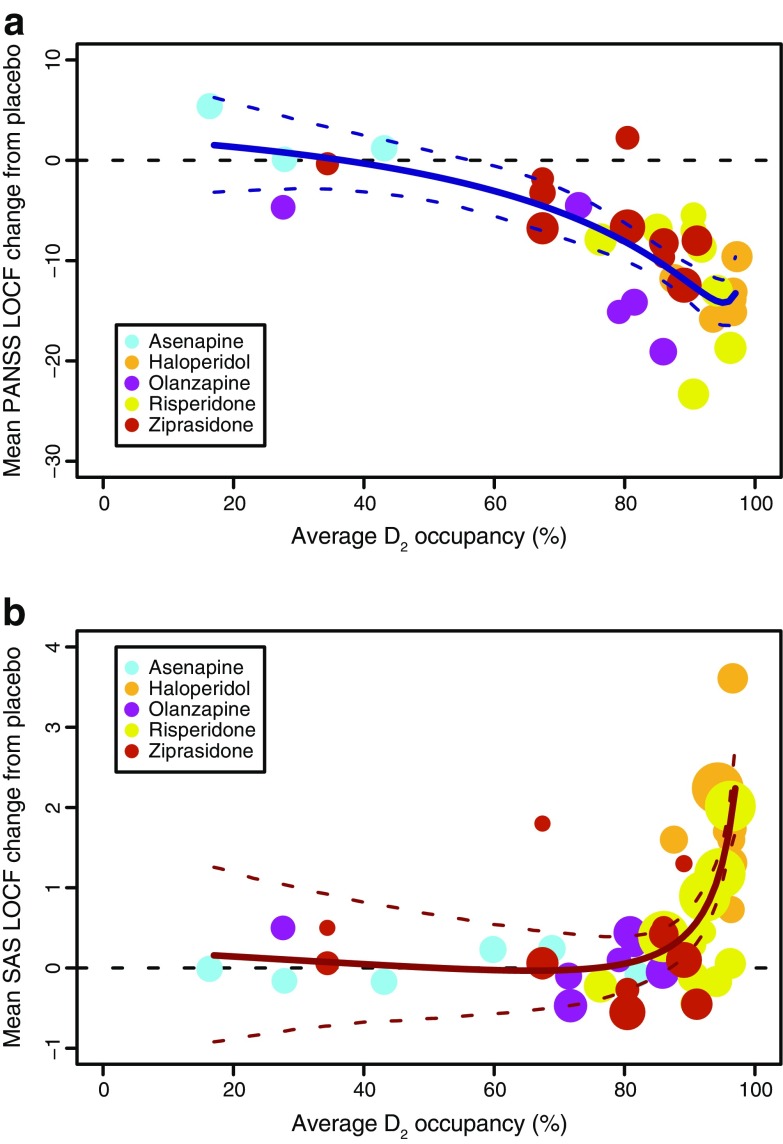
The fit of PANSS total score versus D2 occupancy using the Fixed Emax scenario is depicted in Fig 5a. The PANSS–D2 relationship was described using a cubic polynomial:
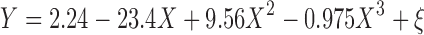 |
where Y is the change from placebo at week 6 for PANSS total score, X is the log-transformed D2 occupancy defined as log(100% − mean percent D2 receptor occupancy), and ξ is the residual error. The model predicts greater improvement in PANSS score compared with placebo at week 6, with increasing D2 occupancy up to a value of approximately 90%, where the relationship plateaus.
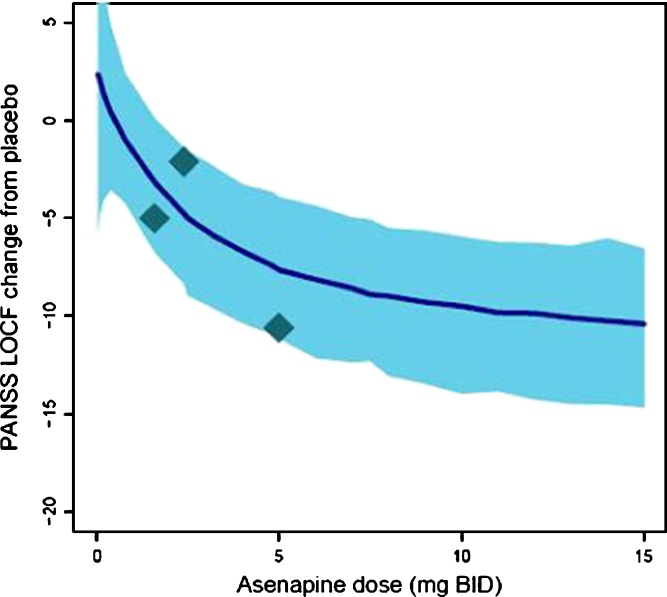
Fig. 5.
