Abstract
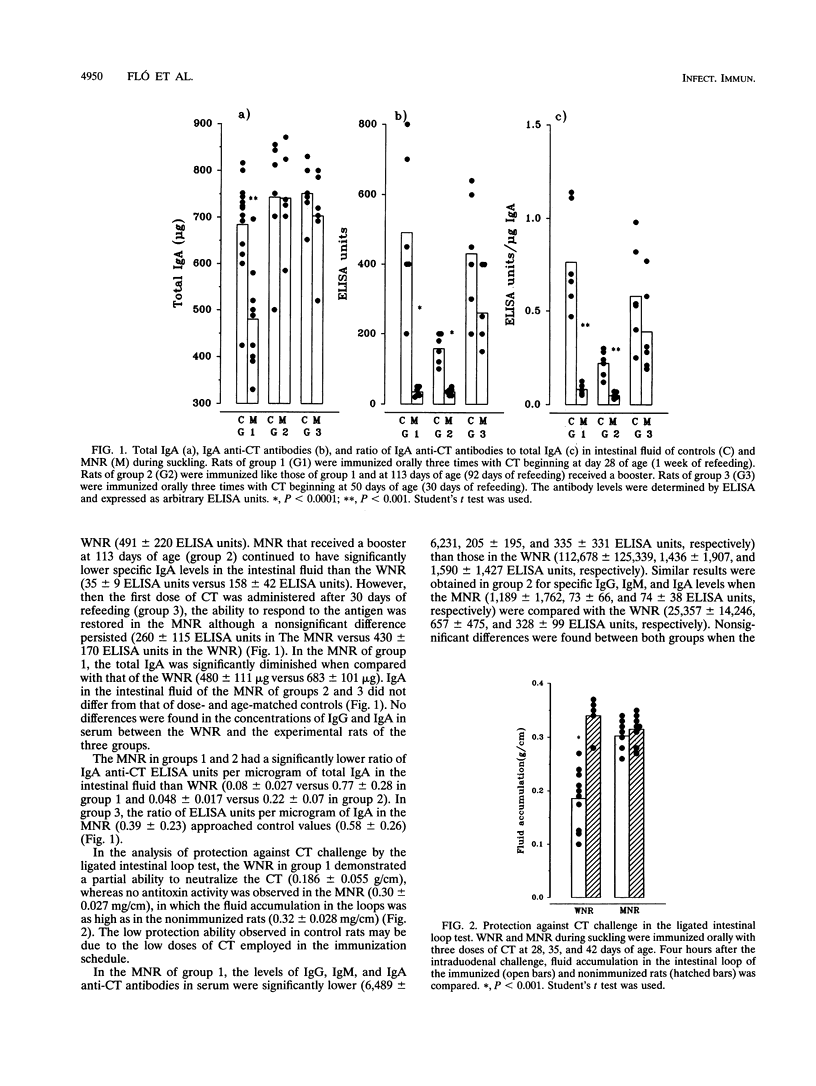
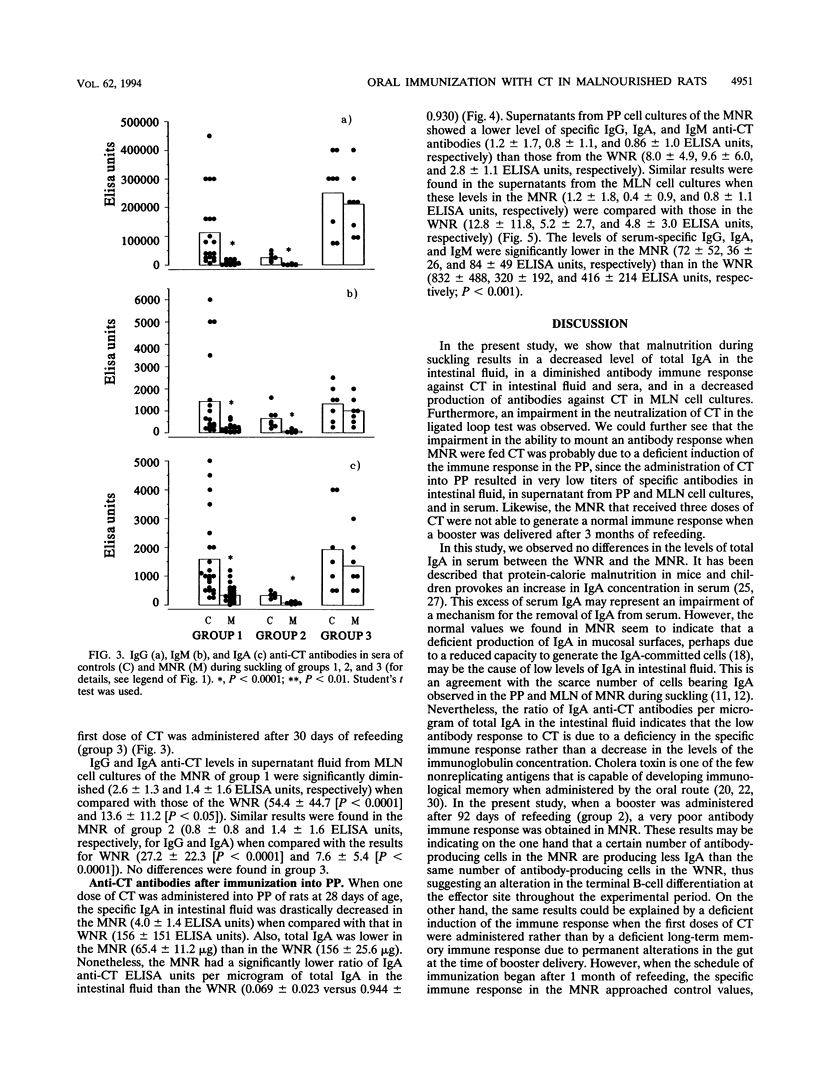
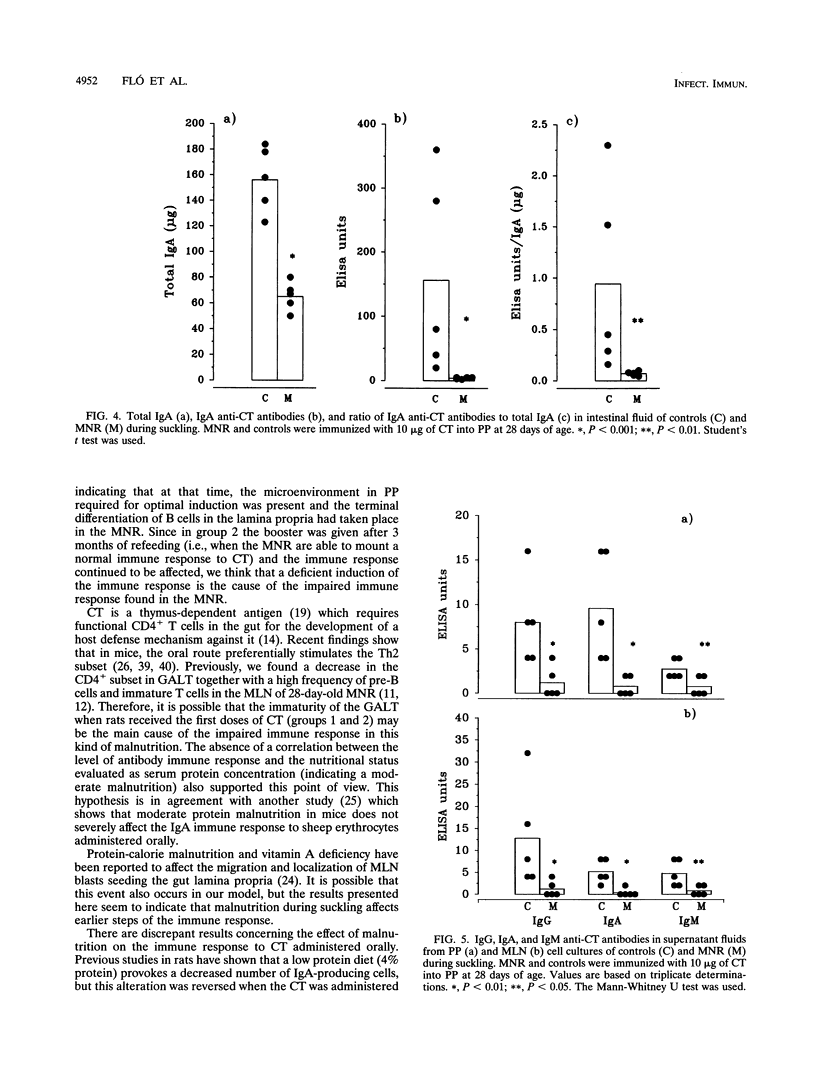
Malnourished rats during suckling were orally immunized with cholera toxin (CT) after different periods of refeeding. Intestinal fluids, sera, and supernatant fluids from cultured mesenteric lymph node (MLN) cells were obtained after rats were given three doses of CT and analyzed by enzyme-linked immunosorbent assay (ELISA) to evaluate the specific antibody response. Serum-specific immunoglobulin G (IgG), IgA, and IgM were severely diminished in malnourished rats immunized with three doses of CT after 1 week of refeeding when compared with those of controls. Also, a decreased IgA ELISA titer of the intestinal fluids and abrogation of the capacity to neutralize the CT in the intestinal ligated loop test were found. When a booster was given at 113 days of age, the immune response continued to be affected in the serum and the intestinal fluid. The results from the analysis of the supernatant fluids from cultured MLN cells were coincident with those mentioned above. When one dose of CT was administered into Peyer's patches (PP) after 1 week of refeeding, an impaired immune response was found in the intestinal fluid of malnourished rats during suckling compared with that of controls. This result together with the analysis of supernatant from MLN and PP cell cultures suggests that antigen triggering in the PP was affected. When the refeeding period was extended to 30 days and then the first dose of CT was administered, the antibody immune responses in intestinal fluid serum and supernatant fluid approached control values. These observations reinforce the fact that the gut-associated lymphoid tissue immaturity of the rats when they received the first CT dose (at 28 days old) was the main reason for the decreased immune response observed in the experimental group.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry W. S., Pierce N. F. Protein deprivation causes reversible impariment of mucosal immune response to cholera toxoid/toxin in rat gut. Nature. 1979 Sep 6;281(5726):64–65. doi: 10.1038/281064a0. [DOI] [PubMed] [Google Scholar]
- Betancourt M., Ortíz R., González C. Proliferation index in bone marrow cells from severely malnourished rats during lactation. Mutat Res. 1992 Nov;283(3):173–177. doi: 10.1016/0165-7992(92)90104-p. [DOI] [PubMed] [Google Scholar]
