Abstract
Highly crosslinked UHMWPE has become the bearing surface of choice in total hip arthroplasty. First generation crosslinked UHMWPEs, clinically introduced in the 1990s, show significant improvements compared to gamma sterilised, conventional UHMWPE in decreasing wear and osteolysis. These crosslinked UHMWPEs were thermally treated (annealed or melted) after irradiation to improve their oxidation resistance. While annealing resulted in the retention of some oxidation potential, post-irradiation melted UHMWPEs had reduced fatigue strength due to the crystallinity loss during melting. Thus, the stabilisation of radiation crosslinked UHMWPEs by the diffusion of the antioxidant vitamin E was developed to obtain oxidation resistance with improved fatigue strength by avoiding post-irradiation melting. A two-step process was developed to incorporate vitamin E into irradiated UHMWPE by diffusion to obtain a uniform concentration profile. Against accelerated and real-time aging in vitro, this material showed superior oxidation resistance to UHMWPEs with residual free radicals. The fatigue strength was improved compared to irradiated and melted UHMWPEs crosslinked using the same irradiation dose. Several adverse testing schemes simulating impingement showed satisfactory behaviour. Peri-prosthetic tissue reaction to vitamin E was evaluated in rabbits and any effects of vitamin E on device fixation were evaluated in a canine model, both of which showed no detrimental effects of the inclusion of vitamin E in crosslinked UHMWPE. Irradiated, vitamin E-diffused, and gamma sterilised UHMWPEs have been in clinical use in hips since 2007 and in knees since 2008. The clinical outcome of this material will be apparent from the results of prospective, randomised clinical studies.
Introduction
Osteolysis triggered mainly by ultrahigh molecular weight polyethylene (UHMWPE) wear particles has been one of the major problems in total hip arthroplasty along with instability/dislocation and infection [7]. Highly crosslinked UHMWPEs were developed using high-dose irradiation (50-100 kGy) to decrease the wear rate of UHMWPE [44, 46] and have become the standard-of-care after their first decade of service, especially in North America [61].
Irradiation causes crosslinking in the amorphous phase of the UHMWPE [33], but also initiates the formation of free radicals in the crystalline phase [23], unable to recombine due to structural limitations, they become trapped for long periods of time [29]. These residual free radicals are believed to migrate to the crystalline/amorphous interface and cause oxidative degradation in the material [16–18, 59, 69] through a cascade of reactions with oxygen [32, 71].
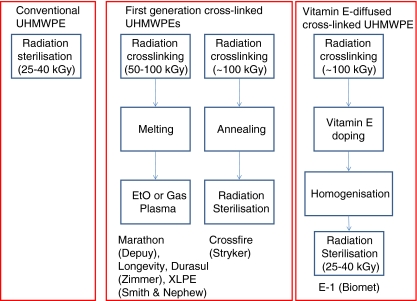
In first generation crosslinked UHMWPEs [34], post-irradiation thermal treatment was used to increase the oxidation resistance (Fig. 1). One approach was to anneal the irradiated UHMWPE below its peak melting point [63], reducing the free radical concentration by providing more energy to the free radicals and allowing them to recombine to some extent. The free radicals were reduced by annealing but this implant was also terminally gamma sterilised, which introduced additional free radicals. In the long-term, there was significant oxidation in irradiated and annealed UHMWPE in vivo [21, 35, 72]. Despite this at intermediate follow-up (up to eight years), the penetration rates of highly crosslinked annealed UHMWPE are less than those for conventional UHMWPE [43, 64], and the clinical effects of this elevated oxidation have yet to manifest themselves [35]. One study showed fatigue damage at the oxidised rim of explanted irradiated and annealed UHMWPE acetabular liners although none were revised due to rim damage [21]. The second approach was post-irradiation re-melting of the crystals to allow the free radicals to recombine completely (Fig. 1). This method reduced the radiation-induced residual free radical concentrations to undetectable levels as measured by electron spin resonance and resulted in very low measurable oxidation in accelerated aging tests [49]. At the same time, re-crystallisation was hindered by the newly formed crosslinks, the crystallinity was reduced and therefore the fatigue strength of radiation crosslinked UHMWPE was further reduced [53]. The in vitro wear rate [47] and the penetration rates of re-melted UHMWPE in the intermediate term (five years) have been very low [22, 45], but there are some concerns about the increased incidence of rim fracture under impingement and adverse loading conditions due to the lowered fatigue strength of this material [6, 27, 70].
Fig. 1.
Schematic depiction of processing of crosslinked ultrahigh molecular weight polyethylene (UHMWPE)
Thus, vitamin E stabilisation of UHMWPE was introduced as an alternative method to provide oxidation resistance without sacrificing some of the fatigue strength [55]. There are two methods by which vitamin E can be incorporated into radiation crosslinked UHMWPE. The first is blending of the liquid antioxidant with UHMWPE resin powder, consolidating the mixture by compression molding and irradiating the consolidated blend for crosslinking [8, 36, 52, 58]. Because vitamin E can also act as a free radical scavenger during irradiation, the crosslinking efficiency of UHMWPE is lowered in the presence of vitamin E [52, 58] and the vitamin E concentration in the blend is limited to less than 0.3 wt% [51]. In addition, the radiation dose has to be optimised as it is necessary to expose a vitamin E blend to a higher dose irradiation than virgin UHMWPE to obtain a desired crosslinking or wear rate. Nevertheless, this vitamin E-stabilised UHMWPE is promising in decreasing oxidation of irradiated UHMWPE [36, 39] and can have low wear rates if the vitamin E concentration and radiation dose are optimised [51, 52]. Recently, medical grade UHMWPE resin supplier Ticona (Florence, KY) announced its large-scale production of vitamin E-containing resin for various ultimate applications, and a 0.1 wt% vitamin E-containing, 91-kGy irradiated UHMWPE acetabular cup was implanted in Switzerland in 2009 (Mathys). The second approach, which we will describe in detail in this review, is diffusing vitamin E into already consolidated and radiation crosslinked UHMWPE. Using this method, the amount of incorporated vitamin E is not limited by the crosslink density, but the saturation limit of the crosslinked polymer at body temperature, which is approximately 0.7 wt%. Total hip implants manufactured from 100-kGy irradiated, vitamin E-diffused and terminally gamma sterilised UHMWPEs have been clinically used since 2007 (Fig. 2). Prospective, randomised clinical studies, including an RSA study based at our institution are underway.
