Abstract
Ceramic-on-ceramic coupling is thought to be a durable alternative to metal- or alumina-on-polyethylene pairing. No evidence exists suggesting superior clinical and radiological results for hydroxyapatite-coated stems versus uncoated stems. The aim of this study is to report the performance of an alumina-on-alumina bearing cementless total hip arthroplasty and to compare stems with a tapered design with and without hydroxyapatite coating. We prospectively analysed the results of cementless tapered femoral stems (40 hydroxyapatite-coated versus 22 uncoated stems), a metal-backed fibre mesh hydroxyapatite-coated socket and alumina-on-alumina pairing. Of 75 hips studied, 62 were available for follow-up (mean of 10.5 years after surgery). The average Harris hip score was 90. Only one hydroxyapatite-coated stem was revised for aseptic loosening. One instance of non-progressive osteolysis was detected around a screw of a cup. All other components showed radiographic signs of stable ingrowth. Hydroxyapatite coating of the stem had no significant impact on the clinical or radiological results. Total hip arthroplasty with the presented implant and pairing provides a durable standard for all patients requiring hip joint replacement against which all newer generations of cementless implants should be judged.
Introduction
Polyethylene wear debris is thought to induce osteolysis after total hip arthroplasty (THA) [1]. Ceramic-on-ceramic bearing with a metal-backed socket is an alternative coupling with minimal wear and the potential to reduce polyethylene debris-associated problems after THA [2, 3]. Problems with alumina ceramic reported in the past seem to have been overcome with improved design and manufacture [4]. Short- and intermediate-term results with contemporary ceramic bearings have been published with promising clinical and radiological outcomes [4–6].
Hydroxyapatite coating in THA is believed to enhance initial fixation and osseous integration, thus promoting stable bony ingrowth between the implant and the surrounding bone [7, 8]. Human histomorphometric studies have shown that the hydroxyapatite coating resorbs with time [9]. While hydroxyapatite is not involved in polyethylene-induced inflammatory bone reaction, the concern has been raised whether debonded hydroxyapatite might increase polyethylene wear [9]. Reports of the Swedish Hip Arthroplasty Register have shown an increased risk of revision of acetabular cups coated with hydroxyapatite [10]. Numerous studies have reported excellent survivorship with cementless hydroxyapatite-coated stems as well as uncoated stems [11–13]. Independent of good long-term clinical results, high rates of proximal osteolysis have been observed, usually associated with increased polyethylene wear [12, 14]. The meta-analysis of Gandhi et al. [3], which included only randomised controlled trials or comparative observational studies comparing hydroxyapatite-coated and uncoated stems, found no clinical benefits in the use of hydroxyapatite coating. In a matched series study of cementless stems with and without hydroxyapatite coating (polyethylene inlays were used in all cases) the prevalence of radiographic findings of femoral osteolysis was 16% in stems with hydroxyapatite coating compared to 43% in stems without hydroxyapatite coating [12].
To our knowledge, minimum ten-year results of a contemporary stem with tapered design and a hydroxyapatite-coated press-fit cup with alumina-on-alumina pairing have not been published. Furthermore, there is no prospective study that clinically and radiographically compares two cohorts of patients with cementless THA and alumina-on-alumina bearing differing exclusively with respect to the presence of hydroxyapatite coating of the stem.
The study had two objectives: First, the clinical and radiological performance of a consecutive series with a contemporary alumina-on-alumina bearing cementless THA is described after a minimum follow-up of ten years. Second, we prospectively analysed whether the clinical and radiographic performance of the cohort of patients with hydroxyapatite-coated stems compared favourably to the outcome in patients with non-coated stems.
Patients and methods
Between 1997 and 1999, we performed 75 consecutive unselected primary THA in 71 patients with primary and secondary osteoarthritis using the cementless Cerafit hip system (Ceraver Osteal, Roissy, France) with alumina-on-alumina pairing. For the first 25 THA non-hydroxyapatite-coated stems were used; the following 50 implanted stems were hydroxyapatite coated with otherwise identical design differing only in the hydroxyapatite coating.
