Abstract
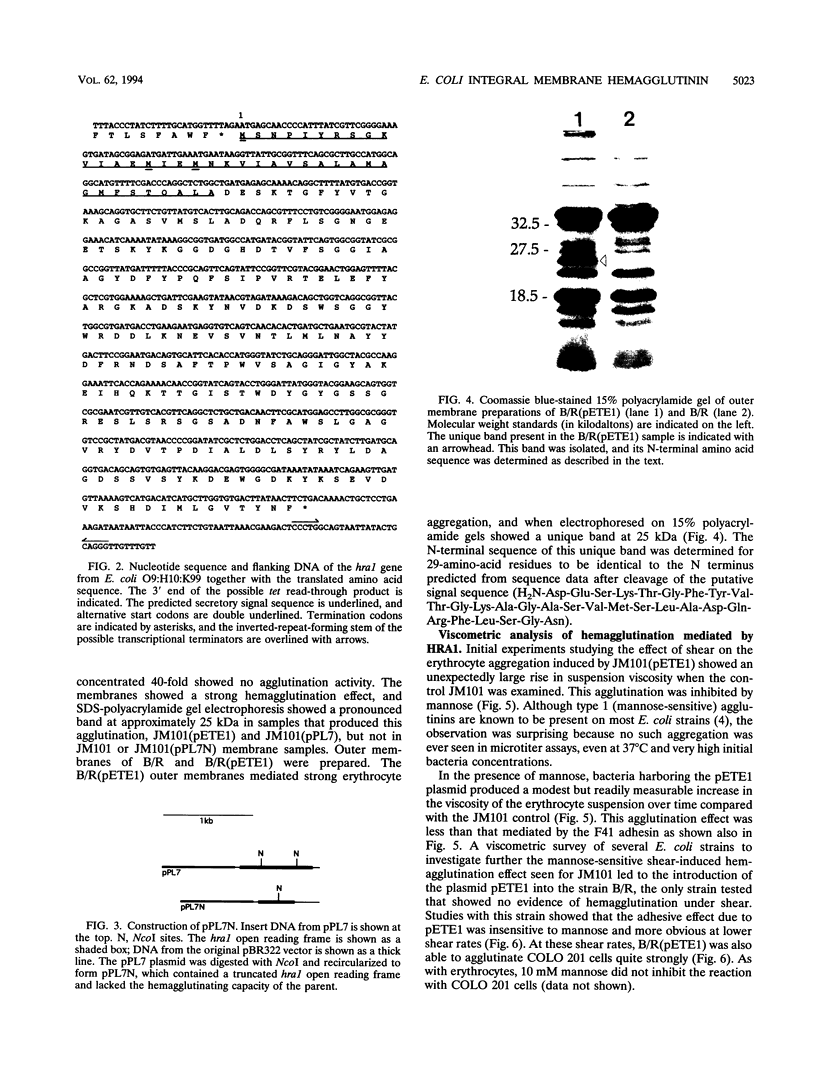
The gene encoding a mannose-resistant hemagglutinating protein was cloned from Escherichia coli O9:H10:K99. The hemagglutinin is different from two other mannose-resistant hemagglutinins in this strain, K99 and F41. The agglutinin, named heat-resistant agglutinin 1 (HRA1) since heating to 70 degrees C does not destroy its aggregative properties, strongly agglutinates human, pig, and dog erythrocytes, shows little or no affinity towards cow and chicken erythrocytes, but agglutinates human colon adenocarcinoma 201 (COLO 201) cells. The hra1 gene present on the recombinant plasmid pETE1 was localized by subcloning, and its nucleotide sequence was determined. The gene consists of a 792-bp open reading frame coding for a putative protein of 29 kDa with a predicted N-terminal secretory signal sequence. HRA1 shares no significant identity with data base protein sequences. HRA1 is strongly associated with the bacterial membrane, resisting sonication and isolation attempts based upon standard adhesin purification techniques. N-terminal sequencing of a unique 25-kDa band present in polyacrylamide gels of outer membrane preparations of bacteria harboring pETE1 correlated with the predicted N-terminal amino acid sequence of HRA1 after cleavage of the signal peptide. A viscometric agglutination assay sensitive to the strength of bacterial adhesion shows that the agglutination mediated by bacteria expressing HRA1 is weaker than that of bacteria bearing the F41 adhesin, probably because of the high-molecular-weight, multivalent nature of the latter adhesin. Our observations suggest that HRA1 is a monomeric outer membrane agglutinin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brooks D. E., Cavanagh J., Jayroe D., Janzen J., Snoek R., Trust T. J. Involvement of the MN blood group antigen in shear-enhanced hemagglutination induced by the Escherichia coli F41 adhesin. Infect Immun. 1989 Feb;57(2):377–383. doi: 10.1128/iai.57.2.377-383.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D. E., Trust T. J. Interactions of erythrocytes with bacteria under shear. Ann N Y Acad Sci. 1983;416:319–331. doi: 10.1111/j.1749-6632.1983.tb35196.x. [DOI] [PubMed] [Google Scholar]
- Cassidy J. T., Jourdian G. W., Roseman S. The sialic acids. VI. Purification and properties of sialidase from Clostridium perfringens. J Biol Chem. 1965 Sep;240(9):3501–3506. [PubMed] [Google Scholar]
- DUGUID J. P., SMITH I. W., DEMPSTER G., EDMUNDS P. N. Non-flagellar filamentous appendages (fimbriae) and haemagglutinating activity in Bacterium coli. J Pathol Bacteriol. 1955 Oct;70(2):335–348. doi: 10.1002/path.1700700210. [DOI] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Eshdat Y., Speth V., Jann K. Participation of pili and cell wall adhesion in the yeast agglutination activity of Escherichia coli. Infect Immun. 1981 Dec;34(3):980–986. doi: 10.1128/iai.34.3.980-986.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Goldhar J., Perry R., Golecki J. R., Hoschutzky H., Jann B., Jann K. Nonfimbrial, mannose-resistant adhesins from uropathogenic Escherichia coli O83:K1:H4 and O14:K?:H11. Infect Immun. 1987 Aug;55(8):1837–1842. doi: 10.1128/iai.55.8.1837-1842.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith H. L., Takamura K., Bell D. Shear-induced collisions between human blood cells. Ann N Y Acad Sci. 1983;416:299–318. doi: 10.1111/j.1749-6632.1983.tb35195.x. [DOI] [PubMed] [Google Scholar]
- Guinée P. A., Veldkamp J., Jansen W. H. Improved minca medium for the detection of K99 antigen in calf enterotoxigenic strains of Escherichia coli. Infect Immun. 1977 Feb;15(2):676–678. doi: 10.1128/iai.15.2.676-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfman D. M., Hughes S. H. Use of antibodies to screen cDNA expression libraries prepared in plasmid vectors. Methods Enzymol. 1987;152:451–457. doi: 10.1016/0076-6879(87)52053-5. [DOI] [PubMed] [Google Scholar]