- Chandra R. K. Antibody formation in first and second generation offspring of nutritionally deprived rats. Science. 1975 Oct 17;190(4211):289–290. doi: 10.1126/science.1179211. [DOI] [PubMed] [Google Scholar]
- Chandra R. K. Mucosal immune responses in malnutrition. Ann N Y Acad Sci. 1983 Jun 30;409:345–352. doi: 10.1111/j.1749-6632.1983.tb26882.x. [DOI] [PubMed] [Google Scholar]
- Chandra R. K. Reduced secretory antibody response to live attenuated measles and poliovirus vaccines in malnourished children. Br Med J. 1975 Jun 14;2(5971):583–585. doi: 10.1136/bmj.2.5971.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christadoss P., Talal N., Lindstrom J., Fernandes G. Suppression of cellular and humoral immunity to T-dependent antigens by calorie restriction. Cell Immunol. 1984 Oct 1;88(1):1–8. doi: 10.1016/0008-8749(84)90046-7. [DOI] [PubMed] [Google Scholar]
- Elson C. O. Cholera toxin as a mucosal adjuvant: effects of H-2 major histocompatibility complex and lps genes. Infect Immun. 1992 Jul;60(7):2874–2879. doi: 10.1128/iai.60.7.2874-2879.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Ealding W., Lefkowitz J. A lavage technique allowing repeated measurement of IgA antibody in mouse intestinal secretions. J Immunol Methods. 1984 Feb 24;67(1):101–108. doi: 10.1016/0022-1759(84)90089-9. [DOI] [PubMed] [Google Scholar]
- Fló J., Massouh E. J., Roux M. E. Long term effects of malnutrition during lactation on GALT. Reg Immunol. 1993 Mar-Apr;5(2):100–105. [PubMed] [Google Scholar]
- Glass R. I., Svennerholm A. M., Stoll B. J., Khan M. R., Huda S., Huq M. I., Holmgren J. Effects of undernutrition on infection with Vibrio cholerae O1 and on response to oral cholera vaccine. Pediatr Infect Dis J. 1989 Feb;8(2):105–109. [PubMed] [Google Scholar]
- Hörnqvist E., Goldschmidt T. J., Holmdahl R., Lycke N. Host defense against cholera toxin is strongly CD4+ T cell dependent. Infect Immun. 1991 Oct;59(10):3630–3638. doi: 10.1128/iai.59.10.3630-3638.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M., Stiehm E. R. Host defense in malnutrition. Pediatrics. 1977 Apr;59(4):490–495. [PubMed] [Google Scholar]
- Koster F., Pierce N. F. Effect of protein deprivation on immunoregulatory cells in the rat mucosal immune response. Clin Exp Immunol. 1985 Apr;60(1):217–224. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lange S., Holmgren J. Protective antitoxic cholera immunity in mice: influence of route and number of immunizations and mode of action of protective antibodies. Acta Pathol Microbiol Scand C. 1978 Aug;86C(4):145–152. doi: 10.1111/j.1699-0463.1978.tb02572.x. [DOI] [PubMed] [Google Scholar]
