Abstract
Alumina-on-alumina bearings in total hip arthroplasty have been developed in an attempt to minimise debris and the occurrence of osteolytic lesions. The outstanding tribological properties of this bearing system are explained by low surface roughness, high hardness for major scratch resistance, and high wettability. Since the 1970s, technological improvements in the manufacturing process of alumina components together with a better understanding of Morse taper technology have provided a surgical grade material with high density, high purity and small grains. Published studies on the outcome of total hip arthroplasty performed with this new generation of implants showed high survivorship especially in young and active patients, with survival rates free of revision of 90.8% to 97.4% at ten years. However, concern remains over ceramic liner fracture and squeaking, which has been noted recently with increasing prevalence. This review will discuss the current knowledge on the use of alumina-on-alumina bearings.
Introduction
Beginning with the early work of Boutin in France [1], alumina-on-alumina (al-al) implants have become a reliable alternative to metal on polyethylene bearings because of decreased wear production and lower rates of osteolytic lesions. During their initial use, until 1979, ceramic implants resulted in a high rate of aseptic loosening of the cemented socket and risk of component fracture, which were mainly related to bad design and flaws in the material. Incremental improvements in the manufacturing process, design, and quality control have since significantly decreased the risk of fracture to approximately 0.02% to 0.1% [2]. Also, minimal wear rates together with limited osteolysis can be expected for up to 20 years, provided that sound fixation of the acetabular component is achieved. During recent decades, different solutions have been explored in our department to address the problem of socket fixation, including the use of cement (from 1977 to 1983) [3], smooth screwed threaded Ti shells (from 1983 to 1989) [4], press-fit bulk alumina cups [5], and finally press-fit Ti shells that allowed better and durable fixation of the implants. Ti shells were initially covered with a pure Ti grid (from 1989 to 1997), and then replaced by hydroxyapatite-coated Ti shells with alumina modular inserts (starting in 1997) (Fig. 1).
Fig. 1.
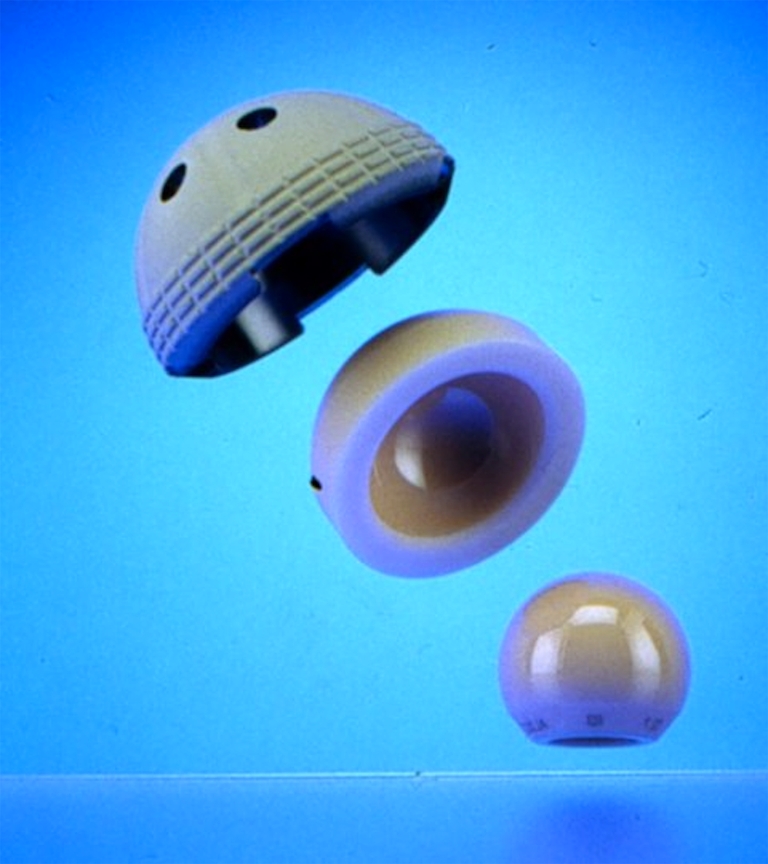
Current design of the socket: Hydroxyapatite-coated Ti shell with an alumina modular insert. Courtesy of Ceraver Osteal (Roissy, France)
With this new generation of implants, the alumina-on-alumina bearing represents a very promising option, especially in young and active patients, with survival rates free of revision of 90.8% to 97.4% at ten years [6, 7]. When compared to metal-on-highly cross-linked polyethylene, the alumina-on-alumina bearing appears to be as reliable and durable at mid-term follow-up [8]. However, a number of authors have recently raised concerns regarding the use of titanium acetabular components coupled with ceramic press-fit liners including malseating of the ceramic liner, fracture of the liner [9], and the occurrence of noise [10–12].
