Abstract
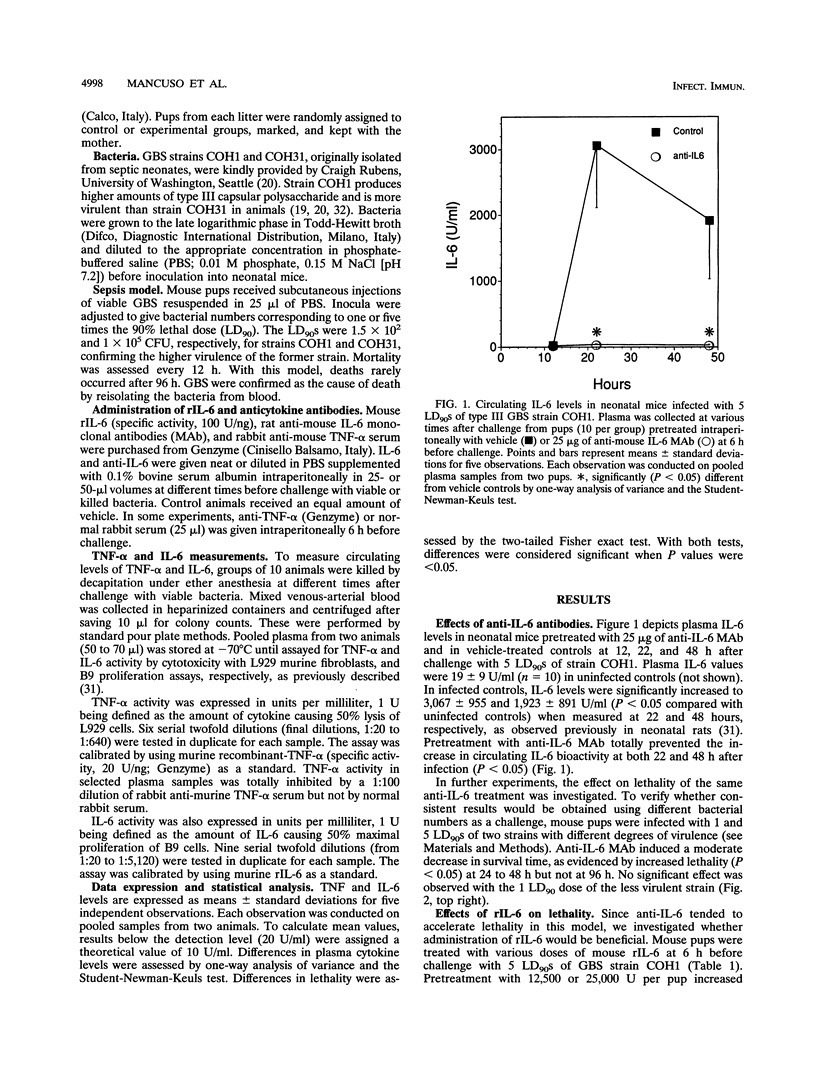
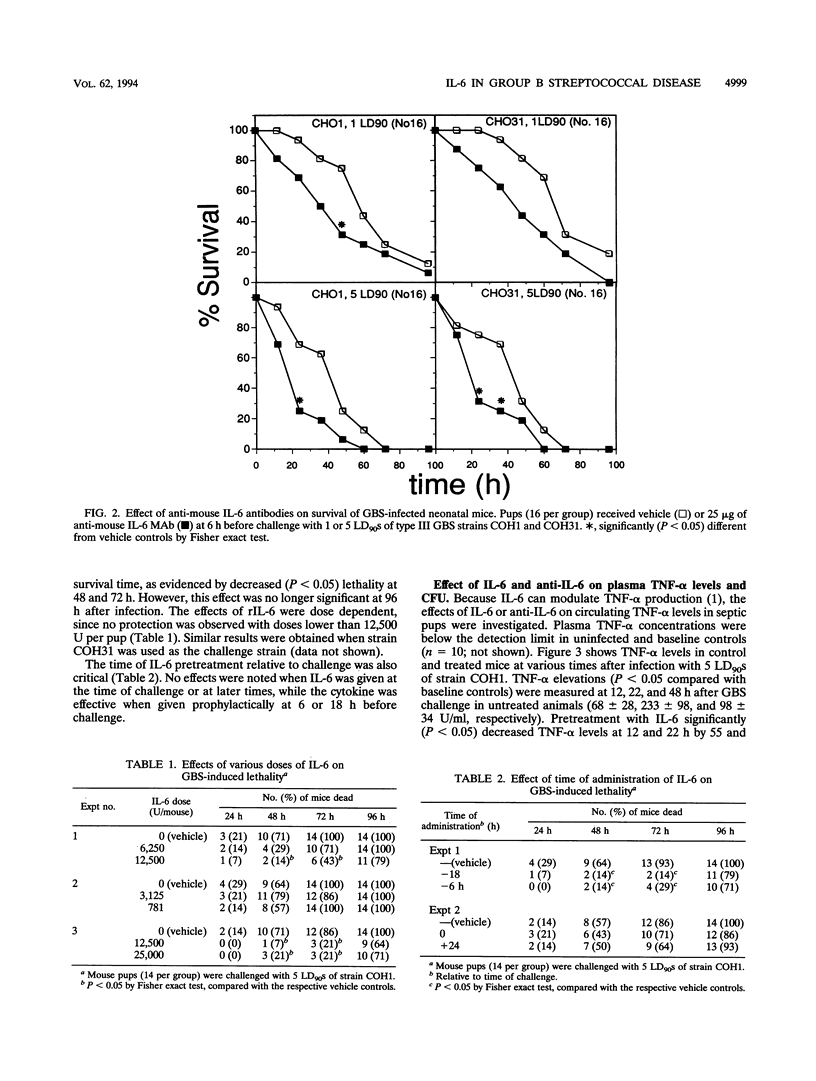
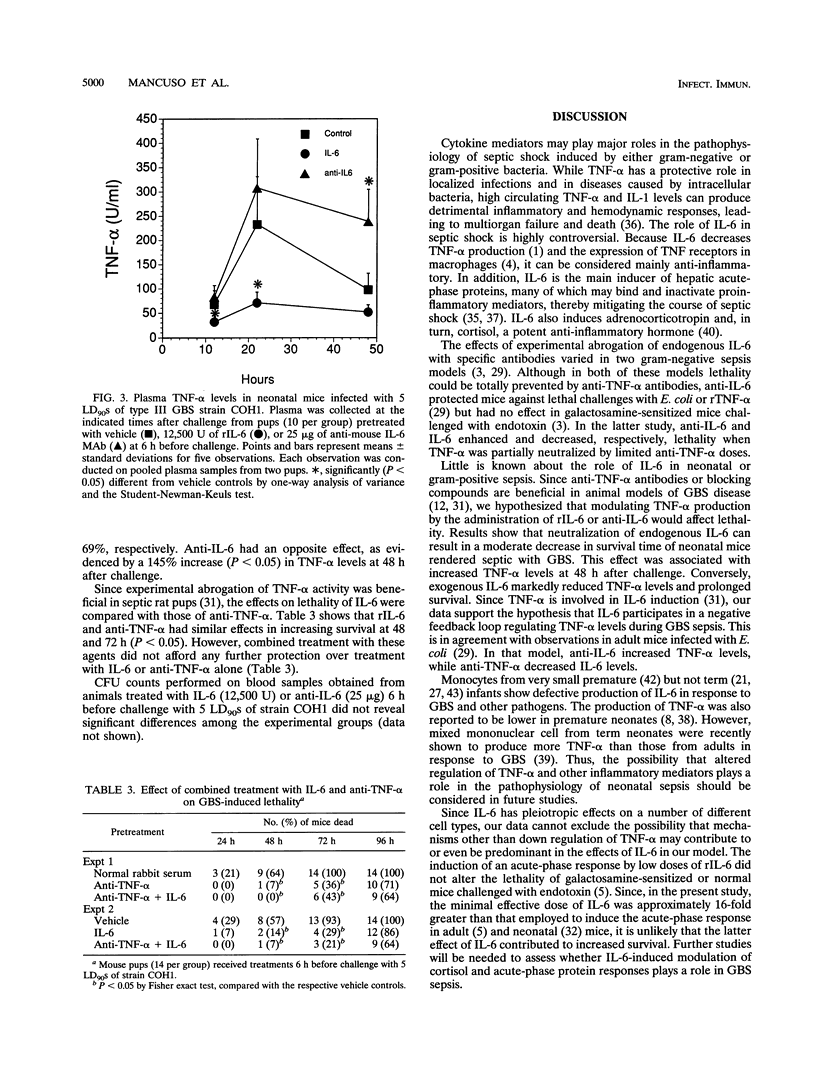
Previous studies have shown that tumor necrosis factor alpha (TNF-alpha) plays a pathophysiologic role in sepsis induced in rat pups by group B streptococci (GBS). In this model, TNF-alpha is also partially responsible for the induction of interleukin-6 (IL-6). The present study was undertaken to investigate the role of IL-6 in neonatal BALB/c mice infected with type III GBS. The effect of anti-IL-6 monoclonal antibodies and recombinant IL-6 on lethality and TNF-alpha production was investigated. In mouse pups infected with GBS strain COH1, plasma IL-6 reached levels of 3,067 +/- 955 and 1,923 +/- 891 U/ml when measured at 22 and 48 h, respectively (P < 0.05 compared with uninfected controls). Pretreatment with 25 micrograms of anti-IL-6 antibodies totally prevented the increase in circulating IL-6 bioactivity at both 22 and 48 h after infection (P < 0.05). Treatment with anti-IL-6 also induced a moderate decrease in survival time of mice infected with lethal doses of strains COH1 and COH31, as evidenced by increased lethality (P < 0.05) at 24 to 48 h but not at 96 h. Mouse recombinant IL-6 (12,500 U) given 6 h before challenge with strains COH1 and COH31 consistently increased survival time, as evidenced by decreased (P < 0.05) lethality at 48 to 72 h but not at 96 h. The effects of IL-6 pretreatment were dose dependent, since no protection was observed with doses lower than 12,500 U. In addition, no effects on lethality were noted when IL-6 was given at the time of challenge or at later times. TNF-alpha elevations (P < 0.05 compared with uninfected controls) were measured at 12, 22, and 48 h after challenge with strain COH1 (68 +/- 28, 233 +/- 98, and 98 +/- 34 U, respectively). Pretreatment with IL-6 significantly (P < 0.05) decreased plasma TNF-alpha levels at 12 and 22 h, with 55 and 69% inhibitions, respectively. Anti-IL-6 had an opposite effect, as evidenced by a 145% increase (P < 0.05) in TNF-alpha levels at 48 h after challenge. Collectively, our data are compatible with the hypothesis that IL-6 is involved in negative feedback regulation of plasma TNF-alpha levels in experimental GBS sepsis. In this model, IL-6 pretreatment can increase survival time. Future studies will be needed to investigate the mechanisms underlying this effect.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Le J. M., Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989 Dec 1;143(11):3517–3523. [PubMed] [Google Scholar]