- Lycke N., Eriksen L., Holmgren J. Protection against cholera toxin after oral immunization is thymus-dependent and associated with intestinal production of neutralizing IgA antitoxin. Scand J Immunol. 1987 Apr;25(4):413–419. doi: 10.1111/j.1365-3083.1987.tb02208.x. [DOI] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Intestinal mucosal memory and presence of memory cells in lamina propria and Peyer's patches in mice 2 years after oral immunization with cholera toxin. Scand J Immunol. 1986 May;23(5):611–616. doi: 10.1111/j.1365-3083.1986.tb01995.x. [DOI] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Long-term cholera antitoxin memory in the gut can be triggered to antibody formation associated with protection within hours of an oral challenge immunization. Scand J Immunol. 1987 Apr;25(4):407–412. doi: 10.1111/j.1365-3083.1987.tb02207.x. [DOI] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986 Oct;59(2):301–308. [PMC free article] [PubMed] [Google Scholar]
- Lycke N., Lindholm L., Holmgren J. Cholera antibody production in vitro by peripheral blood lymphocytes following oral immunization of humans and mice. Clin Exp Immunol. 1985 Oct;62(1):39–47. [PMC free article] [PubMed] [Google Scholar]
- McGee D. W., McMurray D. N. The effect of protein malnutrition on the IgA immune response in mice. Immunology. 1988 Jan;63(1):25–29. [PMC free article] [PubMed] [Google Scholar]
- McGhee J. R., Kiyono H. New perspectives in vaccine development: mucosal immunity to infections. Infect Agents Dis. 1993 Apr;2(2):55–73. [PubMed] [Google Scholar]
- McMurray D. N., Watson R. R., Reyes M. A. Effect of renutrition on humoral and cell-mediated immunity in severely malnourished children. Am J Clin Nutr. 1981 Oct;34(10):2117–2126. doi: 10.1093/ajcn/34.10.2117. [DOI] [PubMed] [Google Scholar]
- Mengheri E., Nobili F., Crocchioni G., Lewis J. A. Protein starvation impairs the ability of activated lymphocytes to produce interferon-gamma. J Interferon Res. 1992 Feb;12(1):17–21. doi: 10.1089/jir.1992.12.17. [DOI] [PubMed] [Google Scholar]