Mean change from placebo in total PANSS (a)or SAS score (b) at endpoint with LOCF versus predicted average D2 occupancy across antipsychotics. Circle diameter reflects trial size. LOCF last observation carried forward, PANSS Positive and Negative Syndrome Scale, SAS Simpson–Angus Scale
The transformation of the D2 occupancies allowed the model fit to be more strongly influenced by occupancies in the range of 60– 95%. The appropriateness of using the PANSS scores derived from the BPRS data was evaluated by fitting the model both with and without these data. The two fitted models were very similar. Thus, it was decided that the increased gain in precision of the model parameters outweighed the concerns over the imperfect mapping between the BPRS and PANSS scales. Overall, uncertainty in the model parameters was high (SE > 100%), and as such, the model could be considered overparameterized. However, this parameter uncertainty was to be included in the dose response simulations and therefore was accounted for in the overall predictions.
D2 Occupancy–SAS Model
The fit for the SAS versus D2 occupancy using the Fixed Emax scenario is depicted in Fig. 5b. The SAS–D2 relationship was described using a cubic polynomial:
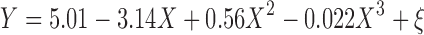 |
where Y is the change from placebo in SAS score, X is the log-transformed D2 occupancy, and ξ is the residual error. As D2 receptor occupancy increased, mean SAS scores also increased, steeply at occupancy values above 90%. A sensitivity analysis, like that conducted for the PANSS–BPRS mapping, indicated that the inclusion of ESRS data did not have a significant impact on the fitted model, but did improve the precision of the model parameters. Thus, again, it was considered of value to include the ESRS-derived SAS scores. Overall, uncertainty in model parameters was high (SE > 50%), but, as for the D2–PANSS model, was to be accounted for in overall model-based predictions.
Simulations
The predicted dose response for asenapine on the change from placebo in PANSS total score in a typical patient is presented in Fig. 6. Simulations were based on the Fixed Emax scenario (Emax set to 100%) and assumed average (instead of maximum) D2 occupancy to be the driver of efficacy. The confidence interval around the mean prediction is the net result of the uncertainty in the parameter estimates for the three model components used in the simulations. The prediction was performed prior to the availability of data from phase II studies investigating asenapine dose levels of 1.6, 2.4, and 5 mg BID. For reference, the endpoint data from these studies have been added to Fig. 6, indicating that the model adequately predicted the dose response of asenapine.
Fig. 6.
Predicted dose response of asenapine on PANSS total change from placebo. Solid line reflects median predicted dose response. Shaded area reflects 95% confidence interval of the prediction. Diamonds reflect the actual outcomes from phase II studies at dose regimens of 1.6, 2.4, and 5 mg BID
DISCUSSION
It is hypothesized that D2 occupancy plays a critical role in determining the efficacy and EPS liability associated with antipsychotic treatment. For example, D2 receptor occupancy levels 48 h after the cessation of 2 weeks of haloperidol treatment predicted short-term clinical response, as well as hyperprolactinemia and EPS, in patients with schizophrenia (21). Generally, it is thought that efficacy is optimal when D2 occupancy levels range from 65% to 80% (4–6), with EPS risk substantially increasing as D2 occupancy levels exceed 80% (7). To the best of our knowledge, this is the first study to quantify the relationship between D2 receptor occupancy, antipsychotic efficacy, and EPS across a range of doses of asenapine and several other antipsychotic drugs (haloperidol, olanzapine, risperidone, ziprasidone).