The aim of this review paper is to provide an overview of current knowledge on the use of alumina-on-alumina bearings in total hip arthroplasty.
Wear performance of al-al bearings
Ceramics were introduced in total hip arthroplasty because of their low friction and high wear resistance [13]. Alumina ceramic is a highly oxidised ceramic, obtained from alumina oxide powder with bonding and lubricating agents, pressed and sintered at 1600°C. The outstanding tribological properties of the alumina couple are explained by a low surface roughness because of the low grain size (Ra = 0.02 μm), a high hardness responsible for major scratch resistance, a high wettability, and fluid-film lubrication [14]. Also, sphericity and clearance between the alumina femoral head and cup must be almost perfect to avoid abnormal wear of al-al bearings, as reported in the past by German groups [15–17]. In vitro wear studies have shown that the optimal deviation from sphericity was ±1 μm, and clearance between the two components was 20–50 μm. More will result in increased friction coefficient and less in breakage of the fluid film lubrication. Also, high contact stresses should be avoided because they are associated with increased wear and wear particle shedding. It was found that high contact stresses were associated with excessive sphericity clearance (more than 50 μm) and impingement due to tilting of the socket or inadequate positioning during surgery [14]. Clinical retrievals of al-al bearings have indicated steady-state wear rates of alumina bearings to be a few microns per year [16, 18], and sphericity, surface roughness, and wear volume to be directly related to alumina grain size. In some instances, wear was as low as a few microns for a 15-year period in use, which is 2000 times less than a regular metal-on-polyethylene sliding couple and 100 times less than a metal-on-metal prosthesis [19]. However, in abnormal situations massive wear of alumina components may be observed, mainly due to the use of old aluminas with a low density, and a very coarse microstructure [20]. During the last 20 years, alumina quality has improved progressively, which has resulted in a correlated decrease of the microscopic wear and better survival rates of implanted hips.
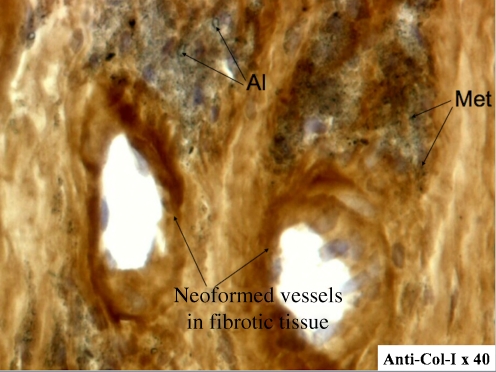
From a biological perspective, the usual reaction to alumina wear debris is fibrocytic with very few macrophages and no giant cells (Fig. 2). In vitro studies have shown that ingested particles will activate macrophage production of proinflammatory cytokines, but in a lower quantity than with polyethylene particles [21]. Macrophage apoptosis occurred sooner and was more extensive with alumina particles than with polyethylene particles of the same size. This may explain the lower inflammatory response to alumina particles and the lower incidence of osteolysis around hip implants [21, 22].
Fig. 2.
Immunohistochemical study of type I collagen expression in periprosthetic tissues around aseptically loosened al-al implants showed mainly a fibrocytic reaction with very few macrophages and lymphocytes. Al alumina particles, Met metallic particles
Clinical outcome
The extensive literature reporting the outcome after total hip replacement with the al-al combination must be interpreted with caution, depending upon the period of implantation, design of the prosthesis, and the method of femoral and actebular fixation. Our initial experience with the cemented plain alumina socket gave disappointing results, with a survival rate at ten years averaging 88.6% [3]. Failures were mainly due to isolated aseptic loosening of the acetabular component by acute debonding at the implant–cement interface. This was confirmed in a longer follow-up of patients operated by Pierre Boutin [23]. The series was composed of 118 consecutive alumina-on-alumina total hip arthroplasties done in 106 patients between 1978 and 1980. The prostheses combined a 32-mm alumina head and an all-alumina socket. Both components were cemented in 85 hips, cementless in 29, and the stem was cemented and the socket was cementless in four. The mean age of the patients at the time of the index arthroplasty was 62.2 years (range, 32–89 years). At the 20-year follow-up evaluation, 45 patients (51 hips) were still alive and had not been revised, 25 patients (25 hips) had revision of either one or both components, 27 patients (30 hips) had died from unrelated causes, and nine patients (12 hips) were lost to follow-up. The mean Merle d’Aubigné hip score was 16.2 ± 1.8 at the latest follow-up. Survival of the cup at 20 years with revision for any reason was 85.6% (95% confidence interval range, 72.2–99%) for cementless cups versus 61.2% (95% confidence interval range, 46.8–75.6%) for cemented cups. Survival of the stem at 20 years with revision for any reason was 84.9% (95% confidence interval range, 71.1–98.8%) for