Abstract
The Reelin gene (RELN) encodes a secretory glycoprotein critical for brain development and synaptic plasticity. Post-mortem studies have shown lower Reelin protein levels in the brains of patients with schizophrenia and bipolar disorder (BP) compared with controls. In a recent genome-wide association study of schizophrenia, the strongest association was found in a marker within RELN, although this association was seen only in women. In this study, we investigated whether genetic variation in RELN is associated with BP in a large family sample. We genotyped 75 tagSNPs and 6 coding SNPs in 1,188 individuals from 318 nuclear families, including 554 affected offspring. Quality control measures, transmission-disequilibrium tests (TDTs), and empirical simulations were performed in PLINK. We found a significant overtransmission of the C allele of rs362719 to BP offspring (OR = 1.47, P = 5.9 × 10–4); this withstood empirical correction for testing of multiple markers (empirical P = 0.048). In a hypothesis-driven secondary analysis, we found that the association with rs362719 was almost entirely accounted for by overtransmission of the putative risk allele to affected females (ORFemale = 1.79, P = 8.9 × 10–5 vs. ORMale = 1.12, P = 0.63). These results provide preliminary evidence that genetic variation in RELN is associated with susceptibility to BP and, in particular, to BP in females. However, our findings should be interpreted with caution until further replication and functional assays provide convergent support.
Keywords: genetics, bipolar disorder, schizophrenia, overlap, neurodevelopment
BACKGROUND
The Reelin gene (RELN) encodes an extracellular matrix protein critical in neuronal migration, cortical lamination, and overall brain development [Fatemi, 2005]. Reelin is secreted into the extracellular space, where it binds to one of two receptors (VLDL-receptor and/or the APOE receptor 2), setting off a phosphorylation cascade that, among many targets, leads to the phosphorylation of glycogen synthase kinase 3 (GSK3), a key component of the Wnt pathway [Herz and Chen, 2006]. In the adult brain, Reelin is strongly expressed in GABAergic interneurons of the hippocampus, where it has been found to play an important role in neuroplasticity and long-term potentiation, by modulating and recruiting the glutamatergic NMDA receptor to the post-synaptic cleft [Chen et al., 2005].
How to Cite this Article.
Goes FS, Willour VL, Zandi PP, Belmonte PL, MacKinnon DF, Mondimore FM, Schweizer B, National Institute of Mental Health Genetics Initiative Bipolar Disorder Consortium, DePaulo JR Jr, Gershon ES, McMahon FJ, Potash JB. 2010. Sex-Specific Association of the Reelin Gene With Bipolar Disorder.
Am J Med Genet Part B 153B:549–553.
The RELN gene, which spans 517.7 kb on chromosome 7q22 and contains 65 annotated exons, is a putative candidate for involvement in both schizophrenia and bipolar disorder (BP). The chromosomal region of 7q22 has been implicated in a joint meta-analysis of BP and schizophrenia linkage studies [Badner and Gershon, 2002]. Studies have found lower Reelin mRNA expression and protein levels in GABAergic neurons of patients with schizophrenia and BP [Fatemi et al., 2000; Guidotti et al., 2000; Veldic et al., 2007], which have suggested that lower levels of Reelin expression may be associated with susceptibility to both disorders. In addition, upstream of the RELN promoter sequence is a CpG island that has been observed to be hypermethylated in the post-mortem prefrontal cortex of cases with schizophrenia compared with controls [Grayson et al., 2005], which could explain the reduced expression; notably, pharmacological agents used in the treatment of schizophrenia (clozapine [Dong et al., 2008]) and BP (valproic acid [Dong et al., 2007]) have been shown to reverse this hypermethylation. We note, however, that some recent studies have failed to replicate the observation of RELN hypermethylation in schizophrenia [Tochigi et al., 2008].