Fig. 2.
An E-1™ acetabular liner (Biomet, Inc.) fabricated from highly crosslinked, vitamin E doped/homogenised, terminally gamma sterilised ultrahigh molecular weight polyethylene (UHMWPE) (Courtesy of Dave Schroeder, Biomet)
Rationale for the use of vitamin E
Vitamin E is the most abundant and effective chain-breaking antioxidant present in the human body, whose major physiological role is to react with free radicals in cell membranes and protect polyunsaturated fatty acids from degradation due to oxidation [57]. Oxidation of polyunsaturated fatty acids results in active free radicals (LOO•, LO•. The antioxidant activity of vitamin E (RRR-α-tocopherol in vivo) is due to hydrogen donation from the phenolic OH group on the chroman ring to a free radical on the oxidised lipid chain. Hydrogen abstraction results in a tocopheryl free radical, which can combine with another free radical. Therefore, tocopherol can theoretically prevent two peroxy free radicals from attacking other fatty acid chains and producing more free radicals [9, 10, 31]. Therefore, the cascading nature of oxidation is stopped, and oxidative damage can be prevented.
Oxidation reactions in polyethylene, which has a lipid-like molecular structure, also follow a similar mechanism of oxidation as lipids in vivo [1, 19, 24]. In irradiated UHMWPE, the carbon free radicals resulting from the breakage of the C-H bonds are prevalent [11]. When oxygen is present in irradiated polyethylene, it reacts with the primary free radicals to form peroxy free radicals [12, 38]. These peroxy radicals, in the absence of an antioxidant such as vitamin E, abstract a hydrogen atom from other polyethylene chains, creating new primary free radicals, which can then react with oxygen to further this chain of reactions [1, 60]. When peroxy free radicals react with hydrogen, they form hydroperoxides, which are not stable and degrade into oxidation products, mainly ketones, esters, and acids [2, 3, 16, 17]. The formation of these oxidation products is accompanied by chain scission and a decrease in the molecular weight of polyethylene, reducing its mechanical properties [14, 37]. In an irradiated polyethylene containing vitamin E, peroxy free radicals presumably abstract a hydrogen atom from vitamin E, forming hydroperoxides without the formation of new free radicals. Vitamin E is also effective in reacting with alkyl radicals [66], but the reaction rate with peroxy free radicals is higher than any other radical [9, 31]. The oxidation cascade in irradiated polyethylene is therefore hindered in the presence of vitamin E.
Preparation of radiation crosslinked, vitamin E-diffused, and gamma sterilised UHMWPE
For vitamin E to be able to protect UHMWPE against oxidation, the antioxidant has to be distributed throughout the entire thickness of the component. Polyethylene can be doped with vitamin E simply by allowing it to sit in a bath of pure or diluted vitamin E. Because vitamin E is highly hydrophobic, it diffuses readily into polyethylene, especially at elevated temperature; however, in the application of this concept, there are several factors to be considered.
First, it is important not to melt the sample substantially during the diffusion process and thereby to avoid the loss of crystallinity during post-irradiation melting, which causes a decrease in mechanical strength [53]. The melting range of UHMWPE starts at 100°C with a peak melting point of about 140°C; more crystallinity is lost as the diffusion temperature nears the peak melting point. Increased temperature also increases the surface concentration and the saturation concentration of vitamin E at the surface of UHMWPE [56, 74]. Dimensional changes can occur both due to the thermal release of residual stresses from previous processing steps and also due to the swelling of the surface with vitamin E. A slightly oversized preform can be processed and machined as a terminal step before sterilisation to overcome changes in dimensions.
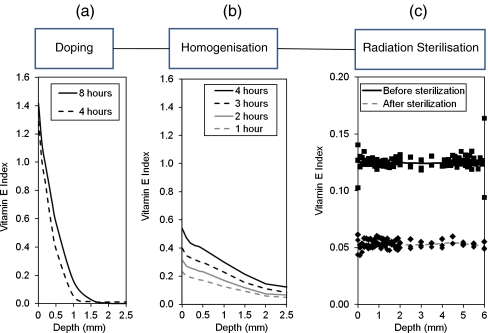
To obtain a uniform vitamin E concentration profile throughout components of various thickness, a two-step diffusion process at elevated temperatures below the melting point was developed involving doping of UHMWPE by soaking in vitamin E with subsequent homogenisation at an elevated temperature below the melting point [56]. The doping step was restricted to 120°C because there were significant volumetric and weight changes above this temperature. When the high surface concentration of vitamin E had been obtained (Fig. 3a), the homogenisation of the surface vitamin E throughout the irradiated component could then be achieved at a higher temperature below the melting point because there was no additional diffusion of vitamin E into the component (Fig. 3b).
Fig. 3.
Examples of the effects of process steps on vitamin E concentration profile. Doping of 85-kGy irradiated ultrahigh molecular weight polyethylene (UHMWPE) at 120°C as a function of time (a); doping at 120°C followed by homogenisation at 120°C for 24 hours (b); doped and homogenised UHMWPE before and after sterilisation (c)
A secondary effect of exposing the polyethylene to high temperature during doping and homogenisation would be to decrease the amount of residual free radicals caused by radiation crosslinking. But, a terminal sterilisation by gamma irradiation is used currently in clinically available implants prepared using this method (Fig. 3c), resulting in substantially detectable free radicals in this material (3.5 × 1016 spins/g compared to 11.7 × 1016 spins/g for 100-kGy irradiated UHMWPE; [54]). Despite the presence of these free radicals, vitamin E-stabilised UHMWPE has shown great oxidative stability when subjected to accelerated aging as will be discussed later (Fig. 4a).