The models described in this report quantified the PANSS–D2 and EPS–D2 relationships as cubic polynomials. There was no physiologic basis for the selection of this particular model; the aim was just to interpolate the data. A cubic polynomial was selected because more complex models did not improve the fit and less complex models offered a poorer fit. Similar type models, such as splines or generalized additive models, are likely to have yielded a similar fit. However, it is important to note that the present model permitted a possible reduction of the PANSS change at the highest D2 occupancy, unlike, for example, an Emax type model.
Generally, the assumptions made during the development of the models appeared reasonable. For the efficacy and EPS models, respectively, the conversion of BPRS scores to PANSS scores and of ESRS scores to SAS scores seemed beneficial. This allowed a larger pool of studies and patients to be considered, resulting in increased model precision, with point estimates of the model parameters being similar to the fits where these derived data were excluded. The use of both daily peak D2 occupancy and average D2 occupancy mitigated pharmacokinetic differences across agents. It was also demonstrated that the effects of low-dose antipsychotics on EPS could be assumed to be indistinguishable from the effects of placebo treatment without introducing any noticeable bias, similar to when data were converted from ESRS to SAS (as for risperidone). A similar inclusion of low-dose data was not employed for PANSS modeling because the mere presence of a placebo arm in a trial was expected to have more influence on efficacy outcomes than on EPS.
The validity of the overall approach to empirically link D2 occupancy time course information to clinical data was confirmed by the efficacy results of the phase II trials being well in line with the independently predicted efficacy dose response. The availability of such external validation also motivated the use of the model framework (extended to include EPS) for dose selection decisions for the pivotal phase III trials. As illustrated in Fig. 6, the uncertainty in the model prediction is relatively high as a result of the additive effects of the uncertainty in the three model components underlying this simulation. Nevertheless, a clear dose response trend emerges from this prediction, and the overall shape has been further confirmed by the phase III data in which the 10-mg BID dose did not show increased efficacy compared to the 5-mg BID dose (22).
One potential limitation of this model is that only D2 receptor occupancy, which relates mainly to efficacy in treating positive symptoms, was used. All of the included compounds interact with several other receptor (sub)types besides the D2 receptor. Asenapine has high affinity for multiple serotonin (5-HT) receptor subtypes (5-HT1A, 5-HT1B, 5-HT2A, 5-HT2C, 5-HT6, and 5-HT7), as well as for dopamine D3 receptors (10). It has been suggested that serotonergic activity or the ratio of serotonergic activity to dopaminergic activity plays an important role in mediating the efficacy of atypical antipsychotics (23). Blockade of 5-HT2A, 5-HT2C, and D3 receptors may play an important role in alleviating symptoms in schizophrenia (23–26). As such, differential activity at receptors other than the D2 receptor may account for some of the variance in the efficacy model, if not in the EPS model. Relatively high affinity at any of these alternative receptor subtypes could potentially enable a compound to produce antipsychotic efficacy at lower levels of D2 occupancy. The present dataset, however, did not allow such differentiation between the five included agents. In this regard, it should also be noted that clozapine and quetiapine were not included in the analyses because estimates of their D2 occupancy levels from PET studies at effective doses are generally lower than those for other agents (27–29).
The modeling of these data revealed some issues for which no clear answer is available. First, it remains unclear what the optimal time course of receptor occupancy is for maximum clinical benefit. Therefore, there was some uncertainty as to the best receptor occupancy variable to incorporate into the model, average or maximum occupancy during the dosing interval. Our findings also demonstrated that Emax estimates based on PET data varied as a function of D2 binding affinity across agents. It is unlikely that this represents a true difference in the maximum occupancy level attainable by each agent. More likely, it is related to artifacts associated with the PET studies. In particular, the choice of radioligand could be a critical factor. Seeman and Tallerico (30) have noted that agents with low binding affinity are less able to compete with high-affinity radioligands. As a result, differences in affinity across active agents in relation to the affinity of 11C-raclopride may have contributed to differences in the estimation of Emax. In our opinion, the Fixed Emax scenario is most appropriate because D2 receptors will eventually become saturated as the drug concentration increases.