- Isaacson R. E. K99 surface antigen of Escherichia coli: antigenic characterization. Infect Immun. 1978 Nov;22(2):555–559. doi: 10.1128/iai.22.2.555-559.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. A., Venema J., Leeven R., van Pelt-Heerschap H., de Graaf F. K. Inhibition of adhesive activity of K88 fibrillae by peptides derived from the K88 adhesin. J Bacteriol. 1987 Feb;169(2):735–741. doi: 10.1128/jb.169.2.735-741.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. A., van den Berg P. A., Bak H. J., de Graaf F. K. Localization of lysine residues in the binding domain of the K99 fibrillar subunit of enterotoxigenic Escherichia coli. Biochim Biophys Acta. 1986 Jul 25;872(1-2):92–97. doi: 10.1016/0167-4838(86)90151-2. [DOI] [PubMed] [Google Scholar]
- Jones C. H., Jacob-Dubuisson F., Dodson K., Kuehn M., Slonim L., Striker R., Hultgren S. J. Adhesin presentation in bacteria requires molecular chaperones and ushers. Infect Immun. 1992 Nov;60(11):4445–4451. doi: 10.1128/iai.60.11.4445-4451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Klose M., Schwarz H., MacIntyre S., Freudl R., Eschbach M. L., Henning U. Internal deletions in the gene for an Escherichia coli outer membrane protein define an area possibly important for recognition of the outer membrane by this polypeptide. J Biol Chem. 1988 Sep 15;263(26):13291–13296. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Miller H. Practical aspects of preparing phage and plasmid DNA: growth, maintenance, and storage of bacteria and bacteriophage. Methods Enzymol. 1987;152:145–170. doi: 10.1016/0076-6879(87)52016-x. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane. DNA sequence of the osmoregulated ompC gene. J Biol Chem. 1983 Jun 10;258(11):6932–6940. [PubMed] [Google Scholar]
- Nowicki B., Svanborg-Edén C., Hull R., Hull S. Molecular analysis and epidemiology of the Dr hemagglutinin of uropathogenic Escherichia coli. Infect Immun. 1989 Feb;57(2):446–451. doi: 10.1128/iai.57.2.446-451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Birch-Andersen A., Duguid J. P., Stenderup J., Orskov F. An adhesive protein capsule of Escherichia coli. Infect Immun. 1985 Jan;47(1):191–200. doi: 10.1128/iai.47.1.191-200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoll T., Hoschützky H., Morschhäuser J., Lottspeich F., Jann K., Hacker J. Analysis of genes coding for the sialic acid-binding adhesin and two other minor fimbrial subunits of the S-fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1989 Dec;3(12):1735–1744. doi: 10.1111/j.1365-2958.1989.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Semple T. U., Quinn L. A., Woods L. K., Moore G. E. Tumor and lymphoid cell lines from a patient with carcinoma of the colon for a cytotoxicity model. Cancer Res. 1978 May;38(5):1345–1355. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H., Knutton S., Brown M. G., Candy D. C., McNeish A. S. Characterization of nonfimbrial mannose-resistant protein hemagglutinins of two Escherichia coli strains isolated from infants with enteritis. Infect Immun. 1984 Jun;44(3):592–598. doi: 10.1128/iai.44.3.592-598.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Roorda I. Production, purification, and characterization of the fimbrial adhesive antigen F41 isolated from calf enteropathogenic Escherichia coli strain B41M. Infect Immun. 1982 May;36(2):751–758. doi: 10.1128/iai.36.2.751-758.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zijderveld F. G., Westenbrink F., Anakotta J., Brouwers R. A., van Zijderveld A. M. Characterization of the F41 fimbrial antigen of enterotoxigenic Escherichia coli by using monoclonal antibodies. Infect Immun. 1989 Apr;57(4):1192–1199. doi: 10.1128/iai.57.4.1192-1199.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