Fig. 4.
Oxidation (a) and free radical concentration (b) as a function of time of real-time aging in water at 40°C for 150-kGy irradiated ultrahigh molecular weight polyethylene (UHMWPE) without stabilisation and 150-kGy irradiated, vitamin E-diffused and terminally gamma sterilised UHMWPE
During irradiation, vitamin E hinders crosslinking in UHMWPE as a function of increasing concentration [52, 58]. In fact, at vitamin E concentrations above 0.3 wt%, the incremental increase in crosslink density by increasing the radiation dose is minimal and it is not possible to crosslink vitamin E-containing UHMWPE to the same level as that of 100-kGy irradiated and melted UHMWPE even at irradiation doses as high as 200 kGy [51]. The terminal gamma irradiation for sterilisation causes a very small increase in crosslink density. At the same time, some vitamin E is grafted onto the polymer chain by the post-diffusion irradiation, preventing elution of components; therefore, it may be desirable to graft some of the vitamin E in the material.
Oxidative stability
A major factor limiting the maintenance of the wear and mechanical properties of irradiated UHMWPEs in vivo is oxidation [59]. Oxidation is determined by spectroscopic techniques measuring carbonyl moieties on UHMWPE formed as a result of the decay of hydroperoxides [13]. Typically accelerated aging methods are used in vitro to compare the oxidative stability of various polyethylene formulations [34]. While accelerated aging is helpful in comparing the oxidation resistance and oxidation potential of different types of bearing materials, it cannot be used to predict the oxidation timeline or profile of a particular material in vivo.
Nevertheless, it has been shown in several types of accelerated aging studies, carried out at elevated temperatures and/or in the presence of pure oxygen under high pressure, that vitamin-E-containing, irradiated polyethylene is more stable than gamma-sterilised or high-dose irradiated polyethylene [8, 36, 39, 41, 53, 55, 75]. It is proposed that this is due to the reaction of vitamin E with the primary free radicals on the polyethylene chains and also free radicals resulting from their reaction with oxygen. A study looking specifically at the stabilising effect of vitamin E in vitamin-E-diffused, irradiated UHMWPE showed higher oxidative stability than unstabilised, irradiated UHMWPE over seven months in air at room temperature and in water at 40°C [54] (showing similar trends at 36 months; Fig. 4; [65]).
Wear resistance
As mentioned above, a crosslink density equivalent to that of 100-kGy irradiated and melted UHMWPE is desired because this material has been shown in vitro and in vivo to decrease wear significantly compared to conventional UHMWPE [22, 46]. The studies discussed here were performed on UHMWPE with an initial radiation dose of 85 to 100 kGy with a terminal gamma sterilisation dose of ~25– 40 kGy after vitamin E incorporation designed to obtain similar wear resistance to this predicate material.
Wear testing of vitamin-E-diffused, 100-kGy irradiated UHMWPE on a custom-designed pin-on-disc tester in bovine serum showed that the wear rate was comparable to 100-kGy irradiated and melted UHMWPE [55]. In this study, accelerated aging at 80°C for five weeks did not change the wear rate of this UHMWPE despite the fact that vitamin E was diffused only 0.5 mm into the surface of this sample and not throughout the entire thickness. This suggested that the wear region was within this surface layer protected by the vitamin E.
After the development of the diffusion method by doping followed by homogenisation below the melting point, 5-mm thick acetabular liners were irradiated by 85-kGy gamma irradiation, doped with vitamin E, subsequently homogenised to obtain vitamin E throughout, and terminally gamma sterilised. These acetabular liners (28- and 40-mm inner diameter) were wear tested on a hip simulator against CoCr femoral heads using normal gait load and kinematics at 2 Hz in undiluted clean bovine serum for five million cycles and compared to conventional UHMWPE gamma sterilised in inert gas [50]. This study showed in vitro that under normal conditions, the wear reduction in vitamin-E-diffused, highly crosslinked UHMWPE compared to conventional UHMWPE (92% and 90% reduction in wear for 28- and 40-mm cups, respectively, compared to 28-mm conventional UHMWPE) was comparable to that observed previously with irradiated and melted UHMWPE compared to conventional UHMWPE [46, 48]. Similar results were observed when these liners were tested in undiluted bovine serum with third body polymethyl methacrylate (PMMA) and barium sulfate particulate debris (72% and 75% reduction in wear for 28- and 40-mm cups, respectively, compared to 28-mm conventional). The results obtained from these hip simulator studies with vitamin-E-diffused, irradiated highly crosslinked UHMWPE confirmed earlier studies that showed the wear rate of highly crosslinked UHMWPEs were largely independent of femoral head size (Table 1) [48].
Table 1.
Some mechanical and tribological properties of available ultrahigh molecular weight polyethylene (UHMWPE)
| UHMWPE type | UTS (MPa) | Hip simulator wear rate (mg/MC) | Pin-on-disc wear rate (mg/MC) |
|---|---|---|---|
| Conventional UHMWPE | 52 ± 5 | 10 | 6.3 |
| 100-kGy irradiated and melted UHMWPE [47, 48] | 35 ± 5 | 1-2 | 1.6 |
| 100-kGy irradiated and annealed UHMWPE [49, 63] | 46 ± 3 | NA | 1.7 |
| 100-kGy irradiated, vitamin E-diffused UHMWPE [50, 55] | 46 ± 3 | 0.8-1 | NA |
UTS ultimate tensile strength
There are concerns about the ability of highly crosslinked UHMWPEs to withstand especially two clinically adverse situations. One of these is the femoral neck impingement on the rim of an acetabular liner, most often due to the vertical placement of the shell with excessive anteversion [27, 70]. There is increased concern for highly crosslinked UHMWPEs due to their initially lower fatigue strength than unaged conventional UHMWPE and the fact that larger head sizes with thinner polyethylene can now be used, thanks to the independence of the wear rate of highly crosslinked UHMWPEs of femoral head size [40, 48]. In a clinically relevant device fatigue test involving the impingement of the rim of thin polyethylene liners by the neck of the femoral components, simulating the adverse case of vertical malpositioning of the implant, 3.7 mm-thick vitamin-E-diffused, crosslinked acetabular liners with 28- and 40-mm inner diameter were loaded on the rim by the femoral neck for two million cycles. There were no fractures in any of the tested liners, including the conventional UHMWPE liners with 28-mm inner diameter used as controls. No apparent differences between unaged conventional UHMWPE and vitamin-E-diffused, highly crosslinked UHMWPE were observed [50].