- Aiura K., Gelfand J. A., Burke J. F., Thompson R. C., Dinarello C. A. Interleukin-1 (IL-1) receptor antagonist prevents Staphylococcus epidermidis-induced hypotension and reduces circulating levels of tumor necrosis factor and IL-1 beta in rabbits. Infect Immun. 1993 Aug;61(8):3342–3350. doi: 10.1128/iai.61.8.3342-3350.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton B. E., Jackson J. V. Protective role of interleukin 6 in the lipopolysaccharide-galactosamine septic shock model. Infect Immun. 1993 Apr;61(4):1496–1499. doi: 10.1128/iai.61.4.1496-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Wu M., Petrofsky M., Young L. S. Interleukin-6 antagonizes tumor necrosis factor-mediated mycobacteriostatic and mycobactericidal activities in macrophages. Infect Immun. 1992 Oct;60(10):4245–4252. doi: 10.1128/iai.60.10.4245-4252.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucklin S. E., Silverstein R., Morrison D. C. An interleukin-6-induced acute-phase response does not confer protection against lipopolysaccharide lethality. Infect Immun. 1993 Aug;61(8):3184–3189. doi: 10.1128/iai.61.8.3184-3189.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caracciolo D., Clark S. C., Rovera G. Human interleukin-6 supports granulocytic differentiation of hematopoietic progenitor cells and acts synergistically with GM-CSF. Blood. 1989 Feb 15;73(3):666–670. [PubMed] [Google Scholar]
- English B. K., Burchett S. K., English J. D., Ammann A. J., Wara D. W., Wilson C. B. Production of lymphotoxin and tumor necrosis factor by human neonatal mononuclear cells. Pediatr Res. 1988 Dec;24(6):717–722. doi: 10.1203/00006450-198812000-00014. [DOI] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Tatter S. B., Clarick R. H., Santhanam U., Sherris D., May L. T., Sehgal P. B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989 Apr 1;142(7):2321–2324. [PubMed] [Google Scholar]