- Ortiz R., Betancourt M. Cell proliferation in bone marrow cells of severely malnourished animals. J Nutr. 1984 Mar;114(3):472–476. doi: 10.1093/jn/114.3.472. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Sacci J. B., Jr, Craig J. P., Germanier R., Fürer E. Oral immunization against experimental cholera: the role of antigen form and antigen combinations in evoking protection. Ann N Y Acad Sci. 1983 Jun 30;409:724–733. doi: 10.1111/j.1749-6632.1983.tb26911.x. [DOI] [PubMed] [Google Scholar]
- Pierce N. F. Induction of optimal mucosal antibody responses: effects of age, immunization route(s), and dosing schedule in rats. Infect Immun. 1984 Jan;43(1):341–346. doi: 10.1128/iai.43.1.341-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J Exp Med. 1978 Jul 1;148(1):195–206. doi: 10.1084/jem.148.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmucker D. L., Daniels C. K., Wang R. K., Smith K. Mucosal immune response to cholera toxin in ageing rats. I. Antibody and antibody-containing cell response. Immunology. 1988 Aug;64(4):691–695. [PMC free article] [PubMed] [Google Scholar]
- Vajdy M., Lycke N. Y. Cholera toxin adjuvant promotes long-term immunological memory in the gut mucosa to unrelated immunogens after oral immunization. Immunology. 1992 Mar;75(3):488–492. [PMC free article] [PubMed] [Google Scholar]
- Watts T. Thymus weights in malnourished children. J Trop Pediatr (1967) 1969 Dec;15(4):155–158. doi: 10.1093/tropej/15.4.155. [DOI] [PubMed] [Google Scholar]
- Wiedermann U., Hanson L. A., Holmgren J., Kahu H., Dahlgren U. I. Impaired mucosal antibody response to cholera toxin in vitamin A-deficient rats immunized with oral cholera vaccine. Infect Immun. 1993 Sep;61(9):3952–3957. doi: 10.1128/iai.61.9.3952-3957.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winick M., Noble A. Cellular response in rats during malnutrition at various ages. J Nutr. 1966 Jul;89(3):300–306. doi: 10.1093/jn/89.3.300. [DOI] [PubMed] [Google Scholar]
- Xu-Amano J., Aicher W. K., Taguchi T., Kiyono H., McGhee J. R. Selective induction of Th2 cells in murine Peyer's patches by oral immunization. Int Immunol. 1992 Apr;4(4):433–445. doi: 10.1093/intimm/4.4.433. [DOI] [PubMed] [Google Scholar]
- Xu-Amano J., Kiyono H., Jackson R. J., Staats H. F., Fujihashi K., Burrows P. D., Elson C. O., Pillai S., McGhee J. R. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med. 1993 Oct 1;178(4):1309–1320. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rees E. P., Dijkstra C. D., Sminia T. Ontogeny of the rat immune system: an immunohistochemical approach. Dev Comp Immunol. 1990 Winter;14(1):9–18. doi: 10.1016/0145-305x(90)90003-w. [DOI] [PubMed] [Google Scholar]