Peri-prosthetic effects of vitamin E
Wear debris of conventional UHMWPE has been clearly associated with osteolysis and loosening of implants [68]. Currently, there is no conclusion on the differences in the biological activity of the wear debris from highly crosslinked UHMWPE compared to conventional UHMWPE. While the average particle size from highly crosslinked UHMWPEs appears to be smaller than that of conventional UHMWPE, the number of particles is also significantly lower. In addition, conventional UHMWPE has been associated with high oxidation not observed in irradiated and melted UHMWPE; therefore, the activity of the particles may not be the same [15]. Vitamin E may also have a direct role in regulating immune response when associated with UHMWPE particles based on an in vitro study indicating that vitamin E-stabilised unirradiated UHMWPE increased matrix metalloproteinase-9 secretion from granulocytes [62].
Vitamin E is found in foods such as vegetable oils, nuts, seeds, whole grains, leafy green vegetables and avocados [67]. The vitamin E commonly used for the stabilisation of radiation cross-linked UHMWPE is produced synthetically and is certified to be at least 97% pure α-tocopherol. Both synthetic and natural vitamin E are regarded as safe for human consumption by the Food and Drug Administration as additives in prepared food in accordance with current good manufacturing principles [25]. There are no known adverse effects of the consumption of vitamin E naturally occurring in food. One major concern regarding vitamin E-stabilised UHMWPE involves its possible elution from the polymer in vivo and their local/ systemic effects. There was measurable elution of vitamin E from vitamin E-diffused UHMWPE aged in the laboratory in water at 40°C for three years [65]. This was attributed to the surface vitamin E concentration being higher than the saturation concentration of vitamin E in early-phase vitamin E-diffused, irradiated UHMWPE at 40°C. The concentration profile became uniform at a vitamin E level of approximately 0.7 wt% at three years, suggesting that this was the saturation concentration. Nevertheless, these components did not show any detectable oxidation at three years. To quantify the effects of complete elution on the oxidative stability of vitamin E-diffused crosslinked UHMWPE, a clinically irrelevant extraction procedure in boiling hexane was used, after which some vitamin E remained in the samples. These samples were exposed to accelerated aging after extraction and did not oxidise despite the removal of most of the vitamin E. This suggested that under clinically relevant conditions, the complete removal of vitamin E from the components was highly unlikely and that the components would be protected even under adverse conditions. In addition, clinically used components are currently prepared to have a more uniform concentration at around 0.7 wt% before sterilisation, which would minimise the driving force for elution of the components.
Regarding the peri-prosthetic effects of the elution of vitamin E from irradiated UHMWPE, Wolf et al. [73] determined that there were no cellular cytotoxic or genotoxic effects of vitamin E extracted from a vitamin E-blended and terminally gamma sterilised UHMWPE containing 0.8 wt% vitamin E in vitro. In a study investigating the local toxicity of vitamin E, an emulsifying agent (Tween 80™) was used to dissolve vitamin E in an aqueous solution, in order to simulate the mechanism of vitamin E transport conjugated to lipoproteins. Two millilitres of this emulsion (10 mg of vitamin E) was injected to the knee joints of New Zealand white rabbits and the animals were sacrificed at two weeks (n = 3) and at 12 weeks (n = 6). The control knee was injected with the carrier solution without vitamin E. The findings were that regardless of the time in situ, the synovial tissue had a normal appearance at harvest and there were no signs of inflammation or sterile pus. The tissue of all control knees had the same unremarkable appearance [30].
The effect of vitamin E on the device fixation of vitamin E-diffused, irradiated UHMWPE was investigated in a canine hip model [30]. A comparison between bony ingrowth (using histology) of cups with vitamin E-diffused, irradiated UHMWPE liners (n = 14) and irradiated, unstabilised UHMWPE liners (n = 7) showed no significant differences between the two groups after three months of implantation time.
Mechanical properties
As mentioned above, there is a decrease in mechanical and fatigue strength of irradiated and melted UHMWPEs, caused both by crosslinking and the loss of crystallinity during melting, which quenches the residual free radicals [49]. The loss of crystallinity during post-irradiation melting was avoided by doping irradiated UHMWPE with vitamin E [50, 53]. Therefore, the ultimate tensile strength (Table 1), yield strength, and fatigue crack propagation resistance (Fig. 5) were also increased. The value for the ultimate tensile strength of 100 kGy irradiated and melted UHMWPE reported in the literature is approximately 35 MPa [53]; the ultimate tensile strength of vitamin-E-diffused, irradiated UHMWPE was measured to be 46 MPa under the same testing conditions [50].
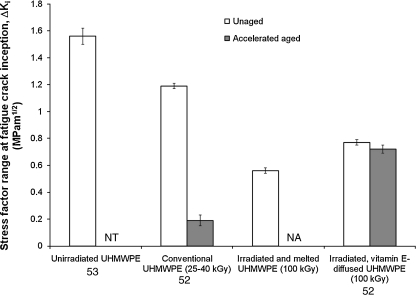
Fig. 5.