For the follow-up analysis after a mean time of 10.5 years (min. 10.1, max. 11.4) there were 27 women (30 hips) and 31 men (32 hips) with an average age of 58 years (34–77 years) at time of surgery. Five patients (five hips) were lost to follow-up and eight patients (eight hips) had died prior to follow-up investigation. Two femoral components were changed: one due to aseptic loosening two years after implantation and the other due to periprosthetic fracture four years after implantation. There were 31 patients rated as Charnley class A, 19 patients as Charnley class B and eight patients as Charnley class C. The demographic data of the hydroxyapatite-coated versus uncoated patient cohorts are shown in Table 1.
Table 1.
Patient collective (patients/hips)
| Hydroxyapatite-coated stem | Non-hydroxyapatite-coated stem | ||
|---|---|---|---|
| Number of patients/hips | 38/40 | 20/22 | |
| Age (years) at surgery | 58.5 (36.0–76.9) | 57.5 (34.1–68.4) | |
| Charnley class | A | 20 (52.6%) | 11 (55%) |
| B | 13 (34.2%) | 6 (30%) | |
| C | 5 (13.2%) | 3 (15%) | |
| Osteoarthritis | 30 (75%) | 18 (81.8%) | |
| Femoral neck fracture | - | 2 (8.1%) | |
| Femoral head necrosis | 5 (12.5%) | - | |
| Dysplasia | 5 (12.5%) | 2 (8.1%) | |
| Follow-up (years) | 10.5 (10.1–10.8) | 10.7 (10.3–11.4) | |
| Women | 14/16 | 13/14 | |
| Men | 24/24 | 7/8 | |
| Lost to follow-up | 4/4 | 1/1 | |
| Deceased | 6/6 | 2/2 | |
| Revision | 1 aseptic loosening | None | |
| 1 periprosthetic fracture | |||
The Cerafit femoral component is a collarless, three-dimensional tapered-wedge stem made of titanium alloy (Ti6Al7Nb) (Ceraver Osteal, Roissy, France). Ribs in the proximal part of the prosthesis are designed to minimise rotational migration. The surface was rough blasted; 50 of the stems used were hydroxyapatite coated [Ca5(PO4)3(OH)] and 25 were uncoated.
The Cerafit acetabular component is a fibre mesh metal-backed cup made of titanium alloy (Ti6Al7Nb) (Ceraver Osteal, Roissy, France). The fibre mesh is coated with hydroxyapatite [Ca3(PO4)2]. We exclusively used an alumina (Al2O3) liner with an alumina (Al2O3) head in all patients.
All procedures were performed by one senior surgeon in a closed air enclosure with laminar air flow. All patients were placed in the supine position and operated up on with a direct transgluteal lateral Bauer approach. The largest stem that provided a stable press-fit was inserted after preparation of the femoral canal with chipped-tooth broaches.
Postoperatively, touch weight-bearing up to 15 kg was allowed for the first six weeks, then progressively increased loading to full weight-bearing within the following two weeks. Preoperatively and at the final follow-up, all patients were assessed clinically using the Harris hip score (HHS).
Plain weight-bearing radiographs in anteroposterior and lateral views were analysed by two independent observers (LAM and AMK) who were unaware of the clinical outcome. Evaluation of the femoral and acetabular components was performed using published criteria [15]. Radiological “stability”, stress shielding, radiolucent lines and cortical hypertrophy of the femoral component were additionally assessed according to the criteria of Engh et al. [16]. We defined “osteolysis” as localised bone resorption or endosteal resorption, as proposed by Willert and coworkers [17]. To estimate correct size of the stem, we determined the canal fill index on the first radiographs postoperatively [18]. To secure comparable results, our cutoff for defining undersizing was a canal fill index of <80%, as previously proposed [19]. Varus or valgus malalignment of the CLS stem was estimated on plain postoperative anteroposterior radiographs as an angle of 3° or more between the femoral axis and stem axis. Stem migration was estimated as previously published [20]. Our cutoff defining vertical migration was set at 3 mm. Location of radiolucent lines, osteolysis and cortical hypertrophy were rated according to the Gruen criteria. For the evaluation of the acetabular component we used the same criteria and the classification according to DeLee and Charnley [21]. For evaluation of heterotopic ossification the Brooker classification was used. Stress shielding was classified as grade 1 when only resorption of the medial edge of the resection line appeared. Grade 2 meant additional proximal medial bone resorption, while grade 3 findings extended more distally [22].