cementless stems versus 87.3% (95% confidence interval range, 77.4–97.1%) for cemented stems. Wear of the prosthetic components was undetectable on plain radiographs. From our institution, many papers have been dedicated to the clinical outcome after total hip replacement in young patients. From 1990 to 1992, 71 hybrid alumina-on-alumina hip replacements were performed in 62 patients with a mean age of 46 years at surgery [24]. The prostheses comprised a cemented, collared, smooth stem of anodised titanium alloy, a 32-mm alumina head and a press-fit, hemispherical, metal-backed socket with an alumina insert. Taking revision for any reason as the endpoint, the overall survival rate was 93.7% at nine years (95% confidence interval range, 87.7–99.7). One socket had a complete radiolucent line less than 1 mm thick. There was no component migration nor osteolysis (Fig. 3). In a recent retrospective study, Petsatodis et al. [25] reported their long-term results of 109 total hip arthroplasties performed with a cementless alumina ceramic-on-ceramic prosthesis. Six hips required revision surgery, and the cumulative survivorship at 20.8 years was 84.4%. This was also confirmed by Kress et al. [26], who showed excellent results with a cementless al-al total hip arthroplasty at 10.5 years, with only one hydroxyapatite-coated stem requiring revision for aseptic loosening, and one showing a non-progressive osteolysis around a screw of a cup. These results show that improvements in socket fixation have been possible when compared with earlier series of alumina-on-alumina arthroplasties and that excellent results can be expected in the long term, especially in younger and more active patients.
Fig. 3.
Twenty-five-year results after implantation of a cemented bulk alumina socket
Low rate of osteolysis
The limited incidence of osteolytic lesions observed with an al-al combination has been confirmed by many authors who gave several different explanations including a lower concentration of wear particles in the periprosthetic tissue around the bearing, which has been evaluated to be many thousand times less than with a regular metal-on-polyethylene couple [27], and the inert nature of the material, which is highly oxidised and very well tolerated both in massive and particulate forms [22, 28]. These data have been supported by clinical studies. At 20 years follow-up, osteolytic lesions were not encountered at the socket nor at the femoral level [23]. Fye et al. [29] reported a very limited osteolysis with the Mittelmeier prosthesis. Similar results were reported by Gualtieri et al. [30] with a cemented al-al prosthesis at eight years follow-up, and Huo et al. [31] with a cementless prosthesis at nine years follow-up. Hernigou et al. [32] recently investigated wear and osteolysis on 28 ceramic-ceramic arthroplasties which had survived 20 years without revision and without loosening. Osteolysis could not be detected on anteroposterior pelvic radiographs but only on 3D volume from CT scans, and was always significantly lower than that observed with a ceramic-polyethylene couple. When present, acetabular bone defects were most often contained cavitary defects that might have been created by removal of the cement from the acetabular anchorage holes when the sockets had been cemented. These defects can be managed by using a component which is 2–4 mm larger or by using an impacted morselised allograft. Acetabular reconstruction with a Kerboull device and bone grafting is seldom necessary when revising an al-al prosthesis. This situation is mainly encountered during the revision of smooth threaded acetabular shells that caused excessive peak forces on the bone, leading to stress-induced bone resorption and early migration of the cup [33]. Among the other factors that might explain osteolytic lesions in al-al bearings, one must also consider that after loosening there are many materials grinding against each other thus producing cement particulates and titanium alloy debris that can trigger a chronic inflammatory response through the enhancement of TNF α production (tumour necrosis factor α) and macrophage activation [21]. Animal studies and histological analysis of pseudomembranes from loosened alumina cups suggest that this unexpected osteolysis was probably induced by metal or cement debris rather than by alumina particles [34]. Recently, Hatton et al. [35] showed that ceramic wear particles generated under microseparation conditions could induce osteolytic cytokine production by human macrophages, but they estimated that the volumetric concentration of the particles needed to generate this response was extremely high and could not be achieved in the clinical setting. We therefore consider that true osteolysis can only be observed in abnormal situations such as inadequate implant positioning, abnormal contact, or with instability of the components.