Fatigue crack propagation resistance (∆Ki) of some available UHMWPEs
The fatigue strength of surgical-grade UHMWPE is typically quantified by determining the resistance to fatigue crack propagation by cyclically loading a specimen designed to concentrate stresses at a crack tip [4]. The stress range that needs to be applied to propagate the crack at a rate of 10−6 mm/cycle is reported as a measure of the resistance to crack propagation. The values for unirradiated or gamma-sterilised UHMWPE, which has high fatigue resistance in its unaged state, have been reported as 1.4–2.0 MPa·m1/2 [5, 55]. In contrast, irradiated and melted highly crosslinked UHMWPEs had values of 0.55–0.69 MPa·m1/2 depending on the radiation dose [5, 55]. The fatigue resistance of vitamin-E-diffused, 100-kGy irradiated UHMWPE was 0.70–0.77 MPa·m1/2 [50, 55]. Also importantly, the strength of vitamin-E-diffused, irradiated UHMWPE remained unchanged when exposed to accelerated aging, while that of conventional, gamma sterilised UHMWPE deteriorated significantly [50, 53]. While conventional UHMWPE has high fatigue strength in its unaged form before exposure to oxygen, its fatigue resistance is severely reduced due to oxidation, to as low as 0.19 MPa·m1/2 [55].
Summary and conclusions
In the hip, highly crosslinked UHMWPEs have now become the standard-of-care as bearing surfaces [61]. It appears that radiation crosslinking has increased the wear resistance of UHMWPE [42] and is likely to decrease the incidence of osteolysis, at least in the first decade [28]. It also appears that using larger head sizes coupled with crosslinked UHMWPE has less of an effect in increasing wear rates than previously observed for conventional UHMWPE [40, 48]. Combined with the belief that using larger head sizes results in less risk of dislocation [20], there is a tendency to use thinner crosslinked UHMWPE components. This may increase the risk of adverse effects such as wear, rim impingement, edge loading and rim fracture, especially given the high prevalence of surgical mal-alignment in the hip (63%-H Malchau personal communication; [26]).
The material discussed here, vitamin E-diffused, irradiated UHMWPE is a second-generation crosslinked bearing surface, which was designed to improve the mechanical and fatigue strength of crosslinked UHMWPE without sacrificing wear and oxidative stability. Thus, its use aims to make crosslinked UHMWPE a more effective and more forgiving bearing surface, which can be used in a variety of indications. In vitro studies have corroborated these concepts; this material showed wear resistance and oxidative stability at least equivalent to the predicate first generation highly crosslinked (irradiated and melted) UHMWPE with improved fatigue strength. It has been available in hips since 2007 and its clinical performance will be determined using prospective, randomised clinical studies, which are under way.
Footnotes
The studies discussed here were performed by research funding in part from NIH/NIAMS AR051142, research grants from Biomet, Inc. (Warsaw, IN) and Zimmer, Inc (Warsaw, IN) and the William H. Harris Foundation. The laboratory and the department also received funding from royalties from Zimmer, Inc. and Biomet, Inc. Dr. Muratoglu received royalties from Biomet, Inc. and Zimmer, Inc. Dr. Oral received royalties from Zimmer, Inc.
References
- 1.Al-Malaika S (1993) Autoxidation. Atmospheric oxidation and antioxidants. Scott G. Amsterdam, Elsevier Science Publishers B.V., pp 45–82
- 2.Al-Malaika S. Perspectives in stabilisation of polyolefins. Adv Polym Sci. 2004;169:121–150. [Google Scholar]
- 3.Assink R, Celina M, Dunbar T, Alam T, Clough R, Gillen K. Analysis of hydroperoxides in solid polyethylene by MAS 13 C NMR and EPR. Macromolecules. 2000;33:4023–4029. doi: 10.1021/ma991970d. [DOI] [Google Scholar]
- 4.ASTM (2008) E647-08 Standard test method for measurement of fatigue crack growth rates. American Society for Testing and Materials, PA, USA
- 5.Baker DA, Bellare A, Pruitt L. The effect of degree of crosslinking on the fatigue crack initiation and propagation resistance of orthopedic-grade polyethylene. J Biomed Mater Res. 2003;66A:146–154. doi: 10.1002/jbm.a.10606. [DOI] [PubMed] [Google Scholar]
- 6.Birman M, Noble P, Conditt M, Li S, Mathis K. Cracking and impingement in ultra-high molecular weight polyethylene acetabular liners. J Arthroplasty. 2005;20(7):87–92. doi: 10.1016/j.arth.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Bozic K, Kurtz S, Lau E, Ong K, Vail T, Berry D. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg. 2009;91:128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 8.Bracco P, Brunella V, Zanetti M, Luda MP, Costa L. Stabilisation of ultra-high molecular weight polyethylene with vitamin E. Polym Degrad Stab. 2007;92:2155–2162. doi: 10.1016/j.polymdegradstab.2007.02.023. [DOI] [Google Scholar]
- 9.Burton G, Ingold K. Autoxidation of biological molecules. 1. The antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J Am Chem Soc. 1981;103:6472–6477. doi: 10.1021/ja00411a035. [DOI] [Google Scholar]