- Freudenberg M. A., Galanos C. Tumor necrosis factor alpha mediates lethal activity of killed gram-negative and gram-positive bacteria in D-galactosamine-treated mice. Infect Immun. 1991 Jun;59(6):2110–2115. doi: 10.1128/iai.59.6.2110-2115.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson R. L., Redding G. J., Henderson W. R., Truog W. E. Group B streptococcus induces tumor necrosis factor in neonatal piglets. Effect of the tumor necrosis factor inhibitor pentoxifylline on hemodynamics and gas exchange. Am Rev Respir Dis. 1991 Mar;143(3):598–604. doi: 10.1164/ajrccm/143.3.598. [DOI] [PubMed] [Google Scholar]
- Girardin E., Grau G. E., Dayer J. M., Roux-Lombard P., Lambert P. H. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1988 Aug 18;319(7):397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
- Hack C. E., De Groot E. R., Felt-Bersma R. J., Nuijens J. H., Strack Van Schijndel R. J., Eerenberg-Belmer A. J., Thijs L. G., Aarden L. A. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989 Oct;74(5):1704–1710. [PubMed] [Google Scholar]
- Havell E. A., Sehgal P. B. Tumor necrosis factor-independent IL-6 production during murine listeriosis. J Immunol. 1991 Jan 15;146(2):756–761. [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Houssiau F. A., Coulie P. G., Olive D., Van Snick J. Synergistic activation of human T cells by interleukin 1 and interleukin 6. Eur J Immunol. 1988 Apr;18(4):653–656. doi: 10.1002/eji.1830180427. [DOI] [PubMed] [Google Scholar]
- Liu Z., Simpson R. J., Cheers C. Recombinant interleukin-6 protects mice against experimental bacterial infection. Infect Immun. 1992 Oct;60(10):4402–4406. doi: 10.1128/iai.60.10.4402-4406.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso G., Tomasello F., von Hunolstein C., Orefici G., Teti G. Induction of tumor necrosis factor alpha by the group- and type-specific polysaccharides from type III group B streptococci. Infect Immun. 1994 Jul;62(7):2748–2753. doi: 10.1128/iai.62.7.2748-2753.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques M. B., Kasper D. L., Pangburn M. K., Wessels M. R. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Infect Immun. 1992 Oct;60(10):3986–3993. doi: 10.1128/iai.60.10.3986-3993.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki N., Saji F., Kameda T., Yoshizaki K., Okada T., Sawai K., Tanizawa O. In vitro and in vivo production of interleukin-6 by fetal mononuclear cells. Clin Immunol Immunopathol. 1990 May;55(2):305–314. doi: 10.1016/0090-1229(90)90106-z. [DOI] [PubMed] [Google Scholar]