Issues related to the use of al-al bearings
Fracture of ceramic implants
Ceramics are known to be brittle materials with no ductility and limited bending strength. As the material has no possibility to deform, implants break without warning. The fracture toughness (K1c value) is used to measure the brittleness of the material. Fracture will occur if the stress-intensity factor K1 of one of the volume elements becomes larger than the fracture toughness. The mode of failure usually is related to the propagation of subcritical cracks, which are favoured by stress concentration zones caused by imperfections either at the surface or within the material including pores inhomogeneities, scratches, and micronotches [36]. Several factors have been reported to increase the risk of failure of ceramic heads including increased body weight, high activity level, hammering of the femoral head, and imprisonment of a foreign body between the morse taper and the ball, but the most important is certainly material quality. Since the 1970s, technical improvements in the manufacturing process of alumina components together with a better understanding of Morse taper technology have offered reliability and higher security, and have resulted in a significant reduction of the ceramic fracture rate [2]. Improvements in conical fixation technology, particularly cone angle accuracy, tolerance, and roughness, have yielded an increase in burst strength to 102 KN. With these improvements the fracture risk has decreased from 1% to less than 0.004% with current-generation alumina femoral heads [36]. Although dramatic, these events have to be compared with other risks of mechanical failure, such as metallic stem fracture, polyethylene wear, and polyethylene liner wear and fractures. In a paper from Heck et al. [37], the incidence of alumina ceramic fracture was less than metallic stem fracture or polyethylene liner fracture.
The management of ceramic fractures is controversial. The results of revision total hip arthroplasty performed in these situations have been quite variable and disappointing. Some authors reported catastrophic metallic head wear and osteolysis caused by third body wear after incomplete synovectomy [38–40]. We observed less dramatic features, even though fracture remains a spectacular mode of failure after total hip replacement. This is supported by Sharma et al. [41], who followed up eight patients in whom a metal-on-polyethylene bearing was implanted after a fracture of the ceramic head. There were no revisions for polyethylene wear, osteolysis, or aseptic loosening at a mean ten years follow-up. It is of paramount importance to recognise ceramic implant fractures early, before catastrophic material deterioration occurs. If revision is performed with some delay, massive destruction of bone and tissues may occur, largely due to metallic debris originated from abrasive effects of alumina particles on the metallic stem, neck or socket.
Specificity of ceramic acetabular liner fractures
Over our 30-year experience with the alumina wear couple, we have observed seven cases of ceramic liner fracture [42]. They occurred soon after surgery (3–10 months), and concerned small component sizes (50 and 52 mm in diameter). Among the seven fractures, three were managed by a single replacement of the alumina liner. Two experienced a refracture of the insert at five months and 88 months after the first revision surgery. Both involved 50-mm diameter sockets, of which the design had been modified by the manufacturer in the year 2000 to diminish the initial overlip of the liner in order to avoid impingement with the femoral neck and improve theoretical range of motion. At refracture, one hip was revised by changing the head and the metal back for a larger one, and one was revised by changing the head and implanting a cemented polyethylene cup. The association of a thin liner and reduction of the overlip acted as a stress riser on the rim of the liner that not only explained the first fracture, but also the second one. We suspect these fractures occur during surgery, as was suggested by the four fractures that occurred during the introduction of the liner in to the metal-back and were replaced immediately. Also, great attention must be paid to cleaning the interior of the metal-backed shell from potential contaminants, and to position the insert correctly before sealing it by gentle hammering. It is likely that the incidence of intra-operative chipping of ceramic inserts will significantly decrease in the next few years, when surgeon education efforts will be put into effect. Given our experience, we recommend the replacement of the acetabular shell when revision is performed for a fracture of the liner, especially in the case of small socket sizes.
Squeaking
With the increasing number of prostheses implanted, audible squeaking has arisen as a new complication. It is a very annoying noise, similar to one emitted from a nonlubricated hinge, and has been reported not only with ceramic-on-ceramic but also with metal-on-metal bearings. The incidence of squeaking varies considerably, ranging from less than 1% to 20% [43–45]. In a recent prospective observational study on 1,486 ceramic-on-ceramic total hip arthroplasties, 6% of the hips developed squeaking but just nine patients required revision surgery [46]. No association could be found between component position and squeaking. The intensity of squeaking decreased in about 30% of the patients but did not resolve completely.
