Abstract
Over the past decade, minimally invasive surgery has gained popularity as a means of optimising early postoperative rehabilitation and increasing patient satisfaction and cosmesis following total hip arthroplasty (THA). However, this surgical exposure has also been associated with increased risk of iatrogenic nerve injury and implant mal-positioning due to limited visibility compared to conventionally larger surgical incisions. The purpose of this meta-analysis was to compare the outcomes of these two surgical exposures. A systematic review of the published and unpublished literature was conducted to include all randomised and non-randomised controlled trials comparing the clinical and radiological outcomes of minimally invasive and conventional THA procedures. In total, 28 studies met the eligibility criteria and included 2,849 hips, i.e. 1,428 minimally invasive compared to 1,421 conventional THAs. The meta-analysis of the current evidence base showed that minimally invasive THA is associated with a significantly increased risk of transient lateral femoral cutaneous nerve palsy (p = 0.006) with no significantly better outcome.
Introduction
Total hip arthroplasty (THA) is the treatment of choice for degenerative changes of the hip joint. The traditional and still most commonly used approaches for primary THA are the posterior approach and direct lateral approach [1–3]. Whilst the recovery and early postoperative outcomes of this procedure have improved over the last 20 years, there remains great interest in accelerated rehabilitation and improving functional outcomes whilst reducing the surgical scar following a THA.
The minimally invasive surgical (MIS) exposure in THA surgery was developed to reduce postoperative bleeding, speed patient recovery and improve the early clinical results [1]. Minimally invasive THA has been defined as an incision length of 10–12 cm or less either with a single or double incision approach [4–8]. Surgeons have suggested that the smaller skin incision, with reduced soft tissue trauma to muscles, tendons and other soft tissues surrounding the hip should result in less postoperative pain, enhance the patient experience and reduce the length of hospital stay [9–12].
Detractors of MIS have suggested that the approach reduces the operative visualisation thus predisposing patients to implant mal-positioning with an increased risk of dislocation, implant loosening and early failure, in addition to an increased risk of neurovascular complications and excessive skin trauma [13, 14].
A previous meta-analysis suggested that there was little difference in the clinical or radiological outcomes following MIS compared to standard exposure THA [15]. However, this study only included randomised and quasi-randomised controlled trials. The purpose of this systematic review was to appraise the entire evidence base to compare the clinical and radiological outcomes of patients who have undergone a traditional exposure to a MIS exposure for THA. The primary aim of the systematic review was to determine whether MIS is superior to a conventional exposure with reference to short- and long-term outcomes.
Materials and methods
Search strategy
All PRISMA compliant searches were performed by TS and CH. The primary search was of published literature using the electronic databases AMED (1985 to April 2010), British Nursing Index (1985 to April 2010), CINHAL (1982 to April 2010), EMBASE (1974 to April 2010) and MEDLINE (1950 to April 2010) using the Ovid search platform. In addition, Scopus, Biomed Central, Zetoc and the Cochrane Library databases were searched. The broad MeSH terms and Boolean operators (“minimally invasive”) AND (“hip”) AND (“replacement” OR “arthroplasty”) were adopted for each database search.
Secondary searches of the unpublished (grey) literature were conducted by searching the electronic databases Open SIGLE (System for Information on Grey Literature in Europe), the WHO International Clinical Trials Registry Platform, Current Controlled Trials, UKCRN Portfolio Database, National Technical Information Service and the UK National Research Register Archive from their inception to April 1, 2010. Conference proceedings were also searched from the British Orthopaedic Association Annual Congress, European Federation of National Associations of Orthopaedics and Traumatology (EFORT) and the British Hip Society to April 2010.
The reference lists from all full text papers included in the review were scrutinised to identify any initially omitted studies. Finally, the corresponding author from each included study was contacted to identify any further studies not previously identified.
Eligibility criteria
All randomised controlled trials (RCTs) and non-randomised controlled trials (nRCT) comparing the clinical and/or radiological outcomes of THA using a standard exposure to a MIS exposure were included. All trials comparing the exposure method, irrespective of whether computer navigation systems were employed in the MIS surgical arm were included. All trials were included irrespective of their publication status, language, sample size, subject age, indication for surgery, duration of follow-up or surgical approach (i.e. lateral or posterior) undertaken. We excluded all cadaver or animal studies, and those studies assessing exposure method with hip resurfacing or hemi-arthroplasty. We also excluded all trials which used multiple incisions for their MIS rather than a single surgical exposure.
Study selection
The title and abstract for each identified citation were independently screened by two reviewers (TS, CH) in relation to the eligibility criteria. Full texts were ordered for those studies which appeared to satisfy these criteria and reviewed independently to determine final inclusion.
Data extraction
Data from the full text reports were extracted by one reviewer (TS) using a standardised data extraction form, and verified by a second reviewer (VB). The data extracted included: sample size, study design, subject age, gender, THA prosthesis, number of surgeons operating, surgical technique, incision approach, clinical, radiological and complication rate results and follow-up period. The corresponding authors from each included study were contacted to obtain any missing data if required.
Outcome
The primary outcome for this study was Harris hip score (HHS). Secondary clinical outcomes included: surgical duration, blood loss, pain, requirement for blood transfusion, length of hospital stay, Oxford hip score (OHS) and Western Ontario and McMaster Universities osteoarthritis index score (WOMAC). Radiological secondary outcomes included: cup inclination angle, stem alignment (varus/valgus) angle, leg length discrepancy, femoral offset, incidence of cup positioning (35–55° valgus) and the incidence of stem positioning (0–5° valgus). Complications included: the incidence of heterotopic ossification, deep and superficial infection, fracture, deep vein thrombosis (DVT), dislocation, haematoma formation, requirement for revision surgery, component loosening, wound complications and the incidence of iatrogenic nerve palsy.
Quality assessment
Study methodological quality was assessed according to the PEDro critical appraisal tool. This 11-item critical appraisal tool is designed to evaluate comparability between the groups, method of randomisation, blinding and statistical analysis of RCTs. This instrument has previously demonstrated reliability and validity [16, 17].
Data synthesis and analysis
All meta-analyses were performed with the Review Manager software (RevMan Version 5.0; Nordic Cochrane Centre, Copenhagen, Denmark) using the Mantel-Haenszel method [18]. Publication bias was assessed using a funnel plot of the most frequently reported outcome.