- 10.Burton GW, Traber MG. Vitamin E: Antioxidant activity, biokinetics, and bioavailability. Annu Rev Nutr. 1990;10:357–382. doi: 10.1146/annurev.nu.10.070190.002041. [DOI] [PubMed] [Google Scholar]
- 11.Carlsson D, Chmela S, Lacoste J. On the structures and yields of the first peroxyl radicals in g-irradiated polyolefins. Macromolecules. 1990;23:4934–4938. doi: 10.1021/ma00225a009. [DOI] [Google Scholar]
- 12.Carlsson D, Dobbin C, Wiles D. Direct observations of macroperoxyl radical propagation and termination by electron spin resonance and infrared spectroscopies. Macromolecules. 1985;18:2092–2094. doi: 10.1021/ma00152a053. [DOI] [Google Scholar]
- 13.Carlsson DJ, Brousseau R, Zhang C, Wiles DM. Polyolefin oxidation: quantification of alcohol and hydroperoxide products by nitric oxide reactions. Polym Degrad Stab. 1987;17:303–318. doi: 10.1016/0141-3910(87)90090-5. [DOI] [Google Scholar]
- 14.Collier J, Sutula L, Currier B, John H, Wooding R, Williams I, Farber K, Mayor M. Overview of polyethylene as a bearing material: Comparison of sterilization methods. Clin Orthop. 1996;333:76–86. [PubMed] [Google Scholar]
- 15.Costa L, Bracco P, Brach del Prever E. Physicochemical and mechanical properties of UHMWPE: 45 years' experience. Interact Surg. 2007;2(3–4):169–173. doi: 10.1007/s11610-007-0052-4. [DOI] [Google Scholar]
- 16.Costa L, Luda MP, Trossarelli L. Ultra-high molecular weight polyethylene: I. Mechano-oxidative degradation. Polym Degrad Stab. 1997;55:329–338. doi: 10.1016/S0141-3910(96)00170-X. [DOI] [Google Scholar]
- 17.Costa L, Luda MP, Trossarelli L. Ultra high molecular weight polyethylene-II. Thermal-and photo-oxidation. Polym Degrad Stab. 1997;58:41–54. doi: 10.1016/S0141-3910(97)00010-4. [DOI] [Google Scholar]
- 18.Costa L, Luda MP, Trossarelli L, Brach del Prever EM, Crova M, Gallinaro P. In vivo UHMWPE biodegradation of retrieved prosthesis. Biomaterials. 1998;19:1371–1385. doi: 10.1016/S0142-9612(98)00013-1. [DOI] [PubMed] [Google Scholar]
- 19.Costa L, Luda MP, Trossarelli L, Brach del Prever EM, Crova M, Gallinaro P. Oxidation in orthopaedic UHMWPE sterilized by gamma radiation and ethylene oxide. Biomaterials. 1998;19:659–668. doi: 10.1016/S0142-9612(97)00160-9. [DOI] [PubMed] [Google Scholar]
- 20.Cuckler J, Moore K, Lombardi A, Jr, McPherson E, Emerson R. Large versus small femoral heads in metal-on-metal total hip arthroplasty. J Arthroplast. 2004;19(8 Suppl. 3):41–44. doi: 10.1016/j.arth.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Currier BH, Currier JH, Mayor MB, Lyford K, Collier JP, Citters DW. Evaluation of oxidation and fatigue damage of retrieved Crossfire polyethylene acetabular cups. J Bone Joint Surg. 2007;89A:2023–2029. doi: 10.2106/JBJS.F.00336. [DOI] [PubMed] [Google Scholar]
- 22.Digas G, Karrholm J, Thanner J, Herberts P. 5-year experience of highly crosslinked polyethylene in cemented and uncemented sockets: Two randomized studies using radiostereometric analysis. Acta Orthop. 2007;78(6):746–754. doi: 10.1080/17453670710014518. [DOI] [PubMed] [Google Scholar]
- 23.Dole M. Free radicals in irradiated polyethylene. The radiation chemistry of macromolecules. New York: Academic Press; 1972. pp. 335–348. [Google Scholar]
- 24.Esterbauer H, Gebicki J, Puhl H, Jurgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992;13:341–390. doi: 10.1016/0891-5849(92)90181-F. [DOI] [PubMed] [Google Scholar]
- 25.FDA DoHaHS (2004) Code of Federal Regulations 184.1890: Direct food substances affirmed as generally recognized as safe-[alpha]-tocopherols. US Food and Drug Administration, Title 21, vol 3
- 26.Gallo J, Havranek V, Zapletalova J. Risk factors for accelerated polyethylene wear and osteolysis in ABG I total hip arthroplasty. Int Orthop. 2010;34:19–26. doi: 10.1007/s00264-009-0731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halley D, Glassman A, Crowninshield R. Recurrent dislocation after revision total hip replacement with a large prosthetic femoral head. J Bone Joint Surg. 2004;86A(4):827–830. doi: 10.2106/00004623-200404000-00025. [DOI] [PubMed] [Google Scholar]
- 28.Hodrick J, Severson E, McAlister D, Dahl B, Hofmann A. Highly crosslinked polyethylene is safe fro use in total knee arthroplasty. Clin Orthop Relat Res. 2008;466:2806–2812. doi: 10.1007/s11999-008-0472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jahan MS, King MC, Haggard WO, Sevo KL, Parr JE. A study of long-lived free radicals in gamma-irradiated medical grade polyethlene. Radiat Phys Chem. 2001;62:141–144. doi: 10.1016/S0969-806X(01)00431-5. [DOI] [Google Scholar]
- 30.Jarrett B, Cofske J, Rosenberg A, Oral E, Muratoglu O, Malchau H (2010) In vivo biological response to vitamin E and vitamin E-doped polyethylene. J Bone Joint Surg 92(13) (in press) [DOI] [PubMed]
- 31.Kamal-Eldin A, Appelqvist L. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31(7):671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- 32.Kashiwabara H, Seguchi T. Radiation induced oxidation of plastics. Processing of polymers. Munich: Carl Hanser Verlag; 1992. pp. 221–254. [Google Scholar]
