Abstract
The Coulter principle can be used for analysis of subvisible particles in protein formulations. The approach has several advantages including: an orthogonal operating principle, high sensitivity, ability to detect very small particles, excellent reproducibility, and high-resolution size information. This minireview discusses some of the important considerations that must be taken into account when utilizing the Coulter principle for subvisible particle analysis in protein formulations.
Key words: Coulter principle, protein aggregates, subvisible, USP 788
INTRODUCTION
Applying the Coulter principle is a well-established method for counting and sizing particles comprised of a wide variety of materials. Several of the American Society for Testing and Materials (ASTM)’s standards are based on the Coulter principle. These diverse applications include aqueous filtration efficiency (1), chromatography media characterization (2), cell counting (3), and ink toner evaluation (4). Recently, industrial and academic investigators have been interested in applying the Coulter principle to subvisible particulates in protein formulations (5–7). The Coulter principle implemented in this way offers advantages over the light obscuration technique used in US Pharmocopeia Protocol 788 (USP 788). These include: an orthogonal approach, high sensitivity, ability to detect very small particles, excellent reproducibility, and high-resolution size information. This minireview will describe the Coulter principle, explain why it is an attractive tool for subvisible particulate analysis, and discuss key experimental design considerations for characterizing subvisible particles in protein formulations.
GENERAL DESCRIPTION OF THE COULTER PRINCIPLE
The Coulter principle relies on the fact that objects placed in an electric field will modify the current flow in that field. In order to turn this observation into a useful tool, some conditions must be met. First, the electric field should be established in a conductive liquid. Next, the current path should be physically constricted so that the presence of particles in the field will cause the current to change in a detectable manner. Furthermore, the particles must be dispersible in the liquid and dilute enough so that only one particle at a time enters the detection area. Typically, the particles have an electrical conductivity different than that of the liquid. In most implementations, suspended particles are forced through a constriction simultaneously with an electric current while the current is measured. As individual particles pass through the constriction, discrete electrical pulses are generated, the magnitude of which are proportional to the particles’ volume. Thus, the approach can allow highly accurate count and size measurements with relatively few restrictions. Interested readers are encouraged to review some of the excellent publications which explain the Coulter principle and its applications in more depth (8–10).
APPLICATION TO SUBVISIBLE PARTICLE ANALYSIS IN PROTEIN FORMULATIONS
There are several features of the Coulter principle that make it attractive for analysis of subvisible particles in protein formulations. First, it is an orthogonal technique that does not rely on the optical properties of protein samples. One limitation of techniques based on imaging or light blockage is that the refractive index of the aggregate of interest must be substantially different from that of the surrounding solution in order to be detected. For subvisible protein aggregates, which can be transparent, some particles may be missed with light-based techniques, particularly in samples with very high protein concentrations. The Coulter principle only requires that the particle occupies volume in the conducting liquid, making it easier to detect small, transparent aggregates.
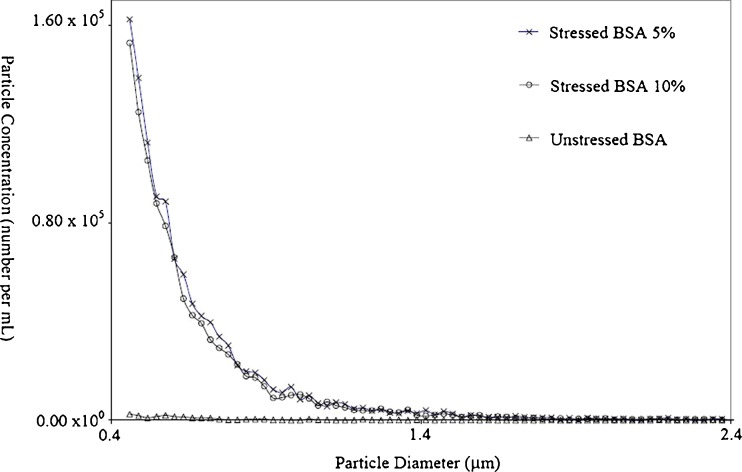
The second attractive feature is the sensitivity, linearity, and reproducibility of the approach. Typical coefficients of variation for repeated measurements using the Coulter principle are about 2%. Furthermore, the linearity and reproducibility of the technique in any liquid of suitable conductivity is well established, and is one reason the Coulter principle is widely used by clinicians for the characterization of patient blood samples. As an example of the linearity of the approach for protein aggregate analysis, Fig. 1 shows the results of experiments with stressed bovine serum albumin (BSA) samples in different dilutions of phosphate-buffered saline (PBS) buffer. In this test, BSA formulations at 10 mg/mL were stressed with vigorous vortexing and then diluted with PBS buffer 1:10 and 1:20. These solutions, along with a control sample were presented to a Beckman Coulter Multisizer™ 4 COULTER COUNTER® for particle count analysis. As the figure shows, there was good agreement between the two different dilutions in this case.