The exact mechanism for squeaking remains unclear. Although several explanations have been proposed, it is likely to be multifactorial and no definite conclusion can be drawn from the reported series. Squeaking can be reproduced experimentally. Taylor et al. [47] demonstrated that squeaking was encountered in association with stripe wear and occurred during subluxation of the head across the insert edge once the stripe wear began to form. He suggested that lubrication conditions and contact stress might also play a role in squeaking, which was confirmed by in vitro bench tests that simulated hip squeaking [48]. In these tests, squeaking only occurred when the film fluid between the two surfaces was disrupted. Walter et al. [49] suggested that squeaking may result from resonance of one or other or both of the metal components especially when there is a mismatch between the shell and the liner during edge loading, which may cause the liner to tilt out of the shell. Nevelos et al. [50] suggested that femoral head microseparation due to femoral neck impingement on an elevated metal rim may cause ceramic pullout and produce 4-mm wide wear stripes. They reported that microseparation was associated with a clicking sound. Finally, in a recent study [51], the prevalence of squeaking was seven times higher for patients who had received a titanium-molybdenum-zirconium-iron-alloy stem as compared to those who received a titanium-aluminum-vanadium-alloy stem, suggesting that different stem alloys may have an impact on the frequency of squeaking following a ceramic-on-ceramic total hip arthroplasty.
Revision of al-al bearings
The revision of an al-al total hip arthroplasty has specific features that are still debated in the literature. Since Pulliam [52] reported on the case of a ceramic head breakage implanted on a used taper, manufacturers have strongly advised against reimplantation of a ceramic head on a used trunnion. Potential damaged areas of the taper may create a stress riser that can be responsible for the initiation and propagation of a crack that ultimately would lead to a fracture of the newly implanted head. Moreover, secure fixation of the head on a damaged taper may be impaired with a theoretical risk of massive wear from abnormal movement between the head and taper. We believe that from a surgeons point of view the removal of a well fixed stem is demanding with respect to blood loss, surgical time and possible later discomfort, and leads to an increased risk of surgical complications that might seriously impair hip function in the long term. Given the lack of clear data available on the revision of ceramic-ceramic implants at the time, during the past 30 years, we reused the same ceramic ball after dissociation in seven hips, and reimplantated a new ceramic ball on a used cone in 54 hips (45 alumina heads, nine zirconia heads) [42]. In all cases, inspection of the Morse taper only showed small scratches, and a protection cap was used during surgery. Among these hips, no ceramic fracture was observed at a mean 88 ± 65 months follow-up. Therefore, we strongly recommend making a visual evaluation of the cone to test the ceramic head and check if there is mobility and retain the stem only if these checks are positive. If there is any uncertainty on the cone, the stem should be definitely replaced. If the Morse taper is damaged, one solution would be to replace the ceramic head with a metallic ball. However, alumina particles remaining in the surrounding tissues might cause third-body wear of the metallic ball. Therefore, when a metallic ball is implanted to replace a fractured ceramic head, care must be taken to remove all ceramic particles from the joint cavity. This includes a thorough lavage and debridement of the hip, a complete synovectomy, and the systematic replacement of a polyethylene cup in which particles can be incorporated. Moreover, it is likely that the use of a chromium-cobalt head, which is tougher than stainless steel, would reduce the risk of metallic head wear.
Conclusion
Over 30 years of experience with al-al bearings has allowed us to draw some conclusions such that improvements in the fabrication of current ceramics have dramatically reduced implant fracture and osteolysis rates which were problematic in the earlier generation of alumina implants. The current alumina-on-alumina bearing system is safe if the material is of high quality, if cone technology is accurate and if the material has a significant thickness. Excellent results may be expected without any physical limitation especially in young and active patients. Revision is easy to perform due to the lack of foreign body reaction and osteolysis. Although al-al bearings have proven their efficacy in the long term, concern remains over some risk of fracture and the cost of the prosthesis. Surgical technique is also highly relevant and effective surgeon education is essential before performing an al-al total hip arthroplasty.