- 33.Kashiwabara H, Shimada S, Hori Y. Free radicals and crosslinking in irradiated polyethylene. Radiat Phys Chem. 1991;37(1):43–46. [Google Scholar]
- 34.Kurtz S, editor. UHMWPE biomaterials handbook. Elsevier: London; 2009. [Google Scholar]
- 35.Kurtz S, Austin M, Azzam K, Sharkey P, MacDonald D, Medel F, Hozack W. Mechanical properties, oxidation and clinical performance of retrieved highly crosslinked Crossfire liners after intermediate-term implantation. J Arthroplasty. 2010;25(4):614–625. doi: 10.1016/j.arth.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurtz S, Dumbleton J, Siskey R, Wang A, Manley M. Trace concentrations of vitamin E protect radiation crosslinked UHMWPE from oxidative degradation. J Biomed Mater Res. 2008;90A:549–563. doi: 10.1002/jbm.a.32122. [DOI] [PubMed] [Google Scholar]
- 37.Kurtz S, Hozack WJ, Marcolongo M, Turner J, Rimnac CM, Edidin A. Degradation of mechanical properties of UHMWPE acetabular liners following long-term implantation. J Arthroplast. 2003;18(7 Supp 1):68–78. doi: 10.1016/S0883-5403(03)00292-4. [DOI] [PubMed] [Google Scholar]
- 38.Kuzuya M, Kondo S, Sugito M, Yamashiro T. Peroxy radical formation from plasma-induced surface free radicals of polyethylene as studied by electron spin resonance. Macromolecules. 1998;31:3230–3234. doi: 10.1021/ma970937t. [DOI] [Google Scholar]
- 39.Lerf R, Zurbrugg D, Delfosse D. Use of vitamin E to protect cross-linked UHMWPE from oxidation. Biomaterials. 2010;31:3643–3648. doi: 10.1016/j.biomaterials.2010.01.076. [DOI] [PubMed] [Google Scholar]
- 40.Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg. 1990;72-A:518–528. [PubMed] [Google Scholar]
- 41.Mallegol J, Carlsson D, Deschenes L. Post-gamma irradiation reactions in vitamin E stabilized and unstabilised HDPE. Nucl Instrum Meth Phys Res B. 2001;185:283–293. doi: 10.1016/S0168-583X(01)00944-2. [DOI] [Google Scholar]
- 42.Manning DW, Chiang PP, Martell JM, Galante JO, Harris WH. In vivo comparative wear study of traditional and highly cross-linked polyethylene in total hip arthroplasty. J Arthroplasty. 2005;20(7):880–886. doi: 10.1016/j.arth.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 43.Martell JM, Verner JJ, Incavo SJ. Clinical performance of a highly cross-linked polyethylene at two years in total hip arthroplasty: a randomized prospective trial. J Arthroplasty. 2003;18(7 Suppl 1):9. doi: 10.1016/s0883-5403(03)00341-3. [DOI] [PubMed] [Google Scholar]
- 44.McKellop H, Shen F-W, Lu B, Campbell P, Salovey R. Development of an extremely wear resistant ultra-high molecular weight polyethylene for total hip replacements. J Orthop Res. 1999;17(2):157–167. doi: 10.1002/jor.1100170203. [DOI] [PubMed] [Google Scholar]
- 45.Mu Z, Tian J, Wu T, Yang J, Pei F. A systematic review of radiological outcomes of highly crosslinked polyethylene versus conventional polyethylene in total hip arthroplasty. Int Orthop. 2009;33:599–604. doi: 10.1007/s00264-008-0716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muratoglu OK, Bragdon CR, O'Connor DO, Jasty M, Harris WH. A novel method of crosslinking UHMWPE to improve wear, reduce oxidation and retain mechanical properties. J Arthroplasty. 2001;16(2):149–160. doi: 10.1054/arth.2001.20540. [DOI] [PubMed] [Google Scholar]
- 47.Muratoglu OK, Bragdon CR, O'Connor DO, Jasty M, Harris WH, Gul R, McGarry F. Unified wear model for highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE) Biomaterials. 1999;20(16):1463–1470. doi: 10.1016/S0142-9612(99)00039-3. [DOI] [PubMed] [Google Scholar]
- 48.Muratoglu OK, Bragdon CR, O'Connor DO, Perinchief RS, Estok DM, Jasty M, Harris WH. Larger diameter femoral heads used in conjunction with a highly cross-linked ultra-high molecular weight polyethylene: A new concept. J Arthroplasty. 2001;16(8 Suppl):24–30. doi: 10.1054/arth.2001.28376. [DOI] [PubMed] [Google Scholar]
- 49.Muratoglu OK, Merrill EW, Bragdon CR, O'Connor DO, Hoeffel D, Burroughs B, Jasty M, Harris WH. Effect of radiation, heat, and aging on in vitro wear resistance of polyethylene. Clin Orthop Relat Res. 2003;417:253–262. doi: 10.1097/01.blo.0000093004.90435.d1. [DOI] [PubMed] [Google Scholar]
- 50.Oral E, Christensen S, Malhi A, Wannomae K, Muratoglu O. Wear resistance and mechanical properties of highly crosslinked UHMWPE doped with vitamin E. J Arthroplasty. 2006;21(4):580–591. doi: 10.1016/j.arth.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oral E, Godleski Beckos C, Malhi A, Muratoglu O. The effects of high dose irradiation on the cross-linking of vitamin E-blended UHMWPE. Biomaterials. 2008;29:3557–3560. doi: 10.1016/j.biomaterials.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oral E, Greenbaum ES, Malhi AS, Harris WH, Muratoglu OK. Characterization of irradiated blends of alpha-tocopherol and UHMWPE. Biomaterials. 2005;26(33):6657–6663. doi: 10.1016/j.biomaterials.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oral E, Malhi A, Muratoglu O. Mechanisms of decrease in fatigue crack propagation resistance in irradiated and melted UHMWPE. Biomaterials. 2006;27:917–925. doi: 10.1016/j.biomaterials.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oral E, Rowell S, Muratoglu O. The effect of alpha-tocopherol on the oxidation and free radical decay in irradiated UHMWPE. Biomaterials. 