It has been hypothesized that differences in dissociation rate (koff) at which antipsychotic agents dissociate from the D2 receptor may lead to functionally different kinds of dopamine blockade and, thereby, to differentiation in clinical effects between antipsychotics (31). The available D2 occupancy data, however, did not allow us to develop a dynamic kon/koff model for the D2 receptor interactions as that would have required repeated occupancy data at short time intervals (within hours). Instead, an equilibrium model (the direct Emax) was applied, which essentially renders the pharmacokinetics of the agents to become the rate-limiting step for the binding dynamics at the D2 receptor. Given the overall purpose of this work, this was considered an acceptable and feasible approach even though it will have limited the ability to differentiate between the different antipsychotic agents included in the analysis.
There are also particular clinical aspects that could act as potential confounders in the models that were developed. It is important to recognize that adverse events could negatively impact efficacy outcomes. If adverse events result in an underestimation of clinical efficacy, the D2 occupancy range associated with efficacy could be artificially narrowed. Conversely, use of antiparkinsonian medications can also introduce bias by decreasing EPS and artificially increasing the lower end of the range of D2 occupancy associated with EPS. However, even though both aspects have not been explicitly accounted for in the models, the model framework can be assumed to apply in the context of clinical trials where these factors play a role.
The use of the PANSS and SAS as the primary endpoints when developing these models seemed appropriate based on their widespread use. It should be noted that our analyses demonstrated that no bias was introduced by the conversion of BPRS scores to PANSS scores, the conversion of ESRS scores to SAS scores, and the assumption that low-dose antipsychotics produce EPS levels that are comparable to those of placebo. However, as a consequence of using these specific scales, these models are specific for PANSS scores and SAS scores and may not translate to other efficacy and EPS rating scales. It should also be noted that these models only predict mean endpoint PANSS and SAS scores in short-term clinical studies and not the individual time course of effect. As a result, models cannot be used to predict individual time courses of response for a given treatment regimen. Finally, the impact of differential dropout patterns cannot be fully accounted for in these models. In the present study, by using LOCF data, we partially accounted for the confounding influence of study discontinuations.
Overall, the presented modeling approach can be classified as an empirical model-based meta-analysis with some mechanistic foundation, through the use of D2 occupancy as the driving parameter for efficacy and safety. This approach will have a more general applicability in situations where a biomarker exists that links to efficacy and/or safety and can be used to leverage data from a compound class to support efficient development of novel drug candidates in that same class. The specific D2-based model framework also could be applied more broadly, among others in situations where new dosing paradigms for existing antipsychotics are to be explored (e.g., intramuscular depot formulations). Integrating data on recently developed depot formulations should help strengthen the model framework and increase the understanding of the required time course of D2 receptor occupancy for an optimal efficacy–safety balance.
CONCLUSION
This work demonstrates that simple empirical models can effectively quantify antipsychotic efficacy and EPS liability, as measured by PANSS scores and SAS scores, respectively, as a function of D2 occupancy level. In regard to asenapine, the models were successfully applied to select dose regimens for full development, and the validity of the models has been confirmed by published clinical trial results showing that asenapine 5 or 10 mg BID manages acute exacerbations of schizophrenia with relatively low risk of EPS (22,32). Despite some uncertainty in model parameters, the overall trends were well captured in each of the models, requiring only a limited set of assumptions. Therefore, these models may be useful for the dose selection of antipsychotics with D2 antagonist properties in the treatment of schizophrenia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 143 872 kb)
Acknowledgments
The authors thank Dr. Shitij Kapur for his insights and direction during the early development of the models. All authors contributed to the development and writing of the paper and are completely responsible for its scientific content. This study was funded by Merck (Whitehouse Station, NJ, USA). Editorial support was provided by Complete Healthcare Communications, Inc., and funded by Merck (Whitehouse Station, NJ, USA).
Conflicts of interest
Rik de Greef and Joep Schoemaker are employees of Merck Sharp & Dohme (Oss, the Netherlands). John Panagides was an employee of Schering-Plough (formerly Organon), now Merck, at the time the study was conducted. Drs. Maloney and Olsson-Gisleskog were employed by Pharsight, A Certara Company, at the time this research was conducted and have no other interests to report.