Fig. 1.
Stressed and unstressed BSA samples characterized using the Coulter principle. The total particle count per milliliter between 0.45 and 2.0 μm for each sample is as follows: stressed BSA 10%, 1.39 × 106; stressed BSA 5%, 1.49 × 106; unstressed BSA, 8.87 × 104; BSA, 8.87 × 104
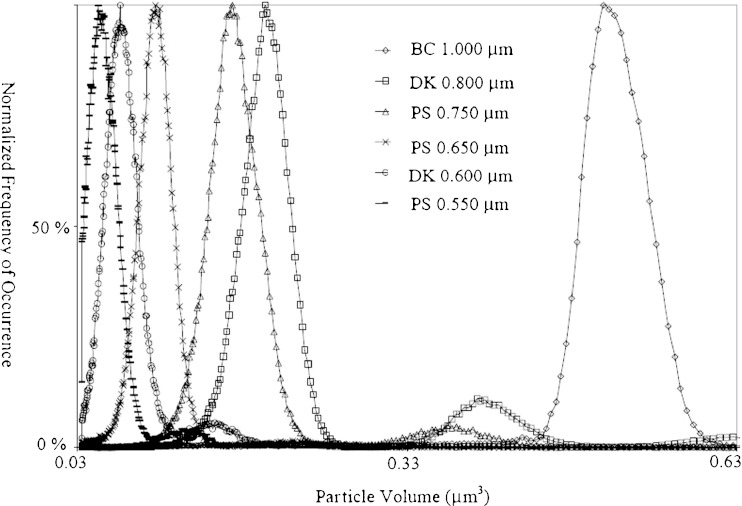
The third attractive feature is the ability to detect very small protein aggregates. Theoretically, if a small enough constriction were available, the Coulter principle could be used to count particles as small as a few nanometers. In fact, some academics have attempted to do just that (11). However, there are many practical limitations with this approach, which are beyond the scope of this discussion. For protein aggregate analysis, the current best implementation of the Coulter principle can count and size particles as small as 0.4 μm. This lower limit is very near the 0.1-μm upper sensitivity limit of size exclusion chromatography. Figure 2 shows the results of analyzing a variety of submicron bead standards with a Multisizer™ 4. The results show that the instrument can detect differences as small as 50 nm in nominal sample diameter, and that a near-complete size distribution of 550-nm beads can be recorded; 500- and 400-nm beads were also measured, however, since a significant proportion of these samples falls below the 400-nm cutoff for the sensitivity of the Multisizer, incomplete distributions were obtained. In Fig. 2, the x-axis is plotted as a function of volume (rather than diameter) to highlight that the small secondary peaks to the right of the major peaks represent doublets of the major peak (the volume at peak center is almost exactly double the major peak). These data show that the Coulter principle can resolve particles well below 1 μm.
Fig. 2.
Submicron standards analyzed in an SEC buffer. Standards from three different manufacturers were used. The abbreviations are as follows BC Beckman Coulter, PS Polysciences, DK Duke Scientific. Size distributions have been normalized to give the most frequently occurring size a value of 100% occurrence
EXPERIMENTAL DESIGN CONSIDERATIONS
Despite its many advantages for subvisible protein aggregate analysis, the Coulter principle is not a perfect tool. Investigators are typically concerned with the large volume requirements of commercial Coulter counters, the need for some minimal conductivity in sample buffers, and the assumed need for dilution. In addition to these obvious concerns, issues such as sample handling and cleanliness of the instrument may affect particulate counts. However, all of these issues can be addressed through development of a detailed operating procedure. Here, we discuss key elements of an optimized procedure for analyzing subvisible particles in protein formulations.
Choice of Coulter Counter®
Several models of Coulter Counters are available, and are each optimized for specific applications. One mistake many investigators make is drawing too much information from a COULTER COUNTER® designed to do the wrong task. Specifically, some models are designed solely for cell counting, and provide some limited average size information. It is critical that investigators choose the model appropriate for the sample to be analyzed. Older instruments or those designed purely for cell counting lack the advanced circuitry and digital pulse analysis needed for accurate count and size of subvisible particulates. Thus, it is advisable to select the COULTER COUNTER® containing the best possible electronics and sample handling system available to ensure high quality, accuracy, and precision.
