Abstract
Filamentous hemagglutinin (FHA) is a major adhesin present on the surface of the gram-negative respiratory pathogen Bordetella pertussis. A number of binding mechanisms have been described for the interaction of FHA with eukaryotic cells. We have focused on its function as a sulfated polysaccharide-binding protein and on identifying potential receptors for FHA on the epithelial cell surface. Using a thin-layer overlay technique, we found that FHA binds specifically to sulfated glycolipids but not to gangliosides or other neutral glycolipids. These results suggest that epithelial cell surface sulfated glycolipids function as receptors for FHA. Further studies demonstrated that a Chinese hamster ovary (CHO) cell strain deficient in glycosaminoglycan expression exhibits greatly diminished attachment to FHA. By FHA-Affi-Gel chromatography, a putative receptor for FHA that has characteristics consistent with a heparan sulfate proteoglycan was isolated from epithelial cell extracts. In addition, by using recombinant FHA fusion proteins, a specific glycosaminoglycan-binding domain located near the N terminus of the FHA molecule was identified. Our results indicate that the B. pertussis adhesin FHA may utilize sulfated glycolipids and proteoglycans commonly found on the surface of human cells and tissues to initiate infection.
Full text
PDF


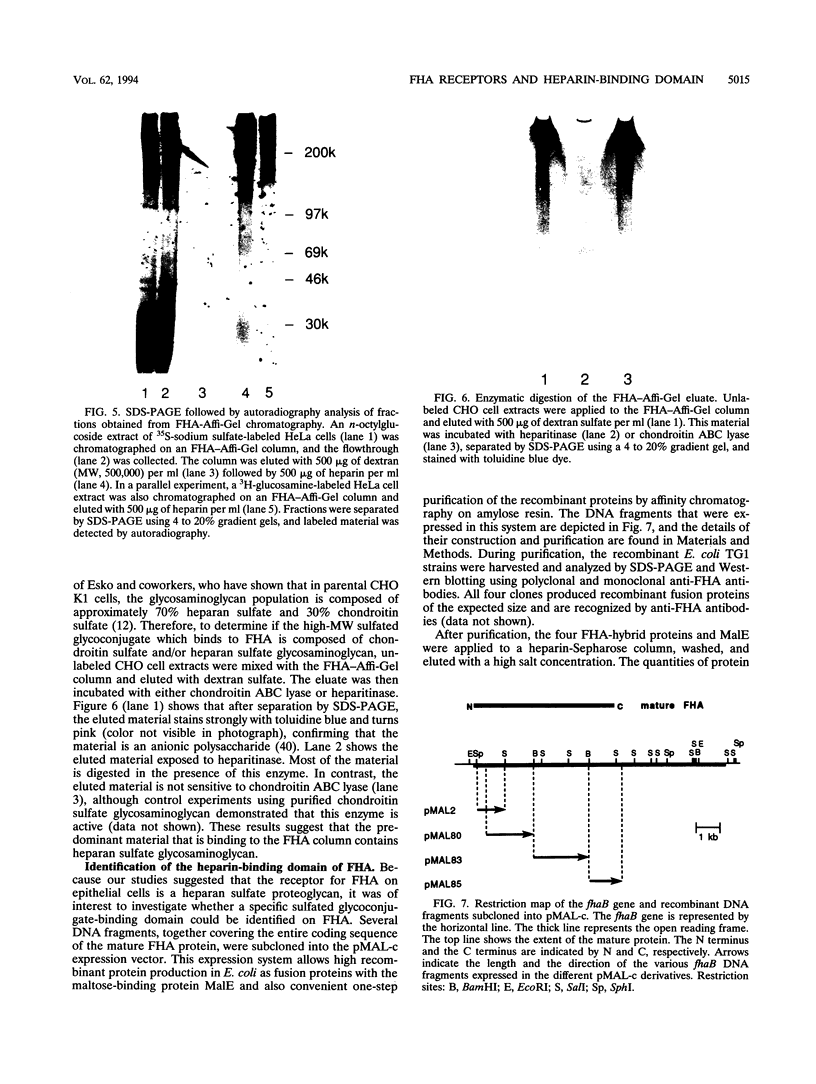
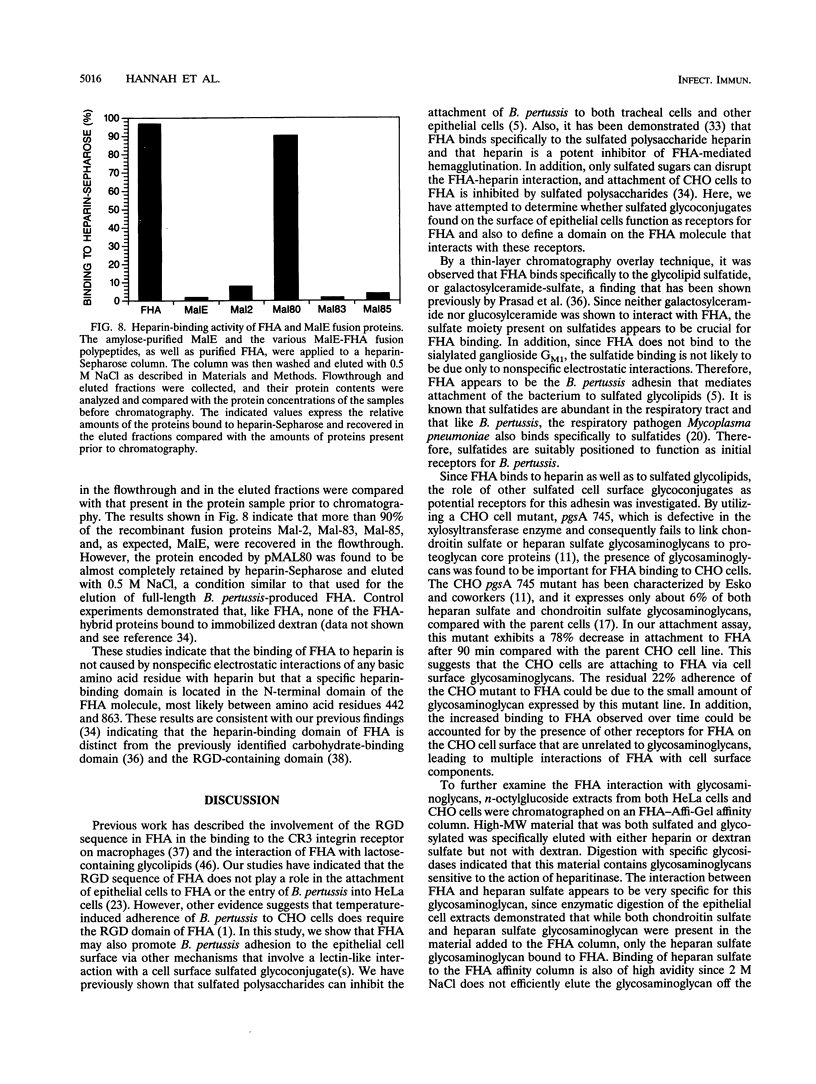



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aricò B., Nuti S., Scarlato V., Rappuoli R. Adhesion of Bordetella pertussis to eukaryotic cells requires a time-dependent export and maturation of filamentous hemagglutinin. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9204–9208. doi: 10.1073/pnas.90.19.9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascencio F., Fransson L. A., Wadström T. Affinity of the gastric pathogen Helicobacter pylori for the N-sulphated glycosaminoglycan heparan sulphate. J Med Microbiol. 1993 Apr;38(4):240–244. doi: 10.1099/00222615-38-4-240. [DOI] [PubMed] [Google Scholar]
- Bergey E. J., Stinson M. W. Heparin-inhibitable basement membrane-binding protein of Streptococcus pyogenes. Infect Immun. 1988 Jul;56(7):1715–1721. doi: 10.1128/iai.56.7.1715-1721.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brennan M. J., Hannah J. H., Leininger E. Adhesion of Bordetella pertussis to sulfatides and to the GalNAc beta 4Gal sequence found in glycosphingolipids. J Biol Chem. 1991 Oct 5;266(28):18827–18831. [PubMed] [Google Scholar]