Convergent support for the involvement of Reelin in psychotic disorders has also emerged from mechanistic studies in the heterozygous reeler mouse. Although the heterozygous mouse does not have marked anatomical abnormalities of the brain, more subtle behavioral abnormalities such as delayed pre-pulse inhibition (PPI) [Barr et al., 2008] and attenuated methamphetamine-induced hyper-locomotion [Matsuzaki et al., 2007] have been reported. Interestingly, PPI abnormalities have been routinely found in schizophrenia [Greenwood et al., 2007] and, to a more modest degree, in BP [Schulze et al., 2007], while methamphetamine-induced hyper-locomotion is a widely used animal model of mania.
While there have been relatively few genetic association studies of RELN, a recent pooled genome-wide association study (GWAS) of schizophrenia found replicated evidence for association with a marker (rs7341475) in intron 4 of the RELN gene [Shifman et al., 2008]. Both the initial study and the subsequent large replication analysis of 2,274 cases and 4,401 controls found this association to be specific to females (ORgenotype = 1.58, combined P = 8.8 × 10–7), raising the possibility that the association of RELN is modified by sex. However, no subsequent replications have been reported.
In addition to the post-mortem and animal studies described, increasing genetic evidence suggests that schizophrenia and BP may share some aspects of common etiology [Potash, 2006]. In this study, we test the hypothesis that common genetic variation in RELN may be associated with BP, by performing a comprehensive linkage disequilibrium (LD)-based study of RELN in a well-characterized BP sample of 1,194 individuals from 319 nuclear families.
MATERIALS AND METHODS
Subjects
We selected nuclear families with affected offspring from three BP family studies: the Chicago, Hopkins, National Institute of Mental Health (NIMH) Intramural Program study [McInnis et al., 2003]; the Clinical Neurogenetics study [Detera-Wadleigh and McMahon, 2006]; and the NIMH Genetics Initiative Bipolar Disorder Collaborative study [1997]. All subjects signed IRB approved informed consent forms prior to enrolling. Affected offspring (237 quads and 80 trios) were diagnosed with bipolar I disorder (N = 489), schizoaffective disorder, bipolar type (N = 26), and bipolar II disorder (N = 39) using DSM-IV or Research Diagnostic Criteria. They included 335 affected females (60.5%) and 219 affected males (39.5%). Among the 554 affected offspring, 341 (61.6%) had psychotic symptoms, defined as a lifetime history of delusions and/or hallucinations.
Genotyping
Using “LD-select” and Phase I data from HapMap [Carlson et al., 2004], we selected 78 tag single nucleotide polymorphisms (tagSNPs) to cover RELN (517.7 kb) and 10 kb of surrounding sequence with r2 ≥ 0.8 and MAF ≥ 0.1. Genotyping was performed on the Illumina BeadArray platform, which had assays for 75 of 78 tagSNPs, as well as for 6 additional coding SNPs. All genotyped markers were in Hardy–Weinberg equilibrium (HWE) and the average missing data rate across the experiment was 0.04%. To attempt to replicate the recent association of rs7341475 in females with schizophrenia, we also genotyped rs7341475 using an ABI 7900HT and a TaqMan assay. This marker was found to be in HWE and had a missing data rate of 0.5%.
Analysis
TDT analysis was performed in PLINK [Purcell et al., 2007]. Empirical P values were derived by performing 10,000 “gene-dropping” permutations using the “–mperm” command. In a post hoc analysis, stratified TDT analyses were performed according to the sex of the affected offspring. To test the statistical significance of the difference in association by sex, we used an implementation of the TDT in STATA 9.2 with sex as a covariate [Cordell et al., 2004]. We formally tested for differences with and without the sex covariate by fitting a conditional logistic regression model that included a term for the covariate by genotype interaction. Likelihood ratio tests (LRTs) were used to test whether the model including the interaction term provided a better fit to the data than a model without the interaction term.
Power calculations using the Genetic Power Calculator [Purcell et al., 2003] showed that under an additive model and with an assumed disease prevalence of 1%, and a Bonferroni corrected α = 0.0007, our sample had >80% power to detect a risk allele of moderate effect (OR = >1.4) over the common range of disease allele frequencies (0.1–0.5).