Choice of Aperture Tube
To implement the Coulter principle, commercial Coulter counters use a glass tube in whose wall is embedded a precisely bored ruby disk that constricts the current and fluid flow. The ruby disks are referred to as apertures and the glass tubes that contain them as aperture tubes. The primary reason for choosing different aperture sizes is that, depending on which COULTER COUNTER® system is in use, the instruments have a maximum dynamic range of about 2–80% of the installed aperture tube diameter. That is, a system equipped with a 100-μm aperture is sensitive to particles between 2 and 80 μm for the most sensitive instrument. In order to count and size particles smaller than 2 μm, smaller apertures must be used.
Importance of Buffer Conductivity
Restrictions on the conductivity of the buffer vary as a function of aperture size. For example, when using a 20-μm aperture, higher conductivity solutions are needed to achieve the same dynamic range than when using a 100-μm aperture. This is due to the reduced total amount of current flowing through the smaller aperture at any given time. If a low conductivity buffer is used with a small aperture, the total resistance to current flow is much greater than when using the same conductivity buffer in a 100-μm aperture. Practically, this means that one would sacrifice some sensitivity at the lower end of the dynamic range of the aperture due to increased electrical noise resulting from increased resistance. Thus, investigators working with buffers containing tens of millimolar salt can expect the lower end of the dynamic range to increase slightly.
It is important to note that not all buffers of equivalent salt concentration have equivalent conductivities. For example, many times protein formulation buffers are titrated with NaOH or HCl to achieve a desired pH, which adds significant ion concentration without increasing the nominal buffer strength. Thus, one sample of 10-mM hisitidine buffer may have a significantly lower limit of detection than another sample of identical salt concentration. It also bears repeating that sensitivity to reduced conductivity is a function of aperture size and the electronics employed inside of a COULTER COUNTER®. Proper experimental design and testing can help identify those conditions that are most acceptable for a particular protein formulation of interest.
Sample Volume
Commercial Coulter counters are typically used with samples that have very high particle concentrations such as red blood cells or industrial abrasives. These samples have tens of millions of particles in each milliliter of suspension whereas protein formulations typically have fewer than 100,000 particles per milliliter. Since the Coulter principle relies on particles traversing the aperture one at a time, the highly concentrated samples must be diluted to a large extent prior to analysis with a COULTER COUNTER®. To accommodate significant dilution, the smallest sample holders currently available for commercial systems are designed to operate with a minimum volume of about 10 mL. Protein formulation scientists have found this to be a rather large volume, particularly since their samples have many fewer particles and need not be diluted. Fortunately, this is only a practical design consideration, and it is possible to adapt Coulter counters for smaller volumes. We have recently developed a procedure that reduces sample volume requirements by 60% to 4 mL.
Table I shows data from a sample containing a mixture of 2, 5, 10, and 20 μm beads. The mixed sample was presented to a Beckman Coulter Multisizer™ 4 COULTER COUNTER® both with the typical 10-mL sample volume in a 20-mL cup and in a newly adapted 5-mL sample vessel filled with 4 mL of sample. Triplicate runs of 100 uL each were drawn from each sample vessel. The CVs presented in Table I show that the variance between the different sample vessels is within the variance measured for repeat runs from the same sample vessel. This indicates that the 5-mL sample vessel is an acceptable substitute for the larger 20-mL vessel. It is important to note that this result is achieved without major modifications to the instrument, and only requires an adapter for the sample stand.
Table I.
Comparison of Triplicate Count Results from 4- to 10-mL Sample Volumes of a Mixed Sample
| Sample volume | 2 μm | 5 μm | 10 μm | 20 μm | ||||
|---|---|---|---|---|---|---|---|---|
| Average | CV (%) | Average | CV (%) | Average | CV (%) | Average | CV (%) | |
| 4 mL | 3,420 | 1.8 | 1,210 | 5.3 | 842 | 2.3 | 258 | 2.8 |
| 10 mL | 3,320 | 1.5 | 1,320 | 2.2 | 880 | 1.9 | 292 | 9.8 |
| Vial comparison | 3,370 | 1.4 | 1,260 | 1.4 | 861 | 2.6 | 275 | 4.6 |
Statistical Precision
Table I also shows that variance tends to increase as absolute particle count decreases. This observation is true for all particle counting techniques, and represents a concern for development of protein counting procedures. Frequency of particulate occurrence in protein formulations is inversely proportional with the size of those particles. That is, larger particles are rarer than smaller particles, which is also the case for the mixed sample used in creating Table I. Since larger particles occur less frequently than smaller particles, it will be generally difficult to obtain precise measurements of their concentration across samples, making development of quality control guidelines difficult. Table II presents a more detailed study of the statistical precision of particle counting by the Coulter principle. The data show that when the total particle count in a given size range is above 10,000, CVs are typically 1% or lower. However, as the total particle count drops below 10,000, CVs increase dramatically to 9% for a total particle count of about 100. While this is simply an example of the principle that error increases as sample size drops, it is an important point to keep in mind as research into particle counting methods for protein solutions grows. Most protein formulations tend to have very low particulate concentrations, which would indicate statistical precision guidelines should have greater tolerances, especially as particle sizes increase.