- Brennan M. J., Li Z. M., Cowell J. L., Bisher M. E., Steven A. C., Novotny P., Manclark C. R. Identification of a 69-kilodalton nonfimbrial protein as an agglutinogen of Bordetella pertussis. Infect Immun. 1988 Dec;56(12):3189–3195. doi: 10.1128/iai.56.12.3189-3195.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. H., Stinson M. W. Purification of a Streptococcus mutans protein that binds to heart tissue and glycosaminoglycans. Infect Immun. 1989 Dec;57(12):3834–3840. doi: 10.1128/iai.57.12.3834-3840.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisse-Gathoye A. M., Locht C., Jacob F., Raaschou-Nielsen M., Heron I., Ruelle J. L., de Wilde M., Cabezon T. Cloning, partial sequence, expression, and antigenic analysis of the filamentous hemagglutinin gene of Bordetella pertussis. Infect Immun. 1990 Sep;58(9):2895–2905. doi: 10.1128/iai.58.9.2895-2905.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenighini M., Relman D., Capiau C., Falkow S., Prugnola A., Scarlato V., Rappuoli R. Genetic characterization of Bordetella pertussis filamentous haemagglutinin: a protein processed from an unusually large precursor. Mol Microbiol. 1990 May;4(5):787–800. doi: 10.1111/j.1365-2958.1990.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Esko J. D. Genetic analysis of proteoglycan structure, function and metabolism. Curr Opin Cell Biol. 1991 Oct;3(5):805–816. doi: 10.1016/0955-0674(91)90054-3. [DOI] [PubMed] [Google Scholar]
- Esko J. D., Stewart T. E., Taylor W. H. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci U S A. 1985 May;82(10):3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko J. D., Weinke J. L., Taylor W. H., Ekborg G., Rodén L., Anantharamaiah G., Gawish A. Inhibition of chondroitin and heparan sulfate biosynthesis in Chinese hamster ovary cell mutants defective in galactosyltransferase I. J Biol Chem. 1987 Sep 5;262(25):12189–12195. [PubMed] [Google Scholar]
- Ewanowich C. A., Melton A. R., Weiss A. A., Sherburne R. K., Peppler M. S. Invasion of HeLa 229 cells by virulent Bordetella pertussis. Infect Immun. 1989 Sep;57(9):2698–2704. doi: 10.1128/iai.57.9.2698-2704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hök M., Kjellén L., Johansson S. Cell-surface glycosaminoglycans. Annu Rev Biochem. 1984;53:847–869. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- Kaesberg P. R., Ershler W. B., Esko J. D., Mosher D. F. Chinese hamster ovary cell adhesion to human platelet thrombospondin is dependent on cell surface heparan sulfate proteoglycan. J Clin Invest. 