Meta-analysis was performed when no substantial heterogeneity in study methodology was observed. Specific statistical heterogeneity was evaluated through Chi2 and I2 statistical tests. When Chi2 was p<0.05, and I2<20% indicating low statistical heterogeneity [19], a fixed effect model was used. A random effect model was adopted when Chi2 was p>0.05, and I2>20%.
Binary data was analysed using risk ratios (RR) with 95% confidence intervals (CI). Continuous data was assessed with mean differences (MD) or, where different scales or tools are used to measure the same outcome, standardised mean differences with 95% CI. A probability of p<0.05 was considered as statistically significant.
Sub-group analyses were conducted to assess outcomes with and without the assistance of computer navigation for MIS compared to traditional exposure. However, since only one study used computer navigation-assisted surgery for a small proportion of their patients, we did not consider it necessary to undertake a sub-group analysis of this variable.
Results
Search results
A total of 534 abstracts and titles were reviewed. Of these 28 satisfied the eligibility criteria and were included in the review (Fig. 1). This included 16 nRCTs and 12 RCTs. Two studies were identified reporting the same cohort. In this instance we included both papers but only analysed the data for each outcome measure once. One study presented the results of two separate surgical approaches using MIS and conventional exposures; both were included in separate analyses [20].
Fig. 1.
PRISMA chart
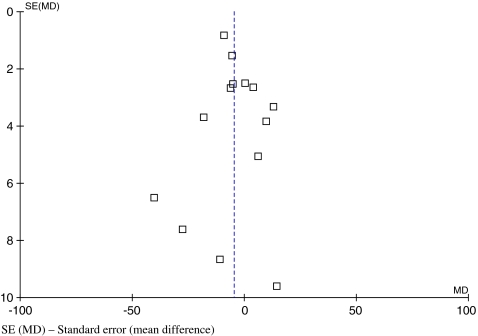
The funnel plot diagram of surgical duration indicated limited evidence of small study exclusion and publication bias with a slightly asymmetrical plot with few studies plotted on the right base of the funnel (Fig. 2).
Fig. 2.
Funnel plot to assess publication for the most frequently reported outcome—surgical duration. SE (MD) standard error (mean difference)
Quality assessment
The results of the PEDro review are presented in Table 1. This indicated that there was considerable variability in the evidence base. Whilst the majority of papers defined their cohorts, as previously stated, only 12 RCTs were identified. Of these, only four concealed the randomisation procedure adequately. A power calculation was used to base the sample size in six studies. Furthermore, only five studies presented both outcome measure and demographic characteristics to allow a full assessment of baseline comparability before the trial began. Only three trials attempted to blind subjects to groups allocation. Whilst surgeon blinding would have been inappropriate in this study design, 16 studies did not blind their assessors to patient group. Ten studies were able to report the outcomes of a minimum of 85% of their starting cohorts; although 13 trials described analysing results through intention-to-treat principles. All studies appropriately used inferential statistics to compare the findings between their experimental cohorts, and all but eight presented both mean and standard deviation or range values to provide an idea of point and variance data from their dataset.
Table 1.
PEDro critical appraisal score
| Study | PEDro criteria | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Yang et al. [49] | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | 9 |
| Lawlor et al. [50] | Y | Y | Y | Y | Y | N | Y | N | Y | Y | Y | 9 |
| Ogonda et al. [35] | Y | Y | Y | Y | Y | N | Y | N | N | Y | Y | 8 |
| Goosen et al. [44] | Y | Y | N | Y | N | N | Y | Y | N | Y | Y | 8 |
| Chimento et al. [6] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 8 |
| Kim [51] | Y | Y | N | Y | N | N | Y | Y | N | Y | Y | 7 |
| Dorr et al. [21] | Y | Y | N | Y | N | N | Y | N | Y | Y | Y | 7 |
| Bennett et al. [22] | N | Y | Y | Y | N | N | Y | N | Y | Y | N | 6 |
| Rittmeister and Peters [52] | Y | N | N | Y | N | N | N | N | Y | Y | Y | 5 |
| Leuchte et al. [53] | Y | N | N | N | N | N | N | N | Y | Y | Y | 5 |
| Kubeš et al. [54] | Y | N | N | Y | N | N | N | Y | N | Y | Y | 5 |
| Chen et al. [26] | Y | N | N | N | N | N | N | Y | N | Y | Y | 5 |
| Szendrõi et al. [55] | Y | N | N | Y | N | N | Y | N | N | Y | Y | 5 |
| Shitama et al. [20] | Y | Y | N | Y | N | N | N | N | N | Y | Y | 5 |
| Sculco et al. [23]-2nd | N | Y | N | Y | N | N | N | Y | N | Y | N | 4 |
| Speranza et al. [32] | Y | Y | N | Y | N | N | N | N | N | Y | N | 4 |
| Vicente et al. [1] | N | N | N | Y | N | N | N | N | Y | Y | Y | 4 |
| Wenz et al. [47] | Y | N | N | N | N | N | N | N | Y | Y | Y | 4 |
| Pospischill et al. [56] | Y | Y | N | N | N | N | N | N | N | Y | Y | 4 |
| Mow et al. [48] | N | N | N | N | N | N | Y | Y | Y | Y | N | 4 |
| Laffosse et al. [45] | N | N | N | Y | N | N | N | N | Y | Y | Y | 4 |
| Laffosse et al. [57] | Y | N | N | N | N | N | N | N | Y | Y | Y | 4 |
| Howell et al. [38] | Y | N | N | N | N | N | N | N | Y | Y | Y | 4 |
| Wohlrab et al. [58] | Y | N | N | Y | N | N | N | N | N | Y | N | 3 |
| Woolson et al. [30] | Y | N | N | N | N | N | Y | N | N | Y | N | 3 |
| Wright et al. [9] | N | N | N | N | N | N | N | Y | N | Y | Y | 3 |
| Sculco et al. [23] | N | N | N | Y | N | N | N | Y | N | Y | N | 3 |
| Pflüger et al. [59] | N | N | N | N | N | N | N | N | Y | Y | N | 2 |
Y Yes, N No
1. Eligibility criteria
2. Random allocation
3. Concealed allocation
4. Baseline comparability
5. Blind subject
6. Blind clinician
7. Blind assessor
8. Adequate follow-up
9. Intention-to-treat analysis
10. Between-group analysis
11. Point estimates and variability
Cohort characteristics
The demographic characteristics of each study cohort are presented in Table 2. The dataset included 2,825 patients who underwent 2,849 THA procedures. This included 1,428 MIS, with a mean age of 61.8 (standard deviation [SD] 3.9) years including 609 males and 602 females; six studies did not document the gender of their cohort. This group was compared to 1,421 conventional exposure THAs with a mean age of 61.5 (SD 4.9) years, consisting of 610 males and 610 females; six papers did not state the gender of their cohorts.