References
- 1.Lieberman JA. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia: efficacy, safety and cost outcomes of CATIE and other trials. J Clin Psychiatry. 2007;68:e04. doi: 10.4088/JCP.0207e04. [DOI] [PubMed] [Google Scholar]
- 2.Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156:286–93. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- 3.Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–44. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- 4.Pani L, Pira L, Marchese G. Antipsychotic efficacy: relationship to optimal D(2)-receptor occupancy. Eur Psychiatry. 2007;22:267–75. doi: 10.1016/j.eurpsy.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Kapur S, Remington G, Jones C, Wilson A, DaSilva J, Houle S, et al. High levels of dopamine D2 receptor occupancy with low-dose haloperidol treatment: a PET study. Am J Psychiatry. 1996;153:948–50. doi: 10.1176/ajp.153.7.948. [DOI] [PubMed] [Google Scholar]
- 6.Nordstrom AL, Farde L, Wiesel FA, Forslund K, Pauli S, Halldin C, et al. Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry. 1993;33:227–35. doi: 10.1016/0006-3223(93)90288-O. [DOI] [PubMed] [Google Scholar]
- 7.Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, et al. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 2006;20:389–409. doi: 10.2165/00023210-200620050-00004. [DOI] [PubMed] [Google Scholar]
- 8.Saphris® (asenapine sublingual tablets). Full prescribing information, Schering Corporation, a subsidiary of Merck & Co., Inc, Whitehouse Station, NJ; 2010.
- 9.European Medicines Agency. Sycrest asenapine. 2010. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001177/human_med_001379.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d124&jsenabled=true. Accessed 28 September 2010.
- 10.Shahid M, Walker GB, Zorn SH, Wong EH. Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol. 2009;23:65–73. doi: 10.1177/0269881107082944. [DOI] [PubMed] [Google Scholar]
- 11.Andree B, Halldin C, Vrijmoed-de Vries M, Farde L. Central 5-HT2A and D2 dopamine receptor occupancy after sublingual administration of ORG 5222 in healthy men. Psychopharmacology Berl. 1997;131:339–45. doi: 10.1007/s002130050301. [DOI] [PubMed] [Google Scholar]
- 12.Data on file. Summit, NJ: Merck; 2010.
- 13.Geodon® (ziprasidone). Full prescribing information, Pfizer Inc., New York, NY; 2009.
- 14.Cheng YF, Paalzow LK, Bondesson U, Ekblom B, Eriksson K, Eriksson SO, et al. Pharmacokinetics of haloperidol in psychotic patients. Psychopharmacology Berl. 1987;91:410–4. doi: 10.1007/BF00216005. [DOI] [PubMed] [Google Scholar]
- 15.Heyden S. Risperidone expert report (Serial No. R64 766). April 1992.
- 16.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 17.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 18.Risperdal® (risperidone). Full prescribing information. Janssen, Division of Ortho-McNeil-Janssen Pharmaceuticals, Inc., Titusville, NJ; 2009.
- 19.Zyprexa. Eli Lilly and Company, Indianapolis, IN; 2010.