The placement of the acetabular component was evaluated by measuring the angle of the cup to the transischial tuberosity line (lateral inclination) on the plain radiograph two weeks postoperatively and at follow-up [23].
Statistics
The Wilcoxon two-sample test was used to evaluate any difference between hydroxyapatite-coated and uncoated stems with respect to stress shielding and the clinical outcome. Furthermore, it was used to test the influence of stress shielding on the clinical result. Implant survival was estimated with a Kaplan-Meier survival curve. A p value of <0.05 was considered significant.
Results
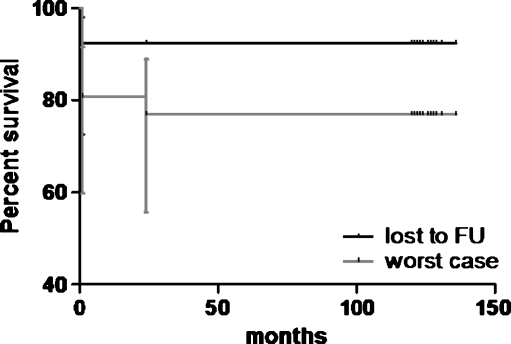
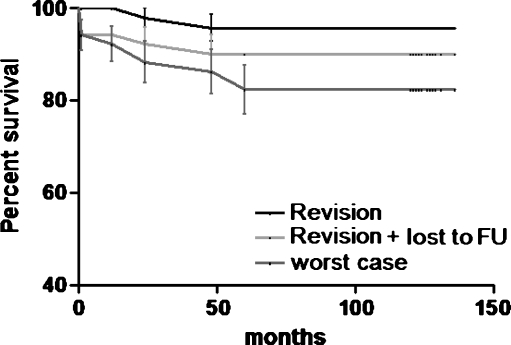
Of the 71 patients (75 hips, 50 coated with hydroxyapatite/25 uncoated) in this study, eight patients (8 hips) had died prior to the time of the follow-up. Intermediate clinical and radiological follow-up ranging from two to six years of four of the deceased patients (four hips, three HA) showed radiological signs of stable ingrowth of all stems without impending failures. There were four patients (four hips, one HA) who had died with their hips intact, but there were no clinical or radiological data beyond the one-year follow-up. At that time the implant was functioning well. Five patients (five hips) were lost to follow-up, three of these patients (three hips) had moved away and were contacted by phone and none of the hips were revised. For two patients (two hips) only a two-month postoperative clinical and radiological follow-up was available with a well-functioning hip. The Kaplan-Meier survival curves for hydroxyapatite-coated and uncoated stems are presented in Figs. 1 and 2.
Fig. 1.
Kaplan-Meier survival curve of uncoated stems with lost to follow-up and worst case (lost to follow-up and deceased patients) scenario
Fig. 2.
Kaplan-Meier survival curve of hydroxyapatite-coated stems with revision, revision + lost to follow-up and worst case (revision + lost to follow-up + deceased patients) scenario
Thus, clinical and radiological data were available for 58 patients (62 hips) after a mean of 10.5 years follow-up (min. 10.1, max. 11.4). Of the 62 implants directly accounted for, two femoral components coated with hydroxyapatite were revised: one for aseptic loosening two years after implantation and the other due to periprosthetic fracture four years after implantation. All other stems showed bony ingrowth; no acetabular component had to be revised. There was no failure of the alumina-on-alumina pairing.