Table 2.
Cohort characteristics
| Study | Design | Sample Size | THA | Mean age (years) | Gender (m/f) | Approach | Follow-up | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pts | THAs | MIS | Conv | MIS | Conv | MIS | Conv | ||||
| Bennett et al. [22] | RCT | 95 | 95 | 43 | 52 | 66.1 | 64.6 | 18/25 | 28/24 | MIS-mini-posterior (≤10 cm) | 5 days |
| Conv-std posterior (16 cm) | |||||||||||
| Chen et al. [26] | nRCT | 166 | 166 | 83 | 83 | 53.5 | 55 | 46/37 | 41/42 | MIS-mini anterolateral | 2 years |
| Conv-std anterolateral | |||||||||||
| Chimento et al. [6] | RCT | 60 | 60 | 28 | 32 | 67.2 | 65.6 | 16/12 | 13/19 | MIS-mini posterolateral | 2 years |
| Conv-std posterolateral | |||||||||||
| Dorr et al. [21] | RCT | 60 | 60 | 30 | 30 | 70.3 | 63.9 | 17/13 | 14/16 | MIS-Mini-posterior with navigation in 27/30 | 6 months |
| Conv-Posterior | |||||||||||
| Goosen et al. [44] | RCT | 120 | 120 | 60 | 60 | 60 | 62 | 30/30 | 29/31 | MIS-mini-anterolateral/mini posterior | 1 year |
| Conv-std anterolateral/posterior | |||||||||||
| Howell et al. [38] | nRCT | 107 | 107 | 50 | 57 | 59.8 | 62.3 | 34/16 | 27/30 | MIS-mini-anterolateral | N/S |
| Conv-std anterolateral | |||||||||||
| Kim [51] | RCT | 140 | 140 | 70 | 70 | 55.6 | 55.6 | 53/17 | 53/17 | MIS-mini-posterolateral | 26.4 months |
| Conv-std posterolateral | |||||||||||
| Kubeš et al. [54] | nRCT | 80 | 80 | 40 | 40 | 67 | 66.1 | 14/26 | 19/26 | MIS-mini anterolateral | 2 years |
| Conv-std anterolateral | |||||||||||
| Laffosse et al. [45] | nRCT | 100 | 100 | 42 | 58 | 57.4 | 59.7 | 24/18 | 33/25 | MIS-mini-anterolateral | 6 months |
| Conv-std posterior | |||||||||||
| Laffosse et al. [57] | nRCT | 110 | 116 | 58 | 58 | 55 | 59.7 | 35/23 | 33/25 | MIS-mini-posterior | 6 months |
| Conv-std posterolateral | |||||||||||
| Lawlor et al. [50] | RCT | 219 | 219 | 109 | 110 | 67.4 | 65.9 | 49/60 | 58/52 | MIS-mini-posterior ≤10 cm | 6 weeks |
| Conv-std posterior 16 cm | |||||||||||
| Leuchte et al. [53] | nRCT | 32 | 32 | 16 | 16 | 59.7 | 62.6 | N/S | N/S | MIS-mini anterolateral | 28 weeks |
| Conv-std lateral | |||||||||||
| Mow et al. [48] | nRCT | 32 | 34 | 20 | 14 | 59 | 63 | 13/6 | 7/6 | MIS-mini-posterior | 24 months |
| Conv-direct lateral | |||||||||||
| Ogonda et al. [35] | RCT | 219 | 219 | 109 | 110 | 67.4 | 65.9 | 49/60 | 58/52 | MIS-mini-posterior ≤10 cm | 6 weeks |
| Conv-std posterior 16 cm | |||||||||||
| Pflüger et al. [59] | nRCT | 100 | 100 | 50 | 50 | N/S | N/S | N/S | N/S | MIS-mini anterolateral | N/S |
| Conv-std anterolateral | |||||||||||
| Pospischill et al. [56] | RCT | 40 | 40 | 20 | 20 | 61.9 | 60.6 | 8/12 | 12/8 | MIS-mini-anterolateral approach | 12 weeks |
| Conv-std lateral approach | |||||||||||
| Rittmeister and Peters [52] | nRCT | 152 | 152 | 76 | 76 | 60 | 65 | 23/53 | 23/53 | MIS-mini-posterior | 4 days |
| Conv-anterolateral | |||||||||||
| Sculco et al. [23] | nRCT | 84 | 84 | 42 | 42 | 67.2 | 65.6 | 12/16 | 19/13 | MIS-mini-anterolateral | 5 years |
| Conv-N/S | |||||||||||
| Sculco et al. [23] - 2nd study | RCT | 60 | 60 | 28 | 32 | N/S | N/S | N/S | N/S | MIS-mini-anterolateral (8 cm) | Min 2 years |
| Conv-N/S (15 cm incision) | |||||||||||
| Shitama et al. PL [20] | RCT | 39 | 39 | 19 | 20 | 58.3 | 61.3 | N/S | N/S | MIS-mini-posterolateral | 6 months |
| Conv-posterolateral | |||||||||||
| Shitama et al. TL [20] | RCT | 23 | 23 | 15 | 8 | 61.7 | 53.4 | N/S | N/S | MIS-mini-translateral | 6 months |
| Conv-translateral | |||||||||||
| Speranza et al. [30] | RCT | 100 | 100 | 50 | 50 | 65 | 66.2 | 20/26 | 23/21 | MIS-mini-lateral (≤8 cm) | 6 months |
| Conv-std lateral (12-14 cm) | |||||||||||
| Szemdrõi et al. [55] | nRCT | 59 | 59 | 38 | 21 | 64 | 57 | N/S | N/S | MIS-mini-lateral (<10 cm) | 3 months |
| Conv-std lateral (>14 cm) | |||||||||||
| Vicente et al. [1] | nRCT | 76 | 76 | 34 | 42 | 50 | 57 | 21/13 | 26/16 | MIS-mini posterior <11 cm | 6 months |
| Conv-direct lateral | |||||||||||
| Wenz et al. [47] | nRCT | 173 | 189 | 124 | 65 | 63 | 65 | 60/64 | 22/43 | MIS-mini-posterior | N/S |
| Conv-direct lateral | |||||||||||
| Wohlrab et al. [58] | nRCT | 50 | 50 | 27 | 23 | 58.8 | 61.9 | 11/26 | 11/12 | MIS-mini posterior | 3 months |
| Conv-std lateral | |||||||||||
| Woolson et al. [30] | nRCT | 135 | 135 | 50 | 85 | 60 | 63 | 29/21 | 31/54 | MIS-mini-posterior | Min 6 months |
| Conv-posterior | |||||||||||
| Wright et al. [9] | nRCT | 84 | 84 | 42 | 42 | 65.0 | 64.2 | N/S | N/S | MIS-mini-posterolateral | 5 years |
| Conv-posterolateral | |||||||||||
| Yang et al. [49] | RCT | 110 | 110 | 55 | 55 | 59 | 56 | 26/29 | 30/25 | MIS-mini-anterolateral | 3 years |
| Conv-posterolateral | |||||||||||
THA total hip arthroplasty, Conv conventional surgery, f females, m males, Min minimum, MIS minimally invasive surgery, nRCT non-randomised controlled trial, N/S not stated, PL posterolateral approach, RCT randomised controlled trial, TL translateral approach
The most commonly used THA MIS approach was the mini-posterior performed in 12 studies. The most commonly adopted conventional THA approach was the standard posterior approach used in seven studies. Computer navigation surgery was performed in some cases in one study [21]. Follow-up period ranged from five days [22] to five years [9, 23].