Footnotes
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Boutin P. Alumina and its use in surgery of the hip. (Experimental study) Presse Méd. 1971;79:639–640. [PubMed] [Google Scholar]
- 2.Hannouche D, Nich C, Bizot P, Meunier A, Nizard R, Sedel L (2003) Fractures of ceramic bearings: history and present status. Clin Orthop Relat Res 19–26 [DOI] [PubMed]
- 3.Nizard RS, Sedel L, Christel P, Meunier A, Soudry M, Witvoet J (1992) Ten-year survivorship of cemented ceramic-ceramic total hip prosthesis. Clin Orthop 53–63 [PubMed]
- 4.Bizot P, Banallec L, Sedel L, Nizard R (2000) Alumina-on-alumina total hip prostheses in patients 40 years of age or younger. Clin Orthop Relat Res 68–76 [DOI] [PubMed]
- 5.Hamadouche M, Nizard RS, Meunier A, Bizot P, Sedel L. Cementless bulk alumina socket: preliminary results at 6 years. J Arthroplasty. 1999;14:701–707. doi: 10.1016/S0883-5403(99)90225-5. [DOI] [PubMed] [Google Scholar]
- 6.Bizot P, Larrouy M, Witvoet J, Sedel L, Nizard R (2000) Press-fit metal-backed alumina sockets: a minimum 5-year followup study. Clin Orthop Relat Res 134–142 [DOI] [PubMed]
- 7.Garcia-Rey E, Cruz-Pardos A, Garcia-Cimbrelo E. Alumina-on-alumina total hip arthroplasty in young patients: diagnosis is more important than age. Clin Orthop Relat Res. 2009;467:2281–2289. doi: 10.1007/s11999-009-0904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bascarevic Z, Vukasinovic Z, Slavkovic N, Dulic B, Trajkovic G, Bascarevic V, Timotijevic S. Alumina-on-alumina ceramic versus metal-on-highly cross-linked polyethylene bearings in total hip arthroplasty: a comparative study. Int Orthop. 2010;34:1129–1135. doi: 10.1007/s00264-009-0899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ha YC, Kim SY, Kim HJ, Yoo JJ, Koo KH. Ceramic liner fracture after cementless alumina-on-alumina total hip arthroplasty. Clin Orthop Relat Res. 2007;458:106–110. doi: 10.1097/BLO.0b013e3180303e87. [DOI] [PubMed] [Google Scholar]
- 10.Ranawat AS, Ranawat CS. The squeaking hip: a cause for concern-agrees. Orthopedics. 2007;30(738):743. doi: 10.3928/01477447-20070901-32. [DOI] [PubMed] [Google Scholar]
- 11.Walter WL, Waters TS, Gillies M, Donohoo S, Kurtz SM, Ranawat AS, Hozack WJ, Tuke MA. Squeaking hips. J Bone Joint Surg Am. 2008;90(Suppl 4):102–111. doi: 10.2106/JBJS.H.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarrett CA, Ranawat AS, Bruzzone M, Blum YC, Rodriguez JA, Ranawat CS. The squeaking hip: a phenomenon of ceramic-on-ceramic total hip arthroplasty. J Bone Joint Surg Am. 2009;91:1344–1349. doi: 10.2106/JBJS.F.00970. [DOI] [PubMed] [Google Scholar]
- 13.Hannouche D, Hamadouche M, Nizard R, Bizot P, Meunier A, Sedel L (2005) Ceramics in total hip replacement. Clin Orthop Relat Res 62–71 [DOI] [PubMed]
- 14.Christel PS (1992) Biocompatibility of surgical-grade dense polycrystalline alumina. Clin Orthop 10–18 [PubMed]
- 15.Hoffinger SA, Keggi KJ, Zatorski LE. Primary ceramic hip replacement: a prospective study of 119 hips. Orthopedics. 1991;14:523–531. doi: 10.3928/0147-7447-19910501-05. [DOI] [PubMed] [Google Scholar]
- 16.Mittelmeier H, Heisel J (1992) Sixteen-years' experience with ceramic hip prostheses. Clin Orthop 64–72 [PubMed]
- 17.Walter IA. On the material and tribology of alumina-alumina couplings for hip joint prostheses. Clin Orthop. 1992;282:31–46. [PubMed] [Google Scholar]
- 18.Boutin P, Christel P, Dorlot JM, Meunier A, Roquancourt A, Blanquaert D, Herman S, Sedel L, Witvoet J. The use of dense alumina-alumina ceramic combination in total hip replacement. J Biomed Mater Res. 1988;22:1203–1232. doi: 10.1002/jbm.820221210. [DOI] [PubMed] [Google Scholar]
- 19.Clarke IC, Good V, Williams P, Schroeder D, Anissian L, Stark A, Oonishi H, Schuldies J, Gustafson G. Ultra-low wear rates for rigid-on-rigid bearings in total hip replacements. Proc Inst Mech Eng H. 2000;214:331–347. doi: 10.1243/0954411001535381. [DOI] [PubMed] [Google Scholar]
- 20.Prudhommeaux F, Hamadouche M, Nevelos J, Doyle C, Meunier A, Sedel L (2000) Wear of alumina-on-alumina total hip arthroplasties at a mean 11-year followup. Clin Orthop Relat Res 113–122 [DOI] [PubMed]