- Miller L. C., Isa S., LoPreste G., Schaller J. G., Dinarello C. A. Neonatal interleukin-1 beta, interleukin-6, and tumor necrosis factor: cord blood levels and cellular production. J Pediatr. 1990 Dec;117(6):961–965. doi: 10.1016/s0022-3476(05)80145-3. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Endotoxins and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- Morrone G., Ciliberto G., Oliviero S., Arcone R., Dente L., Content J., Cortese R. Recombinant interleukin 6 regulates the transcriptional activation of a set of human acute phase genes. J Biol Chem. 1988 Sep 5;263(25):12554–12558. [PubMed] [Google Scholar]
- Ohlsson K., Björk P., Bergenfeldt M., Hageman R., Thompson R. C. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature. 1990 Dec 6;348(6301):550–552. doi: 10.1038/348550a0. [DOI] [PubMed] [Google Scholar]
- Rodewald A. K., Onderdonk A. B., Warren H. B., Kasper D. L. Neonatal mouse model of group B streptococcal infection. J Infect Dis. 1992 Sep;166(3):635–639. doi: 10.1093/infdis/166.3.635. [DOI] [PubMed] [Google Scholar]
- Saito S., Saito M., Kato Y., Maruyama M., Moriyama I., Ichijo M. Production of IL-6 (BSF-2/IFN beta 2) by mononuclear cells in premature and term infants. J Reprod Immunol. 1990 Mar;17(1):17–26. doi: 10.1016/0165-0378(90)90036-6. [DOI] [PubMed] [Google Scholar]
- Saunders B. M., Liu Z., Zhan Y., Cheers C. Interleukin-6 production during chronic experimental infection. Immunol Cell Biol. 1993 Aug;71(Pt 4):275–280. doi: 10.1038/icb.1993.32. [DOI] [PubMed] [Google Scholar]
- Starnes H. F., Jr, Pearce M. K., Tewari A., Yim J. H., Zou J. C., Abrams J. S. Anti-IL-6 monoclonal antibodies protect against lethal Escherichia coli infection and lethal tumor necrosis factor-alpha challenge in mice. J Immunol. 1990 Dec 15;145(12):4185–4191. [PubMed] [Google Scholar]
- Takai Y., Wong G. G., Clark S. C., Burakoff S. J., Herrmann S. H. B cell stimulatory factor-2 is involved in the differentiation of cytotoxic T lymphocytes. J Immunol. 1988 Jan 15;140(2):508–512. [PubMed] [Google Scholar]
- Teti G., Mancuso G., Tomasello F. Cytokine appearance and effects of anti-tumor necrosis factor alpha antibodies in a neonatal rat model of group B streptococcal infection. Infect Immun. 1993 Jan;61(1):227–235. doi: 10.1128/iai.61.1.227-235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Chedid L. A. Strategies for the treatment of endotoxemia: significance of the acute-phase response. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S630–S638. doi: 10.1093/clinids/9.supplement_5.s630. [DOI] [PubMed] [Google Scholar]