Primary outcome analysis
There was no significant difference in HHS recorded for the MIS compared to the conventional exposure THA (MD 1.49; 95% CI −0.08, 3.06; p = 0.06; Fig. 3). A difference of less than 2 points would also not be considered a clinically significant difference [24, 25].
Fig. 3.
Forest plot to illustrate mean difference in Harris hips score between minimally invasive surgery (MIS) and conventional total hip arthroplasty (THA) procedures
Secondary outcome analysis
Clinical outcomes
As anticipated there was a significantly smaller surgical incision length following MIS compared to conventional THA with a mean difference of 8.0 cm (p < 0.0001; Table 3). There was however no statistically significant difference in surgical duration between MIS and conventional exposure (MD 4.65 minutes; 95% CI −9.45, 0.15; p = 0.06). Whilst there was statistically less perioperative blood loss in the MIS group compared to conventional THA (p < 0.001; Table 3), there was no statistically significant difference between the groups in respect to drained postoperative blood loss, total blood loss or requirement for blood transfusion (p > 0.05; Table 3).
Table 3.
Meta-analysis results of clinical outcomes
| Outcome | Groups (n) | Studies (n) | Overall effect | |||
|---|---|---|---|---|---|---|
| MIS | Conv | Effect estimate | 95% CI | p-value | ||
| Incision length | 869 | 803 | 17 | −7.56 | −8.17, −6.95 | <0.0001 |
| Intraoperative blood loss | 621 | 656 | 12 | −42.44 | −60.14, −24.73 | <0.0001 |
| Length of stay | 840 | 856 | 15 | −0.59 | −1.07, −0.12 | 0.01 |
| VAS paina | 359 | 339 | 7 | −0.55 | −0.97, −0.13 | 0.01 |
| Harris hip score | 784 | 797 | 17 | 1.49 | 0.08, 3.06 | 0.06 |
| Surgical duration | 1077 | 1066 | 22 | −4.65 | −9.45, 0.15 | 0.06 |
| Required blood transfusion | 95 | 133 | 7 | 0.75 | −0.56, 1.02 | 0.06 |
| WOMAC score | 402 | 419 | 6 | 2.55 | −0.75, 5.84 | 0.13 |
| Blood loss in drain | 200 | 195 | 6 | −53.46 | −133.55, 26.62 | 0.19 |
| Oxford hip score | 169 | 170 | 2 | −0.92 | −2.62, 0.77 | 0.29 |
| Total blood loss | 738 | 701 | 15 | −43.09 | −135.79, 49.62 | 0.36 |
MIS minimally invasive surgery, Conv conventional surgery, CI confidence interval
aStandardised mean difference
There was no statistically significant difference between the exposure method in respect to WOMAC score (p = 0.13) or OHS (p = 0.29). Although patients who underwent MIS reported lower pain scores on visual analogue scale (VAS) assessment (MD = 0.58; p = 0.02) and a shorter hospital length of stay (MD = 0.59; p = 0.01), these differences were not clinically substantial between the groups.
Radiological outcomes
As Table 4 demonstrates, there was no statistically significant difference between the MIS or conventional THA exposure methods with respect to any radiological measurement recorded in this meta-analysis.
Table 4.
Meta-analysis results of radiological outcomes
| Outcome | Groups (n) | Studies (n) | Overall effect | |||
|---|---|---|---|---|---|---|
| MIS | Conv | Effect estimate | 95% CI | p-value | ||
| Femoral offset | 150 | 150 | 3 | 0.62 | −0.77, 2.01 | 0.38 |
| Leg-length discrepancy | 325 | 300 | 5 | −0.09 | −0.32, 0.43 | 0.42 |
| Femoral positioning (0–5° valgus) | 258 | 177 | 4 | 0.57 | 0.11, 2.85 | 0.49 |
| Cup positioning (35–50° valgus) | 266 | 311 | 5 | 0.82 | 0.42, 1.59 | 0.55 |
| Cup inclination angle | 750 | 730 | 12 | 0.24 | −1.51, 2.00 | 0.79 |
| Stem alignment (varus/valgus) | 314 | 331 | 5 | 0.03 | −0.38, 0.43 | 0.90 |
MIS minimally invasive surgery, Conv conventional surgery, CI confidence interval
Complications
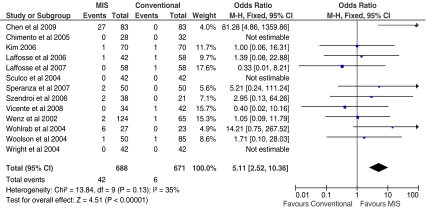
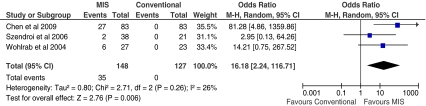
There were no statistically significant differences between the exposure methods during THA for complications such as infection rates, intra- or postoperative fracture, dislocation rate, DVT, haematoma formation, wound complications or component loosening. There was however a statically significant difference in respect to iatrogenic nerve palsy with a five times greater rate of nerve palsy following MIS surgery compared to conventional THA (p < 0.0001; Fig. 4). When assessed individually, the risk of transient lateral femoral cutaneous nerve palsy was significantly higher following MIS (RR = 16.2; p = 0.006; Fig. 5); however, this finding was weighted by a high proportion of cases reported in a cohort study by Chen et al. [26]. There was no statistically significant difference between the groups with respect to the incidence of sciatic nerve palsy (p = 0.11; Table 5).