1989 Mar;83(3):994–1001. doi: 10.1172/JCI113986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Mountzouros K. T., Relman D. A., Falkow S., Cowell J. L. Bordetella pertussis filamentous hemagglutinin: evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infect Immun. 1990 Jan;58(1):7–16. doi: 10.1128/iai.58.1.7-16.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Olson L. D., Barile M. F., Ginsburg V., Roberts D. D. Adhesion of Mycoplasma pneumoniae to sulfated glycolipids and inhibition by dextran sulfate. J Biol Chem. 1989 Jun 5;264(16):9283–9288. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leininger E., Ewanowich C. A., Bhargava A., Peppler M. S., Kenimer J. G., Brennan M. J. Comparative roles of the Arg-Gly-Asp sequence present in the Bordetella pertussis adhesins pertactin and filamentous hemagglutinin. Infect Immun. 1992 Jun;60(6):2380–2385. doi: 10.1128/iai.60.6.2380-2385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leininger E., Probst P. G., Brennan M. J., Kenimer J. G. Inhibition of Bordetella pertussis filamentous hemagglutinin-mediated cell adherence with monoclonal antibodies. FEMS Microbiol Lett. 1993 Jan 1;106(1):31–38. doi: 10.1111/j.1574-6968.1993.tb05931.x. [DOI] [PubMed] [Google Scholar]
- Leininger E., Roberts M., Kenimer J. G., Charles I. G., Fairweather N., Novotny P., Brennan M. J. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):345–349. doi: 10.1073/pnas.88.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang O. D., Ascencio F., Fransson L. A., Wadström T. Binding of heparan sulfate to Staphylococcus aureus. Infect Immun. 1992 Mar;60(3):899–906. doi: 10.1128/iai.60.3.899-906.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C., Bertin P., Menozzi F. D., Renauld G. The filamentous haemagglutinin, a multifaceted adhesion produced by virulent Bordetella spp. Mol Microbiol. 1993 Aug;9(4):653–660. doi: 10.1111/j.1365-2958.1993.tb01725.x. [DOI] [PubMed] [Google Scholar]
- Locht C., Geoffroy M. C., Renauld G. Common accessory genes for the Bordetella pertussis filamentous hemagglutinin and fimbriae share sequence similarities with the papC and papD gene families. EMBO J. 1992 Sep;11(9):3175–3183. doi: 10.1002/j.1460-2075.1992.tb05394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycke E., Johansson M., Svennerholm B., Lindahl U. Binding of herpes simplex virus to cellular heparan sulphate, an initial step in the adsorption process. J Gen Virol. 1991 May;72(Pt 5):1131–1137. doi: 10.1099/0022-1317-72-5-1131. [DOI] [PubMed] [Google Scholar]