2006;27:5580–5587. doi: 10.1016/j.biomaterials.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oral E, Wannomae KK, Hawkins NE, Harris WH, Muratoglu OK. a-Tocopherol doped irradiated UHMWPE for high fatigue resistance and low wear. Biomaterials. 2004;25(24):5515–5522. doi: 10.1016/j.biomaterials.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 56.Oral E, Wannomae KK, Rowell SL, Muratoglu OK. Diffusion of vitamin E in ultra-high molecular weight polyethylene. Biomaterials. 2007;28(35):5225–5237. doi: 10.1016/j.biomaterials.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Packer L, Kagan VE (1993) Vitamin E: The antioxidant harvesting center of membranes and lipoproteins. In Packer L, Fuchs J (eds) Vitamin E in health and disease. Marcel Dekker, Inc., New York, pp 179–192
- 58.Parth M, Aust N, Lederer K. Studies on the effect of electron beam radiation on the molecular structure of ultra-high molecular weight polyethylene under the influence of alpha-tocopherol with respect to its application in medical implants. J Mater Sci Mater Med. 2002;13(10):917–921. doi: 10.1023/A:1019892004830. [DOI] [PubMed] [Google Scholar]
- 59.Premnath V, Harris W, Jasty M, Merrill E. Gamma sterilization of UHMWPE articular implants: an analysis of the oxidation problem. Ultra high molecular weight poly ethylene. Biomaterials. 1996;17:1741–1753. doi: 10.1016/0142-9612(95)00349-5. [DOI] [PubMed] [Google Scholar]
- 60.Rabek J, Ranby B. Photochemical oxidation reactions of synthetic polymers. ESR applications to polymer research. Stockholm: Almqvist-Wiksell Forlag AB; 1973. [Google Scholar]
- 61. CJRR (2008) Hip and knee replacements in Canada. 2008 annual report. Canadian Joint Replacement Registry
- 62.Reno F, Bracco P, Lombardi F, Boccafoschi F, Costa L, Cannas M. The induction of MMP-9 release from granulocytes by vitamin E in UHMWPE. Biomaterials. 2004;25(6):1001. doi: 10.1016/S0142-9612(03)00623-9. [DOI] [PubMed] [Google Scholar]
- 63.Ries M, Pruitt L. Effect of crosslinking on the microstructure and mechanical properties of ultra-high molecular weight polyethylene. Clin Orthop Relat Res. 2005;440:149–156. doi: 10.1097/01.blo.0000185310.59202.e5. [DOI] [PubMed] [Google Scholar]
- 64.Rohrl S, Li M, Nilsson K, Nivbrant B. Very low wear of non-remelted highly crosslinked polyethylene cups: An RSA study lasting up to 6 years. Acta Orthop. 2007;78(6):739–745. doi: 10.1080/17453670710014509. [DOI] [PubMed] [Google Scholar]
- 65.Rowell S, Oral E, Muratoglu O (2010) Comparison of the oxidative stability of vitamin E-stabilized UHMWPE by blending and diffusion. J Orthop Res (in press)
- 66.Scott G (1993) Antioxidants: Chain breaking mechanisms. In Scott G (ed) Atmospheric oxidation and antioxidants. Elsevier Science Publishers B.V., pp 121–160
- 67.Sheppard AJ, Pennington JA, Weihrauch JL (1993) Analysis and distribution of vitamin E in vegetable oils and foods. In Packer L, Fuchs J (eds) Vitamin E in health and disease. Marcel Dekker, Inc., New York, pp 9–31
- 68.Sochart DH. Relationship of acetabular wear to osteolysis and loosening in total hip arthroplasty. Clin Orthop Relat Res. 1999;363:135–150. doi: 10.1097/00003086-199906000-00018. [DOI] [PubMed] [Google Scholar]
- 69.Stea S, Antonietti B, Baruffaldi F, Visentin M, Bordini B, Sudanese A, Toni A. Behavior of Hylamer polyethylene in hip arthroplasty: comparison of two gamma sterilization techniques. Int Orthop. 2006;30(1):35–38. doi: 10.1007/s00264-005-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tower SS, Currier JH, Currier BH, Lyford KA, Citters DW, Mayor MB. Rim cracking of the cross-linked longevity polyethylene acetabular liner after total hip arthroplasty. J Bone Joint Surg Am. 2007;89(10):2212–2217. doi: 10.2106/JBJS.F.00758. [DOI] [PubMed] [Google Scholar]
- 71.Tudos F, Iring M. Polyolefine oxidation. Acta Polym. 1988;39(1/2):19–26. doi: 10.1002/actp.1988.010390105. [DOI] [Google Scholar]
- 72.Wannomae K, Bhattacharyya S, Freiberg A, Estok D, Harris W, Muratoglu O. In vivo oxidation of retrieved crosslinked UHMWPE acetabular components with residual free radicals. J Arthroplasty. 2006;21(7):1005–1011. doi: 10.1016/j.arth.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 73.Wolf C, Lederer K, Muller U. Tests of biocompatibility of a-tocopherol with respect to the use as a stabilizer in ultrahigh molecular weight polyethylene for articulating surfaces in joint endoprostheses. J Mater Sci Mater Med. 2002;13:701–703. doi: 10.1023/A:1015750112343. [DOI] [PubMed] [Google Scholar]
- 74.Wolf C, Maninger J, Lederer K, Fruhwirth-Smounig H, Gamse T, Marr R. Stabilisation of crosslinked ultra-high molecular weight polyethylene (UHMW-PE)-acetabular components with alpha-tocopherol. J Mater Sci Mater Med. 2006;17:1323–1331. doi: 10.1007/s10856-006-0607-7. [DOI] [PubMed] [Google Scholar]
- 75.Yamamoto K, Yamaguchi M, Tani M, Hangyo M, Teramura S, Isu T, Tomita N. Degradation diagnosis of UHMWPE with terahertz-time-domain spectroscopy. Appl Phys Lett. 2004;85(22):5194–5196. doi: 10.1063/1.1827332. [DOI] [Google Scholar]