- 21.Petit A, Catelas I, Antoniou J, Zukor DJ, Huk OL. Differential apoptotic response of J774 macrophages to alumina and ultra-high-molecular-weight polyethylene particles. J Orthop Res. 2002;20:9–15. doi: 10.1016/S0736-0266(01)00077-8. [DOI] [PubMed] [Google Scholar]
- 22.Catelas I, Petit A, Zukor DJ, Marchand R, Yahia L, Huk OL. Induction of macrophage apoptosis by ceramic and polyethylene particles in vitro. Biomaterials. 1999;20:625–630. doi: 10.1016/S0142-9612(98)00214-2. [DOI] [PubMed] [Google Scholar]
- 23.Hamadouche M, Boutin P, Daussange J, Bolander ME, Sedel L. Alumina-on-alumina total hip arthroplasty: a minimum 18.5-year follow-up study. J Bone Joint Surg Am. 2002;84-A:69–77. [PubMed] [Google Scholar]
- 24.Bizot P, Hannouche D, Nizard R, Witvoet J, Sedel L. Hybrid alumina total hip arthroplasty using a press-fit metal-backed socket in patients younger than 55 years. A six- to 11-year evaluation. J Bone Joint Surg Br. 2004;86:190–194. doi: 10.1302/0301-620X.86B2.14026. [DOI] [PubMed] [Google Scholar]
- 25.Petsatodis GE, Papadopoulos PP, Papavasiliou KA, Hatzokos IG, Agathangelidis FG, Christodoulou AG. Primary cementless total hip arthroplasty with an alumina ceramic-on-ceramic bearing: results after a minimum of twenty years of follow-up. J Bone Joint Surg Am. 2010;92:639–644. doi: 10.2106/JBJS.H.01829. [DOI] [PubMed] [Google Scholar]
- 26.Kress AM, Schmidt R, Holzwarth U, Forst R, Mueller LA (2010) Excellent results with cementless total hip arthroplasty and alumina-on-alumina pairing: minimum ten-year follow-up. Int Orthop. doi:10.1007/s00264-010-1150-1 [DOI] [PMC free article] [PubMed]
- 27.Bohler M, Mochida Y, Bauer TW, Plenk H, Jr, Salzer M. Wear debris from two different alumina-on-alumina total hip arthroplasties. J Bone Joint Surg Br. 2000;82:901–909. doi: 10.1302/0301-620X.82B6.9722. [DOI] [PubMed] [Google Scholar]
- 28.Brown C, Williams S, Tipper JL, Fisher J, Ingham E. Characterisation of wear particles produced by metal on metal and ceramic on metal hip prostheses under standard and microseparation simulation. J Mater Sci Mater Med. 2007;18:819–827. doi: 10.1007/s10856-006-0015-z. [DOI] [PubMed] [Google Scholar]
- 29.Fye MA, Huo MH, Zatorski LE, Keggi KJ. Total hip arthroplasty performed without cement in patients with femoral head osteonecrosis who are less than 50 years old. J Arthroplasty. 1998;13:876–881. doi: 10.1016/S0883-5403(98)90193-0. [DOI] [PubMed] [Google Scholar]
- 30.Gualtieri G, Nigrisoli M, Dallari D, Catamo L, Gualtieri I. A study of the mechanical and biological behavior of bioceramic hip arthroplasty followed-up after more than 10 years. Chir Organi Mov. 1993;78:213–222. [PubMed] [Google Scholar]
- 31.Huo MH, Martin RP, Zatorski LE, Keggi KJ. Ceramic total hip replacement done without cement. Long term follow-up study. Orthop Trans. 1995;19:400–401. [Google Scholar]
- 32.Hernigou P, Zilber S, Filippini P, Poignard A. Ceramic-ceramic bearing decreases osteolysis: a 20-year study versus ceramic-polyethylene on the contralateral hip. Clin Orthop Relat Res. 2009;467:2274–2280. doi: 10.1007/s11999-009-0773-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ilchmann T, Neher S, Maurer F, Weise K. Modes of failure of a threaded acetabular cup : a radiographic study with EBRA of 42 revised cups. Int Orthop. 2007;31:211–216. doi: 10.1007/s00264-006-0155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mochida Y, Boehler M, Salzer M, Bauer TW (2001) Debris from failed ceramic-on-ceramic and ceramic-on-polyethylene hip prostheses. Clin Orthop Relat Res 113–125 [DOI] [PubMed]
- 35.Hatton A, Nevelos JE, Matthews JB, Fisher J, Ingham E. Effects of clinically relevant alumina ceramic wear particles on TNF-alpha production by human peripheral blood mononuclear phagocytes. Biomaterials. 2003;24:1193–1204. doi: 10.1016/S0142-9612(02)00510-0. [DOI] [PubMed] [Google Scholar]
- 36.Willmann G (2000) Ceramic femoral head retrieval data. Clin Orthop Relat Res 379:22–28 [DOI] [PubMed]