- Weatherstone K. B., Rich E. A. Tumor necrosis factor/cachectin and interleukin-1 secretion by cord blood monocytes from premature and term neonates. Pediatr Res. 1989 Apr;25(4):342–346. doi: 10.1203/00006450-198904000-00006. [DOI] [PubMed] [Google Scholar]
- Williams P. A., Bohnsack J. F., Augustine N. H., Drummond W. K., Rubens C. E., Hill H. R. Production of tumor necrosis factor by human cells in vitro and in vivo, induced by group B streptococci. J Pediatr. 1993 Aug;123(2):292–300. doi: 10.1016/s0022-3476(05)81706-8. [DOI] [PubMed] [Google Scholar]
- Woloski B. M., Smith E. M., Meyer W. J., 3rd, Fuller G. M., Blalock J. E. Corticotropin-releasing activity of monokines. Science. 1985 Nov 29;230(4729):1035–1037. doi: 10.1126/science.2997929. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Clark S. C. Multiple actions of interleukin 6 within a cytokine network. Immunol Today. 1988 May;9(5):137–139. doi: 10.1016/0167-5699(88)91200-5. [DOI] [PubMed] [Google Scholar]
- Yachie A., Takano N., Ohta K., Uehara T., Fujita S., Miyawaki T., Taniguchi N. Defective production of interleukin-6 in very small premature infants in response to bacterial pathogens. Infect Immun. 1992 Mar;60(3):749–753. doi: 10.1128/iai.60.3.749-753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachie A., Takano N., Yokoi T., Kato K., Kasahara Y., Miyawaki T., Taniguchi N. The capability of neonatal leukocytes to produce IL-6 on stimulation assessed by whole blood culture. Pediatr Res. 1990 Mar;27(3):227–233. doi: 10.1203/00006450-199003000-00005. [DOI] [PubMed] [Google Scholar]
- de Bont E. S., Martens A., van Raan J., Samson G., Fetter W. P., Okken A., de Leij L. H. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 plasma levels in neonatal sepsis. Pediatr Res. 1993 Apr;33(4 Pt 1):380–383. doi: 10.1203/00006450-199304000-00013. [DOI] [PubMed] [Google Scholar]
- van Vugt H., van Gool J., de Ridder L. Alpha 2 macroglobulin of the rat, an acute phase protein, mitigates the early course of endotoxin shock. Br J Exp Pathol. 1986 Jun;67(3):313–319. [PMC free article] [PubMed] [Google Scholar]