Fig. 4.
Forest plot to illustrate odds ratio for incidence of iatrogenic nerve injury between minimally invasive surgical (MIS) and conventional total hip arthroplasty (THA) procedures
Fig. 5.
Forest plot to illustrate odds ratio for incidence of transient lateral femoral cutaneous nerve injury between minimally invasive surgical (MIS) and conventional total hip arthroplasty (THA) procedures
Table 5.
Meta-analysis results of complications
| Outcome | Groups (n) | Studies (n) | Overall effect | |||
|---|---|---|---|---|---|---|
| MIS | Conv | Effect estimate | 95% CI | p-value | ||
| Iatrogenic nerve palsy | 650 | 650 | 13 | 5.27 | 2.55, 10.91 | <0.0001 |
| Transient lateral femoral cutaneous nerve injury | 148 | 127 | 3 | 16.18 | 2.24, 116.71 | 0.006 |
| Haematoma formation | 270 | 201 | 4 | 2.48 | 0.85, 7.21 | 0.09 |
| Sciatic nerve palsy | 160 | 195 | 3 | 4.38 | 0.70, 27.20 | 0.11 |
| Wound complication | 112 | 141 | 3 | 2.99 | 0.62, 14.35 | 0.17 |
| Deep vein thrombosis | 529 | 509 | 9 | 0.49 | 0.18, 1.35 | 0.17 |
| Dislocation | 929 | 918 | 16 | 0.65 | 0.33, 1.26 | 0.20 |
| Intraoperative fracture | 563 | 568 | 10 | 1.52 | 0.76, 3.04 | 0.23 |
| Heterotrophic ossification | 85 | 85 | 2 | 0.32 | 0.05, 2.10 | 0.24 |
| Acetabular component loosening | 316 | 324 | 6 | 2.40 | 0.52, 10.98 | 0.26 |
| Periprosthetic fracture | 212 | 223 | 4 | 0.67 | 0.27, 1.69 | 0.40 |
| Required revision surgery | 230 | 250 | 5 | 0.72 | 0.15, 3.50 | 0.68 |
| Deep infection | 594 | 540 | 9 | 0.82 | 0.22, 2.97 | 0.76 |
| Superficial wound infection | 620 | 609 | 10 | l1.08 | 0.35, 3.37 | 0.89 |
| Femoral component loosening | 222 | 222 | NE | NE | NE | NE |
MIS minimally invasive surgery, Conv conventional surgery, CI confidence interval, NE not estimatable
Discussion
The findings of this review of the current evidence base suggest that MIS THA results in a significantly increased risk of lateral femoral cutaneous nerve palsy. There was no clinically significant reduction in total blood loss or hip scores at final follow-up with no difference in radiological outcomes at final review compared to a conventional approach. Whilst hospital stay and pain scores were lower in the MIS group, this was not a clinically significant difference.
The PEDro appraisal identified a number of methodological limitations to the current evidence base. These were largely cited as poor concealment of randomisation, permitting selection and allocation bias, not blinding patients and assessors to their surgical exposure, allowing further expectation and assessor bias, and not recruiting sample sizes based on an appropriate power calculation, allowing the potential for type II statistical error from impacting on the findings of these clinical studies [27, 28]. Accordingly, whilst the findings of this meta-analysis should be considered as appropriate, based on the best available literature, these methodological shortcomings should be considered when interpreting the findings.
A major finding reported by the overall meta-analysis was the significantly greater risk of iatrogenic nerve injury during MIS compared to conventional procedures. One suggestion for this is related to retractor position. Yoon et al. [29] suggested that femoral nerve palsy, for instance, may be associated with retractor position [29]. The anterior retractor should be underneath the rectus femoris muscle to prevent this. Similarly, reduced operative visibility may increase the potential for nerve injury due to the added difficulty in identifying nerves during dissection.
Although not included in this meta-analysis, Woolson et al. [30] reported acetabular and femoral prostheses were more frequently mal-positioned in MIS compared to conventional approaches. Similarly, they reported a significantly higher percentage of cementless stems in the MIS cohort had a poor fit and fill with less than 2 mm between the distal portion of the stem and the femoral cortex (p = 0.004). They related this to the reduced visualisation of the acetabulum and proximal femur from the small incision. Given the findings of this study, such issues in implant positioning do not seem to be supported by the literature. Nonetheless, since we were unable to distinguish the results between experienced and in-experienced MIS surgeons, it remains unclear whether this factor was important when generalising this complication to general clinical practice. Furthermore, since the longest follow-up period documented was five years [9, 23], it remains unclear whether the affect of implant positioning has any longer-term effect on prosthesis survival. Future surveillance studies of longer follow-up will enlighten as to whether this is a potential feature of MIS THA procedures.
Although this meta-analysis reported no statistically significant difference between surgical exposure method and wound healing complications (p = 0.17), the effect size was substantial between the groups with nearly a three times greater risk following MIS compared to conventional THA in the overall analysis. As Table 5 demonstrates, this outcome was measured in a small number of subjects. Accordingly, this conclusion may be attributed to type II statistical error [28]. Such an effect size for this outcome may be attributed to the extensive use of retractors in MIS procedures. Noble et al. [31] reported that during MIS THA, large pressures can develop between the retractors and the wound edges, predisposing to wound healing complications. They recommended that surgeons should consider using the largest possible incision within the realms of MIS principles, and that the precise anatomical placement should be carefully considered to minimise the duration of tissue compression whilst ensuring adequate visualisation of the surgical field [31].
There is a lack of consensus over the actual definition of MIS and the relationship between skin incision and soft tissue trauma. Speranza et al. [32] and Procyk [33] suggested that the ideal MIS is that of a procedure which has little tissue disruption without cutting muscles and tendons and therefore less pain to provide a significantly shorter rehabilitation with longer-term outcomes which are equal or better to a conventional approach. Accordingly, the little difference in outcomes reported in this meta-analysis may be attributed to similarities in the operative procedure after the skin incision for traditional and MIS procedures.
An increase in perioperative cytokine level has been demonstrated to correlate with surgical trauma [34]. Both Ogonda et al. [35] and Chimento et al. [6] reported no significant difference between the minimally invasive and conventional THA exposure for cytokine level suggesting that whilst the skin incision may be reduced, tissue trauma is similar between the groups for this procedure. Nonetheless, these findings may however be dependent on the surgical approach adopted and the degree of soft tissue dissection. For instance, in a mini-posterior if the femoral head can be excised without dislocating the hip, there will be less trauma to muscles and capsule unlike the anterolateral approach [36, 37]. Further assessment of surgical approach and dissection is therefore warranted.