- 37.Heck DA, Partridge CM, Reuben JD, Lanzer WL, Lewis CG, Keating EM. Prosthetic component failures in hip arthroplasty surgery. J Arthroplasty. 1995;10:575–580. doi: 10.1016/S0883-5403(05)80199-8. [DOI] [PubMed] [Google Scholar]
- 38.Kempf I, Semlitsch M. Massive wear of a steel ball head by ceramic fragments in the polyethylene acetabular cup after revision of a total hip prosthesis with fractured ceramic ball. Arch Orthop Trauma Surg. 1990;109:284–287. doi: 10.1007/BF00419946. [DOI] [PubMed] [Google Scholar]
- 39.Allain J, Goutallier D, Voisin MC, Lemouel S. Failure of a stainless-steel femoral head of a revision total hip arthroplasty performed after a fracture of a ceramic femoral head. A case report. J Bone Joint Surg Am. 1998;80:1355–1360. doi: 10.1302/0301-620X.80B4.8659. [DOI] [PubMed] [Google Scholar]
- 40.Allain J, Roudot-Thoraval F, Delecrin J, Anract P, Migaud H, Goutallier D. Revision total hip arthroplasty performed after fracture of a ceramic femoral head. A multicenter survivorship study. J Bone Joint Surg Am. 2003;85-A:825–830. doi: 10.2106/00004623-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Sharma V, Ranawat AS, Rasquinha VJ, Weiskopf J, Howard H, Ranawat CS. Revision total hip arthroplasty for ceramic head fracture: a long-term follow-up. J Arthroplasty. 2010;25:342–347. doi: 10.1016/j.arth.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Hannouche D, Delambre J, Zadegan F, Sedel L, Nizard R. Is there a risk in placing a ceramic head on a previously implanted trunion? Clin Orthop Relat Res. 2010;468:3322–3327. doi: 10.1007/s11999-010-1505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baek SH, Kim SY. Cementless total hip arthroplasty with alumina bearings in patients younger than fifty with femoral head osteonecrosis. J Bone Joint Surg Am. 2008;90:1314–1320. doi: 10.2106/JBJS.G.00755. [DOI] [PubMed] [Google Scholar]
- 44.Keurentjes JC, Kuipers RM, Wever DJ, Schreurs BW. High incidence of squeaking in THAs with alumina ceramic-on-ceramic bearings. Clin Orthop Relat Res. 2008;466:1438–1443. doi: 10.1007/s11999-008-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schroder D, Bornstein L, Bostrom MP, Nestor BJ, Padgett DE, Westrich GH (2010) Ceramic-on-ceramic total hip arthroplasty: incidence of instability and noise. Clin Orthop Relat Res. Sep 18 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 46.Restrepo C, Matar WY, Parvizi J, Rothman RH, Hozack WJ. Natural history of squeaking after total hip arthroplasty. Clin Orthop Relat Res. 2010;468:2340–2345. doi: 10.1007/s11999-009-1223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor S, Manley MT, Sutton K. The role of stripe wear in causing acoustic emissions from alumina ceramic-on-ceramic bearings. J Arthroplasty. 2007;22:47–51. doi: 10.1016/j.arth.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 48.Chevillotte C, Trousdale RT, Chen Q, Guyen O, An KN. The 2009 Frank Stinchfield Award: “Hip squeaking”: a biomechanical study of ceramic-on-ceramic bearing surfaces. Clin Orthop Relat Res. 2010;468:345–350. doi: 10.1007/s11999-009-0911-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walter WL, Insley GM, Walter WK, Tuke MA. Edge loading in third generation alumina ceramic-on-ceramic bearings: stripe wear. J Arthroplasty. 2004;19:402–413. doi: 10.1016/j.arth.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 50.Nevelos J, Ingham E, Doyle C, Streicher R, Nevelos A, Walter W, Fisher J. Microseparation of the centers of alumina-alumina artificial hip joints during simulator testing produces clinically relevant wear rates and patterns. J Arthroplasty. 2000;15:793–795. doi: 10.1054/arth.2000.8100. [DOI] [PubMed] [Google Scholar]
- 51.Restrepo C, Post ZD, Kai B, Hozack WJ. The effect of stem design on the prevalence of squeaking following ceramic-on-ceramic bearing total hip arthroplasty. J Bone Joint Surg Am. 2010;92:550–557. doi: 10.2106/JBJS.H.01326. [DOI] [PubMed] [Google Scholar]
- 52.Pulliam IT, Trousdale RT. Fracture of a ceramic femoral head after a revision operation. A case report [see comments] J Bone Joint Surg Am. 1997;79:118–121. doi: 10.2106/00004623-199701000-00013. [DOI] [PubMed] [Google Scholar]