- 20.Friberg LE, de Greef R, Kerbusch T, Karlsson MO. Modeling and simulation of the time course of asenapine exposure response and dropout patterns in acute schizophrenia. Clin Pharmacol Ther. 2009;86:84–91. doi: 10.1038/clpt.2009.44. [DOI] [PubMed] [Google Scholar]
- 21.Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157:514–20. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 22.Kane JM, Cohen M, Zhao J, Alphs L, Panagides J. Efficacy and safety of asenapine in a placebo- and haloperidol-controlled trial in patients with acute exacerbation of schizophrenia. J Clin Psychopharmacol. 2010;30:106–15. doi: 10.1097/JCP.0b013e3181d35d6b. [DOI] [PubMed] [Google Scholar]
- 23.Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1159–72. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds GP, Yao Z, Zhang X, Sun J, Zhang Z. Pharmacogenetics of treatment in first-episode schizophrenia: D3 and 5-HT2C receptor polymorphisms separately associate with positive and negative symptom response. Eur Neuropsychopharmacol. 2005;15:143–51. doi: 10.1016/j.euroneuro.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Lieberman JA, Mailman RB, Duncan G, Sikich L, Chakos M, Nichols DE, et al. Serotonergic basis of antipsychotic drug effects in schizophrenia. Biol Psychiatry. 1998;44:1099–117. doi: 10.1016/S0006-3223(98)00187-5. [DOI] [PubMed] [Google Scholar]
- 26.Lane HY, Lee CC, Liu YC, Chang WH. Pharmacogenetic studies of response to risperidone and other newer atypical antipsychotics. Pharmacogenomics. 2005;6:139–49. doi: 10.1517/14622416.6.2.139. [DOI] [PubMed] [Google Scholar]
- 27.Farde L, Nyberg S, Oxenstierna G, Nakashima Y, Halldin C, Ericsson B. Positron emission tomography studies on D2 and 5-HT2 receptor binding in risperidone-treated schizophrenic patients. J Clin Psychopharmacol. 1995;15:19S–23S. doi: 10.1097/00004714-199502001-00004. [DOI] [PubMed] [Google Scholar]
- 28.Gefvert O, Bergstrom M, Langstrom B, Lundberg T, Lindstrom L, Yates R. Time course of central nervous dopamine-D2 and 5-HT2 receptor blockade and plasma drug concentrations after discontinuation of quetiapine (Seroquel) in patients with schizophrenia. Psychopharmacology Berl. 1998;135:119–26. doi: 10.1007/s002130050492. [DOI] [PubMed] [Google Scholar]
- 29.Gefvert O, Lundberg T, Wieselgren IM, Bergstrom M, Langstrom B, Wiesel F, et al. D(2) and 5HT(2A) receptor occupancy of different doses of quetiapine in schizophrenia: a PET study. Eur Neuropsychopharmacol. 2001;11:105–10. doi: 10.1016/S0924-977X(00)00133-4. [DOI] [PubMed] [Google Scholar]
- 30.Seeman P, Tallerico T. Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry. 1998;3:123–34. doi: 10.1038/sj.mp.4000336. [DOI] [PubMed] [Google Scholar]
- 31.Kapur S, Seeman P. Antipsychotic agents differ in how fast they come off the dopamine D2 receptors. Implications for atypical antipsychotic action. J Psychiatry Neurosci. 2000;25:161–6. [PMC free article] [PubMed] [Google Scholar]
- 32.Potkin SG, Cohen M, Panagides J. Efficacy and tolerability of asenapine in acute schizophrenia: a placebo- and risperidone-controlled trial. J Clin Psychiatry. 2007;68:1492–500. doi: 10.4088/JCP.v68n1004. [DOI] [PubMed] [Google Scholar]
- 33.Nordstrom AL, Farde L, Halldin C. Time course of D2-dopamine receptor occupancy examined by PET after single oral doses of haloperidol. Psychopharmacology Berl. 1992;106:433–8. doi: 10.1007/BF02244811. [DOI] [PubMed] [Google Scholar]
- 34.Nyberg S, Farde L, Halldin C. A PET study of 5-HT2 and D2 dopamine receptor occupancy induced by olanzapine in healthy subjects. Neuropsychopharmacology. 1997;16:1–7. doi: 10.1016/S0893-133X(96)00218-7. [DOI] [PubMed] [Google Scholar]
- 35.Remington G, Kapur S, Zipursky R. The relationship between risperidone plasma levels and dopamine D2 occupancy: a positron emission tomographic study. J Clin Psychopharmacol. 1998;18:82–3. doi: 10.1097/00004714-199802000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Nyberg S, Farde L, Eriksson L, Halldin C, Eriksson B. 5-HT2 and D2 dopamine receptor occupancy in the living human brain. A PET study with risperidone. Psychopharmacology Berl. 1993;110:265–72. doi: 10.1007/BF02251280. [DOI] [PubMed] [Google Scholar]