RESULTS
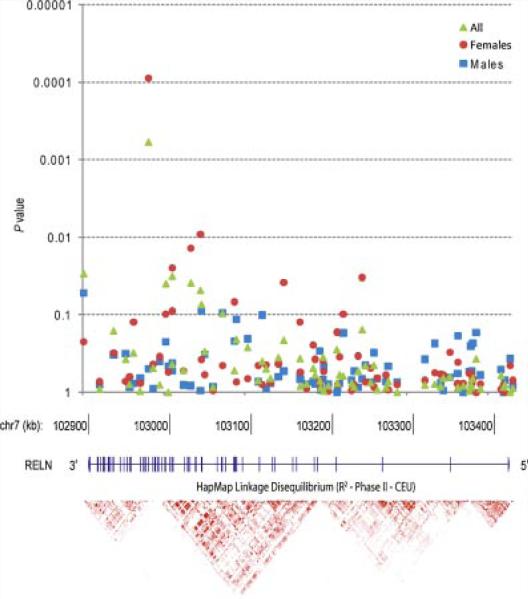
As shown in Table I, single-marker TDT analyses of the BP phenotype revealed six markers with a nominal P < 0.05 (full results are shown in Supplementary Table I). After empirical correction for testing of multiple markers, association remained significant at rs362719 (nominal P = 5.9 × 10–4, empirically corrected P = 0.048), located 86 base pairs from exon 42 of RELN, in a region of low LD (Fig. 1). The common (C) allele was overtransmitted (194 transmitted:132 not transmitted) to affected offspring with an OR of 1.47.
TABLE I.
Nominally Significant TDT Association Findings in the Primary and Sex-Specific Analyses
| All |
Females |
Males |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Marker | Location (build 35) | Transmitted allele (freq) | Transmitted/untransmitted | P-value | OR | Transmitted/untransmitted | P-value | OR | Transmitted/untransmitted | P-value | OR |
| rsl7281921 | 102893131 | G (0.85) | 163:126 | 0.030 | 1.30 (1.02–1.64) | 94:78 | 0.22 | 1.14 (0.89–1.64) | 69:48 | 0.052 | 1.42 (0.99–2.08) |
| rs362719 | 102973112 | C (0.81) | 194:132 | 5.95 × 10–4 | 1.47 (1.18–1.82) | 122:68 | 8.95 × 10–5 | l.79 (1.33–2.44) | 72:64 | 0.49 | 1.12 (0.80–1.59) |
| rs362726 | 102994469 | T (0.60) | 251:207 | 0.040 | 1.22 (1.01–1.45) | 157:129 | 0.098 | 1.22 (0.96–1.54) | 94:78 | 0.22 | 1.20 (0.89–1.64) |
| rs362814 | 103002355 | T (0.27) | 222:179 | 0.032 | 1.24 (1.02–1.51) | 139:104 | 0.025 | 1.34 (1.04–1.72) | 83:75 | 0.52 | 1.11 (0.81–1.51) |
| rs2249372 | 103025445 | A (0.29) | 238:195 | 0.039 | 1.22 (1.01–1.48) | 152:112 | 0.014 | 1.36 (1.06–1.73) | 86:83 | 0.82 | 1.04 (0.77–1.40) |
| rs362642 | 103037106 | T (0.49) | 282:237 | 0.048 | 1.19 (1.00–1.41) | 179:133 | 0.009 | 1.35 (1.08–1.68) | 103:104 | 0.94 | 0.99 (0.75–1.30) |
FIG. 1.
TDT analysis of RELN. The gene and its 65 exons are shown across 517.7 kb on the negative strand of chromosome 7. Above are the 82 SNPs genotyped across the gene with statistical significance of the association on the Y-axis and location on the X-axis. Green triangles show the main analysis with the strongest association at rs362719 (OR = 1.47, nominal P = 0.00059, corrected P = 0.047). Sex-specific associations are shown in red circles (females) and blue squares (males). Below the gene is an illustration of the local linkage disequilibrium using data from the CEPH HapMap parents.