The HHS was taken pre- and postoperatively. Range of hip motion was limited to less than 110° in all cases. At follow-up the average HHS was 89.9 for coated and 90.2 for uncoated stems; 42 hips were rated as excellent (67.7%), 12 as good (19.4%), five as fair (8.1%) and three as poor (4.8%) (Table 2).
Table 2.
Results of the HHS
| Hydroxyapatite-coated stem | Non-hydroxyapatite-coated stem | |
|---|---|---|
| Preoperative HHS | 44 (23–55) | 43 (20–53) |
| Postoperative HHS | 90 (32–100) | 90 (38–100) |
| Excellent | 26 (65%) | 16 (73%) |
| Good | 10 (25%) | 2 (9%) |
| Fair | 3 (7%) | 2 (9%) |
| Poor | 1 (3%) | 2 (9%) |
There was no statistical difference (p = 0.103) between the clinical outcome of hydroxyapatite-coated and uncoated stems.
Femoral component
At the mean 10.5-year follow-up, all stems presented complete bony ingrowth with no signs of definite or probable loosening. No postoperative subsidence was seen. Three stems were undersized, three positioned in slight varus and one in minimal valgus position. Proximal stress shielding was observed in all hips (Fig. 3). Grade 1 and 2 stress shielding was 89% for the hydroxyapatite-coated and 87% for the uncoated stems (p = 0.62). There was no correlation between stress shielding of the stem and the clinical outcome of the patients in either the uncoated group (p = 0.46) or in the hydroxyapatite-coated group (p = 0.52). There was no case of femoral osteolysis (Table 3).
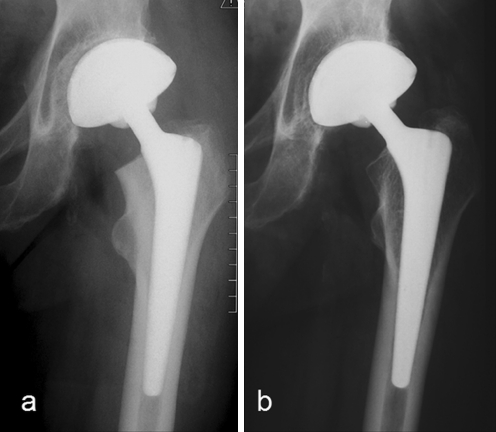
Fig. 3.
A 77-year-old man: postoperative (a) and 10.2-year follow-up (b) with grade 1 stress shielding according to Engh et al.
Table 3.
Radiographic results of the femoral component
| Hydroxyapatite-coated stem | Non-hydroxyapatite-coated stem | ||
|---|---|---|---|
| Undersized | 3 | - | |
| Varus | 2 | 1 | |
| Valgus | 1 | - | |
| Osteolysis | - | - | |
| Stress shielding according to Engh et al. | Grade 1 | 24 (63%) | 7 (32%) |
| Grade 2 | 10 (26%) | 12 (55%) | |
| Grade 3 | 4 (11%) | 3 (14%) | |
| Heterotopic ossifications according to Brooker | Grade I | 5 (13%) | 1 (5%) |
| Grade II | - | - | |
| Grade III | 1 (3%) | 1 (5%) | |
| Pedestal at tip of stem | 17 (47%) | 15 (79%) | |
Cortical hypertrophy of the femur was seen in two hips of the coated group in Gruen zones 3 and 5. There was no relationship between coated and uncoated stems and the HHS at the ten-year follow-up (p = 0.103).
Acetabular component
For all 62 acetabular cementless components fixation was good without change in position at the mean 10.5-year follow-up. The mean lateral inclination at two weeks postoperatively was 37.4° (20–52°) and at the time of follow-up it was 37.5° (22–56°). No acetabular component or alumina liner was revised. One of two components fixed with one screw showed osteolysis (9 × 15 × 9 mm) around the screw.
Seven cups had zone 2 gaps presenting with initial radiolucencies at two weeks; six had closed the gap at the ten-year follow-up and one had a remaining non-progressive zone 2 radiolucency. Three additional cups showed radiolucencies at the ten-year follow-up, one in zone 1 and two in zone 2. At the ten-year follow-up five cups presented with stress shielding in zone 1, eight cups in zone 2, three cups in zones 1 + 2 and five cups in zones 2 + 3.