Whilst we did not assess the difference in outcomes between the different types of MIS, as Table 2 demonstrated a number of different surgical approaches were adopted under the term ‘minimally invasive’. Further study is required to determine whether there is a difference in outcomes between the different MIS procedures used during THA. Nonetheless, theoretically, this factor may be associated with different outcomes and, in particular, the incidence of iatrogenic nerve injury. The anterior approach (as part of the Smith-Peterson approach) may be associated with lateral femoral cutaneous nerve injury which was 17 times more likely to occur with MIS compared to standard incision surgery [29]. Given this important complication, further study is recommended to compare the outcomes of different MIS procedures undertaken in THA surgery to determine the efficacy of each approach taken.
Surgical learning curve is an important variable which may have accounted for the differences in surgical duration between the groups [30, 38–40]. Desser et al. [41] and Pagnano et al. [42] reported that the MIS technique was more difficult than the conventional exposure method, but that complication rates would be expected to decrease with surgical experience [41, 43]. Whilst some authors have reported a low complication rate such as Berry et al. [13] of 2% with four experienced surgeons, others have reported much higher rates such as Pagnano et al. [42] conversely with 14%, which was attributed to surgical experience and the existence of a learning curve [13, 43]. Goosen et al. [44] concluded that relatively inexperienced surgeons should consider carefully the advantages and disadvantages of MIS procedures before adopting such an approach given the long learning curve. Furthermore, Sculco et al. [23] suggested that the posterior approach may be the most appropriate approach to adopt since it is familiar to most surgeons and still allows the easy extension of the wound if operative visibility is insufficient [23, 45].
When considering its application in clinical practice, previous authors have suggested that not all patients are suitable candidates for MIS THA procedures [41]. Those patients who are thin and young, and with a lower risk of peri- or postoperative complication would be most suitable for MIS [46]. However, as Desser et al. [41] commented, these characteristics may not be reflective of the average hip surgeon’s caseload. Sculco et al. [23] suggested that patients with a body mass index greater than 30 should not be considered for MIS due to the difficulty in identifying anatomical landmarks during surgery. Howell et al. [38] also suggested that patients with excessively stiff hips, those with severe dysplasia requiring larger visualisation to manage the distorted acetabular anatomy and correct any femoral shortening or derotation, and those with a marked distortion of the proximal femur may also be unsuitable for MIS THA. For such larger patients a secondary incision may be required distally to allow adequate acetabular reaming [47].
Advocates of the MIS technique have suggested that the smaller skin incision provides improved cosmesis and increased patient satisfaction. Only one study has previously assessed the outcomes of scar cosmesis following conventional or MIS THA [48]. Whilst all patients considered their scars acceptable in appearance, when reviewed by plastic surgeons masked to surgical procedure, the cosmesis of the mini-posterior approach was more frequently reported as poorer than the standard posterior approach. Mow et al. [48] attribute these findings to skin and soft tissue damage caused by the high retractor pressure required for the MIS exposure.
Conclusions
The findings of this study indicate whilst there is little difference in the clinical or radiological outcomes of MIS to conventional THA, MIS procedures pose a significantly increased risk of transient lateral femoral cutaneous nerve palsy than traditional techniques.
Acknowledgements
We thank the library staff at the Norfolk and Norwich University Hospital’s Sir Thomas Browne Library for their assistance in gathering the articles used in this review. We would also like to thank Professor Young-Hoo Kim, MokDong Hospital, Seoul, Korea; Dr J Ricardo Negreiros Vicente, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil; Dr Thomas P. Sculco, Hospital for Special Surgery, New York, USA; Professor Qingsheng Zhu, Xijing Hospital, China; Marie Lawlor, Musgrave Park Hospital, Belfast, Northern Ireland; Mr Lawrence Dorr, Arthritis Institute, Inglewood, California, USA; and Professor Steve Woolson, Stanford University Hospital, Stanford, California, USA, for providing additional data used as part of the meta-analysis and for reviewing the results of our search strategy.
Conflict of interest The authors declare that they have no conflict of interest
Funding No funds were received to undertake or relating to this study.
Ethical approval Ethical approval was not required for this study design.
References
- 1.Vicente JRN, Croci AT, Camargo OP. Blood loss in the minimally invasive posterior approach to total hip arthroplasty: a comparative study. Clinics. 2008;63:351–356. doi: 10.1590/S1807-59322008000300011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore AT. Metal hip joint; a new self-locking vitallium prosthesis. S Med J. 1952;45:1015–1019. doi: 10.1097/00007611-195211000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Hardinge K. The direct lateral approach to the hip. J Bone Joint Surg. 1982;64-B:17–19. doi: 10.1302/0301-620X.64B1.7068713. [DOI] [PubMed] [Google Scholar]
- 4.Beger RA, Jacobs JJ, Meneghini RM, et al. Rapid rehabilitation and recovery with minimally invasive total hip arthroplatsty. Clin Orthop Relat Res. 2004;429:239–247. doi: 10.1097/01.blo.0000150127.80647.80. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein WM, Bransson JJ, Berland KA, Gordon AC. Minimal-incision total hip arthroplasty. J Bone Joint Surg. 2003;85-A(Suppl 4):33–38. doi: 10.2106/00004623-200300004-00004. [DOI] [PubMed] [Google Scholar]
- 6.Chimento GF, Pavone V, Sharrock N, Kahn B, Cahill J, Sculco TP. Minimally invasive total hip arthroplasty: a prospective randomised study. J Arthroplasty. 2005;20:139–144. doi: 10.1016/j.arth.2004.09.061. [DOI] [PubMed] [Google Scholar]