- 37.Nyberg S, Eriksson B, Oxenstierna G, Halldin C, Farde L. Suggested minimal effective dose of risperidone based on PET-measured D2 and 5-HT2A receptor occupancy in schizophrenic patients. Am J Psychiatry. 1999;156:869–75. doi: 10.1176/ajp.156.6.869. [DOI] [PubMed] [Google Scholar]
- 38.Bench CJ, Lammertsma AA, Dolan RJ, Grasby PM, Warrington SJ, Gunn K, et al. Dose dependent occupancy of central dopamine D2 receptors by the novel neuroleptic CP-88, 059–01: a study using positron emission tomography and 11C-raclopride. Psychopharmacology Berl. 1993;112:308–14. doi: 10.1007/BF02244926. [DOI] [PubMed] [Google Scholar]
- 39.Bench CJ, Lammertsma AA, Grasby PM, Dolan RJ, Warrington SJ, Boyce M, et al. The time course of binding to striatal dopamine D2 receptors by the neuroleptic ziprasidone (CP-88, 059–01) determined by positron emission tomography. Psychopharmacology Berl. 1996;124:141–7. doi: 10.1007/BF02245614. [DOI] [PubMed] [Google Scholar]
- 40.Beasley CM, Jr, Tollefson G, Tran P, Satterlee W, Sanger T, Hamilton S. Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology. 1996;14:111–23. doi: 10.1016/0893-133X(95)00069-P. [DOI] [PubMed] [Google Scholar]
- 41.Beasley CM, Jr, Sanger T, Satterlee W, Tollefson G, Tran P, Hamilton S. Olanzapine versus placebo: results of a double-blind, fixed-dose olanzapine trial. Psychopharmacology Berl. 1996;124:159–67. doi: 10.1007/BF02245617. [DOI] [PubMed] [Google Scholar]
- 42.Beasley CM, Jr, Hamilton SH, Crawford AM, Dellva MA, Tollefson GD, Tran PV, et al. Olanzapine versus haloperidol: acute phase results of the international double-blind olanzapine trial. Eur Neuropsychopharmacol. 1997;7:125–37. doi: 10.1016/S0924-977X(96)00392-6. [DOI] [PubMed] [Google Scholar]
- 43.Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry. 1994;151:825–35. doi: 10.1176/ajp.151.6.825. [DOI] [PubMed] [Google Scholar]
- 44.Borison R, et al. Risperidone versus haloperidol versus placebo in the treatment of schizophrenia. Clinical Research Report No. RIS-USA-9001, N83170. Piscataway, NJ: Janssen; 1991.
- 45.Peuskens J. Risperidone in the treatment of patients with chronic schizophrenia: a multi-national, multi-centre, double-blind, parallel-group study versus haloperidol. Risperidone Study Group. Br J Psychiatry. 1995;166:712–26. doi: 10.1192/bjp.166.6.712. [DOI] [PubMed] [Google Scholar]
- 46.Clinical Report: RIS-USA-72. 1997. NDA 20588/S002.
- 47.Daniel DG, Zimbroff DL, Potkin SG, Reeves KR, Harrigan EP, Lakshminarayanan M. Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Ziprasidone Study Group. Neuropsychopharmacology. 1999;20:491–505. doi: 10.1016/S0893-133X(98)00090-6. [DOI] [PubMed] [Google Scholar]
- 48.Keck P, Jr, Buffenstein A, Ferguson J, Feighner J, Jaffe W, Harrigan EP, et al. Ziprasidone 40 and 120 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 4-week placebo-controlled trial. Psychopharmacology Berl. 1998;140:173–84. doi: 10.1007/s002130050755. [DOI] [PubMed] [Google Scholar]
- 49.Goff DC, Posever T, Herz L, Simmons J, Kletti N, Lapierre K, et al. An exploratory haloperidol-controlled dose-finding study of ziprasidone in hospitalized patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 1998;18:296–304. doi: 10.1097/00004714-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Clinical Report: Study 104. 1998. NDA 20825.
- 51.Clinical Report: Study 115. 1998. NDA 20825.
- 52.Arvanitis LA, Miller BG. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. The Seroquel Trial 13 Study Group. Biol Psychiatry. 1997;42:233–46. doi: 10.1016/S0006-3223(97)00190-X. [DOI] [PubMed] [Google Scholar]
- 53.Zimbroff DL, Kane JM, Tamminga CA, Daniel DG, Mack RJ, Wozniak PJ, et al. Controlled, dose–response study of sertindole and haloperidol in the treatment of schizophrenia. Sertindole Study Group. Am J Psychiatry. 1997;154:782–91. doi: 10.1176/ajp.154.6.782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 143 872 kb)