Since a recent GWAS of schizophrenia found a robust association in RELN SNP rs7341475 in affected females [Shifman et al., 2008], we attempted to replicate this association. However, we found no evidence for transmission disequilibrium for rs7341475 in our main sample (P = 0.38), or in our female-stratified sample (P = 0.46). Nevertheless, given that this recently reported association with schizophrenia was specific to females, we performed a secondary TDT analysis across all of our SNPs stratified by sex. As shown in the Table and Figure, this analysis showed that the significant association of rs362719 was almost entirely accounted for by transmission to affected females (ORFemale = 1.79, P = 8.9 × 10–5 vs. ORMale = 1.12, P = 0.63). Evidence for transmission disequilibrium remained significant after empirical permutation to test for multiple markers (P = 0.0073). The difference in ORs was also found to be statistically significant in a LRT (P = 0.037) when a conditional logistic regression model incorporating sex as covariate was compared to a model without the covariate.
Finally, as the initial report of reduced RELN expression levels in BP cases were found in patients with the psychotic form of the illness, we performed an additional family-based association analysis in the 341 affected offspring with psychotic symptoms (delusions and/or hallucinations). Again, the strongest evidence of transmission distortion continued to be in affected females with the rs362719 marker, although the effect size (ORFemale = 1.59) and statistical significance (P = 0.01) were lower than for the full affection phenotype, and no result from this analysis survived empirical correction for multiple testing (Supplementary Table II).
DISCUSSION
This study provides initial evidence, from a family-based study, for association between RELN SNP rs362719 and BP in females from multiply affected pedigrees. Although candidate gene studies of RELN have been performed in schizophrenia and autism, this study represents, to our knowledge, the first focused association study of RELN in BP. Using a carefully characterized sample and a family-based design, we found overtransmission of the derived C allele in the overall sample to affected offspring (OR = 1.47), which was almost entirely accounted for by overtransmission to affected females (OR = 1.79). This finding remained significant after correction for multiple markers.
The Reelin protein is composed of a distinctive N-terminal domain followed by eight tandem repeats, each consisting of 350–390 amino acids. Each repeat comprises an extracellular growth factor (EGF)-like domain surrounded by two subrepeats. rs362719 is located 86 bp upstream of exon 42, which codes for the EGF-like domain of the fifth Reelin repeat [Royaux et al., 1997]. Interestingly, recent in vitro studies have found that both the fifth and sixth Reelin repeats are essential for binding to the two Reelin receptors, ApoER2 and VLDLR [Chameau et al., 2009]. Moreover, addition of the repeat 5–6 fragments was also sufficient to induce phosphorylation of Dab1, an “adapter” molecule responsible for triggering the Reelin intracellular signaling cascade. It is also possible that a potential risk allele is located in the intron itself, perhaps within a regulatory element with sex-specific effects.
Despite its important role in neurodevelopment and plasticity, genetic variation in RELN has only been comprehensively studied by the recent GWASs of BP and schizophrenia, along with a Scandinavian case–control study of schizophrenia [Käahler et al., 2008]. The latter study of 577 cases and 1,136 controls genotyped 71 markers in RELN and found 6 nominal associations (P values of 0.046–0.003) with schizophrenia, although the authors did not report a sex-specific analysis and it is unclear whether rs362719 was assayed. In the BP and schizophrenia GWAS results to date, there has been little support for association in RELN. However, most of these studies have used the Affymetrix platform which does not assay rs362719. As shown in Figure 1, rs362719 lies in a region of low LD that is poorly imputed; indeed, even with all genotypes of the HapMap phases I–III, the most closely linked SNP to rs362719 is insufficiently correlated (r2 = 0.44) to allow imputation with high confidence. To our knowledge, only one published GWAS of BP or schizophrenia has assayed this SNP [Need et al., 2009]. This schizophrenia case–control study found no overall association with rs362719, but did not report sex-specific analyses for this marker.