- Makhov A. M., Hannah J. H., Brennan M. J., Trus B. L., Kocsis E., Conway J. F., Wingfield P. T., Simon M. N., Steven A. C. Filamentous hemagglutinin of Bordetella pertussis. A bacterial adhesin formed as a 50-nm monomeric rigid rod based on a 19-residue repeat motif rich in beta strands and turns. J Mol Biol. 1994 Aug 5;241(1):110–124. doi: 10.1006/jmbi.1994.1478. [DOI] [PubMed] [Google Scholar]
- Mawhinney T. P., Adelstein E., Morris D. A., Mawhinney A. M., Barbero G. J. Structure determination of five sulfated oligosaccharides derived from tracheobronchial mucus glycoproteins. J Biol Chem. 1987 Mar 5;262(7):2994–3001. [PubMed] [Google Scholar]
- McGavin M. J., Raucci G., Gurusiddappa S., Hök M. Fibronectin binding determinants of the Staphylococcus aureus fibronectin receptor. J Biol Chem. 1991 May 5;266(13):8343–8347. [PubMed] [Google Scholar]
- Menozzi F. D., Gantiez C., Locht C. Interaction of the Bordetella pertussis filamentous hemagglutinin with heparin. FEMS Microbiol Lett. 1991 Feb;62(1):59–64. doi: 10.1111/j.1574-6968.1991.tb04417.x. [DOI] [PubMed] [Google Scholar]
- Menozzi F. D., Mutombo R., Renauld G., Gantiez C., Hannah J. H., Leininger E., Brennan M. J., Locht C. Heparin-inhibitable lectin activity of the filamentous hemagglutinin adhesin of Bordetella pertussis. Infect Immun. 1994 Mar;62(3):769–778. doi: 10.1128/iai.62.3.769-778.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Barria E., Pereira M. E. A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell. 1991 Oct 18;67(2):411–421. doi: 10.1016/0092-8674(91)90192-2. [DOI] [PubMed] [Google Scholar]
- Prasad S. M., Yin Y., Rodzinski E., Tuomanen E. I., Masure H. R. Identification of a carbohydrate recognition domain in filamentous hemagglutinin from Bordetella pertussis. Infect Immun. 1993 Jul;61(7):2780–2785. doi: 10.1128/iai.61.7.2780-2785.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D. A., Domenighini M., Tuomanen E., Rappuoli R., Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D., Tuomanen E., Falkow S., Golenbock D. T., Saukkonen K., Wright S. D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990 Jun 29;61(7):1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- Roberts D. D., Ginsburg V. Sulfated glycolipids and cell adhesion. Arch Biochem Biophys. 1988 Dec;267(2):405–415. doi: 10.1016/0003-9861(88)90046-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. C., Choi H. U., Tang L. H., Johnson T. L., Pal S., Webber C., Reiner A., Poole A. R. Isolation of dermatan sulfate proteoglycans from mature bovine articular cartilages. J Biol Chem. 1985 May 25;260(10):6304–6313. [PubMed] [Google Scholar]