Table II.
Statistical Precision of 31 Measurements of 10 μm Latex Beads at Various Concentrations
| Average particle count (n = 31) | Standard deviation | CV (%) |
|---|---|---|
| 106 | 9.5 | 8.9 |
| 1,076 | 31 | 2.9 |
| 9,755 | 86 | 0.9 |
| 10,005 | 85 | 0.9 |
| 10,500 | 99 | 1.0 |
| 49,444 | 221 | 0.5 |
| 98,559 | 234 | 0.2 |
Importance of Robust Cleaning Procedure
In order to ensure consistent results with few artifacts, it is important to ensure the COULTER COUNTER® system is very clean both prior to use and while testing samples. If a system has not been in use for some time and liquid has been allowed to stagnate in the internal fluid handling system, it is advisable to thoroughly clean the system through repeated washes of a strong cleaning solution. This will remove any salt crystals, algae, or other contaminants that may have grown while the system sat idle. Furthermore, it is very important to clean the aperture tube itself regularly during sample analysis. One good way of accomplishing this is with a weak soap solution that can be flushed through the aperture tube in between consecutive samples. In order to test the cleanliness of the aperture tube, investigators can present a well-filtered blank buffer solution to the instrument and measure the particulate counts. If the counts are high in a blank solution, the aperture must be cleaned. However, if the cleaning step is incorporated as part of the standard experimental procedure, accurate and reproducible counts can be expected.
CONCLUSIONS
In summary, the Coulter principle is a well-established technique that is being used in the rapidly expanding area of subvisible particle counting in protein formulations. Its advantages include: its orthogonal approach, sensitivity, linearity, reproducibility, and ability to detect very tiny subvisible aggregates (currently down to 0.4 μm). With proper experimental design, conditions may be chosen that produce informative and reliable particle characterization data in a variety of buffers and with small sample volumes. Thus, the Coulter principle should be used as a complementary, orthogonal technique to USP 788 for analysis of subvisible particulates in protein formulations.
References
- 1.American Society for Testing and Materials. Standard test method for measurement of particle count and size distribution in batch samples for filter evaluation using an electrical resistance particle counter. 1992. Designation F662. ASTM International, West Conshohocken
- 2.American Society for Testing and Materials. Standard test method for particle size distribution of chromatography media by electric sensing zone technique. 1995. Designation E1772. ASTM International, West Conshohocken
- 3.American Society for Testing and Materials. Standard test method for automated analyses of cells—the electrical sensing zone method of enumerating and sizing single cell suspensions. 2001. Designation F2149. ASTM International, West Conshohocken
- 4.American Society for Testing and Materials. Standard test method for particle size measurement of dry toners. 2002. Designation F577. ASTM International, West Conshohocken
- 5.Carpenter JF, Randolph TW, Jiskoot W, Crommelin DJ, Middaugh CR, Winter G, et al. Overlooking subvisible particles in therapeutic protein products: gaps that may compromise product quality. J Pharm Sci. 2009;98(4):1201–1205. doi: 10.1002/jps.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyagi AK, Randolph TW, Dong A, Maloney KM, Hitscherich C, Jr, Carpenter JF. IgG particle formation during filling pump operation: a case study of heterogeneous nucleation on stainless steel nanoparticles. J Pharm Sci. 2009;98(1):94–104. doi: 10.1002/jps.21419. [DOI] [PubMed] [Google Scholar]
- 7.American Association of Pharmaceutical Scientists [Internet]. AAPS Workshop on Methods for Detecting and Characterizing Sub-visible Particulates. Presentations Available from: www.aapspharmaceutica.com/meetings/files/153/agenda.pdf. Accessed 21 June 2009
- 8.Coulter WH. Means for counting particles suspended in a fluid, U.S. Patent 2,656,508. 20 Oct 1953.
- 9.Coulter WH. High speed automatic blood cell counter and cell size analyzer. Proc Natl Electron Conf. 1956;12:1034–1042. [Google Scholar]
- 10.Graham MD. The Coulter principle: foundation of industry. J Assoc Lab Autom. 2003;8(6):72–81. doi: 10.1016/S1535-5535(03)00023-6. [DOI] [Google Scholar]
- 11.Henriquez RR, Ito T, Sun L, Crooks RM. The resurgence of Coulter counting for analyzing nanoscale objects. Analyst. 2004;129(6):478–482. doi: 10.1039/b404251b. [DOI] [PubMed] [Google Scholar]