Notably, our female-specific finding with RELN parallels the results from a recent schizophrenia GWAS [Shifman et al., 2008], albeit with a different marker. Although sex-specific associations may be vulnerable to higher rates of type I error [Patsopoulos et al., 2007], our analyses were hypothesis driven and within the context of several converging lines of evidence suggesting a sex-specific effect for Reelin. For example, rat studies have provided evidence that the female hormone progesterone strongly upregulates the expression of Reelin in peripheral nerves [Roglio et al., 2008]. In addition, studies of the heterozygous reeler mouse have found significant cell loss in the cerebellum of males, with no evident effect in females [Hadj-Sahraoui et al., 1996]. The heterozygous reeler mouse was also found to have diminished expression of the oxytocin receptor [Liu et al., 2005], which is key to one of the most well characterized sexually dimorphic signaling pathways in the brain [de Vries, 2008]. While such mechanistic data are not available in humans, a meta-analysis of 12 post-mortem brain expression studies found RELN levels to be moderately, but significantly, lower in females compared with males (–1.10 change, P-value = 1.5 × 10–4), as well as lower in BP cases compared with controls (–1.11 fold change; P-value = 0.03), however, data on diagnosis-sex interaction is not available [Stanley Medical Research Institute Online Genomics Database, 2009]. We further note that there have been several prior sex-specific genetic association findings in BP including those in GPR50 [Thomson et al., 2005]. Such findings might reflect genetic correlates of well-known clinical variation in illness patterns between the sexes, such as those associated with the female reproductive cycle [Payne et al., 2007].
The strengths of our study include the rigorous characterization of subjects using the DIGS, a well validated diagnostic instrument. Our study also benefits from a family-based design that is robust to spurious findings from population stratification. This is of particular concern for markers like rs362719, which show marked allele frequency differences between populations. However, one limitation of our family-based sample is that we had suboptimal power to detect alleles of small effect (OR < 1.4). A second limitation is that, because the tagSNP selection was based on Phase I of the HapMap project, we did not cover the common variation in RELN as comprehensively as a tagging approach based on the more recent Phase II version of HapMap would have. Third, our sex-specific analyses were exploratory and require confirmation in additional samples.
Supplementary Material
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry. 2002;7:405–411. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- Barr AM, Fish KN, Markou A, Honer WG. Heterozygous reeler mice exhibit alterations in sensorimotor gating but not presynaptic proteins. Eur J Neurosci. 2008;27:2568–2574. doi: 10.1111/j.1460-9568.2008.06233.x. [DOI] [PubMed] [Google Scholar]
- Carlson CS, Eberle MA, Rieder MJ, Yi Q, Kruglyak L, Nickerson DA. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet. 2004;74:106–120. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chameau P, Inta D, Vitalis T, Monyer H, Wadman WJ, van Hooft JA. The N-terminal region of reelin regulates postnatal dendritic maturation of cortical pyramidal neurons. Proc Natl Acad Sci USA. 2009;106:7227–7232. doi: 10.1073/pnas.0810764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Beffert U, Ertunc M, Tang TS, Kavalali ET, Bezprozvanny I, Herz J. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci. 2005;25:8209–8216. doi: 10.1523/JNEUROSCI.1951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell HJ, Barratt BJ, Clayton DG. Case/pseudocontrol analysis in genetic association studies: A unified framework for detection of genotype and haplotype associations, gene-gene and gene-environment interactions, and parent-of-origin effects. Genet Epidemiol. 2004;26:167–185. doi: 10.1002/gepi.10307. [DOI] [PubMed] [Google Scholar]