- 7.Inaba Y, Dorr LD, Wan Z, Sirianni L, Boutary M. Operative and patient care techniques for posterior mini-incision total hip arthroplasty. Clin Orthop Relat Res. 2005;441:104–114. doi: 10.1097/01.blo.0000193811.23706.3a. [DOI] [PubMed] [Google Scholar]
- 8.Dorr LD, Long WT, Inaba Y, Sirianni LE, Boutary M. MIS total hip replacement with a single posterior approach. Semin Arthroplasty. 2005;16:179–185. doi: 10.1053/j.sart.2005.10.003. [DOI] [Google Scholar]
- 9.Wright JM, Crockett HC, Delgado S, et al. Mini-incision for total hip arthroplasty. A prospective, controlled investigation with 5-year follow-up evaluation. J Arthroplasty. 2004;19:538–545. doi: 10.1016/j.arth.2003.12.070. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura S, Matsuda K, Arai N, Wakimoto N, Matsushita T. Mini-incision posterior approach for total hip arthroplasty. Int Orthop. 2004;28:214–217. doi: 10.1007/s00264-004-0570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartzband MA. Posterolateral minimal incision for total hip replacement: technique and early results. Orthop Clin N Am. 2004;34:119–129. doi: 10.1016/S0030-5898(03)00119-6. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien DA, Rorabeck CH. The mini-incision direct lateral approach in primary total hip arthroplasty. Clin Orthop Relat Res. 2005;441:99–103. doi: 10.1097/01.blo.0000193812.31329.3a. [DOI] [PubMed] [Google Scholar]
- 13.Berry DJ, Berger RA, Callaghan JJ, et al. Minimally invasive total hip arthroplasty. Development, early results and a critical analysis. J Bone Joint Surg. 2003;85-A:2235–2246. [PubMed] [Google Scholar]
- 14.Mardones R, Pagnano MW, Nemanich JP, Trousdale RT. Muscle damage after total hip arthroplasty done with the two-incision and mini-posterior techniques. Clin Orthop Relat Res. 2005;441:80–85. doi: 10.1097/01.blo.0000194727.55372.04. [DOI] [PubMed] [Google Scholar]
- 15.Cheng T, Feng JG, Liu T, Zhang XL. Minimally invasive total hip arthroplasty: a systematic review. Int Orthop. 2009;33:1473–1481. doi: 10.1007/s00264-009-0743-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foley NC, Bhogal SK, Teasell RW, Bureau Y, Speechley MR. Estimates of quality and reliability with the physiotherapy evidence-based database scale to assess the methodology of randomized controlled trials of pharmacological and nonpharmacological interventions. Phys Ther. 2006;86:817–824. [PubMed] [Google Scholar]
- 17.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–721. [PubMed] [Google Scholar]
- 18.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 19.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shitama T, Kiyama T, Naito M, Shiramizu K, Huang G. Which is more invasive-mini versus standard incisions in total hip arthroplasty? Int Orthop. 2009;33:1543–1547. doi: 10.1007/s00264-008-0708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorr LD, Maheshwari AV, Long WT, Wan Z, Sirianni LE. Early pain relief and function after posterior minimally invasive and conventional total hip arthroplasty. A prospective, randomized, blinded study. J Bone Joint Surg. 2007;89:1153–1160. doi: 10.2106/JBJS.F.00940. [DOI] [PubMed] [Google Scholar]
- 22.Bennett D, Ogonda L, Elliott D, Humphreys L, Lawlor M, Beverland D. Comparison of immediate postoperative walking ability in patients receiving minimally invasive and standard-incision hip arthroplasty. A prospective blinded study. J Arthroplasty. 2007;22:490–495. doi: 10.1016/j.arth.2006.02.173. [DOI] [PubMed] [Google Scholar]
- 23.Sculco TP, Jordan LC, Walter WL. Minimally invasive total hip arthroplasty: the Hospital for Special Surgery experience. Orthop Clin N Am. 2004;35:137–142. doi: 10.1016/S0030-5898(03)00116-0. [DOI] [PubMed] [Google Scholar]
- 24.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg. 1969;51-A:737–755. [PubMed] [Google Scholar]
- 25.Marchetti P, Binazzi R, Vaccari V, et al. Long-term results with cementless Fitek (or Fitmore) cups. J Arthroplasty. 2005;20:730–737. doi: 10.1016/j.arth.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Chen DW, Hu CC, Chang YH, Yang WE, Lee MS. Comparison of clinical outcome in primary total hip arthroplasty by conventional anterolateral transgluteal or 2-incision approach. J Arthroplasty. 2009;24:528–532. doi: 10.1016/j.arth.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Friedman LF, Furberg CD, DeMets DL. Fundamentals of clinical trials. 3. New York, USA: Springer; 1998. [Google Scholar]
- 28.Bland M. An introduction to medical statistics. 3. Oxford: Oxford University Press; 2006. [Google Scholar]
- 29.Yoon TR, Park KS, Song EK, Seon JK, Seo HY. New two-incision minimally invasive total hip arthroplasty: comparison with the one-incision method. J Orthop Sci. 2009;14:155–160. doi: 10.1007/s00776-008-1305-8. [DOI] [PubMed] [Google Scholar]
- 30.Woolson ST, Mow CS, Syquia JF, Lannin JV, Schurman DJ. Comparison of primary total hip replacements performed with a standard incision or a mini-incision. J Bone Joint Surg. 2004;86:1353–1358. doi: 10.2106/00004623-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Noble PC, Johnston JD, Alexander JA, et al. Making minimally invasive THR safe: conclusions from biomechanical simulation and analysis. Int Orthop. 2007;31(Suppl 1):S25–28. doi: 10.1007/s00264-007-0432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Speranza A, Iorio R, Ferretti M, D’Arrigo C, Ferretti A. A lateral minimal-incision technique in total hip replacement: a prospective, randomised, controlled trial. Hip Int. 2007;17:4–8. doi: 10.1177/112070000701700102. [DOI] [PubMed] [Google Scholar]
- 33.Procyk S. Initial results with a mini-posterior approach for total hip arthroplasty. Int Orthop. 2007;31(Suppl 1):S17–S20. doi: 10.1007/s00264-007-0435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakamoto K, Arakawa H, Mita S, et al. Elevation of circulating interleukin 6 after surgery: factors influencing the serum level. Cytokine. 1994;6:181–186. doi: 10.1016/1043-4666(94)90040-X. [DOI] [PubMed] [Google Scholar]