Discussion
With regard to the two objectives of our study we found that the clinical and radiological performance of the presented cementless THA with alumina-on-alumina bearing was good to excellent after a minimum follow-up of ten years. Hydroxyapatite-coated stems did not out perform the non-coated stems. The absence of measurable wear and only one case of osteolysis in this series seems to underline the benefit of alumina-on-alumina coupling compared to other bearing surfaces [14]. Only two stems (aseptic loosening in one case and a periprosthetic fracture in the other) had to be revised. All other stems and cups showed signs of stable ingrowth after a mean follow-up of 10.5 years (minimum 10.1 years).
Femoral fixation
Numerous studies have reported excellent survivorship with cementless hydroxyapatite-coated and uncoated stems [11, 13, 14]. Independent of good long-term clinical results, high rates of proximal osteolysis have been observed, usually associated with increased polyethylene wear [12, 14].
The meta-analysis of Gandhi et al. [3] found no clinical benefits in the use of hydroxyapatite coating of the stem. Two studies comparing hydroxyapatite-coated and uncoated stems with a follow-up period comparable to our study have been published; both found no significant differences in the clinical results, radiographic results or the survival distributions with regard to the presence or absence of hydroxyapatite-coated and uncoated stems [24, 25]. Higher rates of femoral osteolysis were found for uncoated (43%) compared to coated (16%) stems; a polyethylene insert was used in all cases [12]. We found no demonstrable advantages of hydroxyapatite coating of the stem with regard to the clinical outcome [25]. We observed no significant difference in pain relief, improvement of function or proximal stress shielding between the hydroxyapatite-coated and uncoated group.
In contrast to the observations of Parvizi et al. [24], Yoon et al. [25] and Sanchez-Sotelo et al. [12], no evidence of osteolysis of the femur and only one case of non-progressive acetabular osteolysis around a screw was seen in either group. The low incidence of osteolysis might be attributed to the alumina-on-alumina bearing used in our study. The concern that debonded hydroxyapatite might increase polyethylene wear and thus osteolysis seems irrelevant with the alumina-on-alumina bearing [6, 9]. The known resorption of hydroxyapatite coating with time has had no radiologically visible impact on the stable bony ingrowth (spot welds) between the implant and the surrounding bone in our study [9].
Acetabular fixation
Acetabular fixation is the major problem compromising the longevity of cementless THA [8, 26, 27]. While numerous studies with excellent results for cemented and uncemented stems have been reported, all have inferior results for the acetabular components with the use of polyethylene inlays (with either metal or ceramic heads) in common [3, 8, 11, 26].
A report of the Norwegian Arthroplasty Register showed that hydroxyapatite-coated cups with polyethylene inlays showed higher revision rates and more radiological signs of aseptic loosening compared to the cemented polyethylene Charnley cup [27]. After a mean follow-up of 81 months Blacha and Gagala [8] predict a survival rate of 86 ± 7% at ten years for hydroxyapatite-coated hemispherical cups with polyethylene inserts. After a mean 81-month follow-up, four of 60 cups had to be revised because of osteolysis, and in another six cups osteolysis was seen [8]. The Swedish Hip Arthroplasty Register has shown an increased risk of revision of acetabular cups coated with hydroxyapatite [10]. None of our acetabular components needed revision; additionally, only one of our cups showed non-progressive osteolysis. Hydroxyapatite coating did not seem to have a negative effect on our fibre mesh cup.
Good long-term survivorship rates of the fibre mesh cementless Harris-Galante porous I and II acetabular components have been reported, but eccentric wear of the polyethylene inlay and osteolysis remained a problem [28]. We attribute the lack of wear and only one case of non-progressive osteolysis in our group to the alumina-on-alumina bearing surface.
Conclusion
Durable femoral and acetabular fixation and good clinical results were achieved independent of femoral coating. The absence of measurable wear and only one case of osteolysis in this series seems to outline the benefit of alumina-on-alumina coupling compared to other bearing surfaces but remains speculative at this stage.
Acknowledgments
Conflict of interest The authors declare that they have no conflict of interest.
Footnotes
Alexander M. Kress and Rainer Schmidt have contributed equally to this paper.
References
- 1.Harris WH. The problem is osteolysis. Clin Orthop Relat Res. 1995;311:46–53. [PubMed] [Google Scholar]
- 2.Garcia-Cimbrelo E, Garcia-Rey E, Murcia-Mazón A, Blanco-Pozo A, Martí E. Alumina-on-alumina in THA: a multicenter prospective study. Clin Orthop Relat Res. 2008;466(2):309–316. doi: 10.1007/s11999-007-0042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandhi R, Davey JR, Mahomed NN. Hydroxyapatite coated femoral stems in primary total hip arthroplasty: a meta-analysis. J Arthroplasty. 2009;24(1):38–42. doi: 10.1016/j.arth.2008.01.299. [DOI] [PubMed] [Google Scholar]
- 4.Sedel L. Evolution of alumina-on-alumina implants: a review. Clin Orthop Relat Res. 2000;379:48–54. doi: 10.1097/00003086-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Nizard R, Pourreyron D, Raould A, Hannouche D, Sedel L. Alumina-on-alumina hip arthroplasty in patients younger than 30 years old. Clin Orthop Relat Res. 2008;466(2):317–323. doi: 10.1007/s11999-007-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bascarevic Z, Vukasinovic Z, Slavkovic N, Dulic B, Trajkovic G, Bascarevic V, Timotijevic S. Alumina-on-alumina ceramic versus metal-on-highly cross-linked polyethylene bearings in total hip arthroplasty: a comparative study. Int Orthop. 2009 doi: 10.1007/s00264-009-0899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Søballe K, Hansen ES, B-Rasmussen H, Jørgensen PH, Bünger C. Tissue ingrowth into titanium and hydroxyapatite-coated implants during stable and unstable mechanical conditions. J Orthop Res. 1992;10(2):285–299. doi: 10.1002/jor.1100100216. [DOI] [PubMed] [Google Scholar]
- 8.Blacha J, Gagala J. Clinical and radiological results of hydroxyapatite-coated acetabular cups. Int Orthop. 2004;28(6):362–365. doi: 10.1007/s00264-004-0563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Røkkum M, Reigstad A, Johansson CB. HA particles can be released from well-fixed HA-coated stems: histopathology of biopsies from 20 hips 2–8 years after implantation. Acta Orthop Scand. 2002;73(3):298–306. doi: 10.1080/000164702320155293. [DOI] [PubMed] [Google Scholar]
- 10.Lazarinis S, Kärrholm J, Hailer NP. Increased risk of revision of acetabular cups coated with hydroxyapatite. Acta Orthop. 2010;81:53–59. doi: 10.3109/17453670903413178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajaratnam SS, Jack C, Tavakkolizadeh A, George MD, Fletcher RJ, Hankins M, Shepperd JA. Long-term results of a hydroxyapatite-coated femoral component in total hip replacement: a 15- to 21-year follow-up study. J Bone Joint Surg Br. 2008;90(1):27–30. doi: 10.1302/0301-620X.90B1.19731. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Sotelo J, Lewallen DG, Harmsen WS, Harrington J, Cabanela ME. Comparison of wear and osteolysis in hip replacement using two different coatings of the femoral stem. Int Orthop. 2004;28(4):206–210. doi: 10.1007/s00264-004-0558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swamy G, Pace A, Quah C, Howard P. The Bicontact cementless primary total hip arthroplasty: long-term results. Int Orthop. 2010 doi: 10.1007/s00264-010-1123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reikerås O, Gunderson RB. Excellent results of HA coating on a grit-blasted stem: 245 patients followed for 8–12 years. Acta Orthop Scand. 2003;74(2):140–145. doi: 10.1080/00016470310013851. [DOI] [PubMed] [Google Scholar]
- 15.Johnston RC, Fitzgerald RH, Jr, Harris WH, Poss R, Müller ME, Sledge CB. Clinical and radiographic evaluation of total hip replacement. A standard system of terminology for reporting results. J Bone Joint Surg Am. 1990;72(2):161–168. [PubMed] [Google Scholar]
- 16.Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br. 1987;69(1):45–55. doi: 10.1302/0301-620X.69B1.3818732. [DOI] [PubMed] [Google Scholar]
- 17.Willert HG, Bertram H, Buchhorn GH. Osteolysis in alloarthroplasty of the hip. The role of ultra-high molecular weight polyethylene wear particles. Clin Orthop Relat Res. 1990;258:95–107. [PubMed] [Google Scholar]
- 18.Noble PC, Alexander JW, Lindahl LJ, Yew DT, Granberry WM, Tullos HS. The anatomic basis of femoral component design. Clin Orthop Relat Res. 1988;235:148–165. [PubMed] [Google Scholar]
- 19.Aldinger PR, Jung AW, Breusch SJ, Ewerbeck V, Parsch D. Survival of the cementless Spotorno stem in the second decade. Clin Orthop Relat Res. 2009;467(9):2297–2304. doi: 10.1007/s11999-009-0906-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spotorno L, Schenk RK, Dietschi C, Romagnoli S, Mumenthaler A. Personal experiences with uncemented prostheses (in German) Orthopade. 1987;16(3):225–238. [PubMed] [Google Scholar]
- 21.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed] [Google Scholar]
- 22.Engh CA, McGovern TF, Bobyn JD, Harris WH. A quantitative evaluation of periprosthetic bone-remodeling after cementless total hip arthroplasty. J Bone Joint Surg Am. 1992;74(7):1009–1020. [PubMed] [Google Scholar]
- 23.Manaster BJ. From the RSNA refresher courses. Total hip arthroplasty: radiographic evaluation. Radiographics. 1996;16(3):645–660. doi: 10.1148/radiographics.16.3.8897629. [DOI] [PubMed] [Google Scholar]
- 24.Parvizi J, Sharkey PF, Hozack WJ, Orzoco F, Bissett GA, Rothman RH. Prospective matched-pair analysis of hydroxyapatite-coated and uncoated femoral stems in total hip arthroplasty. A concise follow-up of a previous report. J Bone Joint Surg Am. 2004;86-A(4):783–786. doi: 10.2106/00004623-200404000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Yoon KS, Kim HJ, Lee JH, Kang SB, Seong NH, Koo KH. A randomized clinical trial of cementless femoral stems with and without hydroxyapatite/tricalcium-phosphate coating: an 8- to 12-year follow-up study. J Arthroplasty. 2007;22(4):504–508. doi: 10.1016/j.arth.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Eskelinen A, Remes V, Helenius I, Pulkkinen P, Nevalainen J, Paavolainen P. Total hip arthroplasty for primary osteoarthrosis in younger patients in the Finnish arthroplasty register. 4,661 primary replacements followed for 0–22 years. Acta Orthop. 2005;76(1):28–41. doi: 10.1080/00016470510030292. [DOI] [PubMed] [Google Scholar]
- 27.Havelin LI, Espehaug B, Engesaeter LB. The performance of two hydroxyapatite-coated acetabular cups compared with Charnley cups. From the Norwegian Arthroplasty Register. J Bone Joint Surg Br. 2002;84(6):839–845. doi: 10.1302/0301-620X.84B6.12492. [DOI] [PubMed] [Google Scholar]
- 28.Anseth SD, Pulido PA, Adelson WS, Patil S, Sandwell JC, Colwell CW., Jr Fifteen-year to twenty-year results of cementless Harris-Galante porous femoral and Harris-Galante porous I and II acetabular components. J Arthroplasty. 2010;25:687–691. doi: 10.1016/j.arth.2009.05.033. [DOI] [PubMed] [Google Scholar]