- de Vries GJ. Sex differences in vasopressin and oxytocin innervation of the brain. Prog Brain Res. 2008;170:17–27. doi: 10.1016/S0079-6123(08)00402-0. [DOI] [PubMed] [Google Scholar]
- Detera-Wadleigh SD, McMahon FJ. G72/G30 in schizophrenia and bipolar disorder: Review and meta-analysis. Biol Psychiatry. 2006;60:106–114. doi: 10.1016/j.biopsych.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Dong E, Guidotti A, Grayson DR, Costa E. Histone hyperacetylation induces demethylation of reelin and 67-kDa glutamic acid decarboxylase promoters. Proc Natl Acad Sci USA. 2007;104:4676–4681. doi: 10.1073/pnas.0700529104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Nelson M, Grayson DR, Costa E, Guidotti A. Clozapine and sulpiride but not haloperidol or olanzapine activate brain DNA demethylation. Proc Natl Acad Sci USA. 2008;105:13614–13619. doi: 10.1073/pnas.0805493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH. Reelin glycoprotein: Structure, biology and roles in health and disease. Mol Psychiatry. 2005;10:251–257. doi: 10.1038/sj.mp.4001613. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle JA, McMenomy T. Reduction in reelin immunore-activity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry. 2000;5:654–663. 571. doi: 10.1038/sj.mp.4000783. [DOI] [PubMed] [Google Scholar]
- Genomic survey of bipolar Genomic survey of bipolar illness in the NIMH genetics initiative pedigrees: A preliminary report. Am J Med Genet. 1997;74:227–237. doi: 10.1002/(sici)1096-8628(19970531)74:3<227::aid-ajmg1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Grayson DR, Jia X, Chen Y, Sharma RP, Mitchell CP, Guidotti A, Costa E. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci USA. 2005;102:9341–9346. doi: 10.1073/pnas.0503736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Mintz J, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Schork NJ. Initial heritability analyses of endophenotypic measures for schizophrenia: The consortium on the genetics of schizophrenia. Arch Gen Psychiatry. 2007;64:1242–1250. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: A postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Hadj-Sahraoui N, Frederic F, Delhaye-Bouchaud N, Mariani J. Gender effect on purkinje cell loss in the cerebellum of the heterozygous reeler mouse. J Neurogenet. 1996;11:45–58. doi: 10.3109/01677069609107062. [DOI] [PubMed] [Google Scholar]
- Herz J, Chen Y. Reelin, lipoprotein receptors and synaptic plasticity. Nat Rev Neurosci. 2006;7:850–859. doi: 10.1038/nrn2009. [DOI] [PubMed] [Google Scholar]
- Käahler AK, Djurovic S, Kulle B, Jönsson EG, Agartz I, Hall H, Opjordsmoen S, Jakobsen KD, Hansen T, Melle I, Werge T, Steen VM, Andreassen OA. Association analysis of schizophrenia on 18 genes involved in neuronal migration: MDGA1 as a new susceptibility gene. Am J Med Genet Part B. 2008;147B(7):1089–1100. doi: 10.1002/ajmg.b.30726. [DOI] [PubMed] [Google Scholar]
- Liu W, Pappas GD, Carter CS. Oxytocin receptors in brain cortical regions are reduced in haploinsufficient (+/–) reeler mice. Neurol Res. 2005;27:339–345. doi: 10.1179/016164105X35602. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Minabe Y, Nakamura K, Suzuki K, Iwata Y, Sekine Y, Tsuchiya KJ, Sugihara G, Suda S, Takei N, Nakahara D, Hashimoto K, Nairn AC, Mori N, Sato K. Disruption of reelin signaling attenuates methamphetamine-induced hyperlocomotion. Eur J Neurosci. 2007;25:3376–3384. doi: 10.1111/j.1460-9568.2007.05564.x. [DOI] [PubMed] [Google Scholar]
- McInnis MG, Lan TH, Willour VL, McMahon FJ, Simpson SG, Addington AM, MacKinnon DF, Potash JB, Mahoney AT, Chellis J, Huo Y, Swift-Scanlan T, Chen H, Koskela R, Stine OC, Jamison KR, Holmans P, Folstein SE, Ranade K, Friddle C, Botstein D, Marr T, Beaty TH, Zandi P, DePaulo JR. Genome-wide scan of bipolar disorder in 65 pedigrees: Supportive evidence for linkage at 8q24, 18q22, 4q32, 2p12, and 13q12. Mol Psychiatry. 2003;8:288–298. doi: 10.1038/sj.mp.4001277. [DOI] [PubMed] [Google Scholar]