- 35.Ogonda L, Wilson R, Archbold P, et al. A minimal-incision technique in total hip arthroplasty does not improve early postoperative outcomes. A prospective, randomized, controlled trial. J Bone Joint Surg. 2005;87:701–710. doi: 10.2106/JBJS.D.02645. [DOI] [PubMed] [Google Scholar]
- 36.Laffosse JM, Chiron P, Molinier F, Bensafi H, Puget J. Prospective and comparative study of the anterolateral mini-invasive approach versus minimally invasive posterior approach for primary total hip replacement. Early results. Int Orthop. 2007;31:597–603. doi: 10.1007/s00264-006-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jerosch J, Theising C, Fadel ME. Antero-lateral minimal invasive (ALMI) approach for total hip arthroplasty technique and early results. Arch Orthop Trauma Surg. 2006;126:164–173. doi: 10.1007/s00402-006-0113-x. [DOI] [PubMed] [Google Scholar]
- 38.Howell JR, Masri BA, Duncan CP. Minimally invasive versus standard incision anterolateral hip replacement: a comparative study. Orthop Clin N Am. 2004;35:153–162. doi: 10.1016/S0030-5898(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 39.Archibeck MJ, White RE., Jr Learning curve for the two-incision total hip replacement. Clin Orthop Relat Res. 2004;429:232–238. doi: 10.1097/01.blo.0000150272.75831.2f. [DOI] [PubMed] [Google Scholar]
- 40.Swanson TV. Posterior single-incision approach to minimally invasive total hip arthroplasty. Int Orthop. 2007;31(Suppl 1):S1–S5. doi: 10.1007/s00264-007-0436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desser DR, Mitrick MF, Ulrich SD, Delanois RE, Mont MA. Total hip arthroplasty: comparison of two-incision and standard techniques at an AOA-accredited community hospital. J Am Osteopath Assoc. 2010;110:12–15. [PubMed] [Google Scholar]
- 42.Pagnano MW, Leone J, Lewallen DG, Hanssen AD. Two-incision THA had modest outcomes and some substantial complications. Clin Orthop Relat Res. 2005;441:86–91. doi: 10.1097/01.blo.0000191275.80527.d6. [DOI] [PubMed] [Google Scholar]
- 43.Bal BS, Haltorn D, Aleto T, Barrett M. Early complications of primary total hip replacement performed with a two-incision minimally invasive technique. J Bone Joint Surg. 2005;87-A:2432–2438. doi: 10.2106/JBJS.D.02847. [DOI] [PubMed] [Google Scholar]
- 44.Goosen JH, Kollen BJ, Castelein RM, Kuipers BM, Verheyen CC (2010) Minimally invasive versus classic procedures in total hip arthroplasty: a double-blind randomized controlled trial. Clin Orthop Relat Res. March 30 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 45.Laffosse JM, Chiron P, Accadbled F, Molinier F, Tricoire JL, Puget J. Learning curve for a modified Watson-Jones minimally invasive approach in primary total hip replacement: Analysis of complications and early results versus the standard-incision posterior approach. Acta Orthop Belg. 2006;72:693–701. [PubMed] [Google Scholar]
- 46.Berry DJ, Berger RA, Callaghan JJ, et al. Minimally invasive total hip arthroplasty. Development, early results and a critical analysis. J Bone Joint Surg. 2005;85-A:2235–2245. [PubMed] [Google Scholar]
- 47.Wenz JF, Gurkan I, Jibodh SR. Mini-incision total hip arthroplasty: a comparative assessment of perioperative outcomes. Orthopedics. 2002;25:1031–1043. doi: 10.3928/0147-7447-20021001-14. [DOI] [PubMed] [Google Scholar]
- 48.Mow CS, Woolson ST, Ngarmukos SG, Park EH, Lorenz HP. Comparison of scars from total hip replacements done with a standard or a mini-incision. Clin Orthop Relat Res. 2005;441:80–85. doi: 10.1097/01.blo.0000191317.85422.c3. [DOI] [PubMed] [Google Scholar]
- 49.Yang C, Zhu Q, Han Y et al (2009) Minimally-invasive total hip arthroplasty will improve early postoperative outcomes: a prospective, randomized controlled trial. Ir J Med Sci 179(2):285–289 [DOI] [PubMed]
- 50.Lawlor M, Humphreys P, Morrow E, et al. Comparison of early postoperative functional levels following total hip replacement using minimally invasive versus standard incisions. A prospective randomized blinded trial. Clin Rehabil. 2005;19:465–474. doi: 10.1191/0269215505cr890oa. [DOI] [PubMed] [Google Scholar]
- 51.Kim YH. Comparison of primary total hip arthroplasties performed with a minimally invasive technique or a standard technique. A prospective and randomized study. J Arthroplasty. 2006;21:1092–1098. doi: 10.1016/j.arth.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 52.Rittmeister M, Peters A. Vergleich des hüftgelenkersatzes über eine posteriore miniinzision oder einen klassischen anterolateralen zugang. Orthopade. 2006;35:716–722. doi: 10.1007/s00132-006-0963-5. [DOI] [PubMed] [Google Scholar]
- 53.Leuchte S, Luchs A, Wohlrad D. Measurement of ground reaction forces after total hip arthroplasty using different surgical approaches. Z Orthop. 2007;145:74–80. doi: 10.1055/s-2007-960511. [DOI] [PubMed] [Google Scholar]
- 54.Kubeš J, Landor I, Podškubka A, Majernícek M, Vcelák J. Total hip replacement from a MIS-AL approach (comparison with a standard anterolateral approach) Acta Chir Orthop Traumatol Cech. 2009;76:288–294. [PubMed] [Google Scholar]
- 55.Szendrõi M, Sztrinkai G, Vass R, Kiss J. The impact of minimally invasive total hip arthroplasty on the standard procedure. Int Orthop. 2006;30:167–171. doi: 10.1007/s00264-005-0049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pospischill M, Kranzl A, Attwenger B, Knahr K. Minimally invasive compared with traditional transgluteal approach for total hip arthroplasty: a comparative gait analysis. J Bone Joint Surg. 2010;92-A:328–337. doi: 10.2106/JBJS.H.01086. [DOI] [PubMed] [Google Scholar]
- 57.Laffosse JM, Chiron P, Tricoire JL, Giordano G, Molinier F, Puget J. Prospective and comparative study of minimally invasive posterior approach versus standard posterior approach in total hip replacement. Rev Chir Orthop Reparatrice Appar Mot. 2007;93:228–237. doi: 10.1016/s0035-1040(07)90244-5. [DOI] [PubMed] [Google Scholar]
- 58.Wohlrab D, Hagel A, Hein W. Advantages of minimal invasive total hip replacement in the early phase of rehabilitation. Z Orthop. 2004;142:685–690. doi: 10.1055/s-2004-832447. [DOI] [PubMed] [Google Scholar]
- 59.Pflüger G, Junk-Jantsch S, Schöll V. Minimally invasive total hip replacement via the anterolateral approach in the supine position. Int Orthop. 2007;31(Suppl1):S7–S11. doi: 10.1007/s00264-007-0434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]