- SVENNERHOLM L. THE GANGLIOSIDES. J Lipid Res. 1964 Apr;5:145–155. [PubMed] [Google Scholar]
- Sharon N. Bacterial lectins, cell-cell recognition and infectious disease. FEBS Lett. 1987 Jun 15;217(2):145–157. doi: 10.1016/0014-5793(87)80654-3. [DOI] [PubMed] [Google Scholar]
- Sobel M., Soler D. F., Kermode J. C., Harris R. B. Localization and characterization of a heparin binding domain peptide of human von Willebrand factor. J Biol Chem. 1992 May 5;267(13):8857–8862. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Towbin H., Rosenfelder G., Braun D., Larson G., Hansson G. C., Hill R. Receptor analogs and monoclonal antibodies that inhibit adherence of Bordetella pertussis to human ciliated respiratory epithelial cells. J Exp Med. 1988 Jul 1;168(1):267–277. doi: 10.1084/jem.168.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Weiss A. Characterization of two adhesins of Bordetella pertussis for human ciliated respiratory-epithelial cells. J Infect Dis. 1985 Jul;152(1):118–125. doi: 10.1093/infdis/152.1.118. [DOI] [PubMed] [Google Scholar]
- Urisu A., Cowell J. L., Manclark C. R. Filamentous hemagglutinin has a major role in mediating adherence of Bordetella pertussis to human WiDr cells. Infect Immun. 1986 Jun;52(3):695–701. doi: 10.1128/iai.52.3.695-701.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems R. J., Geuijen C., van der Heide H. G., Renauld G., Bertin P., van den Akker W. M., Locht C., Mooi F. R. Mutational analysis of the Bordetella pertussis fim/fha gene cluster: identification of a gene with sequence similarities to haemolysin accessory genes involved in export of FHA. Mol Microbiol. 1994 Jan;11(2):337–347. doi: 10.1111/j.1365-2958.1994.tb00314.x. [DOI] [PubMed] [Google Scholar]
- WuDunn D., Spear P. G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989 Jan;63(1):52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]
- Yanagishita M., Hascall V. C. Cell surface heparan sulfate proteoglycans. J Biol Chem. 1992 May 15;267(14):9451–9454. [PubMed] [Google Scholar]
- Zhang J. P., Stephens R. S. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell. 1992 May 29;69(5):861–869. doi: 10.1016/0092-8674(92)90296-o. [DOI] [PubMed] [Google Scholar]
- di Guan C., Li P., Riggs P. D., Inouye H. Vectors that facilitate the expression and purification of foreign peptides in Escherichia coli by fusion to maltose-binding protein. Gene. 1988 Jul 15;67(1):21–30. doi: 10.1016/0378-1119(88)90004-2. [DOI] [PubMed] [Google Scholar]