- Need AC, Ge D, Weale ME, Maia J, Feng S, Heinzen EL, Shianna KV, Yoon W, Kasperaviciute D, Gennarelli M, Strittmatter WJ, Bonvicini C, Rossi G, Jayathilake K, Cola PA, McEvoy JP, Keefe RS, Fisher EM, St Jean PL, Giegling I, Hartmann AM, Moller HJ, Ruppert A, Fraser G, Crombie C, Middleton LT, St Clair D, Roses AD, Muglia P, Francks C, Rujescu D, Meltzer HY, Goldstein DB. A genome-wide investigation of SNPs and CNVs in schizophrenia. PLoS Genet. 2009;5:e1000373. doi: 10.1371/journal.pgen.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsopoulos NA, Tatsioni A, Ioannidis JP. Claims of sex differences: An empirical assessment in genetic associations. J Am Med Assoc. 2007;298:880–893. doi: 10.1001/jama.298.8.880. [DOI] [PubMed] [Google Scholar]
- Payne JL, Roy PS, Murphy-Eberenz K, Weismann MM, Swartz KL, McInnis MG, Nwulia E, Mondimore FM, MacKinnon DF, Miller EB, Nurnberger JI, Levinson DF, DePaulo JR, Jr, Potash JB. Reproductive cycle-associated mood symptoms in women with major depression and bipolar disorder. J Affect Disord. 2007;99:221–229. doi: 10.1016/j.jad.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Purcell S. Carving chaos: Genetics and the classification of mood and psychotic syndromes. Harv Rev Psychiatry. 2006;14:47–63. doi: 10.1080/10673220600655780. [DOI] [PubMed] [Google Scholar]
- Purcell S, Cherny SS, Sham PC. Genetic power calculator: Design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–150. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roglio I, Bianchi R, Gotti S, Scurati S, Giatti S, Pesaresi M, Caruso D, Panzica GC, Melcangi RC. Neuroprotective effects of dihydroprogesterone and progesterone in an experimental model of nerve crush injury. Neuroscience. 2008;155:673–685. doi: 10.1016/j.neuroscience.2008.06.034. [DOI] [PubMed] [Google Scholar]
- Royaux I, Lambert de Rouvroit C, D'Arcangelo G, Demirov D, Goffinet AM. Genomic organization of the mouse reelin gene. Genomics. 1997;46:240–250. doi: 10.1006/geno.1997.4983. [DOI] [PubMed] [Google Scholar]
- Schulze KK, Hall MH, McDonald C, Marshall N, Walshe M, Murray RM, Bramon E. P50 auditory evoked potential suppression in bipolar disorder patients with psychotic features and their unaffected relatives. Biol Psychiatry. 2007;62:121–128. doi: 10.1016/j.biopsych.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Shifman S, Johannesson M, Bronstein M, Chen SX, Collier DA, Craddock NJ, Kendler KS, Li T, O'Donovan M, O'Neill FA, Owen MJ, Walsh D, Weinberger DR, Sun C, Flint J, Darvasi A. Genome-wide association identifies a common variant in the reelin gene that increases the risk of schizophrenia only in women. PLoS Genet. 2008;4:e28. doi: 10.1371/journal.pgen.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley Medical Research Institute Online Genomics Database [03-01-09];2009 ( www.stanleygenomic.org)
- Thomson PA, Wray NR, Millar JK, Evans KL, Hellard SL, Condie A, Muir WJ, Blacwood DH, Porteous DJ. Association between the TRAX/DISC locus and both bipolar disorder and schizophrenia in the Scottish population. Mol Psychiatry. 2005;10:657–658. 616. doi: 10.1038/sj.mp.4001669. [DOI] [PubMed] [Google Scholar]
- Tochigi M, Iwamoto K, Bundo M, Komori A, Sasaki T, Kato N, Kato T. Methylation status of the reelin promoter region in the brain of schizophrenic patients. Biol Psychiatry. 2008;63:530–553. doi: 10.1016/j.biopsych.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Veldic M, Kadriu B, Maloku E, Agis-Balboa RC, Guidotti A, Davis JM, Costa E. Epigenetic mechanisms expressed in basal ganglia GABAergic neurons differentiate schizophrenia from bipolar disorder. Schizophr Res. 2007;91:51–61. doi: 10.1